Abstract
Background and purpose: Gabapentin (GBP; 1-(aminomethyl)cyclohexane acetic acid) is used clinically in the treatment of pain. Nevertheless, the sites and mechanisms of action of GBP are poorly defined. Herein, the effects of GBP on brain activation have been studied.
Experimental approach: Changes in blood oxygen level dependent (BOLD) haemodynamic signal following intravenous infusion of GBP (equivalent to 30 mg kg−1 p.o., followed by 100 mg kg−1 p.o.), compared to saline control, were studied in isofluorane anaesthetized rats (n=8 per group). Effects of GBP on mean arterial blood pressure (MAP) were also recorded.
Results: Random effect analysis revealed that the lower dose of GBP produced significant (P<0.001) increases in BOLD signal intensity in several brain regions, including the thalamus and periaqueductal grey (PAG), compared to basal. This dose of GBP also produced significant (P<0.001) decreases in BOLD signal intensity in the amygdala and the entorhinal cortex. Increasing the dose of GBP (100 mg kg−1) produced significantly greater changes in BOLD signal intensity in several brain regions including the thalamus and PAG. MAP was not significantly altered by GBP, compared to saline.
Conclusions and implications: GBP had marked positive and negative effects on BOLD signal intensity in a number of brain regions in naïve rats. The activation of key areas involved in nociceptive processing indicate a supraspinal site of action of GBP and this may contribute to its well-described analgesic effects in animal models of pain and clinical studies.
Keywords: gabapentin, fMRI, periaqueductal grey, thalamus, BOLD, SPM99
Introduction
Gabapentin (GBP; 1-(aminomethyl)cyclohexane acetic acid) is a novel analgesic drug, which was originally developed as an anticonvulsant. GBP has little, or no, effect in models of acute nociception (Hunter et al., 1997; Stanfa et al., 1997; Jun and Yaksh, 1998; Eckhardt et al., 2000). However, GBP does significantly attenuate hyperalgesia (Jones and Sorkin, 1998; Jun and Yaksh, 1998) and allodynia (Hwang and Yaksh, 1997) in models of neuropathy (Chapman et al., 1998; Field et al., 2000; Fox et al., 2003; Lynch et al., 2004; Joshi et al., 2006; Coderre et al., 2007; Ling et al., 2007; Xiao et al., 2007). Clinically, GBP provides pain relief in a number of chronic pain states, including neuropathic pain (Backonja et al., 1998; Rowbotham et al., 1998; Rice and Maton, 2001; Hempenstall et al., 2005; Iannetti et al., 2005; Attal et al., 2006).
Gabapentin binds to the auxiliary α2δ subunit of voltage-sensitive calcium channels (Gee et al., 1996; for review see Dooley et al., 2007), in particular to the α2δ1 and α2δ2 subunits (Marais et al., 2001; Klugbauer et al., 2003). These subunits are expressed in dorsal root ganglia neurons as well as in several regions of the rat brain, including CA1 (field 1 of the hippocampus), subiculum and in regions involved in processing nociceptive information, including the thalamus, PAG and amygdala and the superficial laminae of the dorsal spinal cord (Cole et al., 2005). N-type calcium channels play an important role in the increased central excitability following nerve injury (Yamamoto and Sakashita, 1998; Matthews and Dickenson, 2001) and the α2δ1 subunit is upregulated in the dorsal root ganglia and spinal cord in models of neuropathic pain (Luo et al., 2002; Li et al., 2004). Thus, the ability of GBP to modulate α2δ subunit of voltage-sensitive calcium channels may be the basis for the described antinociceptive effects of GBP in models of persistent, but not acute, pain. L-type Ca2+ channel blockers, such as verapamil or nimodipidine, are unable to attenuate the inhibitory effects of GBP in models of pain, supporting a role of N-type Ca2+ channels in its effects (Cheng et al., 2006). α2δ subunits are, thus, likely to be important sites of action underlying the analgesic effects of GBP, although other mechanisms have also been proposed (Chizh et al., 2000; Shimoyama et al., 2000).
The importance of supraspinal sites mediating the effects of GBP is unclear, but given the distribution of the α2δ subunit in the brain it is likely that GBP modulates activity in these areas. Pharmacological functional magnetic resonance imaging (fMRI) is an ideal non-invasive tool that can be used to determine the effects of drugs on brain activation. A recent fMRI study in humans demonstrated complex effects of GBP on brain activation, with the most pronounced effect being a reduction in stimulus-induced brain deactivation following central sensitization (Iannetti et al., 2005). As the effects of GBP per se on brain activation are unknown, the aim of the present study was to use fMRI to investigate the effects of GBP on blood oxygen level dependent (BOLD) signal intensity, a correlate of neuronal activity, in anaesthetized rats.
Materials and methods
Animal preparation
Animal care and procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986. A total of 16 Sprague–Dawley rats (180–200 g; Charles River Laboratories, Margate, Kent, UK) were housed, three per cage with food and water ad libitum on a 12 h:12 h light–dark schedule, for at least 3 days prior to scanning, to allow for acclimatization.
Animals were anaesthetized with isoflurane (3% induction, 1.5–2% surgery and 1.5% maintenance) in nitrous oxide (0.6 l min−1 induction, 0.4 l min−1 maintenance) and oxygen (0.4 l min−1 induction, 0.2 l min−1 maintenance). Once under anaesthesia, core temperature was recorded with a rectal probe and maintained between 37 and 38 °C. Respiration rate was monitored and maintained around 85 breaths min−1 throughout the scanning period. Rats were implanted with three cannulae for the measurement of blood gases, blood pressure and administration of saline with or without drugs. Blood samples were taken from the femoral artery and arterial blood pH, pO2 and pCO2 were analysed (Radiometer ABL 700 series) after the completion of surgery and at the end of the scanning period. Normal values were determined by plotting results on an acid–base chart (Siggaard-Andersen, 1971). A cannula was inserted into the right femoral artery to record blood pressure (ADInstruments, Oxfordshire, UK) throughout the study. GBP (n=8) or vehicle (n=8) was administered to the animal via a third cannula inserted into the right femoral vein (0.2 ml h−1). Effects of drug treatment on mean arterial pressure (MAP; Chartware software) and rate of respiration (Excel software) were recorded during the study.
fMRI data acquisition
Experiments used a 2.35-T horizontal bore superconducting magnet (40 cm internal diameter) Bruker Biospec Avance MR System equipped with an actively shielded gradient set (200 mT m−1; Bruker, Karlsruhe, Germany). Each animal was placed on a custom-made cradle containing a water system to help maintain core body temperature, and ear bars to secure the head. fMRI signals were received via an electronically decoupled surface coil (Bruker) fixed over the dorsal surface of the animals' head.
Sequential NMR images were acquired using the rapid acquisition relaxation-enhanced sequence (Hennig et al., 1986) from a radio frequency excitation delivered through a birdcage resonator coil (72 mm internal diameter). Sufficient exploratory anatomical images (flip angle 90°; repetition time=5112.5 ms; echo time=62.7 ms; number of excitations=8; field of view=50 mm; acquisition matrix=256 × 256; slice width=1 mm; number of slices=30), using a volume set of high resolution (0.2 mm × 0.2 mm × 1 mm), of cross-sectional slices were performed to obtain optimal brain position prior to functional scans.
Functional scans, to assess whole-brain BOLD responses from the systemic infusion of GBP versus saline, were performed using continuous rapid acquisition relaxation-enhanced spin-echo fMRI sequence images (flip angle 90°; repetition time=4356.0 ms; echo time=62.7 ms; number of excitations=8; field of view=50 mm; acquisition matrix=64 × 64; slice width=1 mm; number of slices=30; in-plane resolution of 0.8 mm × 0.8 mm × 1.0 mm). Scans consisted of 12 baseline volume sets, followed by 48 experimental volume sets during which GBP or saline was administered. Each volume set took approximately 4 min 40 s.
Anatomical and functional data were stored using Paravision 1.4 software (1996; Bruker Medizintechnik GmbH) operating on an XWIN-NMR Silicon Graphics console.
GBP infusions
Effects by GBP on BOLD responses were studied using a two-stepped i.v. infusion: 30 mg kg−1 p.o. (calculated as 65 μM plasma equivalence, referred to as infusion 1) and 100 mg kg−1 p.o. (calculated as 117 μM plasma equivalence, referred to as infusion 2) over a 4-h period (Figure 1). Doses of GBP were selected on the basis of previous studies demonstrating inhibitory effects of these GBP doses in models of pain (Hunter et al., 1997; Field et al., 1999; Hulsebosch et al., 2000; Feng et al., 2003). At the end of the scanning session, a 1 ml blood sample was taken and spun at 7000 r.p.m. for a minute to isolate the plasma, which was frozen (−70 °C) and the systemic concentration of GBP was measured (in house at Pfizer, Sandwich, UK). GBP plasma concentration at the end of the scanning protocol (4 h after the start of the infusion paradigm, n=8) was reported back as 105±24 μM, thus in the range of the equivalence target value (117 μM).
Figure 1.
GBP experimental paradigm: scanning session consisted of first acquiring the anatomical template, then acquisition of 12 baseline volumes immediately followed by two-stepped GBP treatment functional scans over a 4-h period, each taking roughly 2 h. Arrows denote time points at which the concentration of GBP was given according to the concentration depicted: both infusions 1 or 2 consisted of an initial higher dose to achieve a steady-state concentration followed by a lower dose for maintenance. Anat, anatomical; GBP, gabapentin; min, minutes.
Data processing
Processing and analysis of the NMR data were performed using SPM99 (statistical parametric mapping) software (Institute of Neurology, London, UK) and in accordance with previously described methods (Shah et al., 2004, 2005; Moylan Governo et al., 2006). All volume data acquired from each study were motion corrected to a middle volume, to correct for motion artefacts, prior to being masked (Analyze software, version 1.0; Biomedical Imaging Resource, Rochester, MN, USA), the process to remove all non-nervous tissue. During motion correction, any scans exhibiting movements greater than one-third of a voxel (translation) or over 1° (rotation) were discarded. For display purposes of BOLD activity, functional images were co-registered to the corresponding anatomical scans acquired in each experiment to ensure that each structure was overlaid in the same space. Subsequently, the whole group was normalized to an anatomical template, constructed from an average from all 16 anatomical scans according to previously described methods (Shah et al., 2004; Moylan Governo et al., 2006), and Gaussian kernel smoothed (full width at half maximum 1.2 mm, SPM99 software, Institute of Neurology; Worsley and Friston, 1995) resulting in an in-plane resolution of 1.2 mm × 1.2 mm.
First-level contrasts were obtained for each animal by selecting the general linear formulation in SPM99 and modelling the haemodynamic response into a box-car pattern, comparing either greater or smaller differences in signal intensity between the volumes from infusion 1 or 2 periods versus baseline, or infusion 2 versus infusion 1. Group analysis was then carried out by modelling a design matrix that subtracted the contrasts of the saline group from the GBP group, while looking at positive or negative changes in BOLD signal from the three different scanning periods. Resulting images were displayed following a voxel by voxel t-test that was Bonferroni corrected for multiple comparisons and with the probability threshold set at P<0.05. Fixed-effect analysis considers within-subject variance, thus data are averaged across a group.
Activated pixels were subsequently taken to second-order analysis using a random-effects analysis, with probability threshold set at P<0.001. Both fixed and random-effect analyses followed previously described methods (Shah et al., 2004; Dixon et al., 2005; Moylan Governo et al., 2006). The method considers not only within subject variance but also differences between subjects, thus allowing data to be generalized to the population from which the group sample was extracted. Statistically significant changes were defined as t values and Z-score and visualized using a colour-scale gradient. Regional identification was achieved by overlaying the image on a standard stereotaxic atlas (Paxinos and Watson, 1998). The positional accuracy of the localization was estimated at ±1.2 mm according to voxel size and filter width used in this process.
Time-course data are presented as average±s.e.mean and analysis of the effects of GBP was obtained from the coordinates taken from some of the brain regions shown in Figures 3 and 4. These were obtained from group fixed-effect analysis over the course of the scanning period, to demonstrate the temporal profile of GBP/saline infusions. Data were collected by extracting the first eigenvariate from the voxel of interest and exported to a spreadsheet. Percentage changes in BOLD signal intensity for each volume were, in turn, obtained by subtracting the group average for the respective volume by the group average of all baseline values and the result was divided by the group average of all baseline values. Effects between each dose of GBP, versus baseline, or within treatment groups were compared using two-way ANOVA, using the averages taken from the 12 baseline volumes and the 12 volumes from infusion 1 or 2. In addition, infusions 1 and 2 of GBP were compared to baseline using one-way ANOVA followed by Dunnett's multiple comparison post-test. Levels of significance between GBP and corresponding vehicle for each volume were analysed using Student's unpaired t-test. Finally, effects of GBP on respiration rate or MAP were analysed using one-way ANOVA; the averages of the volumes from the time periods described above were compared.
Materials
Gabapentin was kindly supplied by Pfizer and dissolved in saline before use.
Results
Physiological monitoring and the effects of GBP infusion
Blood gas values were within the normal range and were of similar magnitude to those obtained in a previous study in isoflurane-anaesthetized rats (Mackensen et al., 2001). No significant changes in pH, pCO2 or pO2 were recorded between samples taken for analysis at the start and the end of the study (Table 1).
Table 1.
Blood pH, pCO2 and pO2 measured from blood samples taken at the start and the end of each scanning session
| pH | pCO2 (mm Hg) | pO2 (mm Hg) | |
|---|---|---|---|
| Start of scan | 7.41±0.01 | 46.92±3.5 | 156±5.75 |
| End of scan | 7.39±0.01 | 45.65±0.5 | 187.67±5.25 |
Data are presented as average±s.e.mean for the sample group (n=6, taken from the gabapentin (GBP)-injected group).
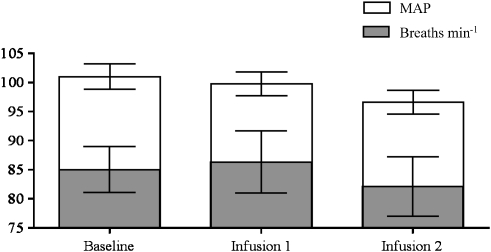
Mean arterial pressure and rate of respiration were monitored throughout each scanning session. Statistical analysis showed that GBP had no significant effect on breathing rates (P=0.8239) or MAP (P=0.3148) (Figure 2). Similarly, infusion of saline had no significant effect on these two measures throughout the study (data not shown).
Figure 2.
Physiological monitoring during the treatment paradigm. There were no significant differences between parameters for the three scanning periods (one-way ANOVA). Data are presented as averages and vertical lines represent s.e.mean.
Effects of GBP on BOLD responses
The design matrix was constructed so that effects of infusion of saline on BOLD signal intensity were subtracted from the effects of infusion of GBP. Random-effect analysis of group data revealed that GBP significantly increased BOLD signal intensity over several nuclei of the rat brain (Figures 3 and 4 and Table 2) compared to basal. As illustrated by the parametric maps, the directions of the changes in the BOLD signal intensity were clearly segregated. Areas showing significant increases in BOLD signal intensity included the thalamus and areas in the midbrain plus brain stem, whereas those exhibiting decreases in BOLD signal intensity were more frequently observed in the cortical regions, with the exception of some nuclei of the hippocampus or external cortex of the inferior colliculus (ECIC) (Figures 3 and 4).
Figure 3.
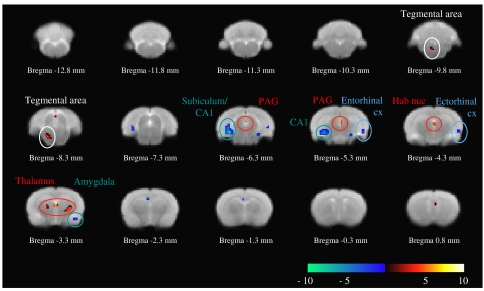
BOLD signal activity after 30 mg kg−1 infusion of GBP compared to saline. Increased BOLD signal intensity (red–white gradient) was observed over the thalamus, habenular nucleus and PAG as well as tegmental area. Strongest decreases in BOLD signal intensity (blue–cyan gradient) were, in turn, found over the subiculum/CA1 and entorhinal/ectorhinal regions of the cortex. BOLD, blood oxygen level dependent; CA1, field 1 of the hippocampus; cx, cortex; GBP, gabapentin; Hab nuc, habenular nucleus; PAG, periaqueductal grey. Circles highlight voxels of interest listed in Table 2.
Figure 4.
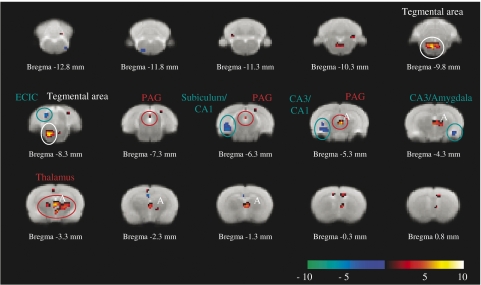
BOLD signal activity after 100 mg kg−1 infusion of GBP compared to saline. At this dose, GBP increased BOLD signal intensity (red–white gradient) over a number of regions of the thalamus and PAG (highlighted by A). Strongest decreases in BOLD signal intensity (blue–cyan gradient) were observed in the subiculum, CA1/CA3 regions of the hippocampus, amygdala and also in the ECIC region. BOLD, blood oxygen level dependent; CA1, field 1 of the hippocampus; CA3, field 3 of the hippocampus; ECIC, external cortex of the inferior colliculus; PAG, periaqueductal grey. Circles highlight voxels of interest listed in Table 2.
Table 2.
Random effect analysis of the effects of GBP on BOLD responses in the brain
| Brain region | Localization | Bregma (mm) | Cluster size (voxel) | Z score | t value |
|---|---|---|---|---|---|
| Infusion 1 versus baseline (from Figure 3) | |||||
| Thalamus | Left | −3.3 | 3 | 3.2 | 4.81 |
| Thalamus | Right | −3.3 | 6 | 3.49 | 6.13 |
| Thalamus | Midline | −3.3 | 29 | 3.75 | 7.21 |
| Habenular nucleus | Midline | −4.3 | ∣ | 3.24 | 5.26 |
| PAG | Midline | −5.3 | ∣ | 3.91 | 7.98 |
| PAG | Midline | −6.3 | ∣ | 3.41 | 5.86 |
| Tegmental area | Left | −8.3 | 14 | 3.59 | 6.52 |
| Tegmental area | Left | −9.8 | ∣ | 3.32 | 5.51 |
| Amygdaloid nucleus | Right | −3.3 | 3 | 3.51 | 6.22 |
| Ectorhinal cortex | Right | −4.3 | 6 | 3.27 | 5.08 |
| Entorhinal cortex | Right | −5.3 | ∣ | 3.25 | 5.28 |
| CA1 | Left | −5.3 | 64 | 3.79 | 7.4 |
| Subiculum/CA1 | Left | −6.3 | 4.27 | 10.19 | |
| Infusion 2 versus baseline (from Figure 4) | |||||
| Thalamus | Midline | −1.3 | 158 (cluster A) | 3.78 | 7.33 |
| Thalamus | Midline | −2.3 | ∣ | 3.28 | 5.39 |
| Thalamus | Right | −2.3 | ∣ | 3.11 | 4.84 |
| Thalamus | Midline | −3.3 | ∣ | 3.99 | 8.46 |
| Thalamus | Right | −3.3 | ∣ | 3.57 | 6.42 |
| Thalamus | Midline | −4.3 | ∣ | 3.69 | 6.96 |
| Thalamus | Right | −4.3 | ∣ | 3.46 | 6.01 |
| PAG | Midline | −5.3 | ∣ | 4.08 | 8.94 |
| Thalamus | Left | −4.3 | 9 | 3.15 | 4.98 |
| PAG | Midline | −6.3 | 6 | 3.29 | 5.44 |
| PAG | Midline | −7.3 | ∣ | 3.22 | 5.19 |
| Tegmental area | Left | −8.3 | 108 | 4.18 | 9.55 |
| Tegmental area | Left | −9.8 | ∣ | 4.42 | 11.24 |
| Amygdaloid nucleus | Right | −4.3 | 5 | 3.32 | 5.52 |
| CA1 | Left | −5.3 | 42 | 3.24 | 5.24 |
| CA3 | Left | −5.3 | ∣ | 3.1 | 4.83 |
| Subiculum/CA1 | Left | −6.3 | ∣ | 3.4 | 5.79 |
| ECIC | Left | −8.3 | 12 | 3.69 | 6.95 |
| Infusion 2 versus infusion 1 | |||||
| Thalamus | Right | −3.3 | 99 (cluster B) | 3.89 | 5.74 |
| Thalamus | Right | −3.3 | ∣ | 3.53 | 6.27 |
| Thalamus | Right | −4.3 | ∣ | 3.25 | 5.27 |
| PAG | Midline | −5.3 | ∣ | 3.78 | 7.36 |
| Tegmental area | Left | −8.3 | 34 | 4.13 | 9.24 |
| Geniculate nucleus | Left | −4.3 | 19 | 3.79 | 7.43 |
| CA3 | Left | −5.3 | 1 | 3.2 | 5.13 |
| ECIC/subiculum | Left | −7.3 | 9 | 3.31 | 5.47 |
Abbreviations: BOLD, blood oxygen level dependent; ECIC, external cortex of the inferior colliculus; GBP, gabapentin; PAG, periaqueductal grey.
Infusion 1 or 2 was compared to baseline and infusion 2 was compared to infusion 1.
Regions in italics refer to areas depicting decreases in BOLD signal intensity.
Random-effect analysis comparing infusion of the low dose of GBP to basal effects revealed significant regions of activation, with the strongest positive changes over the thalamic, PAG and the tegmental areas (P<0.001, uncorrected; Figure 3 and Table 2). This dose of GBP also decreased BOLD signal intensity (significantly to P<0.001, uncorrected, Figure 3 and Table 2) compared to basal, with the greatest changes observed on areas superimposed over the subiculum, CA1, amygdaloid nucleus, entorhinal and ectorhinal cortex.
Following elevation of the dose of GBP (100 mg kg−1), random-effect analysis revealed significantly (P<0.001, uncorrected) greater changes in BOLD signal intensity compared to baseline (Figure 4 and Table 2). The larger cluster (labelled A in Figure 4) demonstrates how the higher dose of GBP produced changes in thalamic and PAG areas that extended beyond that observed with the lower dose of GBP. As with the lower dose of GBP, 100 mg kg−1 GBP produced significant decreases (P<0.001, uncorrected) in BOLD signal intensity in the subiculum, CA1, amygdaloid nucleus and ECIC regions (Table 2).
Comparison of the effects of the lower versus higher dose of GBP with random-effect analysis revealed that increasing the dose of GBP produced a significantly (P<0.001, uncorrected) greater increase in BOLD signal intensity in the thalamic and PAG areas (Table 2). Analysis revealed that the largest cluster extended further over the thalamus and PAG regions. Alternatively, the greater reduction (significant to P<0.001, uncorrected) in BOLD signal intensity attributed to the increase in GBP concentration occurred over the geniculate nucleus, CA3 (field 3 of the hippocampus) and ECIC/subiculum areas.
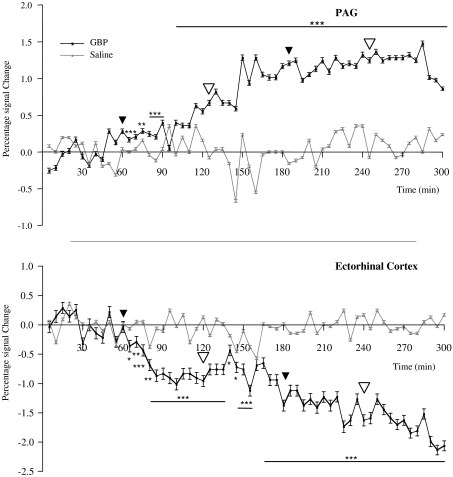
Time-course analyses, extracted from group fixed-effect analysis, of the effects of GBP on BOLD signal intensity in PAG and ectorhinal cortex regions are shown in Figure 5. These demonstrate examples whereby GBP increased (PAG: P=0.008, two-way ANOVA) or decreased (ectorhinal cortex: P<0.0001, two-way ANOVA) BOLD signal intensity compared to saline. In both cases, the effects by GBP were sustained until the end of the scanning session. In addition, effects of infusion 1 or 2 GBP were significant (PAG: P<0.01; ectorhinal: P<0.01, one-way ANOVA with Dunnett's post-test) when compared to the baseline period. On the other hand, comparison of the baseline periods between the GBP- and saline-treated groups revealed no significant difference (comparison used unpaired t-test). In addition, saline infusion had no significant effect compared to baseline, for either brain region studied (PAG: P=0.131; ectorhinal: P=0.059, one-way ANOVA).
Figure 5.
Time-course profile of the i.v. infusion of GBP, extracted from two of the brain regions shown in Figure 3. Two-way ANOVA comparing the lower or higher doses of GBP (represented by the unfilled arrowheads) versus baseline or to the corresponding volumes for the saline group revealed significant (P<0.0001) differences between these two factors—GBP concentration versus baseline and between GBP versus Saline. Post-test analysis also revealed significant (P<0.01, Dunnett's multiple comparison test) differences between both of the two GBP doses versus baseline. Similar post-test analysis for the saline infusion was not significant. Levels of significance between GBP and corresponding values for saline for each volume were analysed using Student's unpaired t-test. *P< 0.05, **P<0.01, ***P<0.001. Each time point represents the average±s.e.mean (n=8). GBP, gabapentin; PAG, periaqueductal grey; black arrowheads denote start of infusion 1 or 2; unfilled arrowheads denote start of maintenance period for each of the infusion concentrations.
Discussion
The aim of our fMRI study was to assess the effects of systemic infusion of GBP on BOLD responses, an index of brain activation in naïve rats. The experimental paradigm consisted of acquiring baseline brain activity followed by a two-stepped infusion equivalent to 30 and 100 mg kg−1 GBP. Any effects of infusion of saline in this experimental paradigm were subtracted so that changes in BOLD signal intensity reflected the effects of GBP alone. This is the first fMRI study investigating the effects of GBP in rats. We showed that GBP alters BOLD signal intensity over several sub- and cortical regions of the rat brain, including the thalamus, PAG, tegmental area, ectorhinal cortex, subiculum and amygdaloid nucleus. Our data also demonstrated that although GBP does not influence acute nociceptive processing in naïve rats (Stanfa et al., 1997; see Introduction) or healthy volunteers (Eckhardt et al., 2000), it does alter BOLD signal intensity, an index of neuronal activity, in a number of brain regions including those involved in nociceptive processing.
Previously, GBP was found not to influence blood pressure (Yoon and Choi, 2003). Our physiological data are in agreement with these studies as neither rate of respiration nor MAP was significantly altered by GBP. Previously, we have shown that isoflurane does not alter MAP (Moylan Governo et al., 2006). Thus, the GBP-induced changes in BOLD responses obtained in our study are not likely to result from indirect effects of GBP on MAP influencing BOLD signal intensity.
The maximal change in BOLD signal intensity observed with the lower dose of GBP was in voxels that, at this resolution, superimpose over the paraventricular/mediodorsal/lateral thalamic areas and dorsomedial PAG. The higher dose of GBP, 100 mg kg−1 (or 117 μM plasma equivalent), in turn resulted in significant increases in BOLD signal intensity extending over other thalamic (ventral anterior, reticular, intermedial or mediodorsal) and PAG (lateral) regions. The thalamus has a role as a central relay area involved in many different processes, one of which is the processing and modulation of noxious inputs (Guilbaud, 1985; Dostrovsky and Guilbaud, 1990; Apkarian and Shi, 1994; Martin et al., 1996; Bordi and Quartaroli, 2000; Abdul Aziz et al., 2005). Indeed, the thalamus receives projections from several regions of the CNS including neurons in lamina I (Craig and Burton, 1981; Jones et al., 1987; also see Gauriau and Bernard, 2002), the termination site of nociceptive-specific primary afferents (Light and Perl, 1979a, 1979b) and deeper laminae (V–VI) of the dorsal horn. The ability of GBP to alter BOLD signal intensity in these brain regions suggests that it is able to alter neuronal activity, either directly or indirectly, at this level in naïve rats.
In the present study, GBP also produced significant increases in BOLD signal intensity that superimpose over the dorsomedial and lateral PAG, two areas that, when experimentally stimulated, produce strong analgesic effects (Fardin et al., 1984a; also see references in Fardin et al., 1984b), suggesting that GBP is able to modulate activity at this level. The PAG plays a pivotal role in the descending inhibitory pain pathway (Mayer et al., 1971; Basbaum and Fields, 1984; Fardin et al., 1984a, 1984b; Morgan et al., 1989, 1991; Behbehani, 1995), as well as sending projections to the thalamus (Krout and Loewy, 2000) and forms part of the spino-parabrachial pain pathway (Gauriau and Bernard, 2002). The PAG also forms reciprocal connections with nuclei involved in cardiovascular regulation (see references in Behbehani, 1995) and, therefore, activity in this region may also represent autonomic responses. Importantly, the effects of GBP on BOLD signal in the PAG, amygdala, hippocampus and entorhinal cortex correlate with the recently reported anxiolytic effects of GBP on fear responses (Nicolas et al., 2007), which are mediated in part by these brain regions.
Previously, we have shown that noxious stimuli such as capsaicin (Moylan Governo et al., 2006) and formalin (Shah et al., 2005) increased BOLD responses in the PAG of anaesthetized rats. The ability of GBP to increase BOLD signal intensity in the PAG is similar to the effects of systemic morphine on BOLD responses at this level under similar experimental conditions (Shah et al., 2005). In the context of nociceptive processing, the PAG has both facilitatory and inhibitory influences on nociceptive processing and disinhibition of GABAergic interneurons plays an important role in the modulation of nociceptive responses by analgesics such as morphine (see references in Fields, 2000). At the present time, the spatial resolution of fMRI is insufficient to allow us to determine which populations of PAG neurons are responsible for blood deoxygenation, and the associated change in BOLD signal and, therefore, the basis for the differential effects of treatments on BOLD responses in the PAG cannot be further determined. Studies on the interaction between morphine and GBP have shown that although GBP does not influence experimental pain scores in healthy volunteers, it does enhance the analgesic effects of morphine (Eckhardt et al., 2000).
In some regions, GBP produced significant decreases in BOLD signal intensity, the greatest change being in voxels that superimposed over the subiculum, amygdaloid and ECIC areas. The exact mechanisms accounting for the phenomenon of negative BOLD still remains undetermined, with several different hypotheses being put forward (see review by Wade, 2002). Nonetheless, at the synaptic level the inhibitory effects by GBP in reducing synaptic transmission is presumed to occur via inhibition of presynaptic calcium influx, as demonstrated in rat hippocampus and neocortex (van Hooft et al., 2002). Similarly, in another study it was found that GBP inhibited potassium-evoked glutamate release from hippocampal and neocortex slices (Dooley et al., 2000). Thus, the occurrence of negative BOLD seen in our study could derive from GBP-mediated suppression of excitatory neurotransmission.
The extracted time-course data of the effects of GBP infusion demonstrate that in some cases infusion of GBP produces a relatively rapid change in BOLD signal intensity, which reaches a plateau after around 2 h of infusion. Pharmacokinetic studies in rats have shown that both blood and brain levels of GBP reach maximum levels within 1–3 h following oral or i.v. administration (Vollmer et al., 1986). On this basis, our data indicate that changes in BOLD signal intensity, produced by our dosing paradigm, can be detected before maximal concentrations of GBP are achieved in the brain. Another important factor that may impact upon our data is that transporter-mediated movement of GBP across the blood–brain barrier has been shown to be saturated at a dose of 100 mg kg−1, the dose given in the second infusion (Luer et al., 1999), which may explain the plateau effect observed.
The major finding of our study is that GBP produces relatively discrete effects on BOLD signal intensity in naïve rats and, therefore, it is important to consider our data in the context of the reported mechanisms of action of GBP (see recent reviews by Cheng and Chiou, 2006; Sills, 2006). Although GBP has been shown to interact with some GABAB isoform receptors (Ng et al., 2001; Parker et al., 2004), the relevance of this mechanism to the pharmacological actions of GBP has been disputed (Lanneau et al., 2001). The most well-described site of action of GBP is at the auxiliary α2δ subunit of voltage-sensitive calcium channels (for review Dooley et al., 2007). The antihyperalgesic effects of GBP have been shown to arise primarily from the interaction of GBP with the α2δ subunit (Gee et al., 1996; review by Bennett and Simpson, 2004). The recently demonstrated distribution of the α2δ1 and α2δ2 subunits in the entorhinal area, amygdala, thalamus, PAG and ECIC (Cole et al., 2005) fits well with the brain regional effects of GBP on BOLD responses observed in the present study. Nevertheless, the effects of GBP on brain regional BOLD responses may also reflect additional sites of action at the level of the spinal cord (Cole et al., 2005), which impact on activity at higher centres.
To date, there are no perfusion studies that have assessed the effects of GBP per se on brain function in humans. The effects of GBP on functional activity have been assessed in an experimental model of pain employing mechanical punctuate stimulation (Iannetti et al., 2005). In this study, GBP reduced punctate mechanical stimulus-induced brain activation in the brainstem, insula, anterior cingulated cortex and thalamus, and inhibited deactivations when the stimulus was combined with a central sensitization paradigm. Although it is difficult to compare the results of these two studies directly, it is worth noting that there was considerable commonality in the brain regions modulated by GBP in our study and the work by Iannetti et al. (2005).
In conclusion, the present study demonstrates that systemic administration of GBP produces significant and discrete changes in brain activation in the anaesthetized rats. The regional distribution of these effects corresponds, at least in part, with the distribution of the auxiliary α2δ subunit of voltage-sensitive calcium channels and areas involved in nociceptive processing.
Acknowledgments
This study was funded by Pfizer, UK. We are also grateful to Dr Malcolm Prior, Brain and Body Centre, University of Nottingham, Nottingham, UK, for his assistance in fMRI acquisition.
Abbreviations
- BOLD
blood oxygen level dependent
- CA1
field 1 of the hippocampus
- ECIC
external cortex of the inferior colliculus
- fMRI
functional magnetic resonance imaging
- GBP
gabapentin
- MAP
mean arterial pressure
- PAG
periaqueductal grey
Conflict of interest
The authors declare that this study was supported by Pfizer and is free of any conflict of interest.
References
- Abdul Aziz AA, Finn DP, Mason R, Chapman V. Comparison of responses of ventral posterolateral and posterior complex thalamic neurons in naive rats and rats with hindpaw inflammation: mu-opioid receptor mediated inhibitions. Neuropharmacology. 2005;48:607–616. doi: 10.1016/j.neuropharm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14:6779–6795. doi: 10.1523/JNEUROSCI.14-11-06779.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Cruccu G, Haanpaa M, Hansson P, Jensen TS, Nurmikko T, et al. EFNS Task Force. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Bennett MI, Simpson KH. Gabapentin in the treatment of neuropathic pain. Palliat Med. 2004;18:5–11. doi: 10.1191/0269216304pm845ra. [DOI] [PubMed] [Google Scholar]
- Bordi F, Quartaroli M. Modulation of nociceptive transmission by NMDA/glycine site receptor in the ventroposterolateral nucleus of the thalamus. Pain. 2000;84:213–224. doi: 10.1016/s0304-3959(99)00205-5. [DOI] [PubMed] [Google Scholar]
- Chapman V, Suzuki R, Chamarette HL, Rygh LJ, Dickenson AH. Effects of systemic carbamazepine and gabapentin on spinal neuronal responses in spinal nerve ligated rats. Pain. 1998;75:261–272. doi: 10.1016/s0304-3959(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Chen CC, Yang JR, Chiou LC. The antiallodynic action target of intrathecal gabapentin: Ca2+ channels, KATP channels or N-methyl-D-aspartic acid receptors. Anesth Analg. 2006;102:182–187. doi: 10.1213/01.ane.0000189550.97536.83. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Chiou LC. Mechanisms of the antinociceptive action of gabapentin. J Pharmacol Sci. 2006;100:471–486. doi: 10.1254/jphs.cr0050020. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Scheede M, Schlutz H. Antinociception and (R,S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid antagonism by gabapentin in the rat spinal cord in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:197–200. doi: 10.1007/s002100000275. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J Neurochem. 2007;100:1289–1299. doi: 10.1111/j.1471-4159.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- Cole RL, Lechner SM, Williams ME, Prodanovich P, Bleicher L, Varney MA, et al. Differential distribution of voltage-gated calcium channel alpha-2 delta (alpha2delta) subunit mRNA-containing cells in the rat central nervous system and the dorsal root ganglia. J Comp Neurol. 2005;491:246–269. doi: 10.1002/cne.20693. [DOI] [PubMed] [Google Scholar]
- Craig AD, Jr, Burton H. Spinal and medullary lamina I projection to nucleus submedius in medial thalamus: a possible pain center. J Neurophysiol. 1981;45:443–466. doi: 10.1152/jn.1981.45.3.443. [DOI] [PubMed] [Google Scholar]
- Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AM. Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology. 2005;48:236–245. doi: 10.1016/j.neuropharm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Mieske CA, Borosky SA. Inhibition of K(+)-evoked glutamate release from rat neocortical and hippocampal slices by gabapentin. Neurosci Lett. 2000;280:107–110. doi: 10.1016/s0304-3940(00)00769-2. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Taylor CP, Donevan S, Feltner D. Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci. 2007;28:75–82. doi: 10.1016/j.tips.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth Analg. 2000;91:185–191. doi: 10.1097/00000539-200007000-00035. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984a;306:105–112. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. II. Differential characteristics of the analgesia induced by ventral and dorsal PAG stimulation. Brain Res. 1984b;306:125–139. doi: 10.1016/0006-8993(84)90361-5. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cui M, Willis WD. Gabapentin markedly reduces acetic acid-induced visceral nociception. Anesthesiology. 2003;98:729–733. doi: 10.1097/00000542-200303000-00023. [DOI] [PubMed] [Google Scholar]
- Field MJ, Hughes J, Singh L. Further evidence for the role of the alpha(2)delta subunit of voltage dependent calcium channels in models of neuropathic pain. Br J Pharmacol. 2000;131:282–286. doi: 10.1038/sj.bjp.0703604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain. 1999;80:391–398. doi: 10.1016/s0304-3959(98)00239-5. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- Fox A, Gentry C, Patel S, Kesingland A, Bevan S. Comparative activity of the anti-convulsants oxcarbazepine, carbamazepine, lamotrigine and gabapentin in a model of neuropathic pain in the rat and guinea-pig. Pain. 2003;105:355–362. doi: 10.1016/s0304-3959(03)00253-7. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Guilbaud G. Thalamic nociceptive systems. Philos Trans R Soc Lond B Biol Sci. 1985;308:339–345. doi: 10.1098/rstb.1985.0034. [DOI] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A'Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig J, Nauerth A, Friedburg H. RARE imaging: a fast imaging method for clinical MR. Magn Reson Med. 1986;3:823–833. doi: 10.1002/mrm.1910030602. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, et al. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol. 1997;324:153–160. doi: 10.1016/s0014-2999(97)00070-8. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. Effect of subarachnoid gabapentin on tactile-evoked allodynia in a surgically induced neuropathic pain model in the rat. Reg Anesth. 1997;22:249–256. doi: 10.1016/s1098-7339(06)80010-6. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Zambreanu L, Wise RG, Buchanan TJ, Huggins JP, Smart TS, et al. Pharmacological modulation of pain-related brain activity during normal and central sensitization states in humans. Proc Natl Acad Sci USA. 2005;102:18195–18200. doi: 10.1073/pnas.0506624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res. 1998;810:93–99. doi: 10.1016/s0006-8993(98)00890-7. [DOI] [PubMed] [Google Scholar]
- Jones MW, Apkarian AV, Stevens RT, Hodge CJ., Jr The spinothalamic tract: an examination of the cells of origin of the dorsolateral and ventral spinothalamic pathways in cats. J Comp Neurol. 1987;260:349–361. doi: 10.1002/cne.902600303. [DOI] [PubMed] [Google Scholar]
- Joshi SK, Hernandez G, Mikusa JP, Zhu CZ, Zhong C, Salyers A, et al. Comparison of antinociceptive actions of standard analgesics in attenuating capsaicin and nerve-injury-induced mechanical hypersensitivity. Neuroscience. 2006;143:587–596. doi: 10.1016/j.neuroscience.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Jun JH, Yaksh TL. The effect of intrathecal gabapentin and 3-isobutyl gamma-aminobutyric acid on the hyperalgesia observed after thermal injury in the rat. Anesth Analg. 1998;86:348–354. doi: 10.1097/00000539-199802000-00025. [DOI] [PubMed] [Google Scholar]
- Klugbauer N, Marais E, Hofmann F. Calcium channel alpha2delta subunits: differential expression, function, and drug binding. J Bioenerg Biomembr. 2003;35:639–644. doi: 10.1023/b:jobb.0000008028.41056.58. [DOI] [PubMed] [Google Scholar]
- Krout KE, Loewy AD. Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;424:111–141. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lanneau C, Green A, Hirst WD, Wise A, Brown JT, Donnier E, et al. Gabapentin is not a GABAB receptor agonist. Neuropharmacology. 2001;41:965–975. doi: 10.1016/s0028-3908(01)00140-x. [DOI] [PubMed] [Google Scholar]
- Li CY, Song YH, Higuera ES, Luo ZD. Spinal dorsal horn calcium channel α2δ-1 subunit upregulation contributes to peripheral nerve injury-induced tactile allodynia. J Neurosci. 2004;24:8494–8499. doi: 10.1523/JNEUROSCI.2982-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observations on the differential termination of coarse and fine fibers. J Comp Neurol. 1979a;186:117–131. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- Light AR, Perl ER. Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol. 1979b;186:133–150. doi: 10.1002/cne.901860203. [DOI] [PubMed] [Google Scholar]
- Ling B, Authier N, Balayssac D, Eschalier A, Coudore F. Behavioral and pharmacological description of oxaliplatin-induced painful neuropathy in rat. Pain. 2007;128:225–234. doi: 10.1016/j.pain.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Luer MS, Hamani C, Dujovny M, Gidal B, Cwik M, Deyo K, et al. Saturable transport of gabapentin at the blood–brain barrier. Neurol Res. 1999;21:559–562. doi: 10.1080/01616412.1999.11740975. [DOI] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, et al. Injury type-specific calcium channel α2δ-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, III, Wade CL, Zhong CM, Mikusa JP, Honore P. Attenuation of mechanical allodynia by clinically utilized drugs in a rat chemotherapy-induced neuropathic pain model. Pain. 2004;110:56–63. doi: 10.1016/j.pain.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Mackensen GB, Sato Y, Nellgard B, Pineda J, Newman MF, Warner DS, et al. Cardiopulmonary bypass induces neurologic and neurocognitive dysfunction in the rat. Anesthesiology. 2001;95:1485–1491. doi: 10.1097/00000542-200112000-00031. [DOI] [PubMed] [Google Scholar]
- Marais E, Klugbauer N, Hofmann F. Calcium channel alpha(2)delta subunits-structure and gabapentin binding. Mol Pharmacol. 2001;59:1243–1248. doi: 10.1124/mol.59.5.1243. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. 1996;16:6601–6611. doi: 10.1523/JNEUROSCI.16-20-06601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Dickenson AH. Effects of spinally delivered N- and P-type voltage-dependent calcium channel antagonists on dorsal horn neuronal responses in a rat model of neuropathy. Pain. 2001;92:235–246. doi: 10.1016/s0304-3959(01)00255-x. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Wolfle TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Gold MS, Liebeskind JC, Stein C. Periaqueductal gray stimulation produces a spinally mediated, opioid antinociception for the inflamed hindpaw of the rat. Brain Res. 1991;545:17–23. doi: 10.1016/0006-8993(91)91264-2. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Sohn JH, Liebeskind JC. Stimulation of the periaqueductal gray matter inhibits nociception at the supraspinal as well as spinal level. Brain Res. 1989;502:61–66. doi: 10.1016/0006-8993(89)90461-7. [DOI] [PubMed] [Google Scholar]
- Moylan Governo RJ, Morris PG, Prior MJ, Marsden CA, Chapman V. Capsaicin-evoked brain activation and central sensitization in anaesthetised rats: a functional magnetic resonance imaging study. Pain. 2006;126:35–45. doi: 10.1016/j.pain.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Ng GY, Bertrand S, Sullivan R, Ethier N, Wang J, Yergey J, et al. Gamma-aminobutyric acid type B receptors with specific heterodimer composition and postsynaptic actions in hippocampal neurons are targets of anticonvulsant gabapentin action. Mol Pharmacol. 2001;59:144–152. [PubMed] [Google Scholar]
- Nicolas LB, Klein S, Prinssen EP. Defensive-like behaviors induced by ultrasound: further pharmacological characterization in Lister-hooded rats. Psychopharmacology. 2007;194:243–252. doi: 10.1007/s00213-007-0838-4. [DOI] [PubMed] [Google Scholar]
- Parker DA, Ong J, Marino V, Kerr DI. Gabapentin activates presynaptic GABAB heteroreceptors in rat cortical slices. Eur J Pharmacol. 2004;495:137–143. doi: 10.1016/j.ejphar.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: New York; 1998. [Google Scholar]
- Rice AS, Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224. doi: 10.1016/S0304-3959(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–1842. doi: 10.1001/jama.280.21.1837. [DOI] [PubMed] [Google Scholar]
- Shah YB, Haynes L, Prior MJ, Marsden CA, Morris PG, Chapman V. Functional magnetic resonance imaging studies of opioid receptor-mediated modulation of noxious-evoked BOLD contrast in rats. Psychopharmacology (Berl) 2005;180:761–773. doi: 10.1007/s00213-005-2214-6. [DOI] [PubMed] [Google Scholar]
- Shah YB, Prior MJ, Dixon AL, Morris PG, Marsden CA. Detection of cannabinoid agonist evoked increase in BOLD contrast in rats using functional magnetic resonance imaging. Neuropharmacology. 2004;46:379–387. doi: 10.1016/j.neuropharm.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Shimoyama M, Shimoyama N, Hori Y. Gabapentin affects glutamatergic excitatory neurotransmission in the rat dorsal horn. Pain. 2000;85:405–414. doi: 10.1016/S0304-3959(99)00283-3. [DOI] [PubMed] [Google Scholar]
- Siggaard-Andersen O. An acid–base chart for arterial blood with normal and pathophysiological reference areas. Scand J Clin Lab Invest. 1971;27:239–245. doi: 10.3109/00365517109080214. [DOI] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Stanfa LC, Singh L, Williams RG, Dickenson AH. Gabapentin, ineffective in normal rats, markedly reduces C-fibre evoked responses after inflammation. Neuroreport. 1997;8:587–590. doi: 10.1097/00001756-199702100-00002. [DOI] [PubMed] [Google Scholar]
- van Hooft JA, Dougherty JJ, Endeman D, Nichols RA, Wadman WJ. Gabapentin inhibits presynaptic Ca(2+) influx and synaptic transmission in rat hippocampus and neocortex. Eur J Pharmacol. 2002;449:221–228. doi: 10.1016/s0014-2999(02)02044-7. [DOI] [PubMed] [Google Scholar]
- Vollmer KO, von Hodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–839. [PubMed] [Google Scholar]
- Wade AR. The negative BOLD signal unmasked. Neuron. 2002;36:993–995. doi: 10.1016/s0896-6273(02)01138-8. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited—again. Neuroimage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Xiao W, Boroujerdi A, Bennett GJ, Luo ZD. Chemotherapy-evoked painful peripheral neuropathy: analgesic effects of gabapentin and effects on expression of the alpha-2-delta type-1 calcium channel subunit. Neuroscience. 2007;144:714–720. doi: 10.1016/j.neuroscience.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sakashita Y. Differential effects of intrathecally administered N- and P-type voltage-sensitive calcium channel blockers upon two models of experimental mononeuropathy in the rat. Brain Res. 1998;794:329–332. doi: 10.1016/s0006-8993(98)00306-0. [DOI] [PubMed] [Google Scholar]
- Yoon MH, Choi JI. Hemodynamic effects of gabapentin in rats. J Korean Med Sci. 2003;18:478–482. doi: 10.3346/jkms.2003.18.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]