Cell migration is a fundamental process, playing a central role in embryonic development, wound healing, inflammation, and tumor metastasis. To migrate, cells must form a leading and trailing edge, apply coordinated force in the direction of movement, and both adhere and release their hold on the substrate as they travel (1–5). To perform these feats, migrating cells respond to a variety of factors such as extracellular matrix molecules and growth factors, which engage cell surface receptors to initiate and maintain migration. One such family of receptors, the integrins, plays an important role in migration, in part by adhering to the extracellular matrix, and activating intracellular cascades that promote actin polymerization involved in lamellipodial extension (1–5). Integrins are heterodimeric receptors that consist of α- and β-subunits with large extracellular ligand-binding domains, and smaller cytoplasmic domains that initiate intracellular signaling (1–5). One mechanism of integrin-mediated cell migration involves the ability of integrins to regulate the activity of the Rho family of small G proteins. For example, localized Rac activation, important for lamellipodial extension, is promoted at the leading edge of cells by α4β1 integrin phosphorylation and consequent unbinding of the signaling adapter protein paxillin and is inhibited at the trailing edge by α4β1 dephosphorylation, binding of paxillin, and inactivation of Rac (1, 4). The closely related α9β1 integrin also is able to enhance cell migration, but its mechanism of action was not known. In an elegant study from the Sheppard laboratory published in this issue of PNAS (6), deHart et al. identify a novel pathway by which α9β1 integrins increase cell migration by modulation of polyamine metabolism and activation of potassium channels.
Most members of the integrin family are able to mediate cell migration, but two related members, α4β1 and α9β1 integrins, are able to both increase migration and inhibit cell spreading (7, 8). Previous studies demonstrated that α4β1 and α9β1 integrins bind paxillin through their C-terminal domain, which is involved in their ability to inhibit cell spreading (7, 8). However, their mechanism for enhancing cell migration differs and, for α9β1 integrin, was shown to be paxillin-independent (8). By using yeast two-hybrid screening to identify potential signaling proteins and then coimmunoprecipitation to confirm their results, Chen et al. (9) discovered that the polyamine catabolic enzyme spermidine/spermine N1-acetyltransferase (SSAT) is a specific binding partner of the cytoplasmic domain of α9 integrin. They demonstrated that interaction of SSAT with the α9 cytoplasmic domain is required for α9β1-mediated migration, although whether this involves increased local recruitment of SSAT or increased activity of the enzyme is not known. Sheppard and colleagues (9) further demonstrated that SSAT was necessary and sufficient for α9β1-mediated enhanced cell migration. Overexpression of SSAT enhanced migration, and siRNA knockdown of SSAT inhibited α9β1-dependent migration without affecting cell adhesion or migration mediated by other integrins (9).
Spermidine/spermine N1-acetyltransferase (SSAT) is a catabolic enzyme that acetylates the high-order polyamines spermine and spermidine (10). It is considered to be the rate-limiting step in the conversion of spermine (valence +4) and spermidine (valence +3) to the lower-order polyamine putrescine (valence +2) (10). The decrease in positive charge on the polyamine results in a decrease in its ability to bind to acidic macromolecules. The acetylated polyamine products are excreted from the cell or further metabolized, thus decreasing the cellular content of higher-order polyamines (10).
How does activation of SSAT enhance cell migration? The next step in the puzzle is nicely addressed in the article by deHart et al. (6). By using mutant SSAT, they show that catalytically inactive SSAT does not enhance migration, indicating that the enzymatic activity of SSAT is required. deHart et al. further demonstrate that a decrease in the concentration of higher-order polyamine, rather than the presence of acetylated polyamine intermediates, is the signal that initiates α9β1-mediated cell migration. Polyamines have a variety of effects on cells, including effects on cell growth, intracellular signaling pathways, and ion channel activity (10). Polyamines are known from previous studies to influence cell migration, and one mechanism that has been proposed is through effects of polyamines on the small GTPase Rac1 (11). In their present work, deHart et al. have unveiled a new mechanism of cellular migration that involves integrin and polyamines coupling to potassium channels.
Inward rectifier potassium (Kir) channels are blocked by intracellular polyamines and Mg2+. This voltage-dependent block of the channels results in a decrease in outward current at positive membrane potentials, a phenomenon known as inward rectification (reviewed in refs. 12–15). Some polyamines are more potent blockers than others, with the higher-order polyamines spermine and spermidine giving strong channel block, compared with weaker blocking effects by the lower-order polyamine putrescine (12–14). Until now, Kir channels have not been implicated in cell migration, but they play a wide variety of important cellular roles including control of electrical excitability in neurons and muscle, involvement in cardiac action potential repolarization, regulation of development, assisting in myoblast fusion, secretion of insulin, buffering of potassium by glial cells, dilatation of blood vessels, and salt reabsorption in kidney (12, 15).
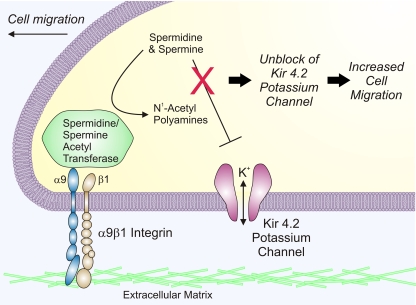
deHart et al. (6) reason that potassium channels are a likely downstream mediator of polyamine metabolism, and in an elegant series of experiments they convincingly demonstrate that both polyamine metabolism and a specific potassium channel, Kir4.2, are involved in cell migration. Their data support a model in which integrin mediates dynamic localized regulation of polyamine levels by SSAT, resulting in unblock of inward rectifier potassium channels, an increase in membrane potassium permeability, and stimulation of cell migration (Fig. 1). In their study, they demonstrate that treatment with barium, an inhibitor of Kir channels, or knockdown of a single potassium channel subunit, Kir4.2, specifically inhibited α9-dependent cell migration (6). In addition, Kir4.2 was concentrated at the leading edge of migrating cells where it partially colocalized in focal adhesions with the more diffusely expressed α9β1 integrin. Significantly, block or knockdown of Kir4.2 reduced the directional persistence of cell locomotion and increased the number of lamellipodial extensions, suggesting that localized changes in K+ efflux may promote cell migration by enhancing the formation of a single dominant lamellipod (6).
Fig. 1.
Model of enhanced cell migration mediated by α9β1 integrins, spermidine/spermine N1-acetyltransferase (SSAT), and inward rectifier potassium channels. The leading edge of a migrating cell is depicted. Engagement of the extracellular domain of α9β1 integrins with specific ligands in the extracellular matrix initiates an increase in cell migration. This process requires SSAT, a polyamine catabolic enzyme that binds to the α9 integrin cytoplasmic tail. SSAT catalyzes the acetylation of the higher-order polyamines spermine (+4 valence) and spermidine (+3 valence), producing acetylated polyamines with a lower charge that may be further degraded or excreted. Inward rectifier Kir 4.2 potassium channels also are required for α9β1 integrin-mediated cell migration. Normally, outward current through Kir 4.2 potassium channels would be blocked by voltage-dependent binding of spermine and spermidine in the channel pore, but acetylation of these polyamines is proposed to relieve the block, allowing increased permeation of K+ ions through the channel. The resulting increase in K+ channel activity, possibly acting through changes in local ion fluxes or membrane potential, or by other downstream channels or effectors, is proposed to enhance cell migration.
It should be noted that some experimental observations are not yet reconciled with the simple model as proposed, and it is suggested that local changes in polyamine concentration and channel activity may not be accurately reflected by global biochemical manipulations of the cell (6). Development of new methods to measure local polyamine concentrations, in particular, at the leading edge of migrating cells, may be required to fully resolve these details.
The studies reported by deHart et al. (6) are important because they provide the first example of a cellular signaling pathway using enzymatic regulation of polyamine metabolism to relay changes to an inward rectifier channel. Previous studies demonstrated proof of principle that physiological polyamine levels could influence potassium channel rectification and thus regulate K+ fluxes in cell lines and in transgenic animals with altered polyamine biosynthesis (16–18), providing important demonstrations that physiological concentrations of polyamines limit the current through the channels.
The study by deHart et al. (6) also is the first to implicate an inward rectifier potassium channel in migration, opening up new understanding of integrin-mediated cell migration, and initiating new avenues for investigation. In particular, it will be of interest to directly explore the effect of integrins on Kir channel electrophysiological activity. Another intriguing question is how integrins regulate SSAT function. Of key significance is how inward rectifier potassium channels might promote migration (19). Potassium channels have a variety of functions in cells because of their ability to regulate transmembrane potassium fluxes and membrane voltage, and their effects are often mediated by downstream influences on other ion channels, calcium, and signaling pathways (12, 15). With persistence, we may migrate toward insightful solutions.
Footnotes
The author declares no conflict of interest.
See companion article on page 7188.
References
- 1.Ridley AJ, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 3.Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 5.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 6.deHart GW, Jin T, McCloskey DE, Pegg AE, Sheppard D. The α9β1 integrin enhances cell migration by polyamine-mediated modulation of an inward-rectifier potassium channel. Proc Natl Acad Sci USA. 2008;105:7188–7193. doi: 10.1073/pnas.0708044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Slepak M, Ginsberg MH. Binding of paxillin to the α9 integrin cytoplasmic domain inhibits cell spreading. J Biol Chem. 2001;276:37086–37092. doi: 10.1074/jbc.M105114200. [DOI] [PubMed] [Google Scholar]
- 8.Young BA, et al. The cytoplasmic domain of the integrin α9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner. Mol Biol Cell. 2001;12:3214–3225. doi: 10.1091/mbc.12.10.3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/spermine N1-acetyltransferase specifically binds to the integrin α9 subunit cytoplasmic domain and enhances cell migration. J Cell Biol. 2004;167:161–170. doi: 10.1083/jcb.200312166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pegg AE. Spermidine/spermine-N1-acetyltransferase: A key metabolic regulator. Am J Physiol Endocrinol Metab. 2008 Mar 18; doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 11.Ray RM, et al. The requirement for polyamines for intestinal epithelial cell migration is mediated through Rac1. J Biol Chem. 2003;278:13039–13046. doi: 10.1074/jbc.M208741200. [DOI] [PubMed] [Google Scholar]
- 12.Nichols CG, Lopatin AN. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 13.Oliver D, Baukrowitz T, Fakler B. Polyamines as gating molecules of inward-rectifier K+ channels. Eur J Biochem. 2000;267:5824–5829. doi: 10.1046/j.1432-1327.2000.01669.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- 15.Conti LR, Leonoudakis D, Vandenberg CA. Inward rectifier potassium channels. In: Adelman G, Smith BH, editors. Encyclopedia of Neuroscience. New York: Elsevier; 2004. [Google Scholar]
- 16.Bianchi L, et al. Regulation by spermine of native inward rectifier K+ channels in RBL-1 cells. J Biol Chem. 1996;271:6114–6121. doi: 10.1074/jbc.271.11.6114. [DOI] [PubMed] [Google Scholar]
- 17.Shyng SL, Sha Q, Ferrigni T, Lopatin AN, Nichols CG. Depletion of intracellular polyamines relieves inward rectification of potassium channels. Proc Natl Acad Sci USA. 1996;93:12014–12019. doi: 10.1073/pnas.93.21.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopatin AN, Shantz LM, Mackintosh CA, Nichols CG, Pegg AE. Modulation of potassium channels in the hearts of transgenic and mutant mice with altered polyamine biosynthesis. J Mol Cell Cardiol. 2000;32:2007–2024. doi: 10.1006/jmcc.2000.1232. [DOI] [PubMed] [Google Scholar]
- 19.Schwab A, Nechyporuk-Zloy V, Fabian A, Stock C. Cells move when ions and water flow. Pflügers Arch. 2007;453:421–432. doi: 10.1007/s00424-006-0138-6. [DOI] [PubMed] [Google Scholar]