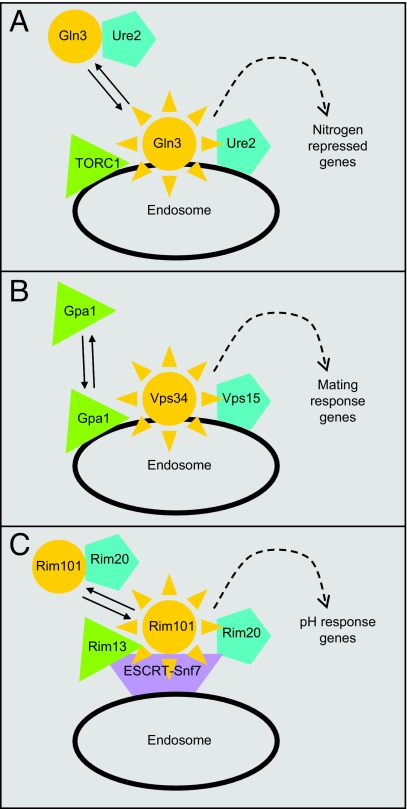
The vesicle trafficking system has long been known to influence signal transduction through its role in biogenesis and degradation of membrane receptors (1). There are also well known examples of vesicle-associated regulatory proteins, such as Ire1 and SREBP, that control responses closely tied to vesicle properties or function (2, 3). But a new paradigm is emerging, as illustrated by a study in this issue of PNAS (4), in which vesicle-associated signal transduction (VAST) may not be related to trafficking of a transmembrane receptor. Rather, vesicles serve as staging areas for regulatory proteins, facilitating interactions that ultimately lead to activation or effector interaction (Fig. 1).
Fig. 1.
New vesicle-associated regulatory interactions. (A) The Gln3 system (4, 6), as described in this issue of PNAS (4). Transcription factor Gln3, perhaps in association with inhibitor Ure2, is conveyed by Golgi-derived vesicles to endosomes, where its regulator TORC1 resides. Trafficking to the endosome is required for Gln3 to enter the nucleus and activate nitrogen-repressed genes. (B) The Gpa1 (Gα subunit) system (8, 9). Activation of Gpa1 releases it from Gβγ association at the plasma membrane and permits binding to Vps34 at the endosome membrane, stimulating phosphatidylinositol 3-kinase activity that promotes mating response gene expression. (C) The Rim101 system (10, 15). Transcription factor Rim101 binds to Rim20, which is associated with the ESCRT (endosomal sorting complex required for transport). Protease Rim13, also ESCRT-associated, activates Rim101 by cleavage, permitting expression of pH-response genes.
The report by Puria et al. in this issue of PNAS (4) focuses on yeast Gln3, a transcription factor that is activated by TORC1 kinase inhibition (5) or by growth on poor nitrogen sources (6). A set of vesicle trafficking proteins were recently implicated in TORC1 kinase function (7). The new study shows that these vesicle trafficking proteins—class C and D Vps proteins—are required for Gln3 to translocate into the nucleus and activate transcription specifically in response to the nitrogen signal. These particular Vps proteins were known to be required for Golgi-to-endosome vesicle trafficking. Thus the genetic analysis indicates that Golgi-to-endosome vesicle trafficking is required to relay a specific Gln3-activating signal.
On their own, these observations are consistent with indirect effects of the Vps proteins, such as the possibility that they are required for biogenesis of a plasma membrane receptor. However, the analysis of Gln3 subcellular localization points to a more direct relationship. In wild-type cells, inactive Gln3 is found in both cytoplasmic and membrane fractions. However, in mutants lacking either of two Vps proteins, Gln3 is almost entirely membrane associated. Moreover, sucrose gradient sedimentation analysis and immunofluorescence microscopy confirm that, in wild-type cells, a fraction of inactive Gln3 is associated with the Golgi and endosome marker protein Vps10. The finding that inactive Gln3 is associated with Golgi or endosomal membranes, and that Golgi-to-endosome trafficking defects impair Gln3 activation, together argue that Gln3 trafficking from Golgi to endosome is required for Gln3 activation.
What tethers Gln3 to membranes? Two Gln3 regulators, TORC1 kinase and Gln3 inhibitor Ure2, are both associated with membranes as well. However, deletion derivatives of Gln3 that lack the TORC1-binding or Ure2-binding regions remain membrane associated. Perhaps either interaction is sufficient for Gln3 membrane binding, or an as-yet-unknown binding partner may govern localization.
What is the mechanistic consequence of Gln3-membrane association? Puria et al. (4) speculate that Gln3 (or the Gln3–Ure2 complex) may be conveyed to the endosome-associated TORC1 complex by Golgi-derived vesicles, a crucial step for Gln3 nuclear translocation.
What is the significance of this vesicle-associated regulatory interaction? Binding of each protein to the same membrane surface should stabilize otherwise weak protein–protein interactions by limiting diffusion. But in addition, Puria et al. (4) point to growing evidence that the protein secretory pathway has a more direct role in nutrient sensing, as manifested through nutrient-dependent sorting of the nitrogen-regulated permease Gap1. Thus association of Gln3 with the secretory pathway may be a critical step for transduction of a nitrogen limitation signal.
Transient endosome association has emerged as a pivotal regulatory event in two other signaling pathways. One is the Saccharomyces cerevisiae mating-response pathway (8). A heterotrimeric G protein relays the activating signal, and both the Gα subunit (called Gpa1) and, independently, the Gβγ complex interact with effectors. Slessareva et al. (9) recently identified the Gpa1 effector: endosome-associated Vps34, the lone yeast phosphatidylinositol 3-kinase. Inactive Gpa1 is found predominantly at the plasma membrane, but Slessareva et al. found that an activated mutant Gpa1 is associated with an endosomal compartment along with Vps34 (Fig. 1B). They propose that activation of Gpa1 causes its dissociation from plasma membrane-bound Gβγ, permitting its translocation to endosomal surfaces where it stimulates Vps34.
The other endosome-associated signaling pathway is the fungal Rim101/PacC pathway, which is activated by growth in nonacidic environments (10). Activation results in proteolytic removal of an inhibitory domain from transcription factor Rim101/PacC. The endosome-associated ESCRT complex, including the Snf7 (Vps32) subunit, is required for Rim101 cleavage (11). Snf7 binds to the protease Rim13 as well as the Rim101-binding protein Rim20 (12–14), ultimately placing the protease and substrate in close proximity (Fig. 1C). Recent evidence suggests that Rim20–Snf7 binding is a regulated step in the pathway (15).
Examples of vesicle-associated signal transduction pathways are few, but many connections have yet to be explored. For example, numerous Vps proteins have genetic interactions with DNA integrity functions (16). The examples are thus far confined to the fungi, but detection of these relationships has not been trivial. That is, the critical vesicle associations are transient, and vps mutations may cause severe growth defects in many organisms. How might such vesicle-signaling relationships be detected in other organisms or cells? The relevant clues from these yeast pathways may serve as a guide to detect vesicle-associated signal transduction in other organisms.
(i) Protein interaction. Snf7–Rim20, Snf7–Rim13, Vps34–Gpa1, and Vsp15–Gpa1 interactions were all detectable by two-hybrid or affinity capture binding assays. One could apply these approaches to almost any regulatory protein to test for interaction with a panel of vesicle-associated proteins, to test vesicle-associated protein complexes for presence of a panel of transcription factors. One could also use broader approaches such as mass spectrometry to detect such associations.
Yeast pathways may serve as a guide to detect vesicle-associated signal transduction.
(ii) Functional dependence. The three vesicle–regulator relationships were or could have been discovered through genome-wide mutant screens, in which particular vps mutants were unexpectedly defective in reporter gene expression. This type of assay could be developed with an RNAi screen in mammalian cells, focused to knock down genes that function in vesicle transport or metabolism.
(iii) Microarray analysis. The S. cerevisiae snf7 mutant, defective in endocytic vesicle metabolism, has a microarray expression profile very similar to that of the rim101 transcription factor mutant (17). Thus exploratory microarray studies of mammalian cells with knocked-down vesicle trafficking functions may point to unanticipated regulatory relationships.
(iv) Subcellular localization. The association of each regulator with a class of vesicle has been visualized by colocalization with vesicle marker proteins. Detection is potentially challenging, though, because the novel localization pattern may be evident for only a small fraction of the total protein, and it may occur only transiently upon pathway activation. Such challenges may be mitigated through computational analysis of microscopic images (18), especially with new approaches for dissection of complex images (19).
The three examples discussed above include gene products that represent broadly conserved protein families, such as TOR kinase, the GATA transcription factor Gln3, the Gα subunit Gpa1, and the Bro-domain protein Rim20. It seems likely that the interplay between the vesicle trafficking system and regulator function will be broadly conserved as well.
Acknowledgments.
Work on vesicle-associated signaling in my laboratory is supported by National Institutes of Health Research Grant R01 AI070272.
Footnotes
The author declares no conflict of interest.
See companion article on page 7194.
References
- 1.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein JL, Rawson RB, Brown MS. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch Biochem Biophys. 2002;397:139–148. doi: 10.1006/abbi.2001.2615. [DOI] [PubMed] [Google Scholar]
- 4.Puria R, Zurita-Martinez SA, Cardenas ME. Nuclear translocation of Gln3 in response to nutrient signals requires Golgi-to-endosome trafficking in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2008;105:7194–7199. doi: 10.1073/pnas.0801087105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohde JR, et al. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol. 2008;11:153–160. doi: 10.1016/j.mib.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magasanik B, Kaiser CA. Nitrogen regulation in Saccharomyces cerevisiae. Gene. 2002;290:1–18. doi: 10.1016/s0378-1119(02)00558-9. [DOI] [PubMed] [Google Scholar]
- 7.Zurita-Martinez SA, et al. Efficient Tor signaling requires a functional class C Vps protein complex in Saccharomyces cerevisiae. Genetics. 2007;176:2139–2150. doi: 10.1534/genetics.107.072835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2004;25:1465–1476. doi: 10.1016/j.peptides.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Slessareva JE, et al. Activation of the phosphatidylinositol 3-kinase Vps34 by a G protein alpha subunit at the endosome. Cell. 2006;126:191–203. doi: 10.1016/j.cell.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 10.Penalva MA, Arst HN., Jr Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu Rev Microbiol. 2004;58:425–451. doi: 10.1146/annurev.micro.58.030603.123715. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, et al. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol Biol Cell. 2004;15:5528–5537. doi: 10.1091/mbc.E04-08-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers K, et al. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic. 2004;5:194–210. doi: 10.1111/j.1600-0854.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, et al. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc Natl Acad Sci USA. 2001;98:4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krogan NJ, et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 2006;440:637–643. doi: 10.1038/nature04670. [DOI] [PubMed] [Google Scholar]
- 15.Boysen JH, Mitchell AP. Control of Bro1-domain protein Rim20 localization by external pH, ESCRT machinery, and the Saccharomyces cerevisiae Rim101 pathway. Mol Biol Cell. 2006;17:1344–1353. doi: 10.1091/mbc.E05-10-0949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan X, et al. A DNA integrity network in the yeast Saccharomyces cerevisiae. Cell. 2006;124:1069–1081. doi: 10.1016/j.cell.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Barwell KJ, et al. Relationship of DFG16 to the Rim101p pH response pathway in Saccharomyces cerevisiae and Candida albicans. Eukaryot Cell. 2005;4:890–899. doi: 10.1128/EC.4.5.890-899.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glory E, Murphy RF. Automated subcellular location determination and high-throughput microscopy. Dev Cell. 2007;12:7–16. doi: 10.1016/j.devcel.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhao T, et al. Object type recognition for automated analysis of protein subcellular location. IEEE Trans Image Process. 2005;14:1351–1359. doi: 10.1109/tip.2005.852456. [DOI] [PMC free article] [PubMed] [Google Scholar]