Abstract
The cubic perovskite BaRuO3 has been synthesized under 18 GPa at 1,000°C. Rietveld refinement indicates that the new compound has a stretched Ru–O bond. The cubic perovskite BaRuO3 remains metallic to 4 K and exhibits a ferromagnetic transition at Tc = 60 K, which is significantly lower than the Tc ≈ 160 K for SrRuO3. The availability of cubic perovskite BaRuO3 not only makes it possible to map out the evolution of magnetism in the whole series of ARuO3 (A = Ca, Sr, Ba) as a function of the ionic size of the A-site rA, but also completes the polytypes of BaRuO3. Extension of the plot of Tc versus rA in perovskites ARuO3 (A = Ca, Sr, Ba) shows that Tc does not increase as the cubic structure is approached, but has a maximum for orthorhombic SrRuO3. Suppressing Tc by Ca and Ba doping in SrRuO3 is distinguished by sharply different magnetic susceptibilities χ(T) of the paramagnetic phase. This distinction has been interpreted in the context of a Griffiths' phase on the (Ca Sr)RuO3 side and bandwidth broadening on the (Sr,Ba)RuO3 side.
Keywords: magnetism, compounds, Ruthenate
Transition-metal oxides with perovskite (1) and perovskite-related compounds have been extensively studied in recent years; for example, high-Tc superconductivity in copper oxides, the colossal magnetoresistance effect in manganese oxides, and more recently multiferroic phenomena. Because perovskite has a densely packed crystal structure (2), high-pressure synthesis, which is widely used in the field of geoscience for studying the lower mantle, is very effective and critical, in some cases, to synthesize compounds with the perovskite structure (3). Application of the high-pressure technique enables us to explore new perovskites that are not formed under ambient pressure and have unknown physical properties.
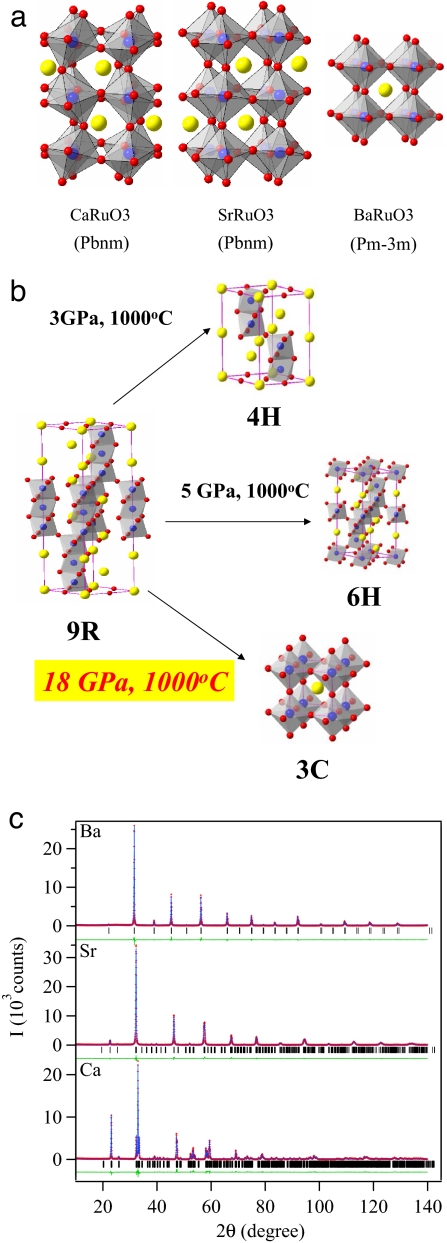
The ruthenium oxides with perovskite-related structure have received growing attention because they exhibit physical properties such as a superconductivity in Sr2RuO4 (4), the meta magnetic metallic behavior of Sr3Ru2O7 (5), and quantum phase transitions in BaRu6O12 (6). The perovskites ARuO3 (A = Sr, Ca) are technically important materials as a conducting buffer layer in electronic devices. Moreover, the cause of the unusual disappearance of ferromagnetism in the Sr1−xCaxRuO3 is currently under hot debate. In the orthorhombic perovskite with Pbnm space group shown in Fig. 1a, the magnetic coupling in these ARuO3 (A = Ca and Sr) perovskites is determined by the Ru–O–Ru bond angle and Ru–O covalent bonding. CaRuO3 remains paramagnetic to the lowest temperature (7, 8), but it is near the verge of ferromagnetic order (9, 10). However, SrRuO3 is an itinerant-electron ferromagnet with a Tc ≈ 164 K (11–16). As a representation of currently popular models about the ferromagnetism in SrRuO3, Mazin and Singh (17) have calculated the band structure of Sr1−xCaxRuO3 by taking into account the cooperative RuO6 site rotation that bends the Ru–O–Ru bond angle from 163° in SrRuO3 to148° in CaRuO3; they found that the increased bending of the Ru–O–Ru bond angle reduces the band degeneracy and, therefore, the density of electronic states at the Fermi energy ρ(εF), perhaps to where the Stoner criterion ρ(εF)U ≈ 1, where ρ(εF) is the electron at the Fermi energy and U is the electron correlation energy, is no longer valid in CaRuO3. This model predicts that the maximum Tc will be achieved as the high-symmetry cubic structure is approached because a higher ρ(εF) is created at high-symmetry points in the Brillouin zone. The striking difference of the magnetic properties between SrRuO3 and CaRuO3 makes cubic perovskite BaRuO3 an interesting candidate since an ideal cubic perovskite is expected because of the larger A-site ionic radius. Mapping out the evolution of the magnetism for the whole perovskite series of ARuO3 from A = Ca across Sr to Ba will shed a new light on the mysterious itinerant-electron ferromagnetism in these ruthenates. However, a literature search shows that BaRuO3 has polytype structures (18–20) depending on how it is synthesized, i.e., the nine-layered rhombohedral (9L), the four-layered hexagonal (4H), and the six-layered hexagonal (6H) as shown in Fig. 1b. All of these polytype structures can be described by different stacking sequences of RuO6 octahedra along the c axis. High-pressure synthesis of an AMO3 compound as a cubic perovskite is necessary where the mismatch between the equilibrium (A–O) and (M–O) bond lengths gives a tolerance factor > 1. The perovskite structure can accommodate a t <1 by a cooperative rotation of the corner-shared MO6 octahedra, which bends the M–O–M bond angle from 180° to (180° − φ) and lowers the symmetry from cubic, but it accommodates to a t > 1 by forming hexagonal polytypes (3). Tabulated ionic radii (21) give room-temperature BaRuO3 a t = 1.0625, and it crystallizes as a 9R polytype with close-packed BaO3 layers stacked cubic-hexagonal-hexagonal-cubic…along the c axis. Because the (M–O) bond is less compressible than the (A–O) bond for most A2+M4+O3 compounds (3), pressure reduces t. Therefore, sintering under high pressure results in the structure sequence in BaRuO3 from 9R to 4H and to 6H polytypes as reported in the literature. The end member in this family, the perovskite structure (3C), could not be synthesized under the highest pressure available in early days. Here, we report that the perovskite BaRuO3 can be obtained under 18 GPa at 1,000°C, nearly the same pressure found at the boundary of the Earth's Upper and Lower mantle. The new compound has the cubic perovskite structure with space group Pm-3m.
Fig. 1.
The crystallography of ARuO3. (a) Schematic drawing of the three perovskites CaRuO3, SrRuO3, and BaRuO3 where red balls stand for O, blue balls for Ru, and yellow balls for alkaline earth Ca, Sr, and Ba. (b) The crystal structures of the BaRuO3 polytypes. Blocks of the face-shared and corner-shared RuO6 octahedra are stacked alternately along the c axis. (c) X-ray powder diffraction spectra of the perovskites CaRuO3, SrRuO3, and BaRuO3 and their best fit with the Rietveld analysis at room temperature.
Results and Discussion
Fig. 1c shows that all x-ray diffraction peaks of the high-pressure product under 18 GPa of BaRuO3 can be indexed with cubic perovskite structure of space group Pm-3m. Results of Rietveld analysis of the x-ray diffraction for perovskite ruthenates ARuO3 (A = Ca, Sr, Ba) are given in Table 1, which illustrates a systematical structural change from CaRuO3 to SrRuO3 and to BaRuO3. The significant structural changes as a function of rA include (i) the bending of the (180° − φ) Ru–O–Ru bonds decreases from 148.6° in CaRuO3 to 162.6 ° in SrRuO3 and finally to 180° in cubic BaRuO3; (ii) the (Ru–O) bond length d = 2.003 Å in cubic BaRuO3 is slightly stretched in comparison with the average Ru–O bond length d = 1.986 Å in SrRuO3 and d = 1.996 Å in CaRuO3; (iii) the octahedral-site distortion as measured by the difference between three Ru–O bond lengths becomes more obvious in CaRuO3, but it remains within the level of intrinsic structural distortion generally found in the orthorhombic perovskite structure with Pbnm space group. The cubic BaRuO3 is stable down to 10 K as checked by x-ray diffraction.
Table 1.
Results of the Rietveld refinement of the x-ray diffraction of Fig. 1c
| CaRuO3(Orthorhombic) | SrRuO3 (Orthorhombic) | BaRuO3(Cubic) | |
|---|---|---|---|
| Space group | Pnma(62) | Pnma(62) | Pm-3m(221) |
| a, Å | 5.35744(3) | 5.57108(4) | 4.0059(2) |
| b, Å | 5.53298(3) | 5.53543(4) | |
| c, Å | 7.66333(4) | 7.85040(7) | |
| Ru-O1 (Å) ×2 | 1.9909(4) | 1.9842(7) | 2.0029 |
| Ru-O2 (Å) ×2 | 2.000(3) | 1.986(7) | |
| Ru-O2 (Å) ×2 | 1.997(2) | 1.988(6) | |
| Ru-O1-Ru, ° | 148.60(2) | 163.08(3) | 180 |
| Ru-O2-Ru, ° | 148.7(1) | 162.4(3) | |
| Rp, % | 8.19 | 5.9 | 7.6 |
| χ 2 | 3.1 | 2.3 | 2.5 |
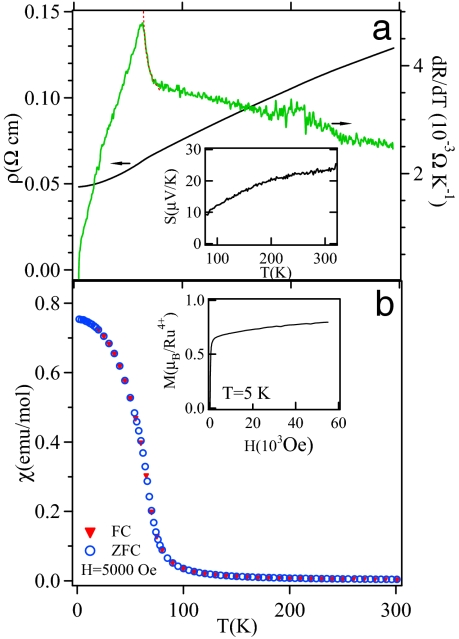
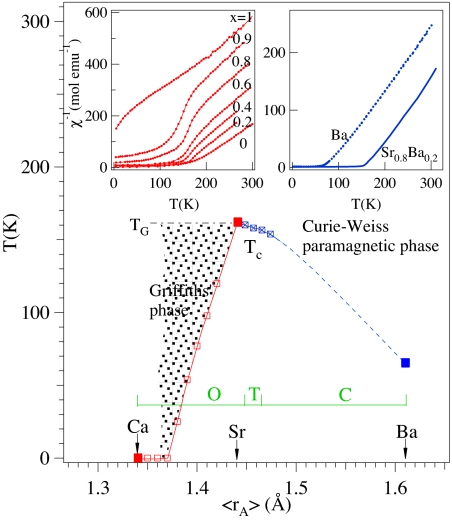
The transport properties of Fig. 2 show that cubic BaRuO3 remains metallic down to 4.2 K. However, the ferromagnetic transition temperature Tc is 60 K, ≈100 K lower than that of SrRuO3, and the saturation magnetization at 5 K in a magnetic field of 5 Tesla remains near 0.8 μB/Ru, far <2.0 μB/Ru expected for a localized electron-spin-only moment for low-spin Ru(IV):t4e0 and significantly lower than the 1.4 μB/Ru in SrRuO3. A small substitution of Ba for Sr in Sr1−yBayRuO3 does not change the magnetization at 5 K; it remains ≈1.4 μB/Ru. However, the effective magnetic moment μeff ≈ 2.6 μB/Ru calculated from the paramagnetic phase, which is close to the spin-only 2.8 μB/Ru for S = 1, is similar for all of the Ba doped Sr1−yBayRuO3 perovskites in this study. The thermoelectric power S(T) (Fig. 2 Inset) of the perovskite BaRuO3 is nearly identical to that of CaRuO3 (22) and it is ≈5 μV/K smaller than that of SrRuO3 at room temperature (15). We have fit dρ/dT of BaRuO3 in the vicinity of Tc with the scaling law |(T − Tc)/Tc|−α. The fitting for T → Tc from above gives an α = 0.11, which matches well theoretical prediction for itinerant-electron ferromagnetism (23). However, the law fails to describe dρ/dT as T → Tc from below. Beyond the critical spin-fluctuation range near Tc, however, ρ(T) of BaRuO3 shows a temperature dependence similar to that of SrRuO3 below and above Tc. As T approaches 4.2 K, ρ(T) is described by the power law ρ(T) − ρ(0) ∼ T2, indicating a Fermi-liquid character. The availability of Sr1−yBayRuO3 with perovskite structure and the literature data for well studied Ca1−xSrxRuO3 allows us to map out completely Tc versus 〈rA〉 for the whole family of ARuO3 perovskites as shown in Fig. 3. Interestingly, Tc peaks out at 〈rA〉 ≈ rSr where it remains within the orthorhombic phase. A preliminary result shows that the transition to a cubic phase occurs at y ≈ 0.15 in Sr1−yBayRuO3. For the compositions with Ba doping y >0.15, the Ru–O bond is stretched from its equilibrium value. One should keep in mind that the size variance (i.e., the composition combination) induced by Ca or Ba doping will also reduce the magnetic transition temperature Tc as has been demonstrated (24). This effect, however, has little to do with a complete suppression of the ferromagnetic transition in CaRuO3 and a dramatic reduction of Tc in cubic BaRuO3. The symmetry argument as mentioned in the introduction also does not work because Tc is reduced in cubic BaRuO3. To identify the mechanism of Tc suppression beyond the size variance induced by the Ca and Ba doping on both sides of SrRuO3, we made a comparison between two compositions of Sr1−xCaxRuO3 and Sr1−yBayRuO3 with approximately the same σ2 = (〈rA2〉 − 〈rA〉2) (a measure to define size variance) (24). As a matter of fact, all compositions of Sr1−yBayRuO3, including y = 0, show typical Curie–Weiss (CW) behavior at T > Tc and Gaussian or critical fluctuations in the vicinity of Tc, whereas compositions of Sr1−xCaxRuO3 show an unusual χ−1(T) as Tc is approached in the paramagnetic phase, as is shown in Fig. 3 Inset. It is clear that the Tc reductions in Sr1−xCaxRuO3 and Sr1−yBayRuO3 appear to be caused by different mechanisms.
Fig. 2.
The primary electric and magnetic properties of BaRuO3 cubic perovskite. (a) Temperature dependence of the resistivity ρ and its derivative dρ/dT of cubic BaRuO3; dashed line on the curve dρ/dT vs. T is a curve fitting to the formula |(T − Tc)/Tc|−α, α = 0.11. (Inset) Shown is the temperature dependence of thermoelectric power. (b) Temperature dependence of the molar magnetic susceptibility of perovskite BaRuO3. (Inset) Shown is the magnetization at 5 K.
Fig. 3.
Phase diagram of the magnetic transition temperatures versus the tabulated average A-site ionic radius 〈rA〉 for Sr1−yBayRuO3 and Sr1−xCaxRuO3. Three members in the phase diagram CaRuO3, SrRuO3, and BaRuO3, in which the A-site size variance is zero, are distinguished by the solid symbols. The shaded area in the phase diagram represents the phase having the characteristic χ−1(T) of the Griffiths' phase. Letters inside the diagram stand for the crystal structure as determined by x-ray diffraction at room temperature, O for orthorhombic, T for tetragonal, and C for cubic. (Inset) Temperature dependence of inverse magnetic susceptibility of Sr1−xCaxRuO3 measured with a magnetic field H = 1 T (data are from ref. 28) and of Sr1−yBayRuO3 measured with a magnetic field H = 0.5 T in this work.
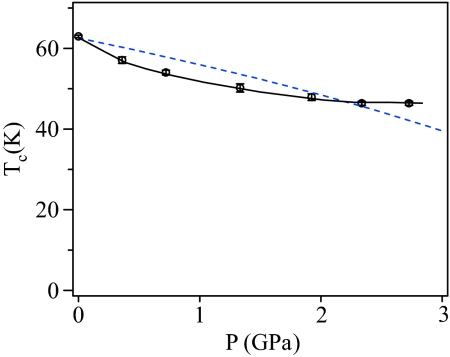
On the Sr1−yBayRuO3 (y ≥0) side, Tc reduction is likely caused by band broadening in a uniform ferromagnetic system because a well defined CW paramagnetism is found at T > Tc (25). Ba doping could change the bandwidth through two opposite effects. Because the A–O interaction in a perovskite ARuO3 competes with the Ru4+ ions for the O-2pπ electrons, the stronger ionic character of the Ba2+, which makes it less competitive for the O-2p orbitals, enhances the covalent admixture of O:2pπ character into the π* bands of primarily 4d-electron character, therefore broadening the bandwidth W associated with the Ru–O bond. However, the Ba doping for y >0.15 in the cubic perovskite phase stretches the Ru–O bond length as obtained from the Rietveld refinement of this work, which reduces the bandwidth. To distinguish which effect plays the dominant role in controlling the bandwidth, we have carried out a structure study and measurement of Tc under pressure on the cubic BaRuO3, as shown in Fig. 4. Pressure does not alter the fact that Ba2+ has strong ionic character, but it does change the Ru–O bond length. The cubic phase is stable up to 100 kbar, the maximum pressure used in this study, and the Ru–O bond length shortens continuously under pressure. Tc drops at a rate dTc/dP ≈ 0.7 K/kbar at low pressures, which is similar to that of SrRuO3 (26) and then gradually approaches a saturated Tc ≈ 50 K at P > 20 kbar. The saturation Tc at high pressures in the cubic BaRuO3 is lower than that of thin-film SrRuO3 found for P > 130 kbar (27). This observation indicates unambiguously that the strong ionic character of Ba2+ broadens W; the broadening is offset slightly by a stretched Ru–O bond length in cubic BaRuO3. High pressure decreases Tc by reducing the Ru–O bond length. It requires a much higher pressure in SrRuO3 than that in the BaRuO3 to lower Tc to a saturation value. The saturation of Tc vs. P curve in both SrRuO3 and the cubic BaRuO3 suggests there is a critical W above which there may be a first-order transition to a Pauli paramagnetic phase. The bandwidth broadening by Ba doping reduces the density of states ρ(εF) near the Fermi energy and, therefore, Tc according to the Stoner model.
Fig. 4.
Pressure dependence of the ferromagnetic transition temperature Tc in cubic BaRuO3. The dashed line is from the prediction of an itinerant-electron ferromagnetism model (34). Tc ≈ (Pc − P)1/2, where Pc (Pc = 5 GPa is used in the plotting) is the critical pressure where Tc vanishes.
In sharp contrast, χ−1(T) for all Sr1−xCaxRuO3 (x > 0) deviates from the CW law at a T ≈ Tc of SrRuO3 with a curvature that is opposite to that caused by critical fluctuations shown in the vicinity of Tc for Sr1−yBayRuO3. Tc reduction and the final vanishing in Sr1−xCaxRuO3 appears to be correlated to the anomalous paramagnetic susceptibility. The peculiar χ−1(T) for all Sr1−xCaxRuO3 (x > 0) (28) is characteristic of a diluted ferromagnetic system (29) in which Griffiths (30) has predicted a nonanalytic χ−1(T) below a TG = Tc of the undiluted parent phase. Moreover, the phase diagram of Tc vs. x on the side of Sr1−xCaxRuO3 matches stunningly well to the prediction based on the model for a diluted Heisenberg ferromagnet (31). The implication of these observations is straightforward: suppression of Tc in Sr1−xCaxRuO3 has little to do with either reducing the ρ(εF) or a competition between ferromagnetic and antiferromagnetic interactions, but is caused by the dilution of ferromagnetic spin–spin coupling across Ru–O–Ru bonds. A thorough study of the magnetic property of the BaRuO3 from the whole series of hexagonal polytypes to the 3C phases helps us to clarify this long-standing puzzle.
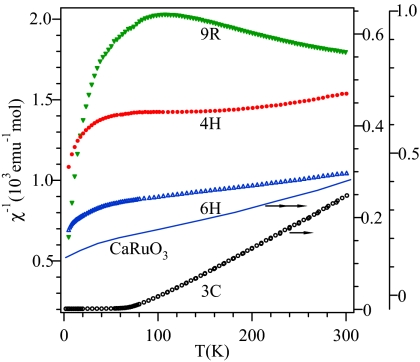
The systematic change in the magnetic susceptibility of the polytype phases of BaRuO3 is shown in Fig. 5. The change of χ(T) can be easily correlated with the characteristics of the polytype structures, which consist of face shared (F) and corner shared (C) RuO6 octahedra. These shared interfaces repeat as CFFCFFCFF, CFCF, FCCFCC, and CCC along the c axis from the 9R, 4H, 6H, to 3C phases. On crossing these interfaces, two Ru–Ru bonds can be defined, i.e., a θ = 180° Ru–[C]–Ru bond of ≈3.9 Å and a θ ≈ 135° Ru–[F]–Ru bond of ≈2.55 Å. The metallic 9R phase shows no magnetic ordering and exhibits neither a CW law nor Pauli paramagnetic susceptibility. Changing the balance of Ru–[F]–Ru bonds vs. Ru–[C]–Ru bonds from the 9R, 4H, 6H, and finally to the 3C phase makes the χ−1(T) evolve from a wiggled curve to the CW law. The relationship between the structural features and a systematic change of χ−1(T) suggests that the Ru–[C]–Ru bond is dominated by the spin–spin interaction, whereas the Ru–[F]–Ru bond has a much reduced magnetic character at low temperatures. In a qualitative discussion, the rhombohedral symmetry of the shared faces splits the threefold-degenerate t orbitals into an a1 and twofold-degenerate e± orbitals carrying an orbital angular momentum. The a1 orbital will be half-filled, forming a Ru–Ru homopolar bond, which leaves a localized hole in the e±3 manifold to give a large orbital angular momentum. A strong spin-orbit λL·S coupling, which is commonly found in the 4d transition-metal compounds (32), would lead at lowest temperature to a state with J = 0 of the Ru4+ ions of a Ru–[F]–Ru pair. The unusual χ(T) of the 9R phase of BaRuO3, where the Ru–[F]–Ru bonds dominate, can indeed be accounted for quantitatively by taking into account the spin–orbit coupling and spin–spin exchange interaction (32). As for the 6H phase in which 2 of 3 blocks consist of the Ru–[C]–Ru bonds, 3D ferromagnetic coupling is not established and spins are not ordered to the lowest temperatures. It is important to note that an approximately linear fitting of χ−1(T) within 90 K < T < 240 K for the 6H phase in Fig. 5 may mean a strong antiferromagnetic coupling, as predicted by a Curie–Weiss law, only at higher temperatures, but a suppression by spin–orbit coupling at lower temperatures of the magnetic moment on the Ru4+ ions of the Ru–[F]–Ru bonds destroys any long-range magnetic order. More interestingly, χ−1(T) of 6H phase of BaRuO3 can be seen in Fig. 5 to resemble the essential feature of the χ−1(T) curve of CaRuO3. This observation suggests that the unusual χ−1(T) found for CaRuO3 and its lack of long-range magnetic order is related to a spin–orbit coupling on the Ru4+ ions that suppresses the ferromagnetic spin–spin coupling across the Ru–O–Ru bonds. Narrowing of the π* band of SrRuO3 by the introduction of the more acidic Ca2+ ion and by reducing the Ru–O–Ru bond angle allows removal of the band degeneracy, which enhances the intraatomic spin–orbit coupling λL·S to where it competes with the interatomic spin-spin interaction across the Ru–O–Ru bonds having a bond angle much reduced from 180°. In the Sr1−xCaxRuO3 system, the reduced bond angle suppresses these Ru–O–Ru interactions to dilute the ferromagnetic interactions so as to give a χ−1(T) behavior typical of a Griffiths' phase.
Fig. 5.
Temperature dependences of the inverse magnetic susceptibility of the 9R, 4H, 6H, and 3C phases of BaRuO3. The χ−1(T) data of the perovskite CaRuO3 are superimposed for comparison.
In conclusion, we have synthesized the new perovskite BaRuO3 under high pressure, which enables us to map out the evolution of magnetic properties for the perovskite ruthenates. The maximum ferromagnetic Curie temperature Tc = 164 K is found for SrRuO3. Substitution of the more ionic and larger Ba2+ ion for Sr2+ straightens the Ru–O–Ru bond angle and broadens the π* bandwidth W even where the Ru–O bond is stretched in BaRuO3. The ferromagnetism of the Sr1−yBayRuO3 system can be well described by the Stoner–Wohlfarth model of band ferromagnetism. Substitution of the more acidic and smaller Ca2+ ion for Sr2+ lowers the Ru–O–Ru bond angles as well as the strength of the interatomic Ru–O–Ru interaction to where the intraatomic spin–orbit λL·S coupling competes with the interatomic spin–spin interactions. As a consequence, the ferromagnetic Ru–O–Ru interactions of SrRuO3 become diluted, and the Sr1−xCaxRuO3 phase behaves as a Griffiths' phase.
Methods
Synthesis.
The starting materials for high-pressure synthesis were prepared by solid-state chemical reaction. The starting materials of BaCO3 (3N), SrCO3 (3N), CaCO3 (3N), and RuO2 (3N) powders were weighed according to the metal-ion ratio and then thoroughly mixed before sintering at ambient pressure to form single-phase precursors. The single phase of orthorhombic SrRuO3 and CaRuO3 were obtained through solid-state reaction at ambient pressure. The well prepared precursors of BaRuO3 and Sr1−yBayRuO3, which have the 9R polytype structure and a mixture of perovskite and the 9R phase, respectively, were further sintered under high pressure and high temperature. The cubic perovskite BaRuO3 was obtained at 18 GPa at 1,100°C with a KAWAI-type multianvil high-pressure apparatus. The Ba-doped perovskites Sr1−yBayRuO3 y ≤ 0.2 were made with a cubic high-pressure apparatus under 6 GPa and 1,000°C. The same apparatus has also been used to synthesize the hexagonal polytypes phase of BaRuO3, i.e., the 4H phase BaRuO3 at 1,000°C under 3 GPa, and the 6H phase of BaRuO3 at 1,000°C under 5 GPa. During high-pressure synthesis the samples were held at the targeting pressure and temperature for 30 min before quenching to room temperature followed by release of the pressure.
Crystal Structure Determination.
Products of the high-pressure synthesis were characterized by x-ray powder diffraction with a Philips X'pert diffractometer. Diffraction data were collected with 0.02° and 15 s per step. Rietveld analysis has been performed by using the program FullPROF software package.
Measurements of Physical Properties.
The magnetic properties of the samples were measured with a superconducting quantum interference device (SQUID) (Quantum Design). The electrical conductivity and thermoelectric power were measured with home-made setups (33). Transport properties under pressure were measured in a piston cylinder high-pressure cell with silicon oil as the pressure medium.
Acknowledgments.
We thank Profs. Z. Fang, G. Cao, and L.Yu for helpful discussions. This work was supported by the National Science Foundation and the Ministry of Science and Technology of China, the National Science Foundation Research Projects 2005CB724402, 2007CB925003, and 50321101, and the Robert A. Welch Foundation of Texas, and the Centers of Excellence project of Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Goodenough JB, Zhou JS. Localized to itinerant electron transitions in transition-metal oxides with the perovskite structure. Chem Mater. 1998;10:2980–2993. [Google Scholar]
- 2.Goodenough JB, Longo JM. Crystallographic and magnetic properties of perovskite and perovskite related compounds. In: Hellwege K-H, Hellwege AM, editors. Landolt-Börnstein Numerical Data and Functional Relationships in Science and Technologym. Vol III/4. Berlin: Springer; 1970. pp. 126–314. [Google Scholar]
- 3.Goodenough JB, Kafalas JA, Longo JM. High pressure synthesis. In: Hagenmuller P, editor. Preparative Methods in Solid State Chemistry. New York: Academic; 1972. pp. 1–238. [Google Scholar]
- 4.Maeno Y, et al. Superconductivity in layered perovskite without copper. Nature. 1994;372:532–534. [Google Scholar]
- 5.Grigera SA, et al. Disorder-sensitive phase formation linked to metamagnetic quantum criticality. Science. 2004;306:1154–1157. doi: 10.1126/science.1104306. [DOI] [PubMed] [Google Scholar]
- 6.Mao ZQ, et al. Quantum phase transition in quasi-one-dimensional BaRu6O12. Phys Rev Lett. 2003;90:186601. doi: 10.1103/PhysRevLett.90.186601. [DOI] [PubMed] [Google Scholar]
- 7.Longo JM, Raccah PM, Goodenough JB. Magnetic properties of SrRuO3 and CaRuO3. J Appl Phys. 1968;39:1327–1328. [Google Scholar]
- 8.Longo JM, Kafalas JA. Pressure-induced structural changes in the system Ba1−xSrxRuO3. Mater Res Bull. 1968;3:687–692. [Google Scholar]
- 9.Klein L, et al. Anomalous spin scattering effects in the badly metallic itinerant ferromagnet SrRuO3. Phys Rev Lett. 1996;77:2774–2777. doi: 10.1103/PhysRevLett.77.2774. [DOI] [PubMed] [Google Scholar]
- 10.Cao G, McCall S, Shepard M, Crow JE, Guertin RP. Thermal, magnetic, and transport properties of single-crystal Sr1−xCaxRuO3. Phys. Rev B. 1997;56:321–329. [Google Scholar]
- 11.Yoshimura K, et al. 17O NMR observation of universal behavior of ferromagnetic spin fluctuations in the itinerant magnetic system Sr1−xCaxRuO3. Phys Rev Lett. 1999;83:4397–4400. [Google Scholar]
- 12.He T, Cava RJ. Disorder-induced ferromagnetism in CaRuO3. Phys Rev B. 2001;63:172403. [Google Scholar]
- 13.Allen PB, et al. Transport properties, thermodynamic properties, and electronic structure of SrRuO3. Phys Rev B. 1996;53:4393–4398. doi: 10.1103/physrevb.53.4393. [DOI] [PubMed] [Google Scholar]
- 14.Kim D, et al. Mean-field behavior with Gaussian fluctuations at the ferromagnetic phase transition of SrRuO3. Phys Rev B. 2003;67:100406. [Google Scholar]
- 15.Klein Y, et al. Insensitivity of the band structure of substituted SrRuO3 as probed by Seebeck coefficient measurements. Phys Rev B. 2006;73 052412. [Google Scholar]
- 16.Shikano M, Huang T-K, Inaguma Y, Itoh M, Nakamura T. Pressure dependence of the magnetic transition temperature for ferromagnetic SrRuO3. Solid State Commun. 1994;90:115–119. [Google Scholar]
- 17.Mazin II, Singh DJ. Electronic structure and magnetism in Ru-based perovskites. Phys Rev B. 1997;56:2556–2571. [Google Scholar]
- 18.Donohue PC, Katz L, Ward R. The crystal structure of barium ruthenium oxide and related compounds. Inorg Chem. 1964;4:306–310. [Google Scholar]
- 19.Hong S-T, Sleight AW. Crystal structure of 4H BaRuO3: High pressure phase prepared at ambient temperature. J Solid State Chem. 1997;128:251. [Google Scholar]
- 20.Rijssenbeek JT, et al. Electrical and magnetic properties of the two crystallographic forms of BaRuO3. Phys Rev B. 1999;59:4561. [Google Scholar]
- 21.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chaleogenides. Acta Crystallogr A. 1976;32:751–767. [Google Scholar]
- 22.Fukunaga F, Tsuda N. On the magnetism and electronic conduction of itinerant magnetic system Ca1−xSrxRuO3. J Phys Soc Jpn. 1994;63:3798–3807. [Google Scholar]
- 23.Fisher ME, Langer JS. Resistive anomalies at magnetic critical points. Phys Rev Lett. 1968;20:665–668. [Google Scholar]
- 24.Attfield JP. A cation control of perovskite properties. Cryst Eng. 2002;5:427. [Google Scholar]
- 25.Hasegawa H. Single-site spin fluctuation theory of itinerant-electron systems with narrow bends. J Phys Soc Jpn. 1980;49:178–188. [Google Scholar]
- 26.Neumeier JJ, Cornelius AL, Schilling JS. Influence of pressure on the ferromagnetic transition temperature of SrRuO3. Physica B. 1994;198:324–328. [Google Scholar]
- 27.Demuer A, Jaccard DJ, Reiner W, Ahn CH, Triscone J-M. Magnetism of SrRuO3 thin films under high hydrostatic pressure. Ann Phys (Leipzig) 2004;13:72–73. [Google Scholar]
- 28.He T, Huang Q, Cava RJ. Comparison of the magnetic properties of isoelectronic Srx(Na0.5La0.5)(1−x)RuO3 and SrxCa1−xRuO3 perovskites. Phys Rev B. 2000;63:24402. [Google Scholar]
- 29.Bray AJ. Nature of the Griffiths phase. Phys Rev Lett. 1987;59:586–589. doi: 10.1103/PhysRevLett.59.586. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths RB. Nonanalytic behavior above the critical point in a random Ising ferromagnet. Phys Rev Lett. 1969;23:17–19. [Google Scholar]
- 31.Stinchcombe RB. Phase Transition and Critical Phenomena. In: Domb C., Lebowitz J.L., editors. Vol 7. London: Academic; 1983. pp. 151–183. [Google Scholar]
- 32.Drillon M, Padel L, Bernier J-C. Effects of spin-orbit coupling and exchange in BaRuO3. J Chem Soc Faraday Trans 2. 1979;75:1193–1198. [Google Scholar]
- 33.Zhou JS, Goodenough JB, Mitchell JF. Unusual thermoelectric power of single-crystal La1.2Sr1.8Mn2O7. Phys Rev B. 1998;58:R579–R582. [Google Scholar]