Abstract
In mammals and insects, pheromones strongly influence social behaviors such as aggression and mate recognition. In Drosophila melanogaster, pheromones in the form of cuticular hydrocarbons play prominent roles in courtship. GC/MS is the primary analytical tool currently used to study Drosophila cuticular hydrocarbons. Although GC/MS is highly reproducible and sensitive, it requires that the fly be placed in a lethal solution of organic solvent, thereby impeding further behavioral studies. We present a technique for the analysis of hydrocarbons and other surface molecules from live animals by using direct analysis in real-time (DART) MS. Cuticular hydrocarbons were sampled from the surface of a restrained, awake behaving fly by using several brief, carefully controlled depressions of the abdomen with a small steel probe. DART mass spectral analysis of the probe detected ions with mass-to-charge ratio (m/z) of the protonated molecule corresponding to many of the previously identified unsaturated hydrocarbons. Six additional cuticular hydrocarbons also were identified. Consistent with previous GC/MS studies, male and female differences in chemical composition were evident. Spatial differences in the expression profile also were observed on males. Sampling from an individual female first as a virgin and then 45 and 90 min after successful copulation showed that mass signals likely to correspond to cis-vaccenyl acetate, tricosene, and pentacosene increased in relative intensity after courtship. This method provides near-instantaneous analysis of an individual animal's chemical profile in parallel with behavioral studies and could be extended to other models of pheromone-mediated behavior.
Keywords: behavior, cis-vaccenyl acetate, courtship, pheromones, Drosophila
A number of volatile chemical signals have been shown in mammals and insects to have profound effects on complex social behaviors such as mate choice (1), kin recognition (2, 3), aggression (4), and aggregation (5). In insects and other arthropods, these signals, many of which are cuticular hydrocarbons, can designate individual roles in social networks in addition to influencing courtship, colony recognition, and aggression (6, 7). In Drosophila melanogaster, several studies have documented the role of hydrocarbons as aphrodisiacs (8–11) or antiaphrodisiacs (11–13). In particular, much focus has been placed on the molecule z-11-octadecenyl acetate [cis-vaccenyl acetate (cVA)] which serves both as a mediator of mate recognition (14–16) and an aggregation factor (8, 9, 14, 17–19). Elucidation of pheromone receptors and the upstream neural pathways has provided a means for delineating the circuitry of complex social behaviors by allowing information flow to be followed from sensory input to motor output (17, 18, 20–23).
The primary method that has been used to characterize hydrocarbons in insects is GC combined with MS (7). Analysis by GC/MS allows quantitation of hydrocarbon content in addition to resolution of individual isomers. Although highly reproducible and sensitive, the method has three limitations. First, the extraction procedure involves placing the animal in hexane or chloroform. These conditions result in lethality and thereby impede further behavioral studies. Second, the solvent and detection conditions are selective for a subset of surface compounds; as a result, other behaviorally relevant cuticular cues may be undetected by using the current protocols. Third, GC/MS analysis can be time-consuming, with typical analysis times ranging from tens of minutes to >1 h.
To address these limitations, we present a method for the analysis of cuticular hydrocarbons and other surface molecules from an awake behaving fly. Ambient MS is a recent technological development that allows mass-to-charge ratio (m/z) measurement with minimal sample preparation (24). One form of ambient MS, direct analysis in real time (DART), uses excited-state helium atoms to desorb and ionize compounds from the surface of the sample directly into the analyzer without the need for chemical extraction or high-vacuum conditions (25–28). The use of DART MS to examine D. melanogaster hydrocarbons improves on previous GC/MS-based techniques by providing rapid analysis of an individual animal's chemical profile while allowing behavioral studies to be performed in-parallel. The ability to track changes in chemical profile before and after social interactions in the same animal provides a control for individual variation of cuticular hydrocarbon expression, which can be significant (29), and also may reveal chemical signatures that could be correlated with behavioral differences observed in individual animals. Using DART MS analysis, it was possible to chemically profile the surface of living flies with high reproducibility, detect differences in male vs. female profiles, detect spatial specificity of male hydrocarbon expression, and monitor hydrocarbon changes in the same individual before and after social interaction.
Results
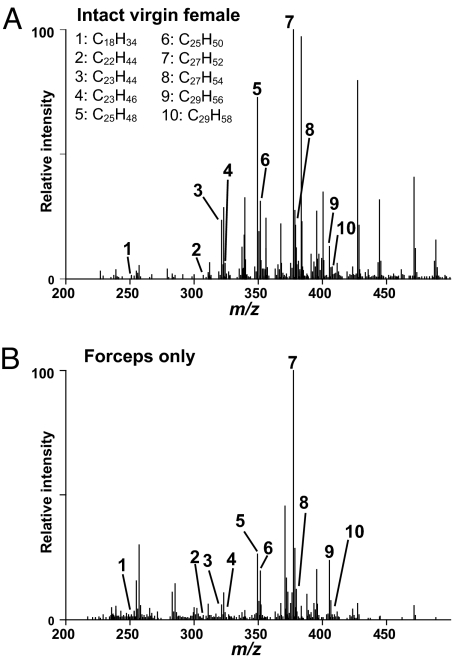
Initial experiments addressed whether the abundance and chemical properties of hydrocarbons on the surface of a single fruit fly allowed detection by DART MS. Stainless-steel forceps were first used to hold an individual fly in the heated helium metastable stream. A robust chemical profile could be obtained with this method (Fig. 1A). Signals with m/z values matching the calculated m/z values of the protonated molecules (MH+) of many of the hydrocarbons previously characterized by GC/MS were detected (Table 1). However, this treatment almost always resulted in severe damage to the fly or lethality. Fortuitously, it was observed that the stainless-steel forceps used to handle the fly provided high-intensity signals, with a profile that showed all of the identified hydrocarbon signals detected on the intact fly (Fig. 1B). Moreover, the relative intensities of these signals to each other also were consistent between the two sampling methods. An attempt was made to sample hydrocarbons from the fly by using alternate surfaces such as a glass capillary tube and filter paper; however, the metal surface produced the greatest signal strength.
Fig. 1.
Comparison of DART mass spectral analysis of an intact fly vs. forceps used to handle a fly. The chemical profile obtained by placing an intact female fly directly in the helium source (A) could be reproduced by analyzing the stainless steel forceps used to handle the female fly (B).
Table 1.
The calculated and observed m/z of each of the labeled ions observed in the spectra shown in Fig. 1
| Spectra | Compound | Formula | Calculated [M + H]+ | Observed [M + H]+ intact fly | Observed [M + H]+ forceps only |
|---|---|---|---|---|---|
| 1 | Octadecadiene | C18H34 | 251.2739 | 251.2755 | 251.2748 |
| 2 | Docasadiene | C22H44 | 307.3365 | 307.3377 | 307.3356 |
| 3 | Tricosadiene | C23H44 | 321.3513 | 321.3491 | 321.3517 |
| 4 | Tricosene | C23H46 | 323.3678 | 323.3689 | 323.3714 |
| 5 | Pentacosadiene | C25H48 | 349.3834 | 349.3848 | 349.3827 |
| 6 | Pentacosene | C25H50 | 351.3991 | 351.3945 | 351.3968 |
| 7 | Heptacosadene | C27H52 | 377.4147 | 377.4098 | 377.4176 |
| 8 | Heptacosene | C27H54 | 379.4304 | (379.4170)* | 379.4306 |
| 9 | Nonacosadiene | C29H56 | 405.4460 | 405.4447 | 405.4476 |
| 10 | Nonacosene | C29H58 | 407.4608 | 407.4604 | 407.4511 |
*Inconsistent mass measurements for heptacosene and (not any of the other compounds) are likely to indicate the presence of an unresolved chemical interference in some of the spectra.
Based on these initial observations, a method was devised for immobilizing and sampling from individual animals with a metal probe that would allow assessment of the chemical profile of the fly in a reproducible and anatomically consistent manner with minimal trauma [Fig. 2A and supporting information (SI) Movie S1]. In all of the following experiments, 4- to 5-day-old socially naïve CantonS flies were used. The fly was probed in the same region with three sampling bouts by using a metal pin guided by a micromanipulator. Each bout consisting of 60 gentle displacements with the micromanipulator to move the pin forward 500–750 μm each time. The pin then was removed from the micromanipulator and placed in the DART helium-metastable source. The mass spectra from each of the three sampling bouts (180 displacements total) were averaged together to produce an overall profile. There was little overall variation observed from bout to bout from the same fly (Fig. S1 and Table S1).
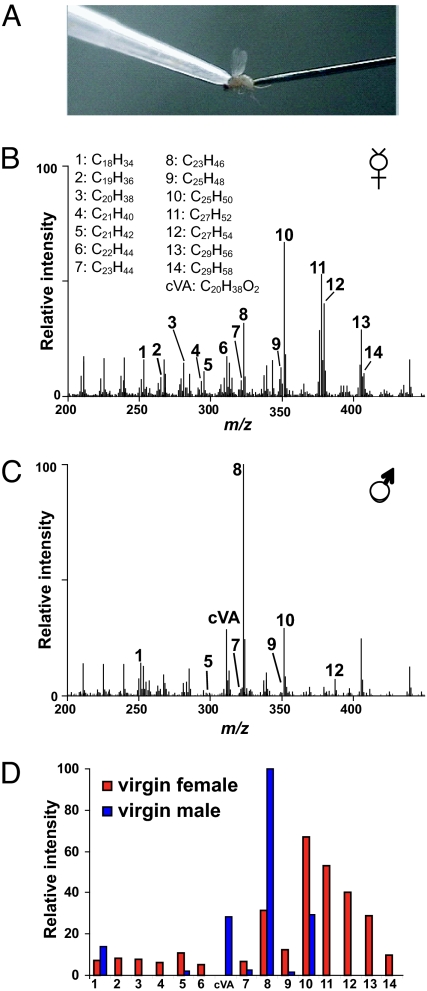
Fig. 2.
Positive mass spectra from DART mass spectral analysis of virgin male and virgin female flies. (A) To profile cuticular compounds from live D. melanogaster, the fly was held by a vacuum applied through a pipette tip and probed with a metal pin. (B and C) The averaged positive-ion mass spectra obtained from DART mass spectral analysis revealed profile differences between a virgin female (B) and male (C). (D) A histogram of the relative intensities of each of the identified hydrocarbon species showed that predominantly longer chain hydrocarbons were detected on the female fly cuticle. In contrast, shorter chain hydrocarbons were detected primarily on the male fly profile, namely cVA, tricosene (peak 8), and pentacosene (peak 10). Numbers in the histogram correspond to the peak labels used in the spectra.
Profiling of Male and Female Flies.
To compare cuticular hydrocarbon analysis by GC/MS to DART ionization, we first analyzed the chemical profile of individually isolated wild-type D. melanogaster males and females. The averaged mass spectra from a socially isolated virgin female and virgin male fly measured with this method are shown in Fig. 2 B and C. The identity of the ions is assigned when the observed m/z of the protonated species is within ± 0.005 of the calculated value (Table 2). The relative intensities of the detected ions corresponding to the major hydrocarbon species in females and males are plotted as a histogram (Fig. 2D). In contrast to analysis by GC/MS, it is not possible to resolve individual isomers with DART-MS; therefore, each ion signal for a hydrocarbon species corresponds to the total signal from all isomers. Despite this difference, DART profiles are consistent with many of the previous findings with GC/MS (6, 9, 30, 31). Both methods show that the male profile is less complex than that of the female, with males expressing shorter chain unsaturated hydrocarbons and females expressing longer chain hydrocarbons with varying degrees of saturation. In the male profile, the most prominent ions observed correspond to tricosene and pentacosene (n = 11). A signal for the pheromone cVA is also detected in males but not in females. In females, the ions with the greatest intensity correspond to pentacosene and heptacosadiene, although the intensity ratios of these two hydrocarbons relative to each other varied between flies (n = 10). Interestingly, the overall signal intensity from males was lower than that of females. There was more variation in overall profiles when comparing fly to fly in both males and females with regard to content and relative intensity ratios of the detected ions (Fig. S2). It is unclear whether the observed differences are caused by natural individual variation, sampling differences that may be less reliable for low-abundance species or regional differences in hydrocarbon expression. Using a probe with a smaller diameter may reduce this variation.
Table 2.
The calculated and observed m/z of each of the labeled ions observed in the spectra shown in Fig. 2
| Spectra | Compound | Formula | Calculated [M + H]+ | Observed [M + H]+ female | Observed [M + H]+ male |
|---|---|---|---|---|---|
| 1 | Octadecadiene | C18H34 | 251.2731 | 251.2716 | 251.2701 |
| 2 | Nonadecadiene | C19H36 | 265.2895 | 265.2876 | n/d |
| 3 | Eicosadiene | C20H38 | 279.3052 | 279.3028 | n/d |
| 4 | Heneicosadiene | C21H40 | 293.3200 | 293.3173 | n/d |
| 5 | Heneicosene | C21H42 | 295.3357 | 295.3345 | 295.3337 |
| 6 | Docasadiene | C22H44 | 307.3365 | 307.3333 | n/d |
| cVA | cis-vaccenyl acetate | C20H38O2 | 311.2950 | n/d | 311.2952 |
| 7 | Tricosadiene | C23H44 | 321.3513 | 321.3516 | 321.3523 |
| 8 | Tricosene | C23H46 | 323.3678 | 323.3645 | 323.3645 |
| 9 | Pentacosadiene | C25H48 | 349.3834 | 349.3819 | 349.3826 |
| 10 | Pentacosene | C25H50 | 351.3991 | 351.3976 | 351.4011 |
| 11 | Heptacosadiene | C27H52 | 377.4147 | 377.4111 | n/d |
| 12 | Heptacosene | C27H54 | 379.4304 | 379.4295 | n/d |
| 13 | Nonacosadiene | C29H56 | 405.4460 | 405.4439 | n/d |
| 14 | Nonacosene | C29H58 | 407.4608 | 407.4603 | n/d |
n/d: not detected.
Detection of Hydrocarbon Species.
In both males and particularly females, DART MS detected ions with m/z corresponding to low-molecular-weight hydrocarbons that have not been previously reported to our knowledge. Using GC/MS, cVA in males (C20) and tricosadiene (C23) in females were the shortest chain hydrocarbons reported. With DART analysis, six compounds with m/z values consistent with the protonated molecules of C18–C22 chain length hydrocarbon species could consistently be detected on the female. The measured m/z values of these ions matched the following chemical structures to within ±0.003 units of the calculated value: C18H34, C19H36, C20H38, C21H40, C21H42, and C22H44. In several samples, two of these hydrocarbons (octadecadiene and eicosadiene) were found in males (Fig. S2). Ions representing higher-molecular-weight triglycerides also could occasionally be observed in both males (Fig. S2H) and females (data not shown). There were no observed sex-specific differences in the triglyceride profile.
Spatial Profiling of Individual Flies.
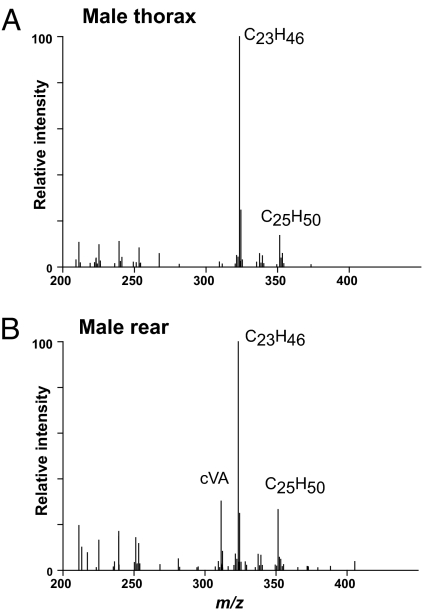
DART MS was used to address whether there are spatial differences in the expression of hydrocarbons by comparing the profiles of individual male flies probed on the lateral thorax vs. the anal–genital region. The signal for cVA was consistently greater from the anal–genital region than the thorax, although the magnitude of this difference varied between flies (n = 3; Fig. 3). These results indicate that a higher concentration of cVA is expressed in the anal–genital region, likely near the tip of the ejaculatory bulb (32). No significant spatial differences in hydrocarbon expression were found when female virgins were profiled (data not shown).
Fig. 3.
DART mass spectral analysis of the thorax (A) and rear (B) of the same male fly reveals spatial differences in hydrocarbon expression. The intensities of the C23H46 (tricosene) and C25H50 (pentacosene) signals are relatively the same between the two regions, but the intensity for cVA is noticeably greater in the anal–genital region vs. the thorax.
Monitoring Chemical Profile Changes in the Same Individual Before and After Courtship.
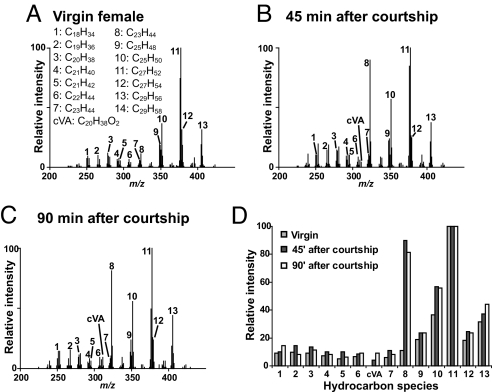
Hydrocarbon expression has been shown to change dramatically after courtship (6, 10, 12, 15). To determine whether these changes could be detected by using DART MS in the same animal, individual female flies were sampled before and at two time points after successful copulation. Consistent with GC/MS studies, changes in the intensity of the peaks representing the ions with m/z values corresponding to the protonated cVA, tricosene, and pentacosene relative to the levels of other hydrocarbons were apparent after copulation at 45 min and persisted until at least 90 min after initiation (n = 4; Fig. 4 and Table 3). The relative intensity of peaks representing other ions in the profile remained similar before and after courtship, indicating that the change observed is likely correlated with copulation and not caused by variation from position effects.
Fig. 4.
Chemical profile changes in the same individual female are observed before and after courtship. (A–C) Comparison of averaged positive ion mass spectra obtained from DART mass spectral analysis of the same female fly before (A) and at 45 min (B) and 90 min (C) after successful copulation. (D) A histogram of the relative intensities of each of the identified hydrocarbon species shows a relative increase in ion signal intensity corresponding to cVA, tricosene (peak 8), and pentacosene (peak 10) after copulation.
Table 3.
The calculated and observed m/z of each of the labeled ions observed in the spectra shown in Fig. 4
| Spectra | Compound | Formula | Calculated [M + H]+ | Observed [M + H]+ virgin female | Observed [M + H]+ 45 min after courtship | Observed [M + H]+ 90 min after courtship |
|---|---|---|---|---|---|---|
| 1 | Octadecadiene | C18H34 | 251.2731 | 251.2692 | 251.2734 | 251.2751 |
| 2 | Nonadecadiene | C19H36 | 265.2895 | 265.2911 | 265.2874 | 265.2911 |
| 3 | Eicosadiene | C20H38 | 279.3052 | 279.2997 | 279.3010 | 279.3039 |
| 4 | Heneicosadiene | C21H40 | 293.3200 | 293.3214 | 293.3177 | 293.3160 |
| 5 | Heneicosene | C21H42 | 295.3357 | 295.3350 | 295.3361 | 295.3349 |
| 6 | Docasadiene | C22H44 | 307.3365 | 307.3317 | 307.3335 | 307.3311 |
| cVA | cis-vaccenyl acetate | C20H38O2 | 311.2950 | n/d | 311.2942 | 311.2925 |
| 7 | Tricosadiene | C23H44 | 321.3513 | 321.3472 | 321.3515 | 321.3509 |
| 8 | Tricosene | C23H46 | 323.3678 | 323.3628 | 323.3644 | 323.3684 |
| 9 | Pentacosadiene | C25H48 | 349.3834 | 349.3778 | 349.3836 | 349.3813 |
| 10 | Pentacosene | C25H50 | 351.3991 | 351.3927 | 351.3985 | 351.3971 |
| 11 | Heptacosadiene | C27H52 | 377.4147 | 377.4090 | 377.4143 | 377.4127 |
| 12 | Heptacosene | C27H54 | 379.4304 | 379.4219* | 379.4287 | 379.4268 |
| 13 | Nonacosadiene | C29H56 | 405.4460 | 405.4417 | 405.4429 | 405.4407 |
| 14 | Nonacosene | C29H58 | 407.4608 | n/d | n/d | n/d |
n/d, not detected.
*Inconsistent mass measurements for heptacosene (and not any of the other compounds) suggest the presence of an unresolved chemical interference in some of the spectra (see also Table 1).
Discussion
Numerous studies have shown the importance of pheromones in shaping behavioral responses and organizing social structures in mammals and insects. Identifying the chemical signals underlying these interactions in addition to correlating the timing of changes in these signals with changes in behavior provides several technical challenges because of issues of detection sensitivity and the disruption of natural behavior. Previous studies of real-time biochemical analysis in conjunction with behavior only took place in mammalian systems where the quantity of analyte was substantial and the size of the brain facilitated implantation of probes used for in situ monitoring of neurotransmitters (33, 34). In this article, we show that biochemical analysis of the fruit fly in parallel with behavior is possible. Although every attempt was made to minimize trauma to the fly, it must be acknowledged that vacuum suspension and repetitive probing are nevertheless highly invasive and unnatural conditions for the animal. Nevertheless, both the profile of hydrocarbons on male and female virgin flies and changes in profile observed on mated female flies were consistent with previous reports of hydrocarbon content measured by GC/MS. Moreover, despite the manipulations, the flies were still capable and interested in behavioral interactions. Thus, it may be concluded that neither the mechanical probing nor additional stress grossly altered the chemical profile of the fly.
Using a different ionization source provided the advantages of potential new biomolecule discovery, increased spatial resolution, improved sensitivity, and greater time resolution. Analysis by DART MS resulted in the identification of six hydrocarbons found on the surface of the fruit fly, several of which were found consistently only on the surface of the female. Moreover, in certain samples, ions with m/z values matching triglycerides also were observed simultaneously with hydrocarbons. These features of DART MS offer possible candidate molecules that may be used in conspecific communication. This method also showed spatial variation in cVA expression on the male fly, with a higher intensity signal found on the rear than on the thorax. Interestingly, variation in cVA signal intensity between individuals was observed despite consistent placement of the probe. The possibility that a smaller diameter probe may reduce this variation should be addressed in future studies although initial experiments with pins 0.15 mm in diameter provided little signal, likely caused by inadequate probe surface area. If differences in hydrocarbon quantities are the product of natural variation, it is intriguing to consider that individual levels of cVA may influence the mating choice of females by possibly serving as one indicator of overall fitness.
Future analytical refinements include the addition of a standard to either the fly or the metal probe to facilitate quantitation and coupling the DART interface with a detector capable of tandem MS to allow structural elucidation and characterization of novel molecules. With these improvements, this rapid sampling method presents the possibility for behavioral MS analysis where dynamic changes in chemical profile may be monitored in parallel with behavior.
Methods
Fly Stocks.
CantonS flies were isolated in the pupal stage and individually placed in glass culture tubes (16 × 100 mm; VWR Scientific) filled with 2 ml of standard fly food. The flies were raised for 4–5 days at 25°C on a standard 12-h light/dark cycle before analysis. Before analysis, the flies were anesthetized by placing the isolation vial in a container of ice-cold water for ≈1 min.
Sample Preparation for DART MS Analysis.
Individual flies were first anesthetized by placing the culture tube briefly in ice. The fly was removed from the tube, positioned on filter paper, and immobilized on the dorsal surface of the thorax by a gel loading pipette tip (10 μl volume; 0.5 mm diameter; Fisher Scientific) attached to a vacuum source (gel dryer vacuum system; Fisher Biotech). If any limbs or wings were caught in the vacuum, the fly was discarded. Once a vacuum seal was established on the surface of the fly, the vacuum line with the attached fly was placed in a micromanipulator (Leitz) with the posterior end of the fly oriented toward a second micromanipulator holding a nickel-plated brass sewing pin (size 17, length 1.0625 in; Dritz). The pin was guided toward the anal–genital region and monitored visually under ×7 magnification. The fly was probed in the same region with three sampling bouts, each bout consisting of 60 gentle displacements, by using the micromanipulator to move the pin forward 500–750 μm each time. The pin then was removed from the micromanipulator and placed in the DART helium source. The mass spectra from each of the three sampling bouts (180 displacements total) were averaged together to produce an overall profile.
Courtship Assay.
Four- to 5-day-old socially isolated virgin male and female flies were used in the courtship assays. Immediately before the courtship assay, an initial averaged DART MS profile of the cuticular surface of the female was obtained as described. After analysis, the female was returned to the original isolation vial. A male fly was then placed in the same vial. Forty-five minutes after successful copulation, the tube was placed on ice, and the female was removed, immobilized, and analyzed. Subsequently, the female was returned to a fresh isolation vial that did not contain a male fly. The female was profiled again 90 min after the first copulation event.
DART MS.
Mass spectrometer.
The atmospheric pressure ionization time-of-flight mass spectrometer (AccuTOF-DART; JEOL USA, Inc.) was equipped with a DART interface and operated in positive-ion mode at a resolving power of 6,000 (FWHM definition). Mass spectra were stored at a rate of one spectrum per s with an acquired m/z range of 60 to 1,000. Calibration for exact mass measurements was accomplished by acquiring a mass spectrum of polyethylene glycol (average molecular weight 600) as an external reference standard in every data file.
DART ion source.
The DART interface was operated in positive-ion mode with helium gas with the gas heater set to 300°C. The glow discharge needle potential was set to 3.5 kV. Electrode 1 was set to +150 V, and electrode 2 (grid) was set to +250 V. It should be noted that protonated molecules are observed because all of the molecules detected by this method contain a heteroatom or at least one site of unsaturation. Protonation is not observed for DART analysis of saturated hydrocarbons, although molecular radical cations may be observed for these compounds under certain conditions.
Supplementary Material
Acknowledgments.
We thank Prof. David Corey, Dr. Alo Basu, and Dr. Siddharthan Govindasamy for help with calibration of the fly sampling device and Drs. Olga Alekseyenko Yick-Bun Chan, Sarah Certel, Adelaine Leung, Jill Penn, and Steven Nilsen of E.A.K.'s laboratory for helpful discussions. This work was supported by National Institutes of Health Grants MH072127 (to J.Y.Y.) and GM067645 and GM074675 (to E.A.K.) and National Science Foundation Grant IBN-0090730 (to E.A.K.).
Footnotes
Conflict of interest statement: DART is a trademark of JEOL USA, Inc.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802692105/DCSupplemental.
References
- 1.Brennan PA, Kendrick KM. Mammalian social odors: Attraction and individual recognition. Philos Trans R Soc London Ser B. 2006;361:2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dapporto L, Romana Dani F, Turillazzi S. Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr Biol. 2007;17:R504–R505. doi: 10.1016/j.cub.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Smith BH. Recognition of female kin by male bees through olfactory signals. Proc Natl Acad Sci USA. 1983;80:4551–4553. doi: 10.1073/pnas.80.14.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamero P, et al. Identification of protein pheromones that promote aggressive behavior. Nature. 2007;450:899–902. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- 5.Sumpter DJ. The principles of collective animal behavior. Philos Trans R Soc London Ser B. 2006;361:5–22. doi: 10.1098/rstb.2005.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferveur JF. Cuticular hydrocarbons: Their evolution and roles in Drosophila pheromonal communication. Behav Genet. 2005;35:279–295. doi: 10.1007/s10519-005-3220-5. [DOI] [PubMed] [Google Scholar]
- 7.Howard RW, Blomquist GJ. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu Rev Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. [DOI] [PubMed] [Google Scholar]
- 8.Bray S, Amrein H. A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 9.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu Rev Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 10.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 11.Ferveur JF, et al. Genetic feminization of pheromones and its behavioral consequences in Drosophila males. Science. 1997;276:1555–1558. doi: 10.1126/science.276.5318.1555. [DOI] [PubMed] [Google Scholar]
- 12.Ferveur JF, Sureau G. Simultaneous influence on male courtship of stimulatory and inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster. Proc Biol Sci. 1996;263:967–973. doi: 10.1098/rspb.1996.0143. [DOI] [PubMed] [Google Scholar]
- 13.Scott D. Sexual mimicry regulates the attractiveness of mated Drosophila melanogaster females. Proc Natl Acad Sci USA. 1986;83:8429–8433. doi: 10.1073/pnas.83.21.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartlelt RJ, Schaner AM, Jackson LL. Cis-vaccenyl acetate as an agregation pheromone in Drosophila melanogaster. J Chem Ecol. 1985;11:1747–1756. doi: 10.1007/BF01012124. [DOI] [PubMed] [Google Scholar]
- 15.Ejima A, et al. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioral responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 17.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 19.Hall JC. Behavioral analysis in Drosophila mosaics. Results Probl Cell Differ. 1978;9:259–305. doi: 10.1007/978-3-540-35803-9_10. [DOI] [PubMed] [Google Scholar]
- 20.Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- 21.Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behavior in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 22.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulac C, Wagner S. Genetic analysis of brain circuits underlying pheromone signaling. Annu Rev Genet. 2006;40:449–467. doi: 10.1146/annurev.genet.39.073003.093937. [DOI] [PubMed] [Google Scholar]
- 24.Cooks RG, Ouyang Z, Takats Z, Wiseman JM. Detection technologies: Ambient mass spectrometry. Science. 2006;311:1566–1570. doi: 10.1126/science.1119426. [DOI] [PubMed] [Google Scholar]
- 25.Cody RB, Laramee JA, Durst HD. Versatile new ion source for the analysis of materials in open air under ambient conditions. Anal Chem. 2005;77:2297–2302. doi: 10.1021/ac050162j. [DOI] [PubMed] [Google Scholar]
- 26.McEwen CN, McKay RG, Larsen BS. Analysis of solids, liquids, and biological tissues using solids probe introduction at atmospheric pressure on commercial LC/MS instruments. Anal Chem. 2005;77:7826–7831. doi: 10.1021/ac051470k. [DOI] [PubMed] [Google Scholar]
- 27.Pierce CY, et al. Ambient generation of fatty acid methyl ester ions from bacterial whole cells by direct analysis in real-time (DART) mass spectrometry. Chem Commun. 2007:807–809. doi: 10.1039/b613200f. [DOI] [PubMed] [Google Scholar]
- 28.Vail T, Jones PR, Sparkman OD. Rapid and unambiguous identification of melamine in contaminated pet food based on mass spectrometry with four degrees of confirmation. J Anal Toxicol. 2007;31:304–312. doi: 10.1093/jat/31.6.304. [DOI] [PubMed] [Google Scholar]
- 29.Foley B, Chenoweth SF, Nuzhdin SV, Blows MW. Natural genetic variation in cuticular hydrocarbon expression in male and female Drosophila melanogaster. Genetics. 2007;175:1465–1477. doi: 10.1534/genetics.106.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Antony C, Jallon J. The chemical basis for sex recognition in Drosophila melanogaster. J Insect Physiol. 1982;28:873–880. [Google Scholar]
- 31.Pechine JM, Perez F, Antony C, Jallon JM. A further characterization of Drosophila cuticular monoenes using a mass spectrometry method to localize double bonds in complex mixtures. Anal Biochem. 1985;145:177–182. doi: 10.1016/0003-2697(85)90344-6. [DOI] [PubMed] [Google Scholar]
- 32.Brieger G, Butterworth FM. Drosophila melanogaster: Identity of male lipid in reproductive system. Science. 1970;167:1262. doi: 10.1126/science.167.3922.1262. [DOI] [PubMed] [Google Scholar]
- 33.Reed B, Zhang Y, Chait BT, Kreek MJ. Dynorphin A(1–17) biotransformation in striatum of freely moving rats using microdialysis and matrix-assisted laser desorption/ionization mass spectrometry. J Neurochem. 2003;86:815–823. doi: 10.1046/j.1471-4159.2003.01859.x. [DOI] [PubMed] [Google Scholar]
- 34.Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT. Capillary liquid chromatography with MS3 for the determination of enkephalins in microdialysis samples from the striatum of anesthetized and freely moving rats. J Mass Spectrom. 2005;40:146–153. doi: 10.1002/jms.733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.