Abstract
The heterodisaccharide lactose (1,4-O-β-d-galactopyranosyl-d-glucose) induces cellulase formation in the ascomycete Hypocrea jecorina (= Trichoderma reesei). Lactose assimilation is slow, and the assimilation of its β-d-galactose moiety depends mainly on the operation of a recently described reductive pathway and depends less on the Leloir pathway, which accepts only α-d-galactose. We therefore reasoned whether galactomutarotase [aldose 1-epimerase (AEP)] activity might limit lactose assimilation and thus influence cellulase formation. We identified three putative AEP-encoding genes (aep1, aep2, aep3) in H. jecorina, of which two encoded intracellular protein (AEP1 and AEP2) and one encoded an extracellular protein (AEP3). Although all three were transcribed, only the aep3 transcript was detected on lactose. However, no mutarotase activity was detected in the mycelia, their cell walls, or the extracellular medium during growth on lactose. Therefore, the effect of galactomutarotase activity on lactose assimilation was studied with H. jecorina strains expressing the C-terminal galactose mutarotase part of the Saccharomyces cerevisiae Gal10. These strains showed increased growth on lactose in a gene copy number-dependent manner, although their formation of extracellular β-galactosidase activity and transcription of the genes encoding the first steps in the Leloir and the reductive pathway was similar to the parental strain QM9414. Cellulase gene transcription on lactose dramatically decreased in these strains, but remained unaffected during growth on cellulose. Our data show that cellulase induction in H. jecorina by lactose requires the β-anomer of d-galactose and reveal the lack of mutarotase activity during growth on lactose as an important key for cellulase formation on this sugar.
Keywords: mutarotase, Leloir pathway, reductive D-galactose catabolism
The heterodisaccharide lactose (1,4-O-β-d-galactopyranosyl-d-glucose) occurs mainly in mammalian milk where it makes up 2–8% of the dry weight. Its hydrolysis by β-galactosidases (= lactases) yields d-glucose and β-d-galactose. In prokaryotes, yeasts, and mammals, the latter is then catabolized via the Leloir pathway (1). However, the first enzyme in this pathway [galactokinase (EC 2.7.1.6)] accepts only α-d-galactose and cannot act on the β-anomer (2). Although the interconversion of the sugar anomers occurs spontaneously in pure water in vitro, efficient in vivo formation of α-d-galactopyranose from β-d-galactopyranose has been shown to depend on the presence of a mutarotase [aldose 1-epimerase (AEP); EC 5.1.3.3], suggesting that the activity of intracellular water is not high enough to permit mutarotation (3).
Galactose mutarotase activity has subsequently been observed in a wide range of organisms, including bacteria (3–5), plants (6, 7), fungi (8), and mammals (9–11) and is present in the cytoplasm of most of these cells. An intriguing case is the Saccharomyces cerevisiae Gal10 where mutarotase occurs as a fusion protein to another enzyme of the Leloir pathway, UDP-glucose 4-epimerase (EC 5.1.3.2) (12). All of these mutarotases have a broad specificity for aldoses, yet, at least in S. cerevisiae, exhibiting highest activity with d-galactose (13). In most bacteria, the enzyme is secreted from the cells (14), whereas the eukaryotic enzyme is mostly intracellular (12).
For the ascomycete Hypocrea jecorina (the teleomorph of the filamentous fungus Trichoderma reesei that is used for the industrial production of cellulases and hemicellulases), lactose, although not being a natural substrate, serves as an important inducer of cellulase formation in industry (15). Interestingly, assimilation of lactose by this fungus depends less on the Leloir pathway of d-galactose catabolism that accepts only α-d-galactose (16, 17), but strongly depends on an alternative pathway in which the d-galactose moiety is first reduced to galactitol, and by a series of subsequent oxidation and reduction steps finally converted to d-fructose (18). The GAL10 protein of H. jecorina and its orthologues in other multicellular ascomycetes have only the UDP-glucose 4-epimerase domain but lack the mutarotase domain (19). Taken together, these data suggest that β-d-galactose mutarotation in H. jecorina (and maybe other filamentous fungi) is absent or inefficient. Direct proof for this hypothesis is lacking, however.
Here, we present evidence that H. jecorina contains three putative mutarotase genes, but indeed lacks mutarotase activity during growth on lactose. Moreover we will show that introduction of mutarotase activity into H. jecorina impairs cellulase induction by lactose. The lack of mutarotase activity is therefore an important trait for industrial cellulase production on lactose.
Results
H. jecorina Has Three aep Genes.
As a prerequisite for this work, we have screened the genome database of H. jecorina (http://genome.jgi-psf.org/Trire2/Trire2.home.html) for genes potentially encoding AEPs. Three loci were found: tre42544 [Protein Data Bank (PDB) ID code 121661], tre44592 (PDB ID code 120784), and tre19341 (PDB ID code 22415). tre43544 encodes a protein with the highest amino acid similarity (35%) to the C terminus of the S. cerevisiae bifunctional Gal10 and exhibits 64% amino acid identity to a hypothetical protein from Gibberella zeae. Similarity of the other two putative AEPs (tre44592 and tre19341) was much weaker (Table 1). We found ESTs for all three genes in the mixed mRNA samples of (20), indicating that none of them is a pseudogene.
Table 1.
Characterization of the three deductive mutarotase proteins of H. jecorina
| Characteristic | Protein |
||
|---|---|---|---|
| AEP1 | AEP2 | AEP3 | |
| Name in genome annotation | tre19341 | tre42544 | tre44592 |
| PDB ID code | 22415 | 121661 | 120784 |
| EST no. | 4 | 4 | 10 |
| Amino acid similarity to S. cerevisiae Gal10, % | 7 | 35 | 23 |
| No. of amino acids | 315 | 342 | 383 |
| Mr | 33,988.2 | 37,074.5 | 42,380.0 |
| Isoelectric point | 4.99 | 5.29 | 6.44 |
| Predicted gene product location | Intracellular | Intracellular | Extracellular |
We have named the three genes aep1, aep2, and aep3, following established nomenclature rules for isoenzymes and starting the numbering with the one whose deduced protein has the lowest isoelectric point (Table 1). Among them, AEP3 contains a predicted signal peptide (SignalP probability 1.00) that is removed with a probability of 0.394 between positions 23 and 24, and therefore constitutes a secreted protein. The location of the other two is predicted to be cytoplasmic.
The Three Mutarotase Genes Are Not Expressed During Growth on Lactose.
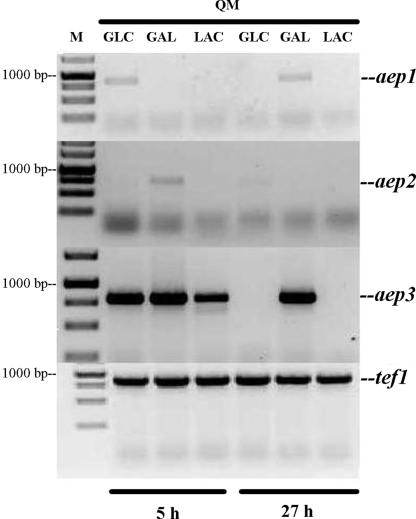
To learn whether any of these three genes could be involved in d-galactose mutarotation during growth of H. jecorina, the fungus was cultivated on d-glucose, d-galactose, and lactose, and the transcripts of aep1, aep2, and aep3 were assayed (Fig. 1). The mRNA of aep3 was most abundant and detected on all three carbon sources during the phase of early growth (5 h), but only on d-galactose during the later stages (27 h) of growth. Traces of the aep1 transcripts were detected on d-glucose at 5 h and d-galactose at 27 h, whereas a higher, but still very low, level of aep2-mRNA was present on d-galactose at 5 h (Fig. 1).
Fig. 1.
Transcription profile of aep1, aep2, and aep3 of H. jecorina QM9414 (QM) during growth on different carbon sources obtained by RT-PCR. Glc, glucose; Gal, galactose; Lac, lactose; M, marker.
The above results would imply that there is no galactomutarotase activity present in H. jecorina when growing on lactose. To independently test this notion, we assayed for mutarotase activity in cell-free extracts, cell walls, and the extracellular culture filtrates. Activities were below the detection limit in all samples, whereas controls with cell-free extracts from S. cerevisiae clearly showed activity (data not shown). The situation was similar on d-galactose. We conclude that H. jecorina, while containing three putative mutarotase genes, does not express such mutarotase activity during growth on lactose.
Overexpression of the S. cerevisiae Gal10 AEP Domain in H. jecorina Increases the Rate of Lactose Utilization.
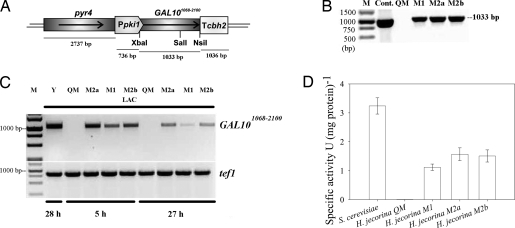
Because of this apparent lack of galactose mutarotase activity in H. jecorina during growth on lactose, we were interested in the effect of overexpression of a mutarotase in the fungus, particularly in its consequences for cellulase induction by lactose. To this end, we used the C-terminal part of the S. cerevisiae Gal10 that is responsible for the d-galactopyranose mutarotation activity (13). A gene fragment spanning from nucleotides 1068 to 2100 (GAL101068–2100) was fused under the expression signals of the constitutive pki1 (pyruvate kinase) promoter and the cbh2 (cellobiohydrolase 2) terminator region (Fig. 2A). Transformants were purified and checked by Southern analysis and PCR (Fig. 2B). Three of them, which exhibited different copy numbers of the construct (i.e., one copy in strains M1 and two copies in strains M2a and M2b), also accumulated correspondingly different amounts of GAL101068–2100 transcripts (Fig. 2C). To ensure that these three transformants in fact also express a functional mutarotase, we assayed cell-free extracts of cultures grown on lactose as a carbon source (Fig. 2D). As clearly seen, all of the mutants contained mutarotase activity, and the activity correlated with the GAL101068–2100 copy number. They were thus chosen for further investigation.
Fig. 2.
Demonstration of the presence and expression of GAL101068-2100 in H. jecorinh mutants. (A) Schematic drawing of the GAL101068–2100 expression cassette used for the transformation of H. jecorina TU-6. (B) Demonstration of the presence of S. cerevisiae GAL101068–2100 in the H. jecorina mutant strains M1, M2a, and M2b by PCR and its absence in QM9414. M, marker; Cont., control (pGAL10Kicsi used as a template). (C) Transcription of the S. cerevisae GAL101068–2100 in H. jecorina strains M1, M2a, and M2b during growth on lactose at 5 and 27 h. Y, S. cerevisiae (positive control); QM, H. jecorina QM9414; M, marker; Lac, lactose. (D) Specific intracellular mutarotase activity of the QM9414- and GAL101068–2100-expressing H. jecorina strains. Samples were taken 12 h after the transferring procedure.
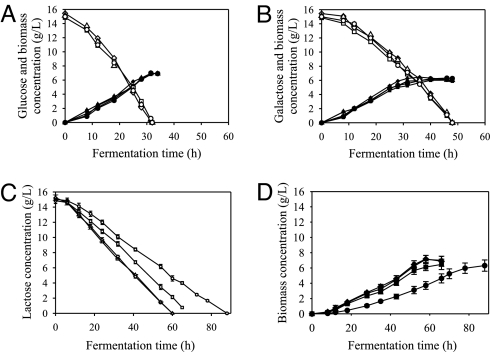
When the reference strain of H. jecorina and the three mutarotase-expressing strains were grown on d-glucose and d-galactose, they exhibited similar growth rates and rates of sugar consumption as the reference strain, indicating that AEP (and thus mutarotase activity in general) is dispensable for the assimilation of both monosaccharides (Fig. 3 A and B). Data consistent with this claim were also obtained for l-arabinose, glycerol, d-xylose, and d-fructose (data not shown). A clear difference, however, was seen on lactose: all of the mutarotase transformants exhibited an enhanced assimilation (Fig. 3C) and a subsequently increased biomass formation (Fig. 3D), and the increase was again positively correlated with the GAL101068–2100 copy number.
Fig. 3.
Fermentation profiles of H. jecorina QM9414 and mutants M1, M2a, and M2b on different carbon sources. (A) Fermentation profile of H. jecorina QM9414 (circles) and the GAL101068–2100-expressing strains M1 (squares), M2a (triangles), and M2b (diamonds) on glucose as a sole carbon source. Open symbols indicate glucose, and filled symbols indicate biomass time profiles. (B) Fermentation profile of H. jecorina QM9414 (circles) and strains M1 (squares) M2a (triangles), and M2b (diamonds) on galactose as a sole carbon source. Open symbols indicate galactose, and filled symbols indicate biomass time profiles. (C) Lactose uptake of H. jecorina QM9414 (○) and the GAL101068–2100-expressing strains M1 (□) M2a (▵), and M2b (◇). (D) Biomass formation of H. jecorina QM9414 (●) and the GAL101068–2100-expressing strains M1 (■), M2a (▴), and M2b (♦) on lactose.
Overexpression of the S. cerevisiae Gal10 AEP Domain in H. jecorina Does Not Affect Extracellular β-Galactosidase Activity.
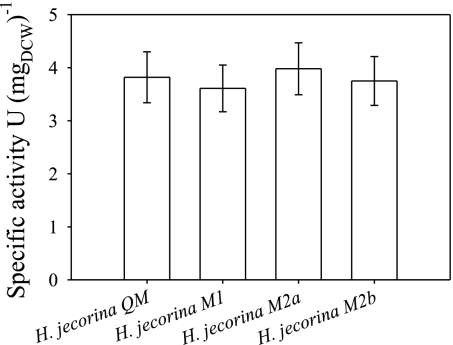
The phenotype of the GAL101068–2100 transformants is reminiscent of that of H. jecorina strains overexpressing the extracellular β-galactosidase BGA1 (21). To test whether the effect of mutarotase overexpression is direct or indirect, we measured the total extracellular β-galactosidase activity and the concentration of free d-galactose in the medium during growth of the reference strain and the three GAL101068–2100-expressing strains on lactose. The data show that extracellular β-galactosidase activity is not different in any of the mutants (Fig. 4), whereas we could not detect any free d-galactose in the medium of any of the H. jecorina strains (data not shown).
Fig. 4.
Specific extracellular β-galactosidase activity of H. jecorina QM9414 and GAL101068–2100-expressing strains M1, M2a, and M2b. Samples were taken 12 h after the transferring procedure.
Overexpression of the S. cerevisiae Gal10 AEP Domain in H. jecorina Does Not Affect the Expression of the Aldose Reductase.
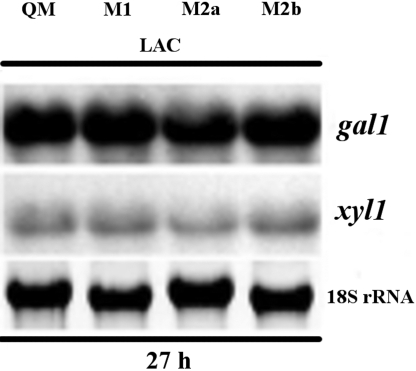
β-d-galactose arising from lactose can be catabolized by H. jecorina via two pathways, i.e., the reductive pathway and after mutarotation via the Leloir pathway (22). Consequently, we were interested to learn whether the activity of the reductive pathway would be reduced in the H. jecorina GAL101068–2100 transformants. Unfortunately, isotope labeling cannot be applied to this problem, because both pathways essentially conserve the individual carbon atoms in d-galactose. We therefore took an indirect means to test this hypothesis, i.e., we analyzed the transcripts of gal1 (galactokinase, which is induced by d-galactose but formed constitutively during growth on lactose), and xyl1 (d-xylose reductase, which is induced by lactose and to a lower level by d-galactose and galactitol and hence shows increased transcript levels when the pathway is in operation). The ratio of the relative transcript abundance of the two genes, however, was essentially similar (Fig. 5). Therefore, both pathways likely cooperate in a similar way in the parent strain and the GAL101068–2100-expressing strains.
Fig. 5.
Expression of the H. jecorina genes gal1 (encoding a galactokinase) and xyl1 (encoding an aldose reductase) on lactose in the QM9414 and GAL101068–2100-expressing H. jecorina strains M1, M2a, and M2b.
Overexpression of the S. cerevisiae Gal10 AEP Domain in H. jecorina Impairs Cellulase Gene Expression.
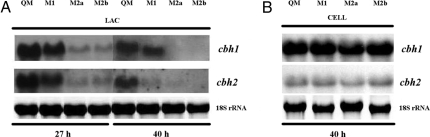
Having established that the overexpression of the Gal10 AEP domain specifically alters the rates of lactose utilization, we have examined whether it would have an effect on cellulase gene expression. To this end, we used the cbh1 and cbh2 genes, which encode the two major cellobiohydrolases of H. jecorina (CEL7A and CEL6A, respectively) and are expressed coordinately with most other cellulases (20) as a model. Fig. 6A shows that transcript abundance of the two cellulases was much lower in the GAL101068–2100-expressing strains and again inversely correlated with the abundance of the GAL101068–2100 transcript in them and their rate of growth on lactose.
Fig. 6.
Expression of H. jecorina cbh1 and cbh2 genes on different carbon sources. (A) Expression of the H. jecorina cellulase genes cbh1 and cbh2 on lactose at 27 h and at 40 h in the QM9414 and GAL101068–2100-expressing H. jecorina strains M1, M2a, and M2b. (B) Expression of the H. jecorina cellulase genes cbh1 and cbh2 on cellulose at 40 h in the QM9414 and GAL101068–2100-expressing H. jecorina strains M1, M2a, and M2b.
To rule out that this effect may be unspecific, we also tested cellulase gene transcription by the transformants during growth on cellulose (Fig. 6B). Under these conditions, cellulase transcript formation by the reference strain and the three transformants was essentially indistinguishable, providing additional evidence that the effect is caused by the impact of the S. cerevisiae Gal10 C terminus on d-galactose mutarotation.
Discussion
Galactose mutarotase activity has been observed in a wide range of organisms, including bacteria (3–5), plants (6, 7), fungi (8), and mammals (9–11). It is present in the cytoplasm of most cells, consistent with the fact that the in vivo rate of uncatalyzed mutarotation is insufficient for the metabolic needs of the organism as shown in Escherichia coli (3). In the present study, H. jecorina was shown to contain genes encoding three different putative mutarotases, of which two are in fact intracellular, whereas the third one (AEP3) is predicted to be a secreted protein. Interestingly, aep3 also shows the highest number of ESTs of all three putative mutarotase genes and is most abundantly transcribed during the early phase of growth on lactose. Transcripts of the other two intracellular mutarotases were not detected on lactose. We therefore conclude that in H. jecorina enzyme catalyzed reaction should have a marginal role in the mutarotation of β-d-galactose. We should note in this context that the strain used throughout these studies is a mutant strain that was selected for enhanced cellulase formation from the WT strain QM6a after two mutagenesis steps. The lack of mutarotase activity may therefore not be valid for the WT strain, but H. jecorina QM6a was not investigated here because of its low level of cellulase formation.
In any case, the present data provide clear evidence that the overexpression of a mutarotase in H. jecorina QM9414 enhances its growth rate on lactose. These findings are consistent with similar reports in E. coli (3) and indicate that the intracellular water activity is not high enough to permit efficient mutarotation in the absence of mutarotase enzyme activity. However, although the maintenance of the anomeric equilibrium of galactose is likely to be a major biochemical role for galactose mutarotase, it may also function in the metabolism of glucose and other sugars. Indeed, mutarotases have been shown to turn over various sugars, including d-glucose, d-fucose, d-quinovose, l-arabinose, and d-xylose (23, 24). It is therefore interesting that our finding of an enhanced rate of assimilation was obtained with lactose, but not with any of the monosaccharides tested (d-glucose, d-galactose, and others). Apart from our finding that the putative mutarotase-encoding aep1 is slightly transcribed on d-galactose, we suppose that in some cases this happens because these sugars already undergo chemical mutarotation before they are taken up by the cells. This notion seems to hold true also for d-galactose, whose β-anomer cannot be catabolized via the Leloir pathway, and yet the mutarotase-overproducing strains did not show any changes in growth on and assimilation of d-galactose. Alternatively, the catabolism of these sugars may proceed at a similar rate with both the α-anomer and β-anomer. In fact, fungal pentose metabolism is initiated by aldose reductase that acts on both sugars with the same affinity and rate (18). Hexokinase, on the other hand, has different affinities for α- and β-d-glucose (25), but both are still <50 μM and thus will unlikely influence the rate of glucose catabolism in a significant way. In the case of lactose, in contrast, β-galactosidase is rate limiting in the assimilation of lactose by H. jecorina (21), and therefore a higher portion of the released β-anomer would be transported into the cells, which is facilitated by the location of part of the β-galactosidase within the cell wall of the fungus and thus physically close to its transport system.
Remaining is the question of why the overexpression of mutarotase activity impairs cellulase gene expression. The fact that this phenomenon is observed on lactose, but not on cellulose, indicates that it is specific and confined to the presence of lactose. We have previously shown that the overexpression of the extracellular β-galactosidase gene bga1 also increased the assimilation of lactose by H. jecorina and at the same time impairs cellulase formation on lactose (21). In the strain overexpressing bga1, however, d-galactose accumulated in the medium, and it is conceivable that this accumulation allowed chemical mutarotation to occur at an increased rate and thus channel d-galactose preferentially through the Leloir pathway. The result is consistent with our findings that cellulase induction on d-galactose can occur only at a low growth rate, but at the same growth rate lactose still induces much higher cellulase expression (26). In contrast, overexpression of the mutarotase in H. jecorina had no effect on extracellular β-galactosidase formation and only a negligible effect on the transcription of gal1 and xyl1 and thus the activity of the two d-galactose-degrading pathways. We consider it therefore unlikely that the growth rate per se is the reason for the impaired cellulase formation. This notion is substantiated by the fact that between 30 and 50 h of growth the transformant with the highest mutarotase activity exhibits a specific growth rate of 0.04 h−1, which is well within the range of growth rates that support cellulase gene expression on lactose (26). We rather speculate that mutarotase specifically interferes with formation of the cellulase inducer from lactose. The chemical nature of this component is still unknown, but it is possible that this yet-to-be-identified molecule must contain d-galactose in the β-anomeric form.
Materials and Methods
Strains.
H. jecorina QM9414 (ATCC 26921) and the uridine-auxotrophic pyr4-negative mutant of it, TU-6 (27), were maintained on malt extract agar supplemented with uridine (10 mM) when required. Strains were grown in 500-ml Erlenmeyer flasks containing 100 ml of medium on a rotary shaker New Brunswick Scientific Co., (250 rpm; gyrotory shaker model G-10) at 30°C in the medium as described (28) with the appropriate carbon source at a final concentration of 15 g/liter.
For transcript analysis (except on cellulose), strains were pregrown on glycerol (1%, wt/vol) for 24 h. Mycelia were then harvested by filtration and washed with autoclaved tap water. Equal amounts of mycelia were transferred to flasks containing the appropriate carbon source (1.5%, wt/vol), and cultivation was continued for the periods indicated. For transcript analysis on cellulose, direct cultivation was used.
For the amplification of yeast mutarotase gene, S. cerevisiae strain S288C (ATCC 204508) was used. Cells were grown in YPD medium (1 g/liter yeast extract, 1 g/liter peptone, and 20 g/liter glucose) for 14 h at 30°C.
E. coli strain JM109 (Promega) was used for plasmid propagation.
Nucleic Acid Isolation and Hybridization.
Fungal mycelia were harvested by filtration, washed with distilled cold water, frozen, and ground under liquid nitrogen. For extraction of genomic DNA, plasmid DNA, and RNA, purification kits (Wizard Genomic DNA Purification Kit, PureYield Plasmid Midiprep System and SV Total RNA Isolation System, respectively; all from Promega) were used according to the manufacturer's protocol. Standard methods were used for electrophoresis, blotting, and hybridization of nucleic acids. A kit was used for performing Southern and Northern analysis (PCR DIG Probe Synthesis kit; Roche), following the manufacturer's protocol. For RT-PCR analysis, first-strand cDNA was synthesized by a RevertAid H minus First Strand cDNA Synthesis Kit (Fermentas) according to the manufacturer's protocol. cDNA was thereafter used as a template for PCR using gene-specific primers (Table 2). Translation-elongation factor-α (tef1) was used as a control.
Table 2.
Primers used for the RT amplification of the three putative H. jecorina mutarotase genes and tef1 (translation elongation factor 1α)
| Gene | Oligonucleotide | Oligonucleotide sequence | Amplicon size, bp |
|---|---|---|---|
| aep1 | tre19341forw | 5′-ATACAACCTCTCTCTCTCTCTG-3′ | 942 |
| tre19341rev | 5′-ACGGTAGATTCACACACATAG-3′ | ||
| aep2 | tre42544 forw | 5′-TCACCTTCTCATCTTCACCAC-3′ | 740 |
| tre42544 rev | 5′-TGGTGGTTGTGAAGCAGC-3′ | ||
| aep3 | tre44592forw | 5′-CTGGCTGTCTTTGCTCTG-3′ | 790 |
| tre44592rev | 5′-TCCCACTCTGCTCAAACC-3′ | ||
| tef1 | tef1forw | 5′-TCATCGTCGCCATCAACAAG-3′ | 877 |
| tef1rev | 5′-TCGACGGCCTTGATGACAC-3′ |
Construction of a Mutarotase Expression Vector.
The S. cerevisiae GAL10 part (nucleotides 1068–2100 relative to the ATG) encoding the mutarotase was expressed under the expression signals of the H. jecorina pki1 (pyruvate kinase) promoter and the cbh2 (cellobiohydrolase 2) terminator region. Therefore the GAL10 part was amplified from S. cerevisiae genomic DNA by PCR using the primers MRfw (5′-GTTATCTAGAATGGAGGCCAGATTTTCC-3′) and MRrev (5′-AGCTATGCATTCAGGAAAATCTGTAGAC-3′), introducing a START codon (ATG) plus an XbaI restriction site, and an NsiI restriction site, respectively. The PCR amplification protocol consisted of an initial denaturation step (2 min, 95°C) followed by 30 cycles with a denaturation step (1 min, 95°C), an annealing step (1 min, 50°C) and an elongation step (1.5 min, 72°C). Reaction was completed with a final 10-min elongation step at 72°C. The resulting 1,043 bp GAL101068–2100 fragment was ligated into the XbaI and NsiI sites of the vector pRLMex30 (29). As a genetic marker the H. jecorina 2.7-kb SalI pyr4 fragment (27) was introduced in the XhoI site of this vector, and the resulting plasmid was designated pGAL10Kicsi. Sequence of the GAL101068–2100 fragment was confirmed by sequencing (MWG).
Construction of a Mutarotase-Expressing H. jecorina Strain.
The expression cassette of GAL101068–2100 (Fig. 2A) was amplified by M13 primers (Promega), and the ≈5.5-kb PCR amplicon was subsequently used for transformation of H. jecorina TU-6 as described (27). Approximately 10 μg of DNA were used for transformation. Twenty-four primary colonies were obtained, of which eight grew repeatedly on uridine-free MM1 medium (30) that was used to select for transformants.
Southern analysis was used to verify the integration of the construct and determine the copy number. Chromosomal DNA was digested with SalI, which has a single restriction site in GAL101068–2100 only (Fig. 2A). pGAL10Kicsi linearized with XhoI was used as a control. Primers MRfw and MRrev (used also for the amplification of GAL101068–2100) were used for probe construction. Copy number (n) of GAL101068–2100 was hence determined by the number of n + 1 SalI fragments, respectively.
Analytical Methods.
Mycelial dry weight was determined by withdrawing 2 × 5-ml aliquots from the culture, doing suction filtration through a preweighed glass wool filter, and drying in an oven at 80°C to constant weight. Data were averaged and deviated by not >14%.
The concentration of d-glucose, d-galactose, and lactose was determined by HPLC analysis, using an H+ exchange column (Bio-Rad Aminex HPX-H+), with 10 mM H2SO4 at 55°C as mobile phase with isocratic elution and a refractive index detection.
The specific activity of the mutarotase was assayed by using the NAD+ and β-d-glucose dehydrogenase coupled assay (13). Specific enzyme activities are related to mg protein, which was determined by a modified Lowry method (31), using BSA for calibration.
The specific activity of the extracellular β-galactosidase was determined with o-nitrophenyl-β-d-galactopyranoside as substrate (21). Specific enzyme activities are related to mg dry cell mass.
Reproducibility.
Analytical data are means of three to five independent experiments. The data were analyzed by Sigmaplot (SPSS), and standard deviations were determined for each procedure. The SD values were always <14% of the means.
Chemicals.
All chemicals were of analytical grade, and, except where noted otherwise, were purchased from Sigma-Aldrich.
Acknowledgments.
This project was carried out under the framework of Austrian–Hungarian Intergovernmental Science and Technology Cooperation Programs 25/2005 and AT-18/2007. L.K. is supported by Hungarian Scientific Research Fund Grants F042602 and K67667 and Bolyai János Research Scholarship BO/00107/06. E.F. is supported by National Office for Research and Technology Grant A2-2006-0017) and Hungarian Scientific Research Fund Grant D048617. Work by B.S. and C.P.K. was financed by Austrian Science Foundation Grant FWF P19690.
Footnotes
The authors declare no conflict of interest.
References
- 1.Caputto R, Leloir LF, Cardini CE, Paladini AC. Isolation of the coenzyme of the galactose phosphate-glucose phosphate transformation. J Biol Chem. 1950;184:333–350. [PubMed] [Google Scholar]
- 2.Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885–43888. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 3.Bouffard GG, Rudd KE, Adhya SL. Dependence of lactose metabolism upon mutarotase encoded in the gal operon in Escherichia coli. J Mol Biol. 1994;244:269–278. doi: 10.1006/jmbi.1994.1728. [DOI] [PubMed] [Google Scholar]
- 4.Wallenfels K, Hucho F, Herrmann K. Enzymatically catalyzed mutarotation of aldoses: Studies on aldose 1-epimerase from E. coli. Biochem Z. 1965;343:307–325. [PubMed] [Google Scholar]
- 5.Erlandson KA, Delamarre SC, Batt CA. Genetic evidence for a defective xylan degradation pathway in Lactococcus lactis. Appl Environ Microbiol. 2001;67:1445–1452. doi: 10.1128/AEM.67.4.1445-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey JM, Fishman PH, Pentchev PG. Mutarotase in higher plants: Distribution and properties. Science. 1966;152:1270–1272. doi: 10.1126/science.152.3726.1270. [DOI] [PubMed] [Google Scholar]
- 7.Bailey JM, Fishman PH, Pentchev PG. Studies on mutarotases. I. Purification and properties of a mutarotase from higher plants. J Biol Chem. 1967;242:4263–4269. [PubMed] [Google Scholar]
- 8.Bentley R, Bhate DS. Mutarotase from Penicillium notatum. I. Purification, assay, and general properties of the enzyme. J Biol Chem. 1960;235:1219–1224. [PubMed] [Google Scholar]
- 9.Keston AS. Occurrence of mutarotase in animals: Its proposed relationship to transport and reabsorption of sugars and insulin. Science. 1954;120:355–356. doi: 10.1126/science.120.3113.355-a. [DOI] [PubMed] [Google Scholar]
- 10.Li LK. A method of purification and certain properties of hog kidney mutarotase. Arch Biochem Biophys. 1965;110:156–162. doi: 10.1016/0003-9861(65)90167-0. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JM, Pentchev PG, Woo J. Distribution of a “mutarotase” activity in rat tissues and possible function in active transport of sugars. Biochim Biophys Acta. 1965;94:124–129. doi: 10.1016/0926-6585(65)90015-4. [DOI] [PubMed] [Google Scholar]
- 12.Thoden JB, Holden HM. The molecular architecture of galactose mutarotase/UDP-galactose 4-epimerase from Saccharomyces cerevisiae. J Biol Chem. 2005;280:21900–21907. doi: 10.1074/jbc.M502411200. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar S, Ghatak J, Mukherji S, Bhattacharjee H, Bhaduri A. UDPgalactose 4-epimerase from Saccharomyces cerevisiae: A bifunctional enzyme with aldose 1-epimerase activity. Eur J Biochem. 2004;271:753–759. doi: 10.1111/j.1432-1033.2003.03974.x. [DOI] [PubMed] [Google Scholar]
- 14.Gatz C, Altschmied J, Hillen W. Cloning and expression of the Acinetobacter calcoaceticus mutarotase gene in Escherichia coli. J Bacteriol. 1986;168:31–39. doi: 10.1128/jb.168.1.31-39.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson I, Tjerneld F, Hahn-Hägerdal B. Fungal cellulolytic enzyme production: A review. Proc Biochem. 1991;26:65–74. [Google Scholar]
- 16.Seiboth B, Hofmann G, Kubicek CP. Lactose metabolism and cellulase production in Hypocrea jecorina: The gal7 gene, encoding galactose-1-phosphate uridylyltransferase, is essential for growth on galactose but not for cellulase induction. Mol Genet Genomics. 2002;267:124–132. doi: 10.1007/s00438-002-0654-9. [DOI] [PubMed] [Google Scholar]
- 17.Seiboth B, et al. The galactokinase of Hypocrea jecorina is essential for cellulase induction by lactose but dispensable for growth on d-galactose. Mol Microbiol. 2004;51:1015–1025. doi: 10.1046/j.1365-2958.2003.03901.x. [DOI] [PubMed] [Google Scholar]
- 18.Seiboth B, Gamauf C, Pail M, Hartl L, Kubicek CP. The d-xylose reductase of Hypocrea jecorina is the major aldose reductase in pentose and d-galactose catabolism and necessary for β-galactosidase and cellulase induction by lactose. Mol Microbiol. 2007;66:890–900. doi: 10.1111/j.1365-2958.2007.05953.x. [DOI] [PubMed] [Google Scholar]
- 19.Seiboth B, Karaffa L, Sándor E, Kubicek CP. The Hypocrea jecorina gal10 (uridine 5′-diphosphate-glucose 4-epimerase-encoding) gene differs from yeast homologues in structure, genomic organization, and expression. Gene. 2002;295:143–149. doi: 10.1016/s0378-1119(02)00834-x. [DOI] [PubMed] [Google Scholar]
- 20.Foreman PK, et al. Transcriptional regulation of biomass-degrading enzymes in the filamentous fungus Trichoderma reesei. J Biol Chem. 2003;278:31988–31997. doi: 10.1074/jbc.M304750200. [DOI] [PubMed] [Google Scholar]
- 21.Seiboth B, et al. Role of the bga1-encoded extracellular β-galactosidase of Hypocrea jecorina in cellulase induction by lactose. Appl Environ Microbiol. 2005;71:851–857. doi: 10.1128/AEM.71.2.851-857.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seiboth B, Pakdaman BS, Hartl L, Kubicek CP. Lactose metabolism in filamentous fungi: How to deal with an unknown substrate. Fung Biol Rev. 2007;21:42–48. [Google Scholar]
- 23.Thoden JB, Kim J, Raushel FM, Holden HM. Structural and kinetic studies of sugar binding to galactose mutarotase from Lactococcus lactis. J Biol Chem. 2002;277:45458–45465. doi: 10.1074/jbc.M208395200. [DOI] [PubMed] [Google Scholar]
- 24.Bailey JM, Fishman PH, Pentchev PG. Studies on mutarotases. 3. Isolation and characterization of a mutarotase from bovine kidney cortex. J Biol Chem. 1969;244:781–788. [PubMed] [Google Scholar]
- 25.Wurster B, Hess B. The reaction of hexokinase with equilibrated d-glucose. Eur J Biochem. 1973;36:68–71. doi: 10.1111/j.1432-1033.1973.tb02885.x. [DOI] [PubMed] [Google Scholar]
- 26.Karaffa L, et al. d-Galactose induces cellulase gene expression in Hypocrea jecorina at low growth rates. Microbiology. 2006;152:1507–1514. doi: 10.1099/mic.0.28719-0. [DOI] [PubMed] [Google Scholar]
- 27.Gruber F, Visser J, Kubicek CP, de Graaff LH. The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr Genet. 1990;18:71–76. doi: 10.1007/BF00321118. [DOI] [PubMed] [Google Scholar]
- 28.Mandels MM, Andreotti RE. The cellulose to cellulase fermentation. Proc Biochem. 1978;13:6–13. [Google Scholar]
- 29.Mach RL, Schindler M, Kubicek CP. Transformation of Trichoderma reesei based on hygromycin B resistance using homologous expression signals. Curr Genet. 1994;25:567–570. doi: 10.1007/BF00351679. [DOI] [PubMed] [Google Scholar]
- 30.Penttilä M, Nevalainen H, Rättö M, Salminen E, Knowles J. A versatile transformation system for the cellulolytic filamentous fungus Trichoderma reesei. Gene. 1987;61:155–164. doi: 10.1016/0378-1119(87)90110-7. [DOI] [PubMed] [Google Scholar]
- 31.Peterson GL. Determination of total protein. Methods Enzymol. 1983;91:86–105. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]