Abstract
EB1 (end-binding protein 1) is a key player in the regulation of microtubule dynamics. In concert with its binding partners, adenomatous polyposis coli and p150glued, EB1 plays a crucial role in a variety of microtubule-based cellular processes. In this study we have identified in a yeast two-hybrid screen the mitotic kinase and chromosome passenger protein Aurora-B as a binding partner of EB1. GST pull-down and immunoprecipitation experiments reveal a specific interaction between Aurora-B and EB1 both in cells and in vitro. Immunofluorescence microscopy shows that these two proteins colocalize on the central spindle in anaphase and in the midbody during cytokinesis. Kinase assays using both immunoprecipitated and purified Aurora-B demonstrate that EB1 is not a substrate of Aurora-B. Rather, EB1 positively regulates Aurora-B kinase activity. EB1 overexpression remarkably enhances Aurora-B activity and knockdown of its expression impairs Aurora-B activity. Our data further show that EB1 is able to protect Aurora-B from dephosphorylation/inactivation by protein phosphatase 2A (PP2A) by blocking PP2A binding to Aurora-B. These findings establish Aurora-B as an EB1-interacting protein and suggest that EB1 stimulates Aurora-B activity through antagonizing its dephosphorylation/inactivation by PP2A.
Keywords: histone H3, yeast two-hybrid
EB1 is a microtubule tip-associated protein that localizes at both the growing plus-ends of microtubules and the centrosome (1, 2). As an important regulator of microtubule dynamics, EB1 is critically involved in many microtubule-mediated cell activities, such as establishment and maintenance of cell polarity, search and capture of chromosomes during mitosis, and positioning of the mitotic spindle during asymmetric cell divisions (2–4). EB1 was originally identified as a protein interacting with the adenomatous polyposis coli (APC) tumor suppressor protein (5). The EB1–APC complex has been shown to increase microtubule polymerization and stabilize microtubule–kinetochore interactions in mitosis (6). In addition, the interaction of EB1 with APC appears to be required for efficient cell migration (7). Another protein that interacts with EB1 is p150glued, a component of the dynein/dynactin complex (8). The EB1–p150glued interaction is important for controlling microtubule dynamic properties and for anchoring microtubules at their nucleation sites (9).
In addition to its role in regulating microtubule dynamics and microtubule-based cellular processes, EB1 has recently been implicated in tumorigenesis. For example, proteomic studies using two-dimensional gel electrophoresis identified EB1 overexpression in human gastric carcinoma and hepatocellular carcinoma (10, 11). In addition, RT-PCR and Western blot analyses revealed the up-regulation of EB1 in esophageal squamous cell carcinoma (ESCC) (12). Elevation of EB1 expression in ESCC has been demonstrated to promote cell proliferation, and this effect has been attributed to the interference with β-catenin–APC interaction, which leads to enhanced β-catenin transcriptional activity (12). However, given the essential role for EB1 in a wide spectrum of cellular processes, it is conceivable that EB1 may promote cancer cell proliferation through alternative mechanisms in addition to the APC/β-catenin pathway.
To better understand EB1 function, we sought to look for additional EB1-interacting proteins. By yeast two-hybrid screening, we have identified Aurora-B, a mitotic kinase and chromosome passenger protein (13), as a binding partner of EB1. Our data reveal that EB1 interacts with Aurora-B both in cells and in vitro and promotes Aurora-B kinase activity through blocking its inactivation by PP2A.
Results
EB1 Interacts with Aurora-B both in Cells and in Vitro.
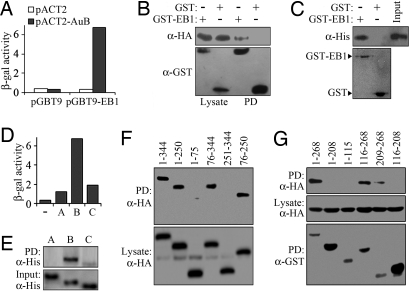
To identify proteins that interact with EB1, we set up a yeast two-hybrid assay with EB1 as bait and screened a HeLa cell cDNA library. From a total of 6 × 106 colonies, we identified 11 cDNAs that allowed yeast cell growth on the selective medium. Five of these positive cDNAs encoded different-length fragments of the mitotic kinase Aurora-B. We found that yeast cells transformed with Aurora-B were able to grow on the selective medium and exhibited robust β-galactosidase activity in an EB1-dependent manner (Fig. 1A), indicating an interaction of Aurora-B with EB1 in yeast.
Fig. 1.
Characterization of the interaction between EB1 and Aurora-B. (A) β-Galactosidase activity in yeast cells transformed with the bait plasmid pGBT9-EB1 or the pGBT9 vector, together with pACT2-Aurora-B or pACT2. (B) 293T cells were transfected with pCMV-HA-Aurora-B and pEBG-EB1 or the pEBG vector. GST pull-down (PD) and Western blotting were then performed to examine the interaction between EB1 and Aurora-B. (C) EB1 and Aurora-B interact in vitro. Bacterially purified His-Aurora-B was incubated with bacterially purified GST-EB1 or GST immobilized on glutathione Sepharose beads. The presence of His-Aurora-B in the pull-down preparation was examined by Western blotting. The levels of GST-EB1 and GST used in the pull-down assay were examined by Coomassie blue staining. (D) β-Galactosidase activity in yeast cells transformed with pGBT9-EB1 and pACT2-Aurora-A, pACT2-Aurora-B, pACT2-Aurora-C, or the pACT2 vector. (E) Purified His-Aurora-A, His-Aurora-B, or His-Aurora-C was incubated with purified GST-EB1 immobilized on glutathione Sepharose beads. The presence of Aurora kinases in the pull-down preparation was examined by Western blotting. (F) Cells were transfected with pEBG-EB1 and plasmids that express various truncated forms of Aurora-B tagged with HA. GST pull-down and Western blotting were then performed to characterize the EB1 interaction domain on Aurora-B. (G) Cells were transfected with pCMV-HA-Aurora-B and plasmids that express various truncated forms of EB1 tagged with GST. GST pull-down and Western blotting were then performed to characterize the Aurora-B interaction domain on EB1.
We next investigated whether EB1 and Aurora-B interact in mammalian cells. Cells were transfected with plasmids expressing HA-Aurora-B and GST-EB1, and cell lysate was then analyzed by GST pull-down assay. As shown in Fig. 1B, GST-EB1 but not GST alone was able to pull down HA-Aurora-B. The interaction of EB1 with Aurora-B was also confirmed by the presence of GST-EB1 in HA-Aurora-B immunoprecipitate (data not shown). To test whether EB1 interacts with Aurora-B in vitro, bacterially purified His-Aurora-B was incubated with bacterially purified GST-EB1 or GST immobilized on glutathione Sepharose beads, and a GST pull-down assay was performed. His-Aurora-B was detected in the pull-down preparation of GST-EB1 but not in that of GST (Fig. 1C). Thus, EB1 and Aurora-B could interact both in cells and in vitro.
We then asked whether EB1 interacts with Aurora-A and Aurora-C, two other members of the Aurora family that are similar to Aurora-B in structure (13). Yeast cells transformed with Aurora-A or Aurora-C together with EB1 were able to grow on the selective medium. However, they exhibited much weaker β-galactosidase activity compared with Aurora-B-transformed cells (Fig. 1D). GST pull-down assay using purified proteins further revealed that, although EB1 interacted strongly with Aurora-B, its interaction with Aurora-C was rather modest, and no EB1 interaction was detected for Aurora-A (Fig. 1E). These data demonstrate a specific interaction of EB1 with Aurora-B.
To identify the EB1 interaction domain on Aurora-B, cells were transfected with plasmids that express various truncated forms of Aurora-B tagged with HA, together with the GST-EB1- expressing plasmid. GST pull-down assay revealed that amino acid sequences 1–344 (full-length), 1–250, 76–344, and 76–250 of Aurora-B were able to interact with GST-EB1 but that sequences 1–75 and 251–344 were unable to interact (Fig. 1F), indicating the importance of the catalytic domain (76–250) of Aurora-B in mediating its interaction with EB1. Similarly, by GST pull-down assay we found that the C-terminal tail (209–268) of EB1 was critical for its interaction with Aurora-B (Fig. 1G).
Endogenous EB1 Interacts with Endogenous Aurora-B.
To investigate whether endogenous EB1 and Aurora-B interact, immunofluorescence microscopy was performed by using specific antibodies against these two proteins. In agreement with previous findings (1, 2, 13), Aurora-B was found to locate at the kinetochore/centromere region of chromosomes in early phases of mitosis, relocate to the central spindle in anaphase, and enrich at the midbody during cytokinesis, and EB1 was found to locate mainly on microtubule ends throughout mitosis (Fig. 2A). Strikingly, Aurora-B colocalized with EB1 on the central spindle in anaphase and in the midbody during cytokinesis (Fig. 2A).
Fig. 2.
Endogenous EB1 interacts with endogenous Aurora-B. (A) Immunofluorescence microscopy showing the localization of EB1 (red), Aurora-B (green), and DNA (blue) in CV1 cells. EB1 and Aurora-B were stained with mouse monoclonal and rabbit polyclonal antibodies, respectively, and DNA was stained with DAPI. (B) HeLa cell lysate was immunoprecipitated (IP) with anti-Aurora-B antibody, anti-EB1 antibody, or IgG control. The interaction between EB1 and Aurora-B was then examined by Western blot analysis of the precipitate. (C) HeLa cells were transfected with control (luciferase), Aurora-B, INCENP, survivin, or borealin siRNAs. The interaction of EB1 with Aurora-B was examined by immunoprecipitation of cell lysate with anti-Aurora-B antibody and Western blot analysis of the precipitate with anti-EB1 antibody.
The interaction between endogenous EB1 and Aurora-B was further investigated by immunoprecipitation. As shown in Fig. 2B, EB1 was detected in the Aurora-B immunoprecipitate, and Aurora-B was also present in the EB1 immunoprecipitate. The coimmunoprecipitation of endogenous EB1 with Aurora-B was significantly compromised by Aurora-B-specific siRNA but not by siRNAs against the chromosome passenger partners of Aurora-B, including inner centromere protein (INCENP), survivin, and borealin (Fig. 2C). These data suggest that the interaction between Aurora-B and EB1 is not mediated by these Aurora-B binding partners.
EB1 Is Not a Substrate of Aurora-B.
Aurora-B is known to function in mitosis primarily through the phosphorylation of substrate proteins (14, 15). For example, Aurora-B is responsible for the phosphorylation of histone H3 on serine-10, one of the classic modifications of chromatin during mitosis (16). In addition to H3, many other proteins have been identified as substrates of Aurora-B, such as CENP-A, myosin II regulatory light chain, and INCENP (13). To investigate whether Aurora-B phosphorylates EB1, cells were transfected with HA-Aurora-B, and cell lysate was immunoprecipitated with anti-HA antibody. Aurora-B kinase assay was then performed on immunoprecipitated HA-Aurora-B using purified GST, GST-EB1, or GST-H3 as the substrate. As shown in Fig. 3A Top, the HA-Aurora-B immunoprecipitate was able to phosphorylate GST-H3 but not GST-EB1 or GST. Similarly, Aurora-B kinase assay performed on bacterially purified His-Aurora-B revealed the ability of Aurora-B to phosphorylate GST-H3 but not GST-EB1 or GST (Fig. 3A, Middle). These results indicate that EB1 is not a substrate of Aurora-B.
Fig. 3.
EB1 is not a substrate of Aurora-B and knockdown of its expression impairs Aurora-B kinase activity. (A) Aurora-B kinase assays were performed on HA-Aurora-B immunoprecipitate (AuB IP) or purified His-Aurora-B (AuB), using GST, GST-EB1, or GST-histone H3 as the substrate. The reaction mixture was subjected to SDS/PAGE and analyzed by autoradiography. The levels of GST-fusion proteins used in the kinase assays were examined by Coomassie blue staining. (B) Western blots showing the decrease of EB1 expression by three different siRNAs, including two targeting the coding sequence (E-1 and E-2) and one targeting the untranslated region (E-3). (C) HeLa cells transfected with control or EB1 siRNAs were examined by immunofluorescence microscopy with a phospho-H3 (serine-10)-specific antibody. Total cell population was detected by staining cells with DAPI. (D) Cells were transfected with the indicated siRNAs and pCMV-HA-Aurora-A, pCMV-HA-Aurora-B, or pCMV-HA-Aurora-C. Kinase assays were then performed on HA-Aurora-A, HA-Aurora-B, or HA-Aurora-C immunoprecipitate, using H3 as the substrate. The reaction mixtures were subjected to SDS/PAGE, and H3 phosphorylation was analyzed by autoradiography. The levels of HA-Aurora-B immunoprecipitate used in the kinase assays were examined by Western blotting with anti-HA antibody. (E) Cells were transfected with control siRNA or EB1 siRNA (E-3) and pCMV-HA-Aurora-B, together with GST or GST-EB1. Kinase assays were performed on HA-Aurora-B immunoprecipitate as in D. The levels of HA-Aurora-B immunoprecipitate used in the kinase assays were examined by Western blotting.
EB1 Promotes Aurora-B Kinase Activity.
The kinase activity of Aurora-B is known to be regulated by its association with binding partners (14, 15). To test whether the association of EB1 also affects the kinase activity of Aurora-B, we inhibited EB1 expression by three different siRNAs (Fig. 3B). In agreement with previous findings (17, 18), we found that EB1 siRNA did not affect mitotic progression although spindle positioning was affected [supporting information (SI) Fig. S1]. Immunofluorescence microscopy was performed to examine the phosphorylation of H3 on serine-10 in EB1 siRNA-treated cells. Using a phospho-H3 (serine-10)-specific antibody, we found that H3 phosphorylation on serine-10 was decreased by all three EB1 siRNAs (Fig. 3C).
In addition to Aurora-B, Aurora-A and Aurora-C are also able to phosphorylate H3 on serine-10 (19). To investigate whether Aurora-B is mainly responsible for the decrease of H3 phosphorylation by EB1 siRNAs, cells were transfected with EB1 siRNAs and plasmids encoding HA-Aurora-A, HA-Aurora-B, or HA-Aurora-C. Kinase assays were then performed on each HA-Aurora immunoprecipitate using purified H3 as the substrate. We found that EB1 siRNAs significantly reduced the kinase activity of Aurora-B but not that of Aurora-A or Aurora-C (Fig. 3D). Furthermore, the decrease of Aurora-B activity by EB1 siRNA targeting the untranslated region could be significantly rescued by reexpression of EB1 (Fig. 3E). These results indicate that EB1 may function as a positive regulator of Aurora-B but not Aurora-A or Aurora-C.
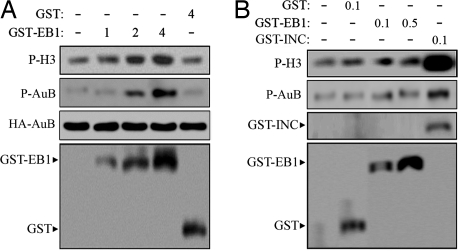
To further investigate the role of EB1 in regulating Aurora-B activity, cells were transfected with HA-Aurora-B and GST-EB1 or GST. Aurora-B kinase assay was performed on HA-Aurora-B immunoprecipitate using H3 as the substrate. GST-EB1 was found to stimulate Aurora-B activity in a dose-dependent manner, whereas GST alone had no effect even at a high dose (Fig. 4A). To investigate whether EB1 promotes Aurora-B activity in vitro, we performed Aurora-B kinase assay on purified His-Aurora-B in the presence of purified GST-EB1, GST-INCENP, or GST. As expected, GST-INCENP significantly increased Aurora-B activity in vitro; in contrast, no obvious effects were detected for GST-EB1 or GST (Fig. 4B). These findings indicate that EB1 promotes Aurora-B activity in cells through an indirect mechanism.
Fig. 4.
EB1 promotes Aurora-B phosphorylation and activity in cells but not in vitro. (A) Cells grown in 10-cm dishes were transfected with 5 μg of pCMV-HA-Aurora-B and 1, 2, or 4 μg of pEBG-EB1 or 4 μg of the pEBG vector. Aurora-B kinase assays were performed on HA-Aurora-B immunoprecipitate using H3 as the substrate. The reaction mixture was subjected to SDS/PAGE and analyzed by autoradiography. The levels of HA-Aurora-B immunoprecipitate used in the kinase assays were examined by Western blotting. The levels of phosphorylated Aurora-B were examined by a phospho-Aurora-B (threonine-232)-specific antibody. The expression of GST-EB1 and GST in cells was examined by Western blotting. (B) Aurora-B kinase assays performed on purified His-Aurora-B in the presence of purified GST (0.1 μg/μl), GST-EB1 (0.1 or 0.5 μg/μl), or GST-INCENP (0.1 μg/μl), using H3 as the substrate. The reaction mixture was subjected to SDS/PAGE and analyzed by autoradiography. The levels of phosphorylated Aurora-B were examined as in A. The levels of GST, GST-EB1, and GST-INCENP used in the kinase assays were examined by Coomassie blue staining.
Aurora-B activity is known to be regulated by phosphorylation (20, 21). In particular, the phosphorylation of Aurora-B on threonine-232 is reported to be important for kinase activity (21). Using a phospho-Aurora-B (threonine-232)-specific antibody, we found that EB1 increased Aurora-B phosphorylation on this site in cells but not in vitro (Fig. 4). This result indicates that EB1 may stimulate Aurora-B activity through increasing its phosphorylation.
EB1 Protects Aurora-B from Dephosphorylation/Inactivation by PP2A.
One possible mechanism for EB1 to promote Aurora-B phosphorylation and activity is that EB1 may stimulate the association of Aurora-B with INCENP and survivin, two positive regulators of Aurora-B (14, 15). It is also possible that EB1 may affect the localization of Aurora-B or its regulators. Immunoprecipitation and immunofluorescence microscopy showed, however, that alteration of EB1 expression did not obviously affect the association of Aurora-B with INCENP or survivin (Fig. S2) or the localization of these proteins (Fig. S3 and Fig. S4), thus ruling out these possibilities.
Another possible way for EB1 to increase Aurora-B phosphorylation and activity is that EB1 may counteract the action of two negative regulators of Aurora-B, PP1 and PP2A (20). To investigate whether EB1 inhibits the negative role of PP1 and PP2A in regulating Aurora-B activity, cells were transfected with plasmids expressing HA-Aurora-B and GST-EB1 and then treated with PP1 or PP2A adenoviruses. In agreement with previous studies (20), we found that Aurora-B phosphorylation level and activity were largely decreased by PP1 and PP2A (Fig. 5A). Importantly, GST-EB1 significantly protected Aurora-B from dephosphorylation and inactivation by PP2A. In contrast, PP1-induced Aurora-B dephosphorylation and inactivation were not affected by GST-EB1 (Fig. 5A). In addition, GST alone and GST-EB1ΔC, which lacks the Aurora-B interaction domain, could not protect Aurora-B from dephosphorylation and inactivation by PP2A (Fig. 5A). Thus, the interaction of EB1 with Aurora-B is necessary for the protection from PP2A.
Fig. 5.
EB1 protects Aurora-B from dephosphorylation and inactivation by PP2A. (A) Cells were transfected for 18 h with pCMV-HA-Aurora-B and pEBG-EB1, pEBG-EB1ΔC, or the pEBG vector. Cells were then treated with PP1 or PP2A adenoviruses for 12 h. Aurora-B kinase assay was performed on HA-Aurora-B immunoprecipitate using H3 as the substrate. The reaction mixture was subjected to SDS/PAGE and analyzed by autoradiography. The levels of HA-Aurora-B immunoprecipitate, phosphorylated Aurora-B, and GST fusion proteins were examined as in Fig. 4A. (B) Cells were transfected for 18 h with pCMV-HA-Aurora-A and pEBG-EB1 or the pEBG vector. Cells were then treated with PP2A adenoviruses for 12 h. Aurora-A kinase assay was performed on HA-Aurora-A immunoprecipitate as in A. The levels of HA-Aurora-A immunoprecipitate and the expression of GST-EB1 and GST were examined by Western blotting. (C) Cell lysate was immunoprecipitated with anti-Aurora-B antibody, anti-EB1 antibody, or IgG control. The interaction between PP2A with Aurora-B and EB1 was then examined by Western blot analysis of the precipitate with anti-PP2A antibody. (D) Cells were transfected with pEBG-EB1, pEBG-EB1ΔC or the pEBG vector. The interaction of PP2A with Aurora-B was examined by immunoprecipitation of cell lysate with anti-Aurora-B antibody and Western blotting of the precipitate with anti-PP2A antibody.
In addition to Aurora-B, Aurora-A is also known to be inactivated by PP2A (22). To investigate whether EB1 also protects Aurora-A from inactivation by PP2A, cells were transfected with plasmids expressing HA-Aurora-A and GST-EB1 or GST. Cells were then treated with PP2A adenoviruses, and kinase assay was performed on HA-Aurora-A immunoprecipitate. As shown in Fig. 5B, GST-EB1 did not inhibit PP2A-induced Aurora-A inactivation. This result demonstrates the specificity of EB1 in protecting Aurora-B from inactivation by PP2A. In addition, the result indicates that EB1 does not directly inhibit PP2A activity. This conclusion was further supported by our immunoprecipitation experiment, which revealed that PP2A does not interact with EB1 in cells (Fig. 5C).
By immunofluorescence microscopy, we found that alteration of EB1 expression did not affect PP2A localization in cells (Fig. S5). Given that the phosphorylation site of Aurora-B, threonine 232, is in the EB1 interaction domain (Fig. 1G), a possible mechanism by which EB1 protects Aurora-B from PP2A is that EB1 may block PP2A binding to Aurora-B. We tested this possibility by immunoprecipitation. As shown in Fig. 5D, the interaction of PP2A with Aurora-B was largely blocked by GST-EB1 but not by GST-EB1ΔC or GST alone. This result thus supports the idea that EB1 protects Aurora-B from PP2A by block PP2A binding to Aurora-B. In addition, the result further demonstrates that the interaction of EB1 with Aurora-B is required for the protection from PP2A.
Discussion
EB1 is well known as a microtubule tip-associated protein. It functions together with the APC tumor suppressor and p150glued to modulate microtubule dynamics and is involved in a variety of microtubule-based cellular processes (1–4). Recent studies have shown that EB1 promotes cancer cell proliferation and have implicated EB1 in tumorigenesis (10–12), but the underlying molecular mechanisms remain elusive. In this study, our data demonstrate a previously unrecognized role for EB1 in promoting the kinase activity of Aurora-B. This is supported by the following evidence: (i) EB1 interacts specifically with Aurora-B both in cells and in vitro; (ii) overexpression of EB1 significantly enhances Aurora-B activity; (iii) knockdown of EB1 expression diminishes Aurora-B activity substantially; and (iv) EB1 protects Aurora-B from inactivation by PP2A. Given that Aurora-B activity is critical for cell proliferation and that increased Aurora-B activity is frequently detected in cancer (23, 24), the observed effect of EB1 in promoting cancer cell proliferation may result in part from its stimulation of Aurora-B activity, in addition to the increase of β-catenin transcriptional activity (12).
As a component of the chromosome passenger complex, Aurora-B is important for chromosome segregation and cytokinesis (13). This protein localizes at different places in the mitotic apparatus depending on the stage of mitosis (13, 14). The data presented in this study show that Aurora-B colocalizes with EB1 on the central spindle in anaphase and in the midbody during cytokinesis. It should be noted, however, that in the cell there is a pool of soluble EB1 not associated with microtubules (1). It is not impossible that a portion of soluble EB1 may interact with Aurora-B at other cellular sites or in other mitotic phases.
Mammalian cells have three Aurora kinases that present similar structures (13). However, the three Aurora kinases have quite different subcellular localizations and functions. In this study, our data reveal a robust interaction of EB1 with Aurora-B but a rather modest interaction with Aurora-C. In contrast, only minor (if there is any) EB1 interaction is found for Aurora-A. The distinct EB1 interaction patterns of the Aurora kinases are also reflected by different effects of EB1 on their kinase activities. Whereas knockdown of EB1 expression significantly decreases Aurora-B activity, it does not obviously affect the activities of Aurora-A and Aurora-C. These findings support the notion that the activities of Aurora kinases may employ fundamentally distinct regulatory mechanisms to perform different functions.
Our finding that EB1 promotes Aurora-B activity in cells but not in vitro suggests that EB1 exerts its effect on Aurora-B through an indirect mechanism. Our data further demonstrate that EB1 increases Aurora-B activity independent of Aurora-B association with INCENP and survivin or the localization of these proteins. Rather, EB1 stimulates Aurora-B activity by counteracting the negative effect of PP2A on Aurora-B activity. This finding reminds us of the action of TPX2 (targeting protein for Xenopus kinesin-like protein 2) in the regulation of Aurora-A kinase activity; TPX2 positively regulates Aurora-A activity by inhibiting its inactivation by PP1 (25). Intriguingly, our data show that EB1 protects Aurora-B from inactivation by PP2A but not PP1. In addition, EB1 does not protect Aurora-A from inactivation by PP2A. These findings, together with the report that TPX2 does not protect Aurora-B from inactivation by PP1, manifest the complexity and specificity of Aurora kinase regulation.
The present study also provides mechanistic insight into how EB1 antagonizes the action of PP2A. Our results show that EB1 does not protect Aurora-A from inactivation by PP2A, suggesting that EB1 is not a direct inhibitor of PP2A. Importantly, our data reveal that the interaction of EB1 with Aurora-B blocks PP2A binding to Aurora-B, which may underlie the ability of EB1 to inhibit Aurora-B inactivation by PP2A. Certainly, alternative mechanisms may exist for this action. In addition, although our results point out a critical role for the PP2A axis in mediating EB1 regulation of Aurora-B activity, it would not be surprising if a PP2A-independent mechanism were uncovered in the future.
Materials and Methods
Materials.
DAPI and antibodies against GST, HA, His, and β-actin were purchased from Sigma–Aldrich. Antibodies against Aurora-B, phospho-Aurora-B (threonine-232), and INCENP were from Abcam. Antibodies against EB1 (BD Transduction Laboratories), survivin (Santa Cruz Biotechnology), PP2A (Upstate Biotechnology), and phospho-histone H3 (serine-10) (Cell Signaling), and horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences), and rhodamine- and fluorescein-conjugated secondary antibodies (Jackson ImmunoResearch) were obtained. The mammalian expression plasmids for GST-EB1 and HA-tagged Aurora-A, Aurora-B, and Aurora-C were constructed by insertion of each individual cDNA in frame into pEBG and pCMV-HA vectors, respectively. The bacterial expression plasmids for GST-EB1 and His-tagged Aurora-A, Aurora-B, and Aurora-C were constructed by insertion of each cDNA into pGEX6P3 and pET21 vectors, respectively. The BL21 (DE3) strain of Escherichia coli was used to express the proteins, and protein purification was carried out by using glutathione Sepharose 4B beads according to the manufacturer's instructions (Promega).
Cells, siRNAs, and Adenoviruses.
Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. siRNA oligonucleotides were synthesized by RiboBio and transfected to cells with the Lipofectamine 2000 reagent (Invitrogen). Adenoviruses encoding PP1 and PP2A were prepared and amplified in low passage human embryonic kidney 293 cells as described previously (26).
Yeast Two-Hybrid Screening.
The yeast two-hybrid assay was conducted using the Matchmaker Gal4 two-hybrid system following the manufacturer's protocol (Clontech). In brief, the yeast strain AH109 was transformed with pGBT9-EB1, a bait vector encoding full-length EB1 fused to the DNA binding domain of Gal4, and a pACT2 vector-based HeLa cell cDNA library, which encodes proteins fused to the activation domain of Gal4. Primary transformants (6 × 106) were screened on the selective medium, and from this Aurora-B was isolated independently three times. The activity of β-galactosidase was measured by standard protocols.
GST Pull-Down and Immunoprecipitation.
Cells were transfected with pEBG-EB1 or the empty vector. The cell lysate was incubated with glutathione Sepharose 4B beads at 4°C for 2 h. The beads were washed extensively and boiled in the SDS loading buffer, and the proteins were detected by SDS/PAGE and Western blotting. For GST pull-down in vitro, GST or GST-EB1 fusion protein immobilized on glutathione Sepharose beads was incubated with purified His-Aurora-A, His-Aurora-B, or His-Aurora-C at 4°C for 2 h. For immunoprecipitation, cell lysate was incubated with antibody-coated agarose beads at 4°C for 2 h. The beads were washed and boiled in the SDS loading buffer, and the precipitated proteins were detected by SDS/PAGE and Western blotting.
Western Blotting.
Proteins were resolved by SDS/PAGE and transferred onto polyvinylidene difluoride membranes (Millipore) as described previously (27). The membranes were blocked in TBS containing 0.05% Tween 20 and 5% fat-free dry milk and incubated first with primary antibodies and then with horseradish peroxidase-conjugated secondary antibodies. Specific proteins were visualized with enhanced chemiluminescence detection reagent according to the manufacturer's instructions (Pierce).
Kinase Assays.
Aurora kinases immunoprecipitated from cells or purified from bacteria were incubated with purified histone H3 for 20 min at 30°C in the kinase assay buffer [20 mM Tris·HCl (pH 7.5), 10 mM MgCl2, 0.5 mM DTT, 0.1 mM EDTA] containing 50 μM ATP and 3 μCi of [γ-32P]ATP. Proteins in the reaction were separated by SDS/PAGE. The gels were dried and 32P was detected by autoradiography.
Immunofluorescence Microscopy.
Cells grown on glass coverslips were fixed with methanol for 3 min at −20°C (for EB1/Aurora-B co-staining) or fixed with 4% paraformaldehyde for 30 min at room temperature followed by permeabilization in 0.5% Triton X-100/PBS for 20 min (for other staining). Cells were then blocked with 2% BSA in PBS and incubated in succession with primary and secondary antibodies followed by staining with DAPI for 5 min. Coverslips were mounted with 90% glycerol in PBS and examined with an Olympus fluorescence microscope.
Supplementary Material
Acknowledgments.
We thank Drs. Jonathan Askham (University of Leeds, Leeds, UK) and Harish Joshi (Emory University, Atlanta, GA) for providing reagents and members of the J.Z. laboratory for discussion. This work was supported by grants from the National Key Scientific Program of China (2007CB914503 and 2007CB914802), the Tianjin Natural Science Foundation (07JCZDJC03000), the New Century Excellent Talents Program, the Ministry of Education (NCET-06-0217), and the National Natural Science Foundation of China (30600313).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710018105/DCSupplemental.
References
- 1.Vaughan KT. TIP maker and TIP marker; EB1 as a master controller of microtubule plus ends. J Cell Biol. 2005;171:197–200. doi: 10.1083/jcb.200509150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison EE. Action and interactions at microtubule ends. Cell Mol Life Sci. 2007;64:307–317. doi: 10.1007/s00018-007-6360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tirnauer JS, Bierer BE. EB1 proteins regulate microtubule dynamics, cell polarity, and chromosome stability. J Cell Biol. 2000;149:761–766. doi: 10.1083/jcb.149.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gundersen GG. Evolutionary conservation of microtubule-capture mechanisms. Nat Rev Mol Cell Biol. 2002;3:296–304. doi: 10.1038/nrm777. [DOI] [PubMed] [Google Scholar]
- 5.Su LK, et al. APC binds to the novel protein EB1. Cancer Res. 1995;55:2972–2977. [PubMed] [Google Scholar]
- 6.Nakamura M, Zhou XZ, Lu KP. Critical role for the EB1 and APC interaction in the regulation of microtubule polymerization. Curr Biol. 2001;11:1062–1067. doi: 10.1016/s0960-9822(01)00297-4. [DOI] [PubMed] [Google Scholar]
- 7.Wen Y, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 8.Berrueta L, Tirnauer JS, Schuyler SC, Pellman D, Bierer BE. The APC-associated protein EB1 associates with components of the dynactin complex and cytoplasmic dynein intermediate chain. Curr Biol. 1999;9:425–428. doi: 10.1016/s0960-9822(99)80190-0. [DOI] [PubMed] [Google Scholar]
- 9.Askham JM, Vaughan KT, Goodson HV, Morrison EE. Evidence that an interaction between EB1 and p150(Glued) is required for the formation and maintenance of a radial microtubule array anchored at the centrosome. Mol Biol Cell. 2002;13:3627–3645. doi: 10.1091/mbc.E02-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishigaki R, et al. Proteomic identification of differentially-expressed genes in human gastric carcinomas. Proteomics. 2005;5:3205–3213. doi: 10.1002/pmic.200401307. [DOI] [PubMed] [Google Scholar]
- 11.Fujii K, et al. Proteomic study of human hepatocellular carcinoma using two-dimensional difference gel electrophoresis with saturation cysteine dye. Proteomics. 2005;5:1411–1422. doi: 10.1002/pmic.200401004. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, et al. Overexpression of EB1 in human esophageal squamous cell carcinoma (ESCC) may promote cellular growth by activating beta-catenin/TCF pathway. Oncogene. 2005;24:6637–6645. doi: 10.1038/sj.onc.1208819. [DOI] [PubMed] [Google Scholar]
- 13.Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- 14.Adams RR, Carmena M, Earnshaw WC. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 2001;11:49–54. doi: 10.1016/s0962-8924(00)01880-8. [DOI] [PubMed] [Google Scholar]
- 15.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: Conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 16.Gurley LR, D'Anna JA, Barham SS, Deaven LL, Tobey RA. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978;84:1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- 17.Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell. 2005;16:4609–4622. doi: 10.1091/mbc.E05-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J. 2006;25:2814–2827. doi: 10.1038/sj.emboj.7601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crosio C, et al. Mitotic phosphorylation of histone H3: Spatio-temporal regulation by mammalian Aurora kinases. Mol Cell Biol. 2002;22:874–885. doi: 10.1128/MCB.22.3.874-885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugiyama K, et al. Aurora-B associated protein phosphatases as negative regulators of kinase activation. Oncogene. 2002;21:3103–3111. doi: 10.1038/sj.onc.1205432. [DOI] [PubMed] [Google Scholar]
- 21.Yasui Y, et al. Autophosphorylation of a newly identified site of Aurora-B is indispensable for cytokinesis. J Biol Chem. 2004;279:12997–13003. doi: 10.1074/jbc.M311128200. [DOI] [PubMed] [Google Scholar]
- 22.Walter AO, Seghezzi W, Korver W, Sheung J, Lees E. The mitotic serine/threonine kinase Aurora2/AIK is regulated by phosphorylation and degradation. Oncogene. 2000;19:4906–4916. doi: 10.1038/sj.onc.1203847. [DOI] [PubMed] [Google Scholar]
- 23.Katayama H, Brinkley WR, Sen S. The Aurora kinases: Role in cell transformation and tumorigenesis. Cancer Metastasis Rev. 2003;22:451–464. doi: 10.1023/a:1023789416385. [DOI] [PubMed] [Google Scholar]
- 24.Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora kinases in mitosis and tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- 25.Eyers PA, Erikson E, Chen LG, Maller JL. A novel mechanism for activation of the protein kinase Aurora A. Curr Biol. 2003;13:691–697. doi: 10.1016/s0960-9822(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, et al. Inhibition of the mitotic kinesin Eg5 up-regulates Hsp70 through the phosphatidylinositol 3-kinase/Akt pathway in multiple myeloma cells. J Biol Chem. 2006;281:18090–18097. doi: 10.1074/jbc.M601324200. [DOI] [PubMed] [Google Scholar]
- 27.Xuan C, et al. Regulation of microtubule assembly and stability by the transactivator of transcription protein of Jembrana disease virus. J Biol Chem. 2007;282:28800–28806. doi: 10.1074/jbc.M702823200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.