Abstract
Body weight is regulated by complex neurohormonal interactions between endocrine signals of long-term adiposity (e.g., leptin, a hypothalamic signal) and short-term satiety (e.g., amylin, a hindbrain signal). We report that concurrent peripheral administration of amylin and leptin elicits synergistic, fat-specific weight loss in leptin-resistant, diet-induced obese rats. Weight loss synergy was specific to amylin treatment, compared with other anorexigenic peptides, and dissociable from amylin's effect on food intake. The addition of leptin after amylin pretreatment elicited further weight loss, compared with either monotherapy condition. In a 24-week randomized, double-blind, clinical proof-of-concept study in overweight/obese subjects, coadministration of recombinant human leptin and the amylin analog pramlintide elicited 12.7% mean weight loss, significantly more than was observed with either treatment alone (P < 0.01). In obese rats, amylin pretreatment partially restored hypothalamic leptin signaling (pSTAT3 immunoreactivity) within the ventromedial, but not the arcuate nucleus and up-regulated basal and leptin-stimulated signaling in the hindbrain area postrema. These findings provide both nonclinical and clinical evidence that amylin agonism restored leptin responsiveness in diet-induced obesity, suggesting that integrated neurohormonal approaches to obesity pharmacotherapy may facilitate greater weight loss by harnessing naturally occurring synergies.
Keywords: pramlintide, metreleptin, adiposity, synergy, leptin resistance
The discovery of leptin in 1994 (1) revolutionized our understanding of the biological basis of body-weight regulation and raised hopes that this adipokine could be a breakthrough treatment for obesity. Although leptin plays a pivotal role in regulating energy homeostasis in rodents and humans, its pharmaceutical development as a stand-alone antiobesity agent has proven unsuccessful (2). Although leptin replacement elicits profound weight loss in leptin-deficient (Lepob/Lepob) mice and humans (3, 4), even high pharmacological doses elicit only marginal weight loss in non-leptin-deficient, diet-induced obese (DIO) rodents and humans (2, 5). The obese state is thus thought to be associated with “leptin resistance,” wherein overweight/obese individuals become insensitive to high circulating leptin concentrations (6). The mechanistic basis for leptin resistance is poorly understood, but rodent data implicate leptin transport saturation (7), leptin receptor down-regulation (8), and reduced hypothalamic postreceptor signaling (9, 10).
Amylin, a 37-aa peptide hormone cosecreted with insulin from pancreatic β-cells (11), binds specific receptors in the hindbrain area postrema (AP) that activate multiple central nervous system (CNS) regions to regulate both glucose and energy homeostasis (12). In obese humans, the amylin analog pramlintide elicited sustained reductions in food intake and body weight (13, 14). Amylin-induced weight loss in DIO rats that was observed to be fat-specific with relative preservation of lean mass was associated with increased proopiomelanocortin (POMC) gene expression in the arcuate nucleus (ARC) and was not accompanied by a compensatory decline in metabolic rate (15, 16).
Leptin is an adipocyte-derived, long-term adiposity signal, whereas islet- and gut-derived peptide hormones, such as amylin, secreted in response to meals generally function as short-term satiety signals. The potential interaction among long-term adiposity and short-term satiety signals is an area of interest.
Here we report a marked synergy for weight loss and decrease in adiposity with leptin and amylin coadministration in DIO rats. Mechanistic studies in rats pretreated with amylin suggest that leptin signaling within the hypothalamus and caudal hindbrain may modulate the observed weight loss synergy. Finally, in a 24-week clinical proof-of-concept study in overweight/obese subjects, coadministration of pramlintide with recombinant human leptin (R-metHuLeptin; metreleptin) yielded significantly greater weight loss than either agent alone.
Results
Amylin Restored Responsiveness to Peripheral Leptin in DIO Rats.
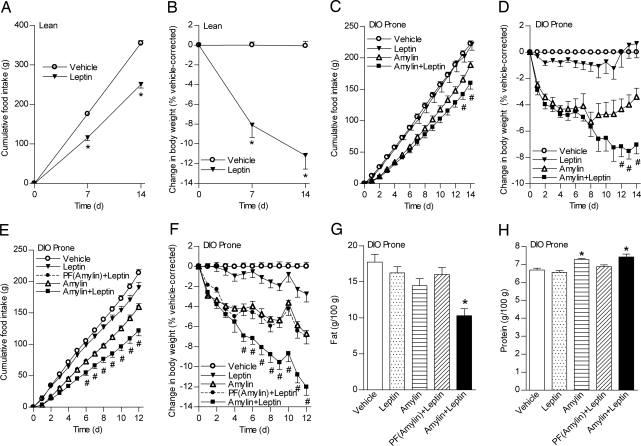
We first administered leptin to lean rats to determine an efficacious dose for suppressing food intake and weight gain. Leptin (500 μg·kg−1·d−1) significantly decreased food intake (≈30%; Fig. 1A) and vehicle-corrected weight gain (≈11%; Fig. 1B). This dose of leptin, which was highly effective in lean animals, had no significant effect on food intake or weight in DIO rats (Fig. 1 C and D). In contrast, amylin [100 μg·kg−1·d−1; ≈ED70 for weight loss (16)] significantly decreased food intake and weight in DIO rats relative to vehicle (P < 0.05; Fig. 1 C and D). Leptin coadministered with amylin further reduced food intake (by an additional ≈16%) and weight (by an additional ≈4%; Fig. 1D).
Fig. 1.
Synergy of amylin and leptin in DIO-prone rats. (A–F) Cumulative food intake (A, C, and E) and changes in body weight (B, D, and F) after administration in HSD rats (n = 4 per group) (A and B) or in DIO-prone rats (C–F) of leptin (filled inverted triangles), vehicle (open circles), amylin (open triangles), PF(amylin)+leptin (filled circles), or amylin+leptin (filled squares) for 14 d (n = 6 per group) (A–D) or for 12 d (n = 7 per group) (E and F). (G and H) Percentage body fat (G) and lean (H) after 12-d treatment in DIO-prone rats. Mean ± SE: *, P < 0.05 vs. vehicle controls; #, P < 0.05 vs. vehicle controls and monotherapies.
Energy expenditure (EE) and respiratory quotient (RQ) were assessed on days 6–8, when the weight curves of amylin- and amylin+leptin-treated animals began to diverge. Dark cycle and 24-hour RQ values were similarly reduced in amylin- and amylin+leptin-treated rats. Light cycle RQ was only decreased in amylin+leptin-treated rats (Table 1). Weight loss was not associated with decreased EE in either group. Despite a 5–6% decrease in weight, dark cycle EE increased significantly (≈10%) in rats treated with the combination (Table 1).
Table 1.
Respiratory quotient and energy expenditure
| Group | RQ |
EE, kcal·h−1·kg−1 |
||||
|---|---|---|---|---|---|---|
| a.m. | p.m. | 24 h | a.m. | p.m. | 24 h | |
| Vehicle | 0.80 ± 0.01 | 0.84 ± 0.01 | 0.82 ± 0.01 | 5.44 ± 0.12 | 6.33 ± 0.13 | 5.89 ± 0.11 |
| Leptin | 0.80 ± 0.01 | 0.84 ± 0.01 | 0.82 ± 0.01 | 5.85 ± 0.15 | 6.58 ± 0.11 | 6.23 ± 0.13 |
| Amylin | 0.78 ± 0.01 | 0.80 ± 0.01* | 0.79 ± 0.01* | 5.54 ± 0.10 | 6.64 ± 0.11 | 6.13 ± 0.08 |
| Amylin+leptin | 0.76 ± 0.01* | 0.80 ± 0.1* | 0.78 ± 0.01* | 5.83 ± 0.11 | 6.87 ± 0.11* | 6.26 ± 0.07 |
*Significant at P < 0.05 vs. vehicle control.
Amylin-Mediated Restoration of Leptin Responsiveness Is Not Explained by Caloric Restriction.
Because decreased food intake is the predominant mechanism of amylin-induced weight loss (15), we examined whether concurrent administration of leptin to animals pair-fed to the daily food intake of the amylin-treated group [PF(amylin)+leptin] might also yield synergistic weight loss. Again, leptin treatment was relatively ineffective in DIO rats, eliciting significant but small decreases in food intake (≈10%) and weight (≈2%). Amylin decreased food intake ≈25% and weight 6.5% relative to vehicle. PF(amylin)+leptin did not induce greater weight loss relative to amylin alone. In contrast, food intake (from day 6) and body weight (from day 5) were significantly reduced in amylin+leptin-treated rats relative to amylin monotherapy or PF(amylin)+leptin (Fig. 1 E and F). Amylin+leptin-treated rats had significantly decreased percentage adiposity (Fig. 1G) and increased percentage lean mass (protein) (Fig. 1H) relative to vehicle. Plasma amylin concentrations were similarly elevated in all amylin-treated groups; plasma leptin concentrations tended to be reduced by amylin and elevated similarly with leptin treatment [supporting information (SI) Table S1].
Other Anorexigenic Peptides Combined With Leptin.
The specificity of the amylin+leptin synergy was examined by combining leptin with PYY3–36 and the GLP-1/exendin-4 analog AC3174 (17)—peptide agonists that induce weight loss in DIO rodents (Fig. S1). By week 2, PYY3–36+leptin further reduced food intake and body weight relative to PYY3–36 alone. Food intake was also significantly reduced by AC3174 and AC3174+leptin but not by leptin alone. Although all three treatments significantly reduced vehicle-corrected body weight, AC3174+leptin did not significantly reduce food intake or body weight relative to AC3174 alone. Overall, neither AC3174 nor PYY3–36 added to leptin in DIO rats resulted in the synergistic weight loss that was observed with amylin.
Weight Loss with Amylin and/or Leptin After Amylin Pretreatment.
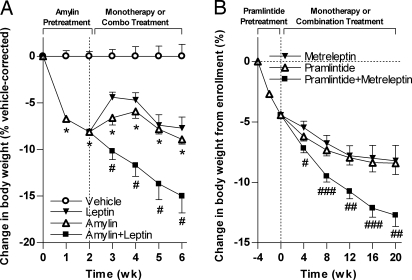
We next examined the effect of pretreatment vs. concurrent treatment with amylin on leptin responsiveness. Pretreatment (14 d) of amylin significantly reduced body weight by 7.0 ± 1.0% relative to vehicle (Fig. 2A). Rats pretreated with amylin and then switched to leptin alone initially regained some weight (to −4.4 ± 0.5%) but then maintained significant weight loss relative to vehicle (−7.7 ± 1.2%). In rats that remained on amylin monotherapy, weight loss reached a plateau (−8.9 ± 0.5% relative to vehicle). Amylin+leptin-treated rats continued to lose weight throughout the experiment, achieving a significantly greater weight loss (−15 ± 1.8%) than with amylin or leptin alone (P < 0.05).
Fig. 2.
Translational research findings: Weight loss effect of combined amylin and leptin agonism in DIO rats and overweight/obese humans. (A) Change in body weight for DIO rats pretreated for 14 d with amylin and then maintained on amylin (open triangles) or switched to either leptin monotherapy (filled inverted triangles) or amylin+leptin combination therapy (filled squares) for an additional 28 d. (B) Change in body weight for 93 evaluable human subjects pretreated with pramlintide for 4 weeks and then treated with pramlintide (open triangles), metreleptin (filled inverted triangles), or pramlintide+metreleptin combination (filled squares). Mean ± SE (A) and LS mean ± SE (B): *, P < 0.05 vs. vehicle controls; #, P < 0.01; ##, P < 0.01; ###, P < 0.001 vs. monotherapies.
Weight Loss with Pramlintide and/or Metreleptin in Overweight/Obese Humans.
Consistent with the weight loss pattern in the aforementioned rodent experiment, combination treatment with pramlintide and metreleptin in our clinical proof-of-concept study led to significantly greater weight loss than treatment with pramlintide or metreleptin alone (Fig. 2B). After a mean weight loss of ≈4.3% during the 4-week lead-in, weight loss from enrollment in the pramlintide and metreleptin monotherapy arms gradually declined, reaching stable weight loss plateaus of 8.4 ± 0.9% (7.9 ± 0.9 kg) and 8.2 ± 1.3% (7.4 ± 1.3 kg), respectively. By comparison, subjects who completed cotreatment with pramlintide+metreleptin continued to lose weight at a more rapid rate throughout the study, achieving a mean weight loss of 12.7 ± 0.9% (11.5 ± 0.9 kg). The difference between the pramlintide and pramlintide+metreleptin arms was statistically significant as early as week 4 and was also statistically significant for the more conservative intent-to-treat (ITT)–last observation carried forward (LOCF) analyses (pramlintide 7.5 ± 0.8%, 7.2 ± 0.7 kg; metreleptin 7.9 ± 1.0%, 7.2 ± 1.0 kg; pramlintide+metreleptin 10.8 ± 0.8%, 9.9 ± 0.7 kg; P < 0.05 vs. monotherapies). Although the rate of weight loss in the pramlintide+metreleptin arm decreased over time, a plateau was not reached by the end of the study. Consistent with previous clinical experience with pramlintide and metreleptin as single agents, the most common side effects seen with pramlintide+metreleptin combination treatment were injection site adverse events and nausea, which were mostly mild to moderate and transient in nature.
Amylin Enhanced Leptin Signaling in the Ventromedial Hypothalamus (VMH) and AP.
Leptin activation of signal transducer and activator of transcription 3 (STAT3) by phosphorylation, an accepted marker of Lepr activation, has been demonstrated in the ARC, VMH, and nucleus tractus solitarii (NTS) (18). High-fat feeding/hyperleptinemia is associated with diminished hypothalamic phospho-STAT3 (pSTAT3) signaling (9), chiefly within the ARC, whereas hindbrain activation is not impaired (10, 18). The synergy between amylin and leptin could be explained in part by amylin restoring hypothalamic sensitivity to leptin and/or augmenting leptin activation in leptin-sensitive regions within the hindbrain. Likewise, because leptin, but not amylin (19, 20), has a direct effect on adipocytes, amylin-induced weight loss could potentiate leptin action at the receptor or postreceptor level of the adipocyte.
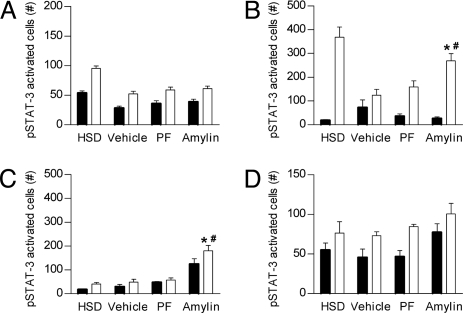
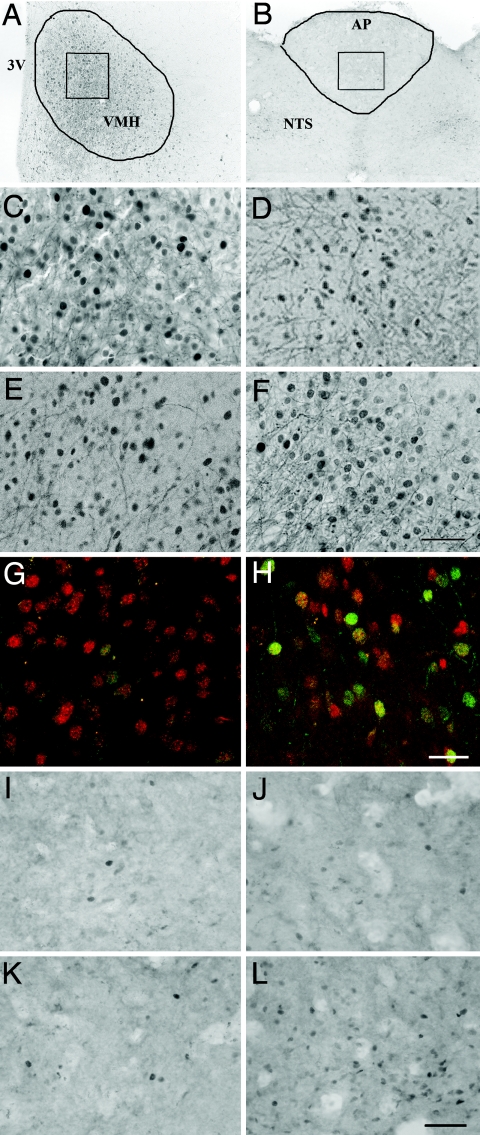
To explore these possibilities, vehicle-, PF(to the amylin group)-, or amylin-pretreated DIO rats (7 d) and a lean rat control group were injected with vehicle or leptin (15 mg/kg i.p., a dose eliciting diminished hypothalamic signaling in DIO-prone vs. DIO-resistant controls) (18). In DIO vs. lean rats, leptin-induced pSTAT3 expression was reduced by 46% in the ARC (Fig. 3A) and 67% in the VMH (Fig. 3B and Fig. 4 C and D). Amylin or PF pretreatment did not increase leptin-stimulated pSTAT3 immunoreactivity (pSTAT3-IR) in the ARC of DIO rats (Fig. 3A). However, amylin pretreatment significantly increased leptin-induced pSTAT3 signaling in VMH by 43% relative to vehicle and PF controls (Fig. 3B and Fig. 4 A and C–F). To clarify the phenotype of leptin-responsive VMH neurons in amylin-pretreated rats, we assessed whether pSTAT3 was colocalized with steroidogenic factor-1 (SF-1), a highly enriched protein in the VMH implicated in weight regulation (21, 22). A moderate population of VMH cells exhibited both pSTAT3 and SF-1 labeling after leptin administration (Fig. 4 G and H).
Fig. 3.
Induction of pSTAT3 expression by leptin (15 mg/kg i.p.; white bars) or vehicle (black bars) in arcuate (A), VMH (B), AP (C), and NTS adjacent to AP (rostral) (D) of lean HSD rats or in vehicle-, PF(to amylin)-, or amylin-pretreated (7 d) DIO-prone rats. Mean ± SE: *, P < 0.05 vs. vehicle; #, P < 0.05 vs. PF(amylin).
Fig. 4.
Effects of amylin pretreatment on leptin-stimulated pSTAT3. (A–F and I–L) Brightfield images of leptin (15 mg/kg i.p.)-stimulated pSTAT3 immunoreactivity (black dots) in VMH (bregma level −3.4 mm) (A and C–F) and AP (bregma level −13.7 mm) (B and I–L). For orientation, A and B show low-magnification representative images of the entire VMH (A) or AP (B). (G and H) Representative confocal fluorescent images show leptin-stimulated pSTAT3 labeling (green), SF-1 labeling (red), and pSTAT3/SF-1 colocalization (yellow-orange) in the VMH. (Scale bar, 25 μm.) Lean HSD rats (A–C and I) or vehicle-pretreated (D, G, and J), PF-pretreated (E and K), or amylin-pretreated (7 d) (F, H, and L) DIO-prone rats are shown. (Scale bars on brightfield images, 50 μm.)
In the AP, leptin treatment did not stimulate pSTAT3-IR in lean rats, vehicle-treated, or PF DIO rats (Fig. 3C and Fig. 4 B and I–K), whereas amylin-treated rats exhibited a significant ≈5-fold pSTAT3-IR induction (Fig. 3C and Fig. 4L). Although basal pSTAT3-IRs in the AP of vehicle and PF controls were similar, pSTAT3-IR was significantly up-regulated in the AP of amylin-treated rats (Fig. 3C). Acute leptin injection significantly increased pSTAT3-IR in amylin-pretreated rats, despite high basal pSTAT3-IR. In the surrounding NTS, basal pSTAT3 expression tended to be higher (nonsignificant) in rats pretreated with amylin (Fig. 3D), whereas at more caudal levels of the NTS, quantification of pSTAT3-IR revealed similar activation irrespective of pretreatment condition (Table S2).
Investigation of peripheral leptin functionality in white adipose tissue (WAT) revealed that, within this range of weight loss, neither pretreatment with amylin nor PF significantly changed mRNA expression levels of Lepr, Lep, or Socs-3 (Table S3). Acute leptin treatment significantly decreased Fas in both liver and fat, but this effect was not augmented by amylin pretreatment. In liver, acute leptin increased Lpl in vehicle, but not amylin-pretreated, animals. In WAT, amylin-mediated weight loss was associated with an induction of Lpl, which was decreased by leptin in both vehicle and amylin-treated groups (Table S3). We were unable to detect increased levels of leptin-induced pSTAT3 in WAT in amylin- or PF-pretreated rats (data not shown). Therefore, leptin regulation of peripheral tissue function does not appear to be augmented by amylin pretreatment.
Discussion
Although leptin plays a pivotal role in the physiological regulation of body weight, efforts to develop recombinant leptin as a stand-alone obesity treatment have proven unsuccessful because DIO rats and obese humans are only minimally responsive to even high, pharmacological doses. Our studies provide preclinical and clinical evidence that leptin responsiveness is at least partially restored by amylin agonism. A dose of exogenous leptin that was highly effective in lean rats had minimal effects on weight or food intake in DIO rats (an accepted surrogate model of leptin-resistant human obesity). Administration of amylin together with leptin resulted in a synergistic, fat-specific reduction in body weight in two independent experiments. This synergism was dissociable from amylin's effects on food intake and was more prominent with the administration of amylin than with two other anorexigenic peptides. Improved leptin signaling in the VMH and AP may be involved in mediating the observed synergistic weight loss effects of amylin and leptin. Finally, our translational clinical research study confirmed that findings in our nonclinical experiments are relevant to human obesity and suggest that metreleptin may have therapeutic potential as an adjunctive treatment to pramlintide in an integrated, neurohormonal approach to obesity pharmacotherapy.
Importantly, the synergy between amylin and leptin was not merely due to the effects of amylin on food intake or weight loss because the addition of leptin did not affect weight loss in PF(amylin) rats. This is consistent with clinical observations that treatment with metreleptin alone does not induce meaningful weight loss in obese subjects, even after a diet lead-in (ref. 2 and data on file at Amylin Pharmaceuticals, Inc.). Weight loss in PF(amylin)+leptin rats was likely due to reduced food intake, implying that weight loss in the amylin+leptin-treated group was mediated by additional mechanisms, such as enhancement of leptin signaling. Amylin+leptin-treated rats had the lowest percentage body fat and, despite achieving twice the weight loss (compared with amylin alone), maintained a significant elevation in percentage protein, indicating fat-specific weight reduction and prevention of a counterregulatory decrease in EE.
In leptin-sensitive lean rodents, other peptide hormones reportedly interact with peripherally administered leptin to induce weight loss, including CCK (23, 24) and exendin-4 (25). We tested in our leptin-resistant DIO model whether synergy was specific to amylin or could be obtained with PYY3–36 or GLP-1 agonism. Although the findings could not rule out the possibility of a weak interaction between leptin and these two agonist classes in DIO rats, weight loss observed with the combination of amylin plus leptin was clearly more robust and pronounced.
We next evaluated whether the amylin/leptin synergy could be explained by amylin (i) restoring hypothalamic sensitivity to leptin, (ii) augmenting leptin-induced activation in leptin-sensitive hindbrain regions, or (iii) potentiating leptin action at the receptor or postreceptor level of the adipocyte. Endogenous leptin and amylin are traditionally thought to exert most of their central effects by means of hypothalamic ARC (leptin) and hindbrain AP/NTS (leptin and amylin)-localized receptors. However, recent observations that selective lesioning of leptin receptors on ARC POMC neurons does not replicate the profoundly obese phenotype of general leptin receptor deficiency (Leprdb/Leprdb) (26) and that diet-induced leptin resistance in the CNS is characterized by a marked diminution of pSTAT3 signaling (not only within the ARC but also in VMH neurons) (10, 18) clearly point to extra-ARC leptin receptors contributing significantly to leptin's weight-regulatory effects. In line with these findings, our DIO rats were less responsive than lean rats to leptin-induced pSTAT3 in both ARC and VMH. Amylin augmented leptin-stimulated signaling by ≈43% in the VMH without improving leptin signaling in the ARC. Within the VMH, enhanced leptin signaling would be expected to positively impact energy regulation because leptin receptors are abundantly expressed on glucose-sensitive VMH neurons, and potent anorexigenic and weight-reducing effects are observed after VMH leptin microinjection (27–29). Leptin-responsive neurons in the VMH also contain SF-1 (22), a transcription factor whose deficiency induces DIO in mice (30). Our colocalization studies revealed that SF-1 VMH neurons are also linked to leptin-induced activation of pSTAT3, further implicating the VMH in modulation and resensitization of leptin action and weight control.
Basal pSTAT3 was elevated 4-fold in the AP of amylin-pretreated rats, with even higher levels achieved with leptin coadministration. This was surprising because long-form Lepr are reported at low density within AP/NTS (31). To what extent basal elevation in pSTAT3 with amylin pretreatment reflects increases in leptin sensitivity and/or receptor number in leptin-responsive AP neurons or increases in sensitivity to other circulating factor(s) that signal through pSTAT3 remains to be elucidated. However, leptin did not stimulate pSTAT3 in the AP of rats not pretreated with amylin. Although leptin actions within the NTS have been implicated in reducing food intake (32), in our research leptin responsiveness within the caudal NTS was unchanged by pretreatment with either amylin or PF. We suggest that amylin-specific effects mediated by amylin receptors within the AP, can, through polysynaptic connections, influence the upstream hypothalamic VMH response to leptin. Amylin is not known to have direct effects on hypothalamic neurons (12); however, amylin binding to the AP activates the NTS, the lateral parabrachial nucleus, and the central nucleus of the amygdala to inhibit fasting-induced activation of the lateral hypothalamic (LH) area (12). LH afferents or direct projections from the amygdala may modulate or enhance VMH signaling (35, 36). Finally, our studies were limited to examining hypothalamic and hindbrain neurons. The extent to which leptin responsiveness in other regions is altered by amylin remains to be determined.
Additional, complementary mechanisms underlying the observed synergy could include amylin potentiating leptin's direct effects at the level of the adipocyte (19, 33, 34). Under the present experimental conditions, amylin pretreatment was not associated with altered Lep, Lepr, or Socs-3 gene expression or pSTAT3 levels in WAT; however, leptin-mediated fat depletion has been shown to follow a complex time course arising from a combination of paracrine, hypothalamic, and sympathetic (catecholaminergic) effects (19, 34). Hence, the interrogation of selected downstream genes/effectors of leptin at a single time point does not exclude the possibility that different durations of amylin or amylin+leptin treatment could enhance direct leptin receptor or postreceptor events at the level of the adipocyte.
Our clinical findings not only add a unique perspective to the study of leptin biology in humans but also may have important therapeutic implications. Previous studies convincingly showed that metreleptin has profound effects in states of leptin deficiency, including humans with homozygous mutations in the ob gene and patients with lipodystrophy (3, 37). By contrast, in numerous clinical studies of general obesity, metreleptin monotherapy (at doses considerably higher than those used here) failed to produce a significant weight loss effect (ref. 2 and data on file at Amylin Pharmaceuticals, Inc.). Our clinical combination study provides evidence that responsiveness to exogenous leptin can be at least partially restored by amylin agonism and represents a robust demonstration of a leptin-mediated weight loss effect in non-leptin-deficient human obesity. Although additional clinical studies in obese subjects are warranted, our findings suggest that metreleptin in combination with pramlintide may have therapeutic potential as part of an integrated, neurohormonal approach to obesity pharmacotherapy.
Materials and Methods
Animals and Drug Administration.
Care of, and procedures with, rats were within the Institutional Animal Care and Use Committee guidelines at Amylin Pharmaceuticals, Inc. Animal care, drug administration, and analysis of plasma leptin and amylin, EE, and body composition have been described previously (15, 16). Male Harlan Sprague–Dawley rats (HSD, ≈375 g; Charles River Laboratories) or DIO-prone rats (≈500 g; Charles River Laboratories) were infused with murine leptin (500 μg·kg−1·d−1 in sterile water; Peprotech), rat amylin (100 μg·kg−1·d−1 in 50% DMSO; Peptisyntha), AC3174 (10 μg·kg−1·d−1 in 50% DMSO), or hPYY3–36 (500 μg·kg−1·d−1 in 50% DMSO).
Perfusion and Tissue Processing.
Isoflourane-anesthetized rats were perfused (20 ml/min for 20 min) transcardially via the ascending aorta with saline, followed by ice-cold 4% paraformaldehyde (PBS, pH 7.5; Electron Microscopy Sciences). Brains were postfixed in perfusate (24 h) and cryoprotected (30% sucrose in 0.02 M KPBS for 24 h). Sections (30 μm collected at 150-μm intervals) were cut on a freezing microtome, cryoprotected, and stored (−20°C) until use. For analyses of mRNA or leptin-induced pSTAT3 in peripheral tissues, epididymal WAT and liver in animals that were not perfused were excised and snap-frozen in liquid nitrogen.
pSTAT3-IR.
Leptin-induced (15 mg/kg i.p. 45 min before killed) pSTAT3-IR was assessed in lean HSD rats and in vehicle-, PF(to the amylin group)-, and amylin-treated (7-d infusion) DIO-prone rats by using an affinity-purified polyclonal p-STAT antiserum (Cell Signaling Technologies) in accordance with a published protocol (38). Immunohistochemical detection was performed on free-floating sections by using avidin–biotin–immunoperoxidase technique with several modifications (18, 39). Sections were pretreated (1% H2O2, 1% NaOH, 0.3% glycine, 0.15% SDS), blocked (4% horse serum, 0.4% Triton for 2 h), incubated with primary antibody (1:1,000, blocking solution for 24 h at 4°C), and visualized by using Vectastain Elite reagents (Vector Laboratories) and a DAB reaction. Colocalization of the pSTAT3 antibody (1:1,000) and an SF-1 mouse monoclonal antibody (10 μg/ml; Perseus Proteomics) were detected with a goat anti-rabbit secondary antibody (Alexa Fluor 488, 1:1,000; Molecular Probes) and a goat anti-mouse secondary antibody (Alexa Fluor 568, 1:1,000; Molecular Probes). Antibody specificity for pSTAT3 was confirmed by demonstrating that the pSTAT3 immunohistochemistry signal was entirely blocked by pSTAT3 blocking peptide (no. 1195; Cell Signaling Technologies) (data not shown) and for the SF-1 antibody by demonstrating central and peripheral staining patterns in rat that were identical to previous reports (40) (data not shown).
Image Acquisition and Processing.
Brightfield and fluorescent images were captured (22.222 × 16.667 in, 72 dpi, and 8 bits per channel) by using a Leica DM5000 upright microscope and a SPOT RT slider digital color camera with Spot Advanced Software (version 4.0.8) (Diagnostic Instruments). pSTAT3-IR cells were hand-counted by a blinded investigator, irrespective of staining intensity. The same region of interest was applied to each nucleus (ARC, VMH, AP and rostral, and NTS), based on cellular morphology. ARC and VMH correspond to bregma −3.4, rostral NTS and AP to bregma −13.7, and caudal NTS to bregma −14.0 in the brain atlas of Paxinos and Watson (38). Results are expressed as cell number per section per animal (DIO, n = 5–7; HSD, n = 4). Fluorescent images were obtained (24 bits per pixel RGB) by using Leica filters L5 (excitation filter BP 480/40) and N3 (excitation filter BP 546/12). Laser scanning confocal microscopy (BioRad Radiance 2100 confocal microscope on a Nikon TE-2000 inverted microscope; 60× n.a. 1.4 oil lens) representative images were acquired by using argon (488 nm) and green HeNe (543 nm) lasers. Z-series consisted of six 1,024 × 1,024-pixel optical sections taken at 1-μm intervals by using Kalman accumulation (n = 3). Images shown are maximum projections. All images were sharpened once. Minor modifications in levels were applied equally across all images in Adobe Photoshop (cs2) and converted to CMYK mode.
Gene Expression and Signaling in Peripheral Tissues.
Total RNA was extracted (TRIzol) from WAT or liver, reverse-transcribed (RETROscript reverse transcription system; Ambion), and gene expression of Lep, leptin receptor (Lepr), suppressor of cytokine signaling-3 (Socs-3), fatty acid synthase (Fas), and lipoprotein lipase (Lpl) mRNA were measured relative to β-actin mRNA by real-time PCR using commercially available assays and Universal PCR master mix (Applied Biosystems). For pSTAT3 activation, WAT was homogenized in 3× tissue volume of cell lysis buffer (Santa Cruz Biotechnology) containing protease inhibitor mixture, phenylmethylsulfonyl fluoride, and sodium orthovanadate (Santa Cruz Biotechnology). After centrifugation (≈12,000 × g for 10 min at 4°C), the protein lysate (supernatant) concentration was determined by BCA assay (Pierce). Protein (50 μg) was separated on a 4–12% Bistris gel. pSTAT3 and pSTAT3-IR was determined by using specific antibodies and chemiluminescence, per the manufacturer's instructions (Cell Signaling Technologies).
Data Analysis.
Data were analyzed by repeated-measures ANOVA, with post hoc comparisons where indicated. One-way ANOVA was used to compare changes in body composition, EE, and neuronal activation. P < 0.05 was considered significant. Graphs were generated by using PRISM 4 software for Windows (GraphPad). Data are presented as mean ± SE.
Clinical Proof-of-Concept Study.
This 24-week, randomized, double-blind, active-drug-controlled, multicenter study enrolled 177 overweight and obese males (age 18 to 55 y) and females (age 18 to 45 y) with a body mass index of 27–35 kg/m2 (Table S4). Study medication included pramlintide and metreleptin and their respective, volume-matched, placebo formulations (placebo-P/L). All study medications were given twice daily (b.i.d.) as separate s.c. injections before breakfast and dinner. For the initial 4 weeks, all subjects were instructed to follow a 40% caloric deficit diet and were treated with pramlintide (180 μg b.i.d., then 360 μg b.i.d.), with a target of achieving a weight loss of 2–8%. Depending on an individual's enrollment weight, height, gender, estimated activity level, and age, the 40% caloric deficit translated into ≈550–1,150 kcal/d deficit, based on the Mifflin equation (41). During the lead-in, 21% of subjects were withdrawn (9% due to insufficient weight loss, 3% for adverse events, and 10% for other reasons) (Table S4). The remaining 139 subjects were randomized 2:2:1 to pramlintide 360 μg+metreleptin 5 mg, pramlintide 360 μg+placebo-L, or metreleptin 5 mg+placebo-P. At the beginning of the 20-week randomized treatment period, all subjects were asked to follow a 20% caloric deficit diet.
The study protocol was approved by the Institutional Review Board (IRB) of each study site or by a centralized IRB and was conducted in accordance with the principles described in the 1964 Declaration of Helsinki, including all amendments up to and including the 1996 South African revision. All patients provided written informed consent before the study.
The ITT population (all randomized subjects who received at least one injection of randomized study medication) was used for all safety analyses. Percentage changes in weight from enrollment were analyzed in the ITT and Evaluable populations (all ITT subjects who remained in the study through week 16 with no major protocol deviations) by using a general linear model that included factors for treatment group, gender, enrollment, BMI stratum (<30, ≥30 kg/m2), and initial lead-in period body weight loss category (<5%, ≥5%). Missing data for the ITT population were imputed by using the LOCF method. P values were based on the least-squares (LS) mean differences between each treatment group in the change from enrollment to each visit. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Carrie Wittmer, Chunli Lei, and Heather Hughes for technical assistance; Lisa Porter, Kim Chen, Julie Mitchell, Amy Halseth, Szecho Lin, Colleen Burns, and Kevin Shan for conduct and statistical analysis of the clinical trial; Tamara Darsow and Nicole Kesty for medical writing and editing; Julie Wilde for graphics support; Elaine Sherman for copyediting; and all of the clinical investigators and subjects for participation in the clinical study.
Footnotes
Conflict of interest statement: The authors declare a conflict of interest (such as defined by PNAS policy). All authors are current or former employees of, and stockholders of, Amylin Pharmaceuticals, Inc. C.M.A. is a former employee of Amylin Pharmaceuticals, Inc., currently employed with Arena Pharmaceuticals, Inc.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0706473105/DCSupplemental.
References
- 1.Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Heymsfield SB, et al. Recombinant leptin for weight loss in obese and lean adults: A randomized, controlled, dose-escalation trial. J Am Med Assoc. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 3.Farooqi IS, et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 4.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 5.Halaas JL, et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94:8878–8883. doi: 10.1073/pnas.94.16.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes. 1996;104:293–300. doi: 10.1055/s-0029-1211457. [DOI] [PubMed] [Google Scholar]
- 7.Banks WA, Farrell CL. Impaired transport of leptin across the blood–brain barrier in obesity is acquired and reversible. Am J Physiol. 2003;285:E10–E15. doi: 10.1152/ajpendo.00468.2002. [DOI] [PubMed] [Google Scholar]
- 8.Martin RL, Perez E, He YJ, Dawson R, Jr, Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein down-regulation. Metabolism. 2000;49:1479–1484. doi: 10.1053/meta.2000.17695. [DOI] [PubMed] [Google Scholar]
- 9.El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 11.Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–976. doi: 10.1172/JCI114528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutz TA. Amylinergic control of food intake. Physiol Behav. 2006;89:465–471. doi: 10.1016/j.physbeh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, et al. Pramlintide treatment reduces 24-h caloric intake and meal sizes and improves control of eating in obese subjects: A 6-week translational research study. Am J Physiol. 2007;293:E620–E627. doi: 10.1152/ajpendo.00217.2007. [DOI] [PubMed] [Google Scholar]
- 14.Aronne L, et al. Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: A phase 2, randomized, placebo-controlled, dose-escalation study. J Clin Endocrinol Metab. 2007;92:2977–2983. doi: 10.1210/jc.2006-2003. [DOI] [PubMed] [Google Scholar]
- 15.Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM. Antiobesity effects of the β-cell hormone amylin in diet-induced obese rats: Effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology. 2006;147:5855–5864. doi: 10.1210/en.2006-0393. [DOI] [PubMed] [Google Scholar]
- 16.Mack CM, et al. Pharmacological actions of the peptide hormone amylin in the long-term regulation of food intake, food preference and body weight. Am J Physiol. 2007;293:R1855–R1863. doi: 10.1152/ajpregu.00297.2007. [DOI] [PubMed] [Google Scholar]
- 17.Hargrove DM, et al. Biological activity of AC3174, a peptide analog of exendin-4. Regul Pept. 2007;141:113–119. doi: 10.1016/j.regpep.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 18.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood–brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol. 2004;286:R143–R150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 19.Park BH, et al. Combined leptin actions on adipose tissue and hypothalamus are required to deplete adipocyte fat in lean rats: Implications for obesity treatment. J Biol Chem. 2006;281:40283–40291. doi: 10.1074/jbc.M607545200. [DOI] [PubMed] [Google Scholar]
- 20.Lupien JR, Young AA. No measurable effect of amylin on lipolysis in either white or brown isolated adipocytes from rats. Diabetes Nutr Metab. 1993;6:13–18. [Google Scholar]
- 21.Segal JP, et al. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci. 2005;25:4181–4188. doi: 10.1523/JNEUROSCI.0158-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhillon H, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 23.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol. 1999;276:R1038–R1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 25.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 26.Balthasar N, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;4:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Jacob RJ, et al. The effect of leptin is enhanced by microinjection into the ventromedial hypothalamus. Diabetes. 1997;46:150–152. doi: 10.2337/diab.46.1.150. [DOI] [PubMed] [Google Scholar]
- 28.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canabal DD, et al. Glucose, insulin and leptin signaling pathways modulate nitric oxide (NO) synthesis in glucose-inhibited (GI) neurons in the ventromedial hypothalamus (VMH) Am J Physiol. 2007;292:R1418–R1428. doi: 10.1152/ajpregu.00216.2006. [DOI] [PubMed] [Google Scholar]
- 30.Majdic G, et al. Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology. 2002;143:607–614. doi: 10.1210/endo.143.2.8652. [DOI] [PubMed] [Google Scholar]
- 31.Elmquist JK, Bjorbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- 32.Huo L, Maeng L, Bjorbaek C, Grill HJ. Leptin and the control of food intake: Neurons in the nucleus of the solitary tract (NTS) are activated by both gastric distension and leptin. Endocrinology. 2007;148:2189–2197. doi: 10.1210/en.2006-1572. [DOI] [PubMed] [Google Scholar]
- 33.Orci L, et al. Rapid transformation of white adipocytes into fat-oxidizing machines. Proc Natl Acad Sci USA. 2004;101:2058–2063. doi: 10.1073/pnas.0308258100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang MY, Orci L, Ravazzola M, Unger RH. Fat storage in adipocytes requires inactivation of leptin's paracrine activity: Implications for treatment of human obesity. Proc Natl Acad Sci USA. 2005;102:18011–18016. doi: 10.1073/pnas.0509001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fahrbach SE, Morrell JI, Pfaff DW. Studies of ventromedial hypothalamic afferents in the rat using three methods of HRP application. Exp Brain Res. 1989;77:221–233. doi: 10.1007/BF00274980. [DOI] [PubMed] [Google Scholar]
- 36.Ono T, Luiten PG, Nishijo H, Fukuda M, Nishino H. Topographic organization of projections from the amygdala to the hypothalamus of the rat. Neurosci Res. 1985;2:221–238. doi: 10.1016/0168-0102(85)90002-1. [DOI] [PubMed] [Google Scholar]
- 37.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- 39.Sawchenko PE, Cunningham ET, Mortrud MT, Pfeiffer SW, Gerfen CR. Phaseolus vulgaris-leucoagglutinin anterograde axonal transport technique. In: Conn PM, editor. Methods in Neuroscience. Vol 3. New York: Academic; 1990. pp. 247–260. [Google Scholar]
- 40.Morohashi K, et al. Functional difference between Ad4BP and ELP, and their distributions in steroidogenic tissues. Mol Endocrinol. 1994;8:643–653. doi: 10.1210/mend.8.5.8058072. [DOI] [PubMed] [Google Scholar]
- 41.Frankenfield DC, Rowe WA, Smith JS, Cooney RN. Validation of several established equations for resting metabolic rate in obese and nonobese people. J Am Diet Assoc. 2003;103:1152–1159. doi: 10.1016/s0002-8223(03)00982-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.