Abstract
Previous studies have shown that chronic opiates may inhibit cell growth and trigger apoptosis leading to impaired cognitive capabilities in both humans and other mammals. In contrast, growth hormone (GH) has been demonstrated to stimulate cell growth and counteract apoptosis. GH has also been shown to improve learning and memory in both human and rodents. In this work, we demonstrate that GH may reverse opiate-induced apoptosis in cells derived from prenatal mouse hippocampus. Primary hippocampal cell cultures derived from 16-day-old fetal mouse neurons were treated with morphine for 7 days during growth in the absence or presence of recombinant human GH (rhGH). The release of lactate dehydrogenase (LDH) into the culture media and the level of cleaved caspase-3 were measured. Results indicate that morphine (15 μM) decreased the cell content in a concentration-dependent manner and increased LDH release and caspase-3 activity. Thus, fetal mouse neurons treated with morphine showed less viability compared with controls. Interestingly, the addition of rhGH (1 μM) counteracted the morphine-induced effect on the cell density. Furthermore, the hormone attenuated the effects on LHD release and caspase-3 activity elicited by morphine. These results suggest that the hormone is capable of preventing or even repairing morphine-induced damage to hippocampal cells.
Keywords: apoptosis, morphine, caspase-3, lactate dehydrogenase
Several investigators (1–7) report that opiates, such as heroin and morphine, impair cognitive functions upon chronic exposure. Heroin is one of the most commonly abused illegal drug, and abuse of this drug is linked to attention deficits and poorer performance on memory tasks compared with controls (1). Chronic exposure to morphine has been shown to deteriorate vigilance, attention, and cognitive capability in pain patients (8, 9) and impair acquisition of reference memory in rats (3). These findings suggest chronic opiate stimulation to induce neurotoxic effects in brain structures related to memory and learning, such as the hippocampus.
The hippocampus is a region located in the limbic area of the brain. This area has been shown to be required for the acquisition of declarative or explicit memory (10). Chronic opiates have been demonstrated to inhibit cell growth and trigger apoptosis, but it is also suggested that opiate-induced toxicity may include impaired regeneration of nerve cells (4–7). The hypothesis of neurogenesis in adult human brains was finally confirmed in 1998 when alteration of new neurons was shown in the dentate gyrus of the hippocampus (11). An impact of these adult-generated neurons on memory and learning has been suggested because training on associative learning tasks doubles these neurons in the rat dentate gyrus (12). Thus, memory dysfunctions after opiate exposure could be caused by decreased adult neurogenesis because opiates inhibit neurogenesis in the adult rat hippocampus (13, 14). The inhibition of neurogenesis might be related to decrease of neural precursors, i.e., increase of apoptosis of newborn neurons. However, in recent years several factors that may increase neurogenesis from preexisting neuronal precursors have been described. For instance, insulin-like growth factor-1 (IGF-1) is shown to be essential for hippocampal neurogenesis (15). This factor is under control by the somatotrophic axis, where growth hormone (GH) has an important role as an activator of IGF-1 and its binding proteins.
Recent studies (for review, see ref. 16) have shown that GH targets many areas of the central nervous system, and GH deficiency has been associated with cognitive impairments, memory loss, and diminished overall well being (17–19). GH replacement therapy in GH-deficient patients has been shown to ameliorate the condition for these patients (17–19). The hormone has further been shown to prevent neuronal loss in the aged rat hippocampus, indicating a neuroprotective effect of GH on old animals (20). Decreased levels of circulating GH and decreasing density of GH-binding sites with age have been shown in several areas of the human brain, including the hippocampus (21, 22). GH has also been shown to increase the expression of the rat hippocampal gene transcript for the NMDA receptor subunit type 2B (NR2B) (23), a subunit earlier shown to enhance cognitive capabilities in an age-dependent manner while overexpressed. In addition, it was recently shown that GH replacement in hypophysectomized rats improves spatial performance and increases the hippocampal gene transcript levels of NMDA receptor subunits and postsynaptic density protein 95 (24). All together, these findings suggest a link between lowered levels of GH in elderly and deterioration of cognitive functions, with a clear indication that GH may improve memory and cognitive capabilities.
The mechanisms by which GH induces its beneficial effect on cognition is poorly known. However, GH has been shown to promote neurogenesis and gliogenesis during brain development in fetal rat (25), presumably through local production of IGF-1. Local production of GH and IGF-1 in certain brain regions has been suggested, because mice with circulating GH and IGF-1 deficiency show normal levels of corresponding mRNAs in the hippocampus (26). Intracerebroventricular infusions of IGF-1 have been shown to reduce age-related decline in hippocampal neurogenesis in rats (27). Peripheral infusions of IGF-1 have also been demonstrated to induce neurogenesis in the adult rat hippocampus (15), and overexpression of IGF-1 is able to promote neurogenesis during postnatal development (28). On the other hand, as mentioned above, opiates exert an inhibitory effect on neurogenesis and previous studies have shown that morphine treatment is able to decrease GH-binding site density in the rat hypothalamus in the acute phase of administration (29). A decrease of the GH receptor gene transcript levels in the rat hippocampus after a single dose of morphine has also been reported (30).
Thus, to examine whether GH may counteract or reverse the opiate-induced apoposis or inhibition of neurogenesis we investigated the effect of morphine and human recombinant GH (rhGH) on primary hippocampal neuronal cultures derived from fetal mice. These cells were treated with morphine and rhGH, and the neuronal cell density was recorded through microscopic studies. To further investigate the effect of morphine and rhGH on cell viability, we also measured the release of lactate dehydrogenase (LDH) and caspase-3 activity, both well known as markers of cell death and apoptosis (31, 32). We found that rhGH may reverse the morphine-induced effects on neuronal cell density and LDH release and caspase-3 activity.
Results
Effects of Morphine and rhGH on Neuronal Cell Density.
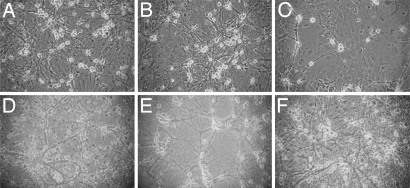
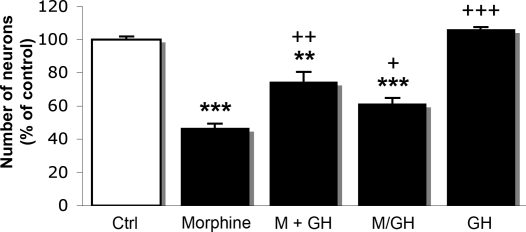
Treatment of hippocampal primary cell cultures for 7 days with morphine in concentrations ranging from 100 nM to 20 μM resulted in a dose-dependent decrease in the neuronal cell density (Fig. 1 A–C) as compared with untreated cells (Fig. 1D). Morphine induced an observable effect at concentrations >1 μM (Fig. 1B). At a concentration of 15 μM the opiate induced a significant reduction (54%; P < 0.001) in the number of neuronal cells, and for this reason this concentration was chosen for further experiments (Figs. 1E and 2). The concentration of rhGH used in the experiments was determined by treating the cells with different concentrations of rhGH (1 nM to 10 μM) for 7 days. A bell-shaped curve was observed in a dose-dependent manner. As maximal response of rhGH was seen at a concentration ≈1 μM, and this concentration was chosen for our continuous experiments. When hippocampal primary cell cultures were treated for 7 days with morphine together with rhGH, the hormone significantly (P < 0.01) prevented the reduction in cell density induced by morphine (Figs. 1F and 2).
Fig. 1.
Effects of morphine and rhGH treatment on the density of neurons in primary cultures of fetal mouse hippocampal neurons. Cells were treated with different concentrations of morphine (A–C and E) alone or in the presence of rhGH (F) for 7 days. Shown are representative light microscopic images. (A) Morphine, 0.1 μM. (B) Morphine, 1 μM. (C) Morphine, 20 μM. (D) Untreated cells. (E) Morphine, 15 μM. (F) Morphine (15 μM) together with rhGH (1 μM). (Magnification: ×20.)
Fig. 2.
Effects of morphine and rhGH treatment on neuronal cell density in primary cultures of fetal mouse hippocampal neurons. Ctrl, control; M, morphine (15 μM) for 7 days; M+GH, morphine (15 μM) together with rhGH (1 μM) for 7 days; M/GH, morphine (15 μM) for 3 days, followed by rhGH (1 μM) for 4 days; GH, rhGH (1 μM) for 7 days. Values represent means from three different experiments in triplicate and are expressed in percent of control ± SE. ***, P < 0.001; **, P < 0.01 vs. control cells; +++, P < 0.001; ++, P < 0.01; + P < 0.02 vs. morphine-treated cells. Note: the M/GH group is compared with cells treated with morphine for 7 days.
Effects of Morphine and rhGH on LDH Release.
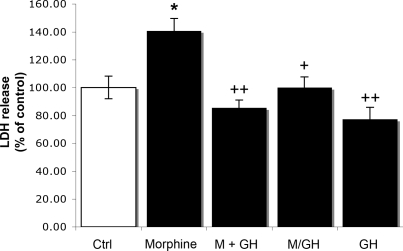
Measurements of LDH release into the culture medium indicated that treatment with morphine (15 μM) for 7 days significantly increased the release of LDH by 41% (P < 0.02) compared with untreated cells (Fig. 3). This increase was reduced to control levels when rhGH (1 μM) was combined with the opiate, and when the hormone was supplemented after 3 days of pretreatment with morphine (Fig. 3). Furthermore, rhGH (1 μM) alone tended to decrease (23%; P = 0.108) the release of LDH (Fig. 3).
Fig. 3.
Effects of morphine and rhGH on LDH release in primary cultures of fetal mouse hippocampal neurons. Ctrl, control; M, morphine (15 μM) for 7 days; M+GH, morphine (15 μM) together with rhGH (1 μM) for 7 days; M/GH, morphine (15 μM) for 3 days, followed by rhGH (1 μM) for 4 days; GH, rhGH (1 μM) for 7 days. Values represent means from four different experiments in triplicate and are expressed in percent of control ± SE. *, P < 0.02 vs. control cells; ++, P < 0.01; +, P < 0.02 vs. morphine-treated cells. Note: the M/GH group is compared with cells treated with morphine for 7 days.
Effects of Morphine and rhGH on Caspase-3 Activity.
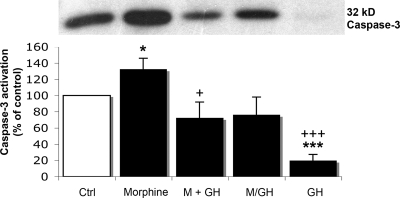
The assessment of the cleavage of caspase-3 indicated that this marker was affected by morphine treatment. The recorded data for the cleaved caspase-3, i.e., the activated caspase-3, are given in Fig. 4. A significant increase (30%; P < 0.05) in caspase-3 activity after 7 days of morphine (15 μM) treatment was observed compared with untreated cells (Fig. 4). This increase was diminished when rhGH was combined with morphine. When the hormone was given after 3 days before treatment with morphine, the caspase-3 immunoreactivity did not differ from control levels. In the absence of morphine, rhGH (1 μM) induced a profound decrease in caspase-3 activity by 80% compared with untreated cells (Fig. 4).
Fig. 4.
Effects of morphine and rhGH on caspase-3 activation in primary cultures of fetal mouse hippocampal neurons. (Upper) Representative Western blots of cleaved caspase-3. (Lower) Histogram representing caspase-3 activation. Ctrl, control; M, morphine (15 μM) for 7 days; M+GH, morphine (15 μM) together with rhGH (1 μM) for 7 days; M/GH, morphine (15 μM) for 3 days, followed by rhGH (1 μM) for 4 days; GH, rhGH, (1 μM) for 7 days. Values represent means from five different experiments in triplicate and are expressed in percent of control ± SE. ***, P < 0.001; *, P < 0.05 vs. control cells; +++, P < 0.001; +, P < 0.05 vs. morphine-treated cells. Note: the M/GH group is compared with cells treated with morphine for 7 days.
Discussion
The unique finding of this study was that opioid receptor activation elicit reduced growth in mouse fetus hippocampal neurons, an effect that was reversed by the addition of rhGH. Moreover, the hormone was found to counteract the morphine-induced effects on the release of LDH and caspase-3 activation, two markers associated with apoptosis and cell death. These results suggest that GH may reverse or restore some opiate-induced cell damage in this area of the brain.
Thus, chronic morphine significantly and dose-dependently reduced neuronal cell density in the cultured hippocampal cells from mouse fetus. The ability of morphine to inhibit cell growth and induce apoptosis is known from previous work. The opiate was seen to elicit apoptosis in human fetal microglia and neurons (7), and apoptosis is also seen to be associated with morphine tolerance (5). The apoptotic effect of morphine was shown to be blocked by naloxone, suggesting an opioid receptor mechanism (7). The effect of morphine is mediated mainly through the μ-opioid peptide (MOP) receptor; although at high concentrations, this opiate also may interact with both δ-opioid (DOP) and k-opioid (KOP) receptors. Moreover, it seems that the various opioid receptor types MOP, DOP, and KOP may regulate different aspects of neuronal development (6). Evidence indicating that the MOP receptor may play an important role in regulating progenitor cell survival was recently described (33).
Morphine is further shown to promote abnormal programmed cell death by enhancing the expression of proapoptotic Fas receptor protein and damping the expression of antiapoptotic Bcl-2 oncoprotein through the sustained activation of opioid receptors (34). Moreover, studies also indicated that opiates inhibit neurogenesis and may alter the hippocampal function (14). Therefore, the decrease in neuronal cell density seen in the present study in the mouse hippocampal primary cell cultures was expected, and a consequence of this decrease should be that markers of apoptosis, such as LDH and caspase-3 activity, are affected. Indeed, both of these enzymes were significantly enhanced (see Figs. 2–4).
The enhanced release of LDH in morphine treated hippocampal cells, as seen in the present study, gives a further indication that morphine induces apoptosis in mouse fetus hippocampal cells. LDH, a mitochondrial dehydrogenase, represents a critical component of the astrocyte-neuron lactate shuttle. It controls the formation and may regulate the turnover of lactate in the cells. Caspase-3 is another enzyme that serves as a marker of apoptosis. We here analyzed the cleaved caspase-3, an activated form of caspase-3 that acts as a lethal protease at the most distal stage of the apoptotic pathway (35). Thus, this enzyme was also examined to explore whether the observed reduction in neuronal cell density involved elements related to apoptosis. In the present study, the level of cleaved caspase-3, as assessed by Western blot analysis, was significantly increased by morphine.
Interestingly, the morphine-induced effects on neuronal cell growth, LDH release, and caspase-3 activation, were all attenuated or reversed after inclusion of rhGH in the incubation media. The effect of morphine on neuronal cell density was counteracted when cells were grown in the presence of rhGH. This observation clearly indicates that rhGH may reverse the opiate-induced inhibitory effect on cell growth. Addition of rhGH, both combined with morphine and added 3 days later, abolished the increase in LDH release, whereas added alone the hormone did not produce any change in the release of LDH. By adding morphine together with rhGH the opiate-induced elevation of caspase-3 activity was significantly decreased and restored to control levels. Moreover, when the rhGH was supplemented 3 days after morphine the opiate-induced increase in cleaved caspase-3 activity was diminished. Interestingly, rhGH alone produced a significant decrease in caspase-3 activity. The marked reduction in activity of caspase-3 caused by this hormone without any alterations in number of surviving cells or in LDH release may reflect an ability of rhGH to counteract spontaneous apoptosis (36), which might explain the comparatively high levels of caspase-3 in untreated cells. Caspase-3 has recently been suggested to be involved in functions besides apoptosis (e.g., cytoskeleton remodeling or synaptic pruning). For example, caspase-3 activation has been shown to be important for astroglial cytoskeleton remodeling after excitotoxic damage (37). It could not be excluded that the reducing effect of rhGH on the caspase-3 activity may include these actions. However, it is more likely that caspase-3 might be exclusively involved in cell death in the present study and that caspase-dependent cell death in untreated cells will occur later than in cells treated with rhGH. From the present study it appears that rhGH may counteract both LDH and caspase-3 activities induced by morphine. Thus, from these observations it can be concluded that the opiate inhibitory effect on neuronal cell growth is reversible by GH, a finding that is further confirmed by the attenuating effect of the hormone on the two enzymes serving as markers of apoptotic activity.
The mechanism by which GH exerts these effects is still not fully clear. It is known that the hormone stimulates the release of IGF-1 in peripheral tissues but also in the brain. In the rat it has been demonstrated that peripheral infusion of this growth factor selectively induces neurogenesis in the adult rat hippocampus (15). IGF-1 is also shown to decrease ischemia-reperfusion induced apoptosis and necrosis in diabetic rats (35). Studies have also shown that GH promotes proliferation of neural precursors, neurogenesis, and gliogenesis during brain development (25). These responses are believed to result from IGF-1 locally produced in the actual brain areas. It was further shown that GH may induce IGF-1 binding protein-3 (IGFBP-3), which also may have a role in the observed responses. It can therefore be concluded that GH may activate the IGF-1/IGFBP-3 system in cerebral cells and thereby induce a physiological action via IGF-1.
Studies on opiate-induced cell damage have recently shown that blockade of the NMDA receptors prevents cell death by apoptosis induced by morphine (38). In contrast, activation of either synaptic or extrasynaptic NR2A-containing NMDA receptors promotes neuronal survival and exerts a neuroprotective action against both NMDA receptor-mediated and non-NMDA receptor-mediated neuronal damage (38). The study suggested that blockade of NR2B or potentiation of NR2A would be effective in reducing neuronal damage (34). On the other hand, studies have also shown that moderate stimulation of NR2B may be involved prosurvival effects of NMDA (39).
In the rat hippocampus rhGH is shown to interact with the NMDA receptor complex (23). The hormone was found to affect the gene transcripts of the NMDA receptor subunits at an extent that is compatible with enhanced cognitive capability (23) as shown from Northern blot analyses and in a behavioral assay for the assessment of memory function (24). Thus, rhGH-treated rats performed significantly better in the spatial memory task than the control animals in the Morris water maze (24). The study indicated that there is a relationship between the NMDA receptor subunits' mRNA expression levels and learning ability, and that learning is improved by rhGH. Thus, it was shown that in old rats rhGH increased the expression of the gene transcripts of the NR1 and NR2A receptor subunits (23, 24). In young rats rhGH increased the hippocampal expression of the NR2B gene transcript (23). These effects of rhGH on the gene transcripts of the NMDA receptor subunits were mimicked by IGF-1 (40), suggesting that the effects by rhGH result after local release of IGF-1. In fact, earlier studies have demonstrated that IGF-1 may induce improved effects on cognitive functions in the rat (41). Moreover, as mentioned above GH is shown to increase neurogenesis and counteract apoptosis (15), and a decline in both GH and IGF-1 have previously been suggested to contribute to the genesis of brain aging (42).
In conclusion, the present data clearly indicate that GH has a powerful counteracting effect on the disturbances of hippocampal cells seen after exposure to morphine. Accordingly, this study demonstrates that the viability of fetal mouse neurons is significantly reduced after chronic treatment with morphine, whereas rhGH treatment may augment viability of these cells and preserve them from morphine-induced toxicity. Consistent results were shown in measurements of LDH release and caspase-3 activity, indicating increased apoptosis in morphine-treated cells. These observations suggest that GH is able to prevent opiate-induced damage to these kinds of cells. This effect of GH, in turn, may open new possibilities with regard to relevant strategies for the treatment of opiate addiction. Activation of the somatotrophic axis in the brain by GH replacement therapy may represent a possible approach for repairing cell damages induced by opiate abuse.
Materials and Methods
Primary Mouse Hippocampal Cell Cultures.
The method used for preparing primary cell cultures was a slight modification of the procedure described by Kim et al. (43). Briefly, pregnant C57BL/6 mice (Scanbur) were anesthetized with carbon dioxide and killed by cervical dislocation. Animal care followed official governmental guidelines. The hippocampi were dissected and removed from brains of the 16-day-old fetal mice and triturated four to five times into individual cells. The dissecting media (HBSS; Invitrogen), was supplemented with 28 mM glucose, 20 mM sucrose, and 4 mM sodium bicarbonate. The cells were plated on 24-well plates (5 × 105 cells per culture well) precoated with 100 μg/ml poly-d-lysine and with the plating media (MEM; Invitrogen) supplemented with 5% FBS, 5% horse serum, 1% glutamine, 20 mM glucose, and 30 mM sodium bicarbonate. The cultures were kept in a tissue culture incubator at 37°C in 5% CO2. After 7–9 days in vitro (DIV 7–9), 10 μM cytosine arabinoside was added to halt growth of glial cells, with experiments starting the next day, at DIV 8–10.
Drug Treatment.
Starting at DIV 8–10, untreated cultures (control) received nutrient media alone, whereas opiate/GH-treated cultures were continuously exposed during 7 days to either 15 μM morphine hydrochloride (Apoteket Production and Laboratories), 1 μM rhGH (Genotropin; Amersham Pharmacia), both 15 μM morphine and 1 μM rhGH or first 15 μM morphine for 3 days and then 1 μM rhGH for 4 days. Before these doses were finally settled on, experiments using different doses of both morphine and rhGH were carried out.
Neuronal Cell Density and Neuronal Cytotoxicity.
Neuronal cell density during drug exposure was observed with a microscope. To determine the relative neuronal cell density in a well, the numbers of neurons in three areas from a representative picture of the well were counted and the mean value in these three areas were calculated. The change in neuronal cell density within the well was compared between different treatments. Neuronal cytotoxicity was evaluated by measurement of LDH release into the culture medium by using a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega) as described by the manufacturer. Data were normalized to the LDH released from control-treated cells (100%) and expressed as a percentage of the control experiments.
Western Blot Analysis.
Cells were washed with PBS three times, lysed on ice in lysis buffer containing 20 mM Hepes (pH 7.5), 10 mM EGTA, 40 mM β-glycerophosphate, 1% Nonidet P-40, 2.5 mM MgCl2, 2 mM orthovanadate, and complete mini protease inhibitors (1 tablet/10 ml buffer; Roche Diagnostics). The lysates were centrifuged at 1,000 × g for 15 min at 4°C, and the supernatant was collected and kept at −20°C until used. Protein concentrations of the lysates were measured by the method of Lowry et al. (44). Equal amounts of proteins (15 μg) were separated on 10% SDS/PAGE gels, and then transferred to a nitrocellulose membrane (Hybond-ECL; GE Healthcare Bio-Science). Membranes were blocked for 1 h at room temperature with 5% nonfat dried milk in Tris-buffered saline [10 mM Tris·HCl (pH 7.6) and 150 mM NaCl] solution containing 0.05% Tween-20, probed with the primary mAb against caspase-3 (3G2 mAb 1:1,000; Cell Signaling Technology) at 4°C overnight, and subsequently incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. Membranes were then exposed to Hyperfilm ECL, and immunoreactivity was detected with ECL Western blotting detection reagents (GE Healthcare Bio-Science). The films were scanned and the intensity of the bands was determined by using the Image J program.
Statistical Analysis.
All experiments were independently repeated a minimum of three times. All data are presented as mean ± SE. Statistical analyses were determined by using one-way ANOVA followed by Fisher's protected least significant difference test as a posthoc test for multiple comparisons. Values of P < 0.05 were considered to be significant.
Acknowledgments.
This study was supported by Swedish Medical Research Council Grant 9459 and the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly.
Footnotes
The authors declare no conflict of interest.
References
- 1.Guerra D, Sole A, Cami J, Tobena A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20:261–270. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]
- 2.Cipolli C, Galliani I. Addiction time and intellectual impairment in heroin users. Psychol Rep. 1987;60:1099–1105. doi: 10.1177/0033294187060003-216.1. [DOI] [PubMed] [Google Scholar]
- 3.Spain JW, Newsom GC. Chronic opioids impair acquisition of both radial maze and Y-maze choice escape. Psychopharmacology (Berl) 1991;105:101–106. doi: 10.1007/BF02316870. [DOI] [PubMed] [Google Scholar]
- 4.Yin D, et al. Morphine promotes Jurkat cell apoptosis through proapoptotic FADD/P53 and antiapoptotic PI3K/Akt/NK-κB pathways. J Neuroimmunol. 2006;174:101–107. doi: 10.1016/j.jneuroim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: Evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22:7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., 3rd Opioids intrinsically inhibit the genesis of mouse cerebellar granule neuron precursors in vitro: Differential impact of m and d receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT. Neuroexcitatory effects of morphine and hydromorphone: Evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol. 2000;27:524–528. doi: 10.1046/j.1440-1681.2000.03290.x. [DOI] [PubMed] [Google Scholar]
- 9.Sjogren P, Thomsen AB, Olsen AK. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J Pain Symptom Manage. 2000;19:100–108. doi: 10.1016/s0885-3924(99)00143-8. [DOI] [PubMed] [Google Scholar]
- 10.Benfenati F. Synaptic plasticity and the neurobiology of learning and memory. Acta Biomed. 2007;78(Suppl 1):58–66. [PubMed] [Google Scholar]
- 11.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 12.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 13.Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci USA. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- 15.Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nyberg F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front Neuroendocrinol. 2000;21:330–48. doi: 10.1006/frne.2000.0200. [DOI] [PubMed] [Google Scholar]
- 17.Bjork S, Jonsson B, Westphal O, Levin JE. Quality of life of adults with growth hormone deficiency: A controlled study. Acta Paediatr Scand Suppl. 1989;356:55–59. doi: 10.1111/j.1651-2227.1989.tb11242.x. [DOI] [PubMed] [Google Scholar]
- 18.Bengtsson BA, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–317. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- 19.Burman P, Deijen JB. Quality of life and cognitive function in patients with pituitary insufficiency. Psychother Psychosom. 1998;67:154–167. doi: 10.1159/000012276. [DOI] [PubMed] [Google Scholar]
- 20.Azcoitia I, et al. Growth hormone prevents neuronal loss in the aged rat hippocampus. Neurobiol Aging. 2005;26:697–703. doi: 10.1016/j.neurobiolaging.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 21.Van Dam PS, et al. Growth hormone, insuline-like growth factor-1, and cognitive function in adults. Growth Horm IGF Res. 2000;10:S69–S73. doi: 10.1016/s1096-6374(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 22.Lai Z, et al. Age-related reduction of human growth hormone-binding sites in the human brain. Brain Res. 1993;621:260–266. doi: 10.1016/0006-8993(93)90114-3. [DOI] [PubMed] [Google Scholar]
- 23.Le Greves M, Steensland P, Le Greves P, Nyberg F. Growth hormone induces age-dependent alteration in the expression of hippocampal growth hormone receptor and N-methyl-d-asparate receptor subunits gene transcripts in male rats. Proc Natl Acad Sci USA. 2002;99:7119–7123. doi: 10.1073/pnas.092135399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Greves M, et al. Growth hormone replacement in hypophysectomized rats affects spatial performance and hippocampal levels of NMDA receptor subunit and PSD-95 gene transcript levels. Exp Brain Res. 2006;173:267–273. doi: 10.1007/s00221-006-0438-2. [DOI] [PubMed] [Google Scholar]
- 25.Ajo R, Cacicedo L, Navarro C, Sanchez-Franco F. Growth hormone action on proliferation and differentiation of cerebral cortical cells from fetal rat. Endocrinology. 2003;144:1086–1097. doi: 10.1210/en.2002-220667. [DOI] [PubMed] [Google Scholar]
- 26.Sun LY, Al-Regaiey K, Masternak MM, Wang J, Bartke A. Local expression of GH and IGF-1 in the hippocampus of GH-deficient long-lived mice. Neurobiol Aging. 2005;26:929–937. doi: 10.1016/j.neurobiolaging.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenwalner RJ, et al. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107:603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 28.O'Kusky JR, Ye P, D'Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai QZ, Lai Z, Yukhananov R, Roos P, Nyberg F. Decreased binding of growth hormone in rat hypothalamus and choroid plexus following morphine treatment. Neurosci Lett. 1995;184:82–85. doi: 10.1016/0304-3940(94)11174-h. [DOI] [PubMed] [Google Scholar]
- 30.Thornwall-Le Greves M, Zhou Q, Lagerholm S, Huang W, Le Greves P, Nyberg F. Morphine decreases the levels of gene transcripts of growth hormone receptor and growth hormone binding protein in the male rat hippocampus and spinal cord. Neurosci Lett. 2001;304:69–72. doi: 10.1016/s0304-3940(01)01757-8. [DOI] [PubMed] [Google Scholar]
- 31.Rami A. Ischemic neuronal death in the rat hippocampus: The caplain-calpastatin-caspase hypothesis. Neurobiol Dis. 2003;13:75–88. doi: 10.1016/s0969-9961(03)00018-4. [DOI] [PubMed] [Google Scholar]
- 32.Kajta M, Trotter A, Lason W, Beyer C. Effect of NMDA on staurosporine-induced activation of caspase-3 and LDH release in mouse neocortical and hippocampal cells. Brain Res Dev Brain Res. 2005;160:40–52. doi: 10.1016/j.devbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Harburg GC, et al. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2007;144:77–87. doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boronat MA, Garcia-Fuster MJ, Garcia-Sevilla JA. Chronic morphine induces up-regulation of proapoptotic Fas receptor and down-regulation of the antiapoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001;134:1263–1270. doi: 10.1038/sj.bjp.0704364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuribayashi K, Mayes PA, El-Deiry WS. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol Ther. 2006;5:763–765. doi: 10.4161/cbt.5.7.3228. [DOI] [PubMed] [Google Scholar]
- 36.Elaut G, Vanhaecke T, Heyden YV, Rogiers V. Spontaneous apoptosis, necrosis, energy status, glutathione levels, and biotransformation capacities of isolated rat hepatocytes in suspension: effect of the incubation medium. Biochem Pharmacol. 2005;69:1829–1838. doi: 10.1016/j.bcp.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Acarin L, et al. Caspase-3 activation in astrocytes following postnatal excitotoxic damage correlates with cytoskeletal remodeling but not with cell death or proliferation. Glia. 2007;55:954–965. doi: 10.1002/glia.20518. [DOI] [PubMed] [Google Scholar]
- 38.Liu Y, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habas A, Kharebava G, Szatmari E, Hetman M. NMDA neuroprotection against a phosphatidylinositol-3 kinase inhibitor, LY294002 by NR2B-mediated suppression of glycogen synthase kinase-3β-induced apoptosis. J Neurochem. 2006;96:335–348. doi: 10.1111/j.1471-4159.2005.03543.x. [DOI] [PubMed] [Google Scholar]
- 40.Le Grevès M, Le Grevès P, Nyberg F. Age-related effects of IGF-1 on the NMDA-, GH- and IGF-1-receptor mRNA transcripts in the rat hippocampus. Brain Res Bull. 2005;65:369–374. doi: 10.1016/j.brainresbull.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 41.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87:559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 42.Sonntag WE, et al. Growth hormone and IGF-I modulate local cerebral glucose utilization and ATP levels in a model of adult-onset growth hormone deficiency. Am J Physiol. 2006;291:E604–E610. doi: 10.1152/ajpendo.00012.2006. [DOI] [PubMed] [Google Scholar]
- 43.Kim EY, et al. Zn2+ entry produces oxidative neuronal necrosis in cortical cell cultures. Eur J Neurosci. 1999;11:327–334. doi: 10.1046/j.1460-9568.1999.00437.x. [DOI] [PubMed] [Google Scholar]
- 44.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]