Abstract
The frontal eye field (FEF) is involved in the transformation of visual signals into saccadic eye movements. Although it is often considered an oculomotor structure, several lines of evidence suggest that the FEF also contributes to visual perception and attention. To better understand the range of behaviors to which the FEF can contribute, we tested whether monkeys could detect activation of their FEF by electrical microstimulation with currents below those that cause eye movements. We found that stimulation of FEF neurons could almost always be detected at levels below those needed to generate saccades and that the electrical current needed for detection was highly correlated with that needed to generate a saccade. This relationship between detection and saccade thresholds can be explained if FEF neurons represent preparation to make particular saccades and subjects can be aware of such preparations without acting on them when the representation is not strong.
The primate frontal eye field (FEF) plays a well established role in the generation of saccades. Single-unit recordings have shown that many neurons in the FEF are active around the time of saccades of specific magnitude and direction and that a topographic representation of saccade vectors exists across the FEF (1). Electrical microstimulation of sites in the FEF can produce saccades of a particular vector (2). Ablating the FEF causes pronounced deficits in generating eye movements to visual targets (3). In humans, functional MRI studies have shown activity in the FEF is correlated with visually guided saccade generation (4), and transcranial magnetic stimulation (TMS) of the FEF has been shown to disrupt saccade generation (5, 6).
Although the FEF is important in oculomotor control, several lines of evidence suggest that it plays a role that goes beyond the initiation and control of eye movements (7). The FEF receives visual inputs from the thalamus and shows extensive reciprocal connectivity with visual cortical areas (1). Single-unit recordings have shown that a substantial fraction of FEF neurons have little or no perisaccadic activity and instead have strong, short-latency visual responses (2). These visually driven neurons can respond selectivity to different stimulus dimensions (8, 9) and distinguish the behavioral relevance of stimuli in an array (10). The activity of these neurons, unlike FEF neurons with perisaccadic activity, is strongly correlated with stimulus onset and only weakly correlated with the timing of saccadic responses (11). Additionally, experiments using electrical microstimulation and TMS of the FEF have shown that FEF activation can produce effects that appear similar to spatial attention (7, 12–15).
To further explore the range of behaviors to which FEF activity can contribute, we have examined whether subjects can detect the activity of FEF neurons at levels that are too low to produce eye movements. Studies using electrical microstimulation of local sites in different areas of visual cortex (16) and somatosensory cortex (17) of non-human primates have shown that activation of neurons with relatively low currents is readily detected. Whether neuronal activity in the FEF can be reported has not been answered. Because the FEF plays a major role in oculomotor control, the activity of its neurons, unlike those in sensory regions, might not be reportable by the subject except indirectly through the detection of eye movements.
We used the responses of trained, behaving rhesus monkeys to measure whether low-current microstimulation of the FEF could be detected. At a given site we compared thresholds for detecting microstimulation directly with the current levels needed to generate a saccade. We found that electric stimulation of the FEF was detected at every site tested, and virtually always at currents lower than those needed to produce an eye movement. Across all sites, the current needed for detection was highly correlated with the current needed to produce an eye movement. These results provide insight into the range of behaviors to which the FEF may contribute.
Results
Detection Thresholds.
Thresholds for detecting electrical microstimulation of the FEF were determined by using a two-alternative forced-choice (2AFC) task. Two rhesus monkeys were trained to fixate a spot on a gray background on a video display while two intervals marked by tones were presented (Fig. 1A). During training, a small, low-contrast, peripheral visual stimulus was displayed during one of the two intervals. Which interval contained the stimulus was determined randomly on each trial. Shortly after the second interval, two response targets appeared 5° above and below the fixation spot, and the animal had to indicate which interval contained the stimulus by making a saccade directly to one of the two targets. The animals reported only whether the stimulus was detected. They did not report on its location or other qualities.
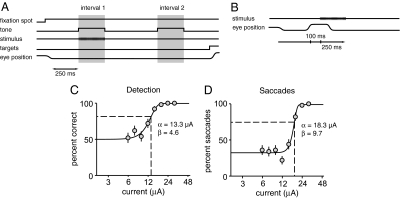
Fig. 1.
Methods for threshold assessment. (A) The animal performed a 2AFC detection task in which an electrical stimulus was delivered during one of two 250-ms intervals that were marked by tones. After the end of the second interval two response targets appeared in fixed locations, and the animal made a direct saccade to one to indicate which interval contained the electrical stimulus. (B) To determine saccadic thresholds, electrical microstimulation was delivered between trials of a fixation task 100 ms after the animal made a spontaneous saccade. (C) At each site 6–10 current levels spanning behavioral threshold were tested 50 times each in a random order. Behavioral data at each site were fit with a sigmoid function, and threshold (dashed lines) and slope were based on parameters of the fit. (D) Saccadic thresholds were determined by stimulating with 6–10 current levels spanning saccadic threshold in a random order, and a sigmoid was fit to the saccade frequencies. The detection and saccade data in C and D are from the same FEF site. Error bars show standard errors based on binomial statistics.
During data collection, in place of a visual stimulus, trains of constant-current electrical pulses were delivered to a site in the FEF through a microelectrode. The current amplitude was varied from trial to trial to produce psychometric detection functions. Each FEF site was tested with 50 repetitions of 6–10 currents spanning detection threshold. Behavioral responses for each site were fit with a sigmoid function (Materials and Methods), and detection threshold was taken as the current where performance was 0.63 of the way from 50% correct to the upper saturation on the performance curve (typically ≈82% of the trials correct; see Materials and Methods, Eq. 1, and Fig. 1C).
Data were collected from sites in the anterior bank of the arcuate sulcus where saccades could be reliably evoked during free viewing by electrical stimulation with 200-Hz trains of 200 μs biphasic current pulses delivered at 50 μA or less. Although electrical stimulation could generate saccades at every site, the animal was required to maintain fixation throughout each trial in the 2AFC task. The animals were usually able to maintain fixation even with currents that were well above detection threshold. On occasional trials with high currents the electrical stimulation would drive a saccadic eye movement, causing the animal to break fixation (see below). These trials were not included in the analyses of detection threshold.
We obtained well formed detection functions at every FEF site tested in both animals (n = 161: 50 from animal 1 and 111 from animal 2). The distributions of detection thresholds for different sites are plotted in Fig. 2A. The median thresholds for the two animals were 13.4 and 14.6 μA. The difference is not statistically significant (P = 0.13 by Kolmogorov–Smirnov test). The lowest detection threshold found at any sites was 6.1 μA, and the highest two thresholds were 47.6 and 32.8 μA. The FEF thresholds showed relatively little variance. The interquartile ranges for the two animals were 6.9 and 9.3 μA, and the coefficients of variation for the detection threshold plots in Fig. 2A are 0.13 and 0.16. This low variance depended in part on using a force-choice design, which effectively eliminates variance from drift in the subject's criterion, and in part from our inclusion of many trials in each threshold measurement (typically 400), such that the 95% confidence intervals on detection thresholds averaged only ±0.12 times threshold. The median detection thresholds for the FEF are slightly higher than those for detection of electrical stimulation of the visual cortex, whereas the coefficients of variation are slightly lower (16) (see Discussion).
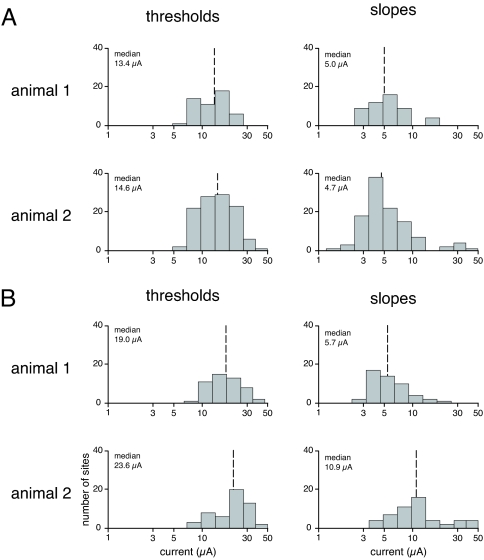
Fig. 2.
Distributions for thresholds and slopes for detection of electrical microstimulation and saccade generation. (A) Median thresholds (dashed lines) and interquartile ranges for detection were as follows: animal 1, 13.4 μA, range 9.5–16.4 μA; animal 2, 14.6 μA, range 10.5–19.8 μA. Median slopes were 5.0 and 4.7 μA. (B) Median thresholds for saccade generation for the two animals were 19.0 and 23.6 μA. Median slopes were 5.7 and 10.9 μA.
The psychometric detection functions are characterized by a slope as well as a threshold. The slope is related to the sensitivity of the detection, with higher slopes corresponding to greater sensitivity. The median slopes for the two animals were 5.0 and 4.7 (Fig. 2A), a difference that was not statistically significant (P = 0.67). The distributions of slope values are similar to those for detection of electrical stimulation of areas in visual cortex (16).
Detection Thresholds Are Not Mediated by Eye Movements.
The electrical microstimulation that was used to measure detection thresholds did not cause overt saccades. The animals were required to hold their gaze within a 1–2° fixation window throughout the stimulation intervals. It would nevertheless be remarkable if activation of an oculomotor structure caused absolutely no eye movements. Because the animals might have detected electrically driven eye movements within the fixation window, rather than neuronal activity that produced no efferent response, we examined eye movements during the stimulation periods.
We measured absolute eye speed because it provides a sensitive measure of eye movement that is independent of potential differences in direction. Because eye position data for 16 sites from animal 2 were lost, this analysis was restricted to 145 of the 161 FEF sites. Fig. 3A shows the overall average absolute eye speeds recorded from both animals during stimulated and unstimulated trial intervals. The traces for stimulated intervals have been binned according to the magnitude of the stimulus current relative to the threshold current for each site. Relative to the average eye speed during unstimulated intervals (black line), there was a small but significant increase in average eye speed for currents starting in a range below behavioral threshold, with stronger currents causing faster speeds. The speed for the strongest currents integrates to a movement of <0.1° more than the unstimulated speed during the stimulus period. Both animals showed this pattern individually (data not shown). These small increases in eye speed raise the possibility that the animals may have detected the electrical stimulus only through the eye movements evoked. Three observations suggest that this is not the case.
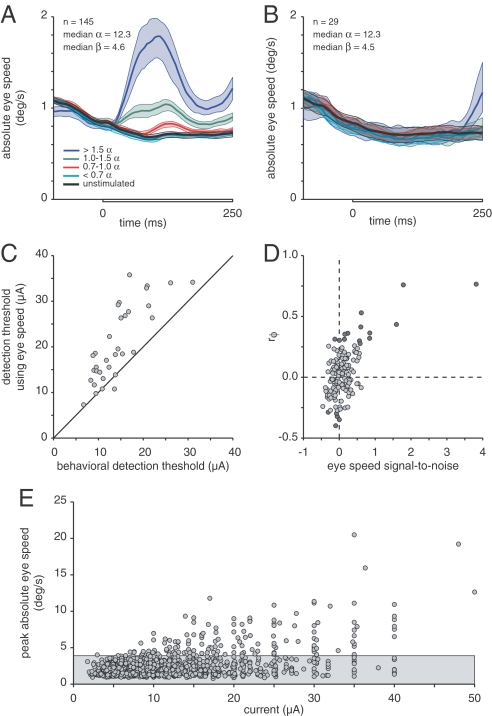
Fig. 3.
Eye movement analysis. (A) The average absolute speed of eye movement was analyzed during the 250-ms stimulus intervals (gray bar on x axis) of the 2AFC task. For 145 FEF sites, the speeds associated with different current strengths were binned according to the current relative to threshold for each site. A separate average was computed for the unstimulated intervals from all trials. Bands around each trace are 95% confidence intervals. Electrical stimulation caused a significant increase in average eye speed even for currents below behavioral threshold. (B) Average eye speeds from a subset of 29 FEF sites (20%) where no eye movements were seen (same format as A). The median threshold and slope for this subset were indistinguishable from the medians for the set of all 145 sites. (C) Behavioral thresholds that would have occurred if the animal based its choice solely on which interval in each trial had the greatest peak eye speed. For 112 of 145 sites, performance never reached threshold for any current. For the remaining 33 sites, behavioral threshold was almost always superior to what it would have been had the animal relied on eye movements. (D) Correlation between the trial interval with the greatest peak eye speed and the interval selected by the animal. The current closest to threshold was used for each site. The phi-correlation coefficient is plotted against the eye speed signal-to-noise ratio for each site. Statistically significant correlations are marked with dark symbols. Only at a handful of sites could subjects have been relying on eye speed to make their decision. (E) Peak eye speed as a function of stimulus current. Each point represents the average peak eye speed from 50 trials at one site during intervals in which a stimulus of a given current was applied. The average peak eye speed from the 50 unstimulated intervals from those trials was also found, and the gray band marks the 99% confidence interval for the distribution of average peak speeds during unstimulated intervals. Significant increases in eye speed are seen for currents starting at <10 μA.
First, the animals achieved excellent performance at sites where the stimulus did not produce even very small eye movements. Fig. 3B shows the average eye speeds recorded for 29 sites (20% of those in Fig. 3A) that were selected for good eye stability throughout the stimulus interval. The average speeds for these sites did not increase shortly after stimulus onset. (There was a rise in eye speed at the end of the stimulation interval for the highest currents, but this is too late to be attributed to a direct effect of FEF stimulation. Instead, this late increase in eye speed is likely to represent preparation for making the response saccade for the detection task.) The thresholds and slopes of the psychometric functions for these sites were statistically indistinguishable from the rest of the sample that contributed to Fig. 3A.
Second, when electrically driven eye movements did occur, they were too unreliable to support the detection thresholds the animals achieved. We computed how well an observer would do by assuming that the stimulus occurred in the interval on each trial that contained the greatest absolute eye speed. The detection thresholds using eye speed were almost invariably poorer than the animal's actual performance. For most of the sites (112/145, 77%), responses based on eye speed did not reach threshold performance (≈82% correct) for any current tested. For the remaining 33 sites we fit psychometric functions to the eye-speed-based performance, exactly as was done with the animals' reports. Fig. 3C shows that eye-speed-based performance almost always would have been poorer than that actually achieved by the animals. Thus, the subjects could not have depended on eye movements in achieving their behavioral thresholds.
Finally, eye movements were generally uncorrelated with the animals' responses. We calculated the phi-correlation between the interval in each trial with the largest peak eye speed and the interval the animal selected. The phi-correlation coefficient is a variant of Pearson's correlation coefficient that is suitable for binary variables (18). For each site, we restricted the analysis to the trials for the current closest to behavioral threshold. Fig. 3D plots the phi-correlation coefficient for each site against the signal-to-noise ratio of the eye speed (see Materials and Methods). Dark symbols mark statistically significant correlations (χ2 test, P < 0.05). For the majority of sites the peak eye speed provided only a weak signal that was not statistically correlated with the animal's response. Only in a handful of cases could the animal have been relying on eye movements.
These observations strongly suggest that the animals did not depend on the small, occasional, electrically evoked eye movements to perform the detection task. FEF microstimulation can also produce head movements at levels below those that produce saccades (19). However, a recent study examining the neck EMG response during FEF stimulation at levels too low to generate overt saccades found that the distribution of EMG thresholds was significantly higher than that of our detection thresholds.† Thus, we believe that it is also unlikely that behavioral detection of FEF microstimulation was based on this type of muscular activity.
Although the increases in eye speed produced by electrical stimulation of the FEF were too unreliable to explain the behavioral thresholds, they occurred in response to surprisingly low currents. Fig. 3E shows the peak absolute eye speed as a function of the current delivered. Each point is the peak absolute eye speed from the average of 50 eye movement traces from trials using one current level at one FEF site. For each set of 50 trials we also found the peak absolute eye speed from the average of the unstimulated intervals. The gray region marks the 99% confidence interval for peak eye speeds from unstimulated trials. Evoked movements were evident for currents as small as 10 μA. Eye movements evoked by such low currents in animals that were highly motivated to suppress eye movements raise questions about attempts to use electrical stimulation that is below movement threshold (see Discussion).
Saccade Thresholds.
We did not use the 2AFC task to measure saccade thresholds because those thresholds are elevated when the animals are electrically stimulated during fixation (20, 21). Instead, we delivered electrical stimuli shortly after spontaneous saccades were made during the free-viewing intertrial interval of a fixation task (Fig. 1B). As with the detection measurements, we presented 50 trials each of 6–10 currents that spanned saccade threshold (Fig. 1D).
We measured saccade thresholds at 102 of the FEF sites (50 for animal 1 and 52 for animal 2). Because FEF sites were selected for generating reliable saccades at currents of 50 μA or lower, each site yielded a saccade threshold. The median thresholds for the two animals were 19.0 and 23.6 μA, which was not a statistically significant difference (P = 0.07 by Kolmogorov–Smirnov test). These median saccade thresholds were 42% and 62% higher than the corresponding median detection thresholds (Fig. 2B). The distributions of the thresholds for detection and saccade differed significantly for each animal (P = 0.001 and P = 6 × 10−6 by Kolmogorov–Smirnov test), but the distributions of the slopes of the detection and saccade functions differed only for animal 2 (P = 0.24 and P = 1.9 × 10−9 by Kolmogorov–Smirnov test).
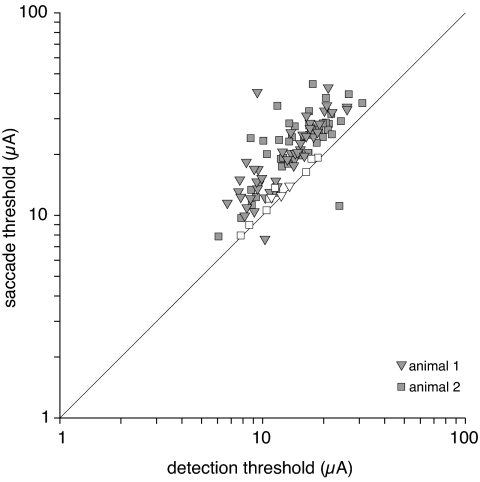
Although the FEF contains different populations of neurons with activity that has been described as visual or perisaccadic, we found no evidence for segregation of neurons into those with lower detection thresholds and those with lower saccade thresholds. To the contrary, in both animals and at most FEF sites, less current was needed to produce a detectable signal than was needed to produce a saccade, and detection and saccade thresholds measured at individual sites had a strong positive correlation. Fig. 4 shows the correlation between detection and saccade thresholds across the sites where both were measured (r = 0.72). Filled symbols indicate sites where the two threshold measurements were significantly different.
Fig. 4.
Detection versus saccade thresholds. Each point represents one site in the FEF. Points for sites with significantly different detection and saccade thresholds are filled. Electrical stimulation thresholds for saccade generation were virtually always higher than detection thresholds at the same site, and the two values were highly correlated.
Discussion
We found that monkeys could detect electrical microstimulation at every site tested in the FEF and that detection thresholds were almost always lower than those needed to evoke saccades at the same site. Detection thresholds did not have to be lower than saccade thresholds. Saccade thresholds might have been the same or lower than detection thresholds. For example, it might have been the case that FEF activity was capable of driving a detection report only when it was strong enough to drive a saccade. Were that the case, we would have been unable to measure behavioral detection thresholds with our experimental design, because our detection task required that the animal not make saccades during delivery of electrical stimulation. Alternatively, detection might have been possible without making a saccade, but only at currents higher than the saccade threshold. That is, when saccades were suppressed during the 2AFC task, the animal might have detected only currents that were much higher than those needed to produce a saccade during the free-viewing periods in which saccade thresholds were measured.
While detection thresholds were lower, the saccade threshold at each site was strongly correlated with the detection threshold. A positive correlation between these effects was not inevitable. If there were local or regional differences in the effects of microstimulation in the FEF, we could have found either no correlation or a negative correlation. For example, single-unit recordings have shown that the FEF contains both visual and perisaccadic neurons. Visual neurons have activity that is closely linked to the onset of visual stimuli (2, 8–10), whereas perisaccadic neurons have activity that is restricted to the time immediately around the execution of saccades (2, 22, 23). Some measurements suggest that these cell types are discrete classes of neurons, rather than ends of a continuum (11). If these neurons contributed differentially to detection thresholds (visual neurons) and saccade thresholds (perisaccadic neurons) but lacked functional architecture and varied randomly in their density from site to site, we might have found wide variability in thresholds and no correlation between the two thresholds. If instead there was a functional architecture that systematically segregated these two types of neurons, we might have found some sites that were enriched with visual neurons, which had detection thresholds lower than saccade thresholds, and other sites that were enriched with perisaccadic neurons, which had saccade thresholds lower than detection thresholds. This would have created an overall negative correlation between the two types of thresholds. We found no evidence for two classes of sites or for an overall negative correlation. Instead, the thresholds had unimodal distributions and a strong positive correlation.
The relationship between detection and saccade thresholds might be explained in different ways. One possibility is that detection was mediated by visual neurons and that saccades were mediated by perisaccadic neurons but that currents needed to activate perisaccadic neurons were consistently higher. This might occur if perisaccadic neurons had systematically different biophysical properties that rendered them less susceptible to extracellular currents or if there were fewer perisaccadic neurons so that a given current always activated more visual neurons. In this case, lower currents would always produce a phosphene (or perhaps some other sensation) and higher currents would be needed to produce a saccade.
Alternatively, the tight positive correlation between the higher saccade thresholds and the lower detection thresholds can be explained if the activity of FEF neurons did not represent two distinct processes but instead represented how prepared the animal is to make a saccade. By preparation we mean a process that increases the probability of a particular action without necessarily causing that action to happen. Thus, preparation to make a particular saccade might occur simultaneously with maintained fixation or with preparation to make saccades to different locations. If FEF activity represents preparation to make a saccade, the effect of our microstimulation might be akin to the artificial generation of an urge to move that has been described by patients undergoing electrical stimulation of various parts of cerebral cortex (24, 25). The detection thresholds might have been based on the animals reporting which interval coincided with a greater readiness, or urge, to make a saccade with a particular vector. If FEF activity represents preparation to move rather than a signal to begin an obligatory saccade, the animal would generally not make a saccade when fixating or otherwise suppressing eye movements, as was required during our detection task. Only occasionally at higher currents, which presumably produced urges so great they were not easily suppressed, would saccades result. During measurements of saccade thresholds the animals were freely viewing the room around them. If the animal were looking at something that held its interest, saccade threshold would be elevated because of spontaneous suppression of most saccades. On the other hand, if the animal were not engaged with anything visual, saccade threshold would approach detection threshold as saccade suppression approached zero. This would result in a positive correlation between detection and saccade thresholds, with saccade thresholds typically somewhat higher. Saccade thresholds would rarely be measured as lower than detection thresholds, because the animal could not act on movement preparation that it could not detect. Several studies have shown that electrical stimulation of the FEF at intensities that do not produce overt eye movements, or TMS, can produce effects similar to spatial attention (7, 12–15). Our results could also be explained in terms of FEF activity corresponding to the degree of attention to a particular location, which would indirectly increase the probability of a saccade to that location. Our threshold measurements cannot distinguish between explanations such as saccade preparation and spatial attention. Although it is consistent with the threshold data, the idea that neurons in FEF represent preparation to make a saccade is not easily reconciled with the two distinct types of FEF single-unit response properties described above. It may be possible to resolve the contributions of visual and perisaccadic cells to detection and saccade thresholds in the future with the development of methods that can selectively stimulate subsets of neurons that are connected with particular targets (26).
In recent years many investigators have electrically stimulated oculomotor structures using currents below those that produce overt eye movements and have reported modulation of activity in visual cortex or behavioral effects in visual tasks (7, 27–34). Although conspicuous eye movements were not produced at the currents used in these studies, stimulation might have been directly detected or may have produced small displacements of eye position (Fig. 3E). The currents used in microstimulation studies are often greater than those that our subjects detected reliably. The interpretation of most of these experiments does not depend greatly on whether the animal detected when an electrical stimulus was delivered. Nevertheless, given the low detection thresholds that have been found in a range of cortical regions, it will be important to construct paradigms in which detection of microstimulation cannot introduce a confound. Although electrical stimuli can be precisely delivered at currents much lower than those typically used, it is possible that most of the neurophysiological and behavioral consequences of microstimulation require a current that is at or above behavioral detection threshold.
The distributions of thresholds for detecting microstimulation in the FEF are only slightly higher than those for detecting microstimulation of areas in visual cortex (16), which were obtained by using the same two animals and the same methods as the current study. Median thresholds increased progressively from ≈6 μA in V1 to ≈11 μA in inferotemporal cortex. The detection thresholds reported here for the FEF (≈14 μA) are a bit higher than those measured in any of the five areas examined in visual cortex. Thus, there is a progressive increase in detection thresholds going from areas closer to sensory input toward those closer to motor output. Although the FEF contains neurons with distinct visual and perisaccadic response properties, thresholds for detecting microstimulation were no more widely distributed than those found in visual cortex, where most areas seem to have a more limited range of response properties. Nevertheless, it is noteworthy that distribution of detection thresholds in the FEF overlaps with those for each of the visual areas, and low thresholds (in the range of 5–30 μA) have been found at every one of hundreds of sites spanning a large range of neocortex. Collectively, these data suggest that activation of relatively small numbers of neurons in any part of neocortex may be behaviorally detectable, with no region more privileged than any other in its ability to support a percept. The readout of a perceptual signal is possible even in areas that have long been thought to be involved primarily in motor functions, such as FEF.
Materials and Methods
Microstimulation was done in two rhesus monkeys (Macaca mulatta) that had previously been used to measure thresholds for detecting microstimulation of areas in visual cerebral cortex (16). A chamber was placed over the arcuate sulcus, as identified by MRI and histological confirmation. Before testing, the FEF was identified based on its stereotaxic location, sulcus location, and response properties. Every site included in the study reliably generated stereotyped saccades using currents no greater than 50 μA. All electrical microstimulation used in these experiments included 200-μs biphasic pulses of constant current delivered at 200 Hz for 250 ms through glass-insulated Pt/Ir electrodes that we made ourselves (≈0.2–1.5 MΩ at 1 kHz). Currents >50 μA were never used.
Each animal required several days of practice detecting electrical stimulation. During this period detection thresholds fell and stabilized. The data reported here were collected after detection thresholds became stable. Behavioral thresholds were determined by using the method of constant stimuli and the 2AFC task described above.
Saccade thresholds were determined by delivering stimulus trains 100 ms after a spontaneous saccade was made during intertrial intervals in a fixation task in a dimly lit recording room. Stimulation was delivered only after a saccade that reached at least 10°/s and had an amplitude between 1° and 10°. If a saccade exceeding 10°/s ensued within 250 ms of stimulus onset, it was counted as an electrically elicited saccade. Electrically elicited saccades were not rewarded. We used a liberal window for detecting saccades to ensure that we did not artificially inflate saccade thresholds. Both animals made some spontaneous saccades within this window, which introduced a pedestal to the saccade-probability functions (e.g., Fig. 1D). This pedestal did not appreciably affect measurements of saccade threshold. Data for saccade thresholds were sometimes collected before detection thresholds and sometimes after.
We fit a cumulative Weibull function (35) to behavioral performance on the 2AFC detection task (Eq. 1) and saccade generation (Eq. 2):
The value of α was taken as the threshold, and β was used for the slope. Ninety-five percent confidence intervals for α and β were taken as the 0.025 and 0.975 values of α and β distributions produced by bootstrapping. Significant differences between detection and saccade thresholds were found by bootstrapping differences between pairs of samples drawn from the bootstrapped α distributions for those two performance functions.
Eye position was recorded every 5 ms by using a scleral search coil. For the analysis of eye speed, horizontal and vertical eye positions were smoothed with a Gaussian filter (σ = 12.5 ms) and differentiated, and the absolute value of the vectorially combined speed was found. Confidence intervals for averages were found by bootstrap. Analyses using the peak of absolute eye speeds took the peak from a period 0–125 ms after the start of the stimulated or unstimulated intervals. The eye speed signal-to-noise ratio for a given stimulus condition was calculated as the peak of the average eye speed during stimulated intervals minus the peak of the average eye speed during unstimulated intervals divided by the standard deviation of the peak eye speed during unstimulated intervals.
Acknowledgments.
We thank William Bosking, Xinmiao Peng, Joonyeol Lee, and Daniel Yoshor for their insights and suggestions during animal training, data collection, and the writing process. We are grateful to Marlene Cohen, Amy Ni, Pierre Pouget, and Jeffrey Schall for helpful comments on drafts of the manuscript. We also thank Vivian Imamura, Dennis Murray, and Tori Williford for their technical assistance. This work was supported by National Institutes of Health Grant R01EY05911. J.H.R.M. is an Investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Elsley J, Nagy B, Rezvani S, Corneil B, Thirty-Sixth Annual Meeting of the Society for Neuroscience, October 14–18, 2006, Atlanta.
References
- 1.Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc London B. 2002;35:1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruce CJ, et al. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 3.Schiller PH, Chou IH. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nat Neurosci. 1998;1:248–253. doi: 10.1038/693. [DOI] [PubMed] [Google Scholar]
- 4.Burke M, Barnes G. Brain and behavior: A task-dependent eye movement study. Cereb Cortex. 2007;18:126–135. doi: 10.1093/cercor/bhm038. [DOI] [PubMed] [Google Scholar]
- 5.Nyffeler T, et al. Repetitive TMS over the human oculomotor cortex: Comparison of 1-Hz and theta burst stimulation. Neurosci Lett. 2006;409:57–60. doi: 10.1016/j.neulet.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Tobler PN, Muri RM. Role of human frontal and supplementary eye fields in double step saccades. NeuroReport. 2002;13:253–255. doi: 10.1097/00001756-200202110-00016. [DOI] [PubMed] [Google Scholar]
- 7.Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- 8.Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381:697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Q, Barborica A, Ferrera VP. Radial motion bias in macaque frontal eye field. Visual Neurosci. 2006;23:49–60. doi: 10.1017/S0952523806231055. [DOI] [PubMed] [Google Scholar]
- 10.Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- 11.DiCarlo JJ, Maunsell JHR. Using neuronal latency to determine sensory-motor processing pathways in reaction time tasks. J Neurophysiol. 2005;93:2974–2986. doi: 10.1152/jn.00508.2004. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Ruff CC, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 14.Silvanto J, Lavie N, Walsh V. Stimulation of human frontal eye fields modulates sensitivity of extrastriate visual cortex. J Neurophysiol. 2006;96:941–945. doi: 10.1152/jn.00015.2006. [DOI] [PubMed] [Google Scholar]
- 15.Neggers SF, et al. TMS pulses on the frontal eye fields break coupling between visuo-spatial attention and eye movements. J Neurophysiol. 2007;98:2765–2778. doi: 10.1152/jn.00357.2007. [DOI] [PubMed] [Google Scholar]
- 16.Murphey DK, Maunsell JHR. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Lafuente V, Romo R. Neuronal correlates of subjective sensory experience. Nat Neurosci. 2005;8:1698–1703. doi: 10.1038/nn1587. [DOI] [PubMed] [Google Scholar]
- 18.Yule GU. On the methods of measuring association between two attributes. J R Stat Soc. 1912:576–642. [Google Scholar]
- 19.Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: Effects of microstimulation. J Neurophysiol. 2007;97:618–634. doi: 10.1152/jn.00256.2006. [DOI] [PubMed] [Google Scholar]
- 20.Tehovnik EJ, Slocum WM. Behavioural state affects saccades elicited electrically from neocortex. Neurosci Biobehav Rev. 2004;28:13–25. doi: 10.1016/j.neubiorev.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg ME, Bushnell MC, Bruce CJ. The effect of attentive fixation on eye movements evoked by electrical stimulation of the frontal eye fields. Exp Brain Res. 1986;61:579–584. doi: 10.1007/BF00237584. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg ME, Bushnell MC. Behavioral enhancement of visual responses in monkey cerebral cortex. II. Modulation in frontal eye fields specifically related to saccades. J Neurophysiol. 1981;46:773–787. doi: 10.1152/jn.1981.46.4.773. [DOI] [PubMed] [Google Scholar]
- 23.Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- 24.Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: Macmillan; 1950. [Google Scholar]
- 25.Fried I, et al. Functional organization of human supplementary motor cortex studied by electrical stimulation. J Neurosci. 1991;11:3656–3666. doi: 10.1523/JNEUROSCI.11-11-03656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagel G, et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burman DD, Bruce CJ. Suppression of task-related saccades by electrical stimulation in the primate's frontal eye field. J Neurophysiol. 1997;77:2252–2267. doi: 10.1152/jn.1997.77.5.2252. [DOI] [PubMed] [Google Scholar]
- 28.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- 30.Opris I, Barborica A, Ferrera VP. Microstimulation of the dorsolateral prefrontal cortex biases saccade target selection. J Cognit Neurosci. 2005;17:893–904. doi: 10.1162/0898929054021120. [DOI] [PubMed] [Google Scholar]
- 31.Cutrell EB, Marrocco RT. Electrical microstimulation of primate posterior parietal cortex initiates orienting and alerting components of covert attention. Exp Brain Res. 2002;144:103–113. doi: 10.1007/s00221-002-1032-x. [DOI] [PubMed] [Google Scholar]
- 32.Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavanaugh J, Alvarez BD, Wurtz RH. Enhanced performance with brain stimulation: Attentional shift or visual cue? J Neurosci. 2006;26:11347–11358. doi: 10.1523/JNEUROSCI.2376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quick RF., Jr A vector-magnitude model of contrast detection. Kybernetik. 1974;16:65–67. doi: 10.1007/BF00271628. [DOI] [PubMed] [Google Scholar]