Abstract
Background and purpose:
Prenatal patency of ductus arteriosus is maintained by prostaglandin (PG) E2 in concert with nitric oxide (NO) and carbon monoxide (CO). Accordingly, we have previously found that NO activity increases upon deletion of either COX. Here, we have examined whether COX inhibition by indomethacin mimics COX deletion in promoting NO.
Experimental approach:
Experiments were performed in vitro and in vivo with wild-type (WT) and eNOS−/−, near-term mouse foetuses. Indomethacin was given p.o. to the mother as single (acute treatment) or repeated (daily for 3 days; chronic treatment) doses within a therapeutic range (2 mg kg−1).
Key results:
Indomethacin promoted eNOS mRNA expression in the WT ductus. Coincidentally, the drug enhanced the contraction of the isolated ductus to the NOS inhibitor, NG-nitro-L-arginine methyl ester, and its effect augmented with the length of treatment. No such enhancement was seen with the eNOS−/− ductus. Chronic indomethacin also increased, albeit marginally, the contraction of the WT ductus to the CO synthesis inhibitor, zinc protoporphyrin. Whether given acutely or chronically, indomethacin induced a little narrowing of the ductus antenatally and had no effect on postnatal closure of the vessel.
Conclusions and implications:
We conclude that activation of NO and, to a much lesser degree, CO mechanisms is an integral part of the indomethacin effect on the ductus. This relaxing influence may oppose the contraction from PGE2 suppression and could explain the failures of indomethacin therapy in premature infants with persistent duct.
Keywords: ductus arteriosus, prostaglandin E2, nitric oxide, carbon monoxide, COX, NOS, haeme oxygenase, indomethacin, fetal and neonatal physiology
Introduction
It is well accepted that patency of the ductus arteriosus in utero is actively sustained by several agents, including prostaglandin (PG) E2, nitric oxide (NO), carbon monoxide (CO) and, possibly, an endothelium-derived hyperpolarizing factor (Smith, 1998; Baragatti et al., 2007). Among them, PGE2 and NO appear to operate in special synergy, with the former being the prime effector and the latter providing any compensatory action. Consistent with a preferential, PGE2–NO, cooperative coupling is the finding of NO upregulation upon deletion of PGE2- but not CO-forming enzymes (Baragatti et al., 2003, 2007). Furthermore, at least in certain species, efficacy of the two agents varies in a reciprocal fashion during development (Momma and Toyono, 1999; Takizawa et al., 2001; Richard et al., 2004). Accordingly, both prenatally and postnatally, in animals as in humans, combined treatment with COX and NOS inhibitors is most effective in promoting closure of the ductus arteriosus (Seidner et al., 2001; Takizawa et al., 2001; Richard et al., 2004; Keller et al., 2005).
From the foregoing, an immediate question is whether pharmacological inhibition of COX mimics COX deletion in causing a rebound activation of the NO system. Available data accord with this possibility. Antenatal exposure to COX inhibitors, whether dual (COX1/COX2) or isoform specific, increases the incidence of patent ductus in the neonate (Norton et al., 1993; Loftin et al., 2002; Suarez et al., 2002), and this outwardly paradoxical finding could reflect the increased importance of a compensatory mechanism. The same explanation may also account for the frequent failure of COX inhibitors, such as indomethacin and ibuprofen, to close a patent ductus in the premature infant despite their adequate concentration in the circulation and a transient constriction to the compounds attesting to their intrinsic efficacy (Ohlsson et al., 2004; Shah and Ohlsson, 2004). In fact, to confirm the point, these patients present an increased urinary excretion of the stable metabolites of NO (Miller et al., 1993). Directly relevant is also the finding that indomethacin, although at a dose far exceeding the conventional range, promotes NO function in the cerebral vasculature of the newborn pig (Zhang and Leffler, 2002).
On the basis of this premise, our objective was to ascertain whether indomethacin, being employed in a therapeutic dosage, promotes NO function in the fetal ductus. A dual inhibitor was preferred not only to mimic the clinical situation, but also to avoid any reciprocal compensation among COX isoforms. The drug was given in single or multiple doses to ascertain how fast any upregulation may develop. As a corollary, to obtain a negative control, the same treatment was carried out in animals lacking the major isoform of NOS (that is, the endothelial isoform, eNOS) (Baragatti et al., 2007). Results validate our hypothesis and demonstrate that NO rebound is an integral part of indomethacin action within the ductus and, conceivably, outside it. The latter notion has conceptual and practical implications for the future use of nonsteroidal anti-inflammatory drugs (NSAIDs).
Methods
Experiments were carried out in wild-type (WT) C57BL/6 mice (litter size, 2–11; mean, 6) and in B6/129 mice with eNOS−/− genotype backcrossed onto the C57BL/6 strain (litter size, 1–11; mean, 7) (line of Leuwerke et al., 2002 from the original stock of Shesely et al., 1996). In the latter case, genotype was confirmed in tail specimens by PCR. Animals were housed in temperature- and humidity-controlled quarters with constant 12:12-h light–dark cycles and were given food and water ad libitum. Surgical procedures and experimental protocols were approved by the Animal Care Committee of the Ministry of Health.
Dosing and measurement of indomethacin
Adult, WT mice (n=14) were given indomethacin (2 mg kg−1, suspended in 5% gum arabic) by orogastric tube at a fixed time of the day (0900 hours) as a single dose or repeated doses over 4 consecutive days. In the former case, blood was withdrawn from the caudal vein in a set sequence after the administration (0.5, 1, 2, 4, 6, 8, 24 h), whereas in the latter case collection was performed once daily at 24-h intervals. For this purpose, a small cut was made in the distal part of the tail and, after collecting a sufficient amount of blood (about 0.1 ml), bleeding was stopped by gently tying a silk thread proximally. Furthermore, no more than four withdrawals were made in any mouse. Hence, two animals were used in parallel with every acute experiment, each yielding blood samples at different time intervals (animal 1: 0.5, 2, 6, 24 h; animal 2: 1, 4, 8 h). Control experiments were carried out in pregnant animals, using a single dose of indomethacin on gestation day 18 (n=2) or repeated daily doses between gestation days 15 and 18 (blood collection, gestation days 16–19) (n=1). In all cases, blood was collected in ice-chilled, polypropylene tubes containing heparin (500 U), and plasma was separated immediately by centrifugation (2000 g; 15 min at 4 °C) to be stored at −20 °C until analysis.
For indomethacin measurement, an aliquot of plasma (60 μl) was added a fixed volume (125 μl) of internal standard in acetonitrile (mefenamic acid, 2 μg ml−1). This mixture was vortexed for 30 s and then centrifuged at 8000 g for an additional 2 min. The resultant supernatant was dried under N2, and the residue was resuspended in phosphoric acid 6 mM-acetonitrile (50:50; v/v) for separation of indomethacin according to the HPLC method of Sato et al. (1997). For this purpose, the sample was passed down a Nova-Pak C18 column (Waters Associates, Milford, DE, USA) using the same acetonitrile-phosphoric acid mixture as the mobile phase (retention time, 9.5 and 16 min for indomethacin and mefenamic acid, respectively). This method separated indomethacin from its metabolites, and plasma samples spiked with known amounts of the drug provided a reference curve. Recovery of indomethacin through the procedure was 96±3% (n=4).
On the basis of the results of this analysis (see Figure 1), in the actual experiment pregnant animals were treated either once on day 18 of gestation (acute treatment) or three times at 24-h intervals from days 16 to 18 of gestation (chronic treatment). With both schedules, foetuses were used on day 19 of gestation 21 h after treatment (single dose or last dose of a sequence), while newborns were studied 3 and 12 h after vaginal delivery. In the latter case, the interval between indomethacin treatment and delivery varied between 20 and 33 h. From published data on the rate of drug transfer across the rodent placenta (Momma and Takao, 1987), we expect with either treatment regimen a fetal/maternal ratio of 0.4. Animals being given vehicle alone served as control. Mortality among litters was exceptional, and no difference was noted in this respect between untreated and treated animals.
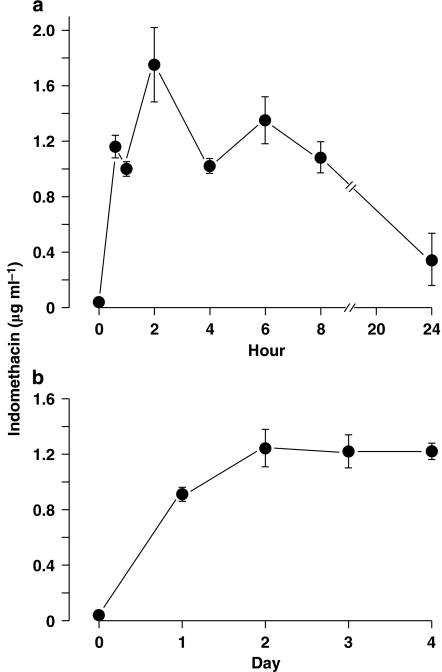
Figure 1.
Nonpregnant wild-type (WT) mouse. Time course of indomethacin concentration in blood following treatment with single (a) (n=5) and repeated (b) (n=9) oral doses (2 mg kg−1). In the latter case, the drug was given at 24-h intervals from days 0 to 3 and blood was collected for analysis just prior to each administration. Note that two animals were used simultaneously with every acute experiment, each yielding blood samples at different time intervals to build a complete concentration curve (for details, see Methods).
In vitro studies
Untreated or indomethacin-treated fetal mice, both WT and eNOS−/−, were delivered by Caesarean section under halothane anaesthesia and were killed by cervical dislocation. Body weight varied between 0.9 and 1.4 g and only one foetus was used in each experiment. The procedure for dissection of the ductus arteriosus, normalization of internal circumference and mechanical record has been described previously (Coceani et al., 1999). In brief, the animal was secured with its left side up in a dissection chamber containing ice-cold Krebs solution gassed with 5% CO2 in N2. Through a thoracotomy, the ductus was exposed, separated from the adjoining large blood vessels and then suspended onto 25-μm tungsten wires (Cooner Wire, Chatsworth, Los Angeles, CA, USA) to be transferred to an organ bath. The fluid in the bath was gassed with a mixture containing 2.5% O2 to mimic the fetal condition. The same mixture was flushed through a hood covering the bath. Preparations were equilibrated (about 60 min at 37 °C) with a minimal stretch being applied (WT, 0.09±0.007 (n=13), 0.07±0.01 (n=5) and 0.07±0.01 (n=12) for control, acute and chronic indomethacin treatments, respectively; eNOS−/−, 0.08±0.01 (n=7) and 0.08±0.01 (n=8) for control and chronic indomethacin treatments, respectively; values in mN mm−1) and the attendant internal circumference (C0) with the related resting dimension (Table 1) served as a reference for choosing the appropriate load. Afterwards, tension was applied to attain an operating circumference (that is, C50) coinciding with the condition in vivo (WT, 0.44±0.006 (n=13), 0.41±0.01 (n=5) and 0.43±0.007 (n=12) for control, acute and chronic indomethacin treatments, respectively; eNOS−/−, 0.46±0.006 (n=7) and 0.45±0.009 (n=8) for control and chronic indomethacin treatments, respectively; values in mN mm−1), and the actual experiment was started following a second, 60–150-min period of equilibration. Notably, indomethacin pretreatment had no obvious effect on the mechanical characteristics of the ductus in either genotype.
Table 1.
Resting dimension of isolated ductus arteriosus from WT and eNOS−/− fetal mice in the absence and presence of indomethacin pretreatment
| Condition | Internal diameter |
Vessel lengtha |
Wall thickness | |
|---|---|---|---|---|
| Short side | Long side | |||
| WT, control (n=13) | 130±1 | 581±20 | 594±20 | 21±0.5 |
| WT, acute indo (n=5) | 120±2* | 503±33 | 512±31 | 19±0.9 |
| WT, chronic indo (n=12) | 128±1 | 525±16 | 540±16 | 20±0.6 |
| eNOS−/−, control (n=7) | 136±1 | 567±13 | 577±12 | 19±0.8 |
| eNOS−/−, chronic indo (n=8) | 132±2 | 537±20 | 546±21 | 20±0.7 |
Abbreviations: eNOS, endothelial NOS; indo, indomethacin; WT, wild type.
Values (μm) are mean±s.e.mean and apply to preparations used for in vitro studies. Pregnant mothers were treated with vehicle alone (control) or indomethacin in single or repeated doses (respectively, acute and chronic treatments; for details, see Methods).
Vessel length of the ductus is uneven because of its insertion into aorta at an angle (short side taken for calculations, see In vitro studies).
*P<0.05 vs WT control and eNOS−/− chronic indo; P<0.001 vs eNOS−/− control (ANOVA with Bonferroni post hoc test).
The study comprised two groups of experiments dealing respectively with WT and eNOS−/− mice. In group 1 (n=30), L-NAME (100 μM) was added to the perfusion fluid and its effect on basal tone, being taken as an index of NO activity, was ascertained in the absence and presence of indomethacin pretreatment (acute and chronic). The selectivity of any NO change was determined by testing under the same condition (chronic indomethacin treatment) the inhibitor of the CO-forming haeme oxygenase, zinc protoporphyrin (ZnPP) (10 μM). Significantly, ZnPP contraction, unlike L-NAME contraction, is not modified by removal of either COX (Baragatti et al., 2003, 2007). In addition, as a separate experiment, indomethacin-pretreated preparations (chronic treatment) were tested again with the same inhibitor (2.8 μM) expecting the response to be reduced in case of persistence of the COX inhibition through the workup procedure. This information would, in turn, indicate whether or not any NO upregulation is limited to the period of COX inhibition. In group 2 (n=15), experiments with L-NAME were repeated with the ductus from untreated vs indomethacin-treated (chronic treatment) eNOS−/− foetuses. In all cases, only one test procedure was employed with each vessel, and the contraction to the TXA2 (thromboxane A2) analogue (0.1 μM) served as a reference.
In vivo studies
Experiments were carried out in WT foetuses (n=15) or newborns (n=21), with the aim of comparing untreated and indomethacin-pretreated animals (acute and chronic treatments). Foetuses were delivered by Caesarean section under halothane anaesthesia, while newborns were used at different intervals after vaginal delivery. Time zero (that is, the time at which delivery was completed) was assessed for each animal in the litter. Throughout the survival period, newborns were kept with the mother. All animals were killed by cervical dislocation.
Ductus calibre was assessed, both prenatally and postnatally, by fixing the vessel in situ with the whole-body freezing technique (Hörnblad and Larsson, 1967; Coceani et al., 1999). Briefly, animals were placed with their right side up in a Petri dish and were covered with an embedding medium (Tissue-Tek optimum cutting temperature compound; Sakura Finetek, Torrance, CA, USA). The dish with the animal was then wrapped with aluminium foil and immersed in liquid N2. Once frozen, specimens were stored at −20 °C for further workup. To prepare a block of tissue with the ductus, the carcass was freed of its embedding in the frozen state. With a razor blade, soft tissues covering the dorsum were removed, making certain that the exposed surface would be parallel to the underlying descending aorta and, hence, perpendicular to the ductus. Serial, transversal sections (5-μm thick), progressing from this cut through the whole length of the vessel, were obtained on a Zeiss freezing microtome (model HM500 OM; Zeiss, Walldorf, Germany) and were stained with 1% methylene blue. Care was taken to collect a complete series of ductus sections. Afterwards, each section was photographed with a CCD solid-state camera (COHU, San Diego, CA, USA), and lumen area of the ductus was measured with a MCID-M4 program (Brock University, St Catharines, ON, Canada). Both maximal and minimal values of this area were retrieved from each series for computation.
Total RNA preparation and quantitative RT-PCR
Ductuses were collected from untreated and indomethacin-pretreated (acute and chronic treatments) foetuses and were pooled in two groups for each condition (38–50 specimens per group). RNA was then isolated as described earlier (Baragatti et al., 2003), and its yield was measured spectrophotometrically. DNase I (Roche, Indianapolis, IN, USA)-treated total RNA (2.5 μg) was reverse transcribed with 1 U of Thermoscript RT (Invitrogen, Carlsbad, CA, USA) in the presence of random hexanucleotide primers according to the manifacturer's instructions. Quantitative reverse transcription (RT)-PCRs (40 cycles) were performed on an ABI Prism 7700 instrument (Applied Biosystems, Foster City, CA, USA) using the TaqMan Universal PCR Master Mix (Applied Biosystems). Primer sequences for eNOS and cyclophilin B (internal standard) were obtained from an online library (Applied Biosystems). Gene expression was quantified in triplicate for each group, using the comparative cycle threshold method and cyclophilin for normalization.
Solutions and drugs
The Krebs medium had the following composition (mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; KH2PO4, 1; MgSO4, 0.9; dextrose, 11.1 and NaHCO3, 25. Depending on the stage of the experiment (see above), the solution was bubbled with gas mixtures containing either no O2 or 2.5% O2 plus 5% CO2 and balance N2. PO2 was measured with a Chiron gas analyzer (mod. 248, Chiron, Halstead, UK) and was 0.96±0.001 and 2.1±0.02 kPa (pH 7.39±0.002) when gas mixtures had, respectively, 0 and 2.5% O2.
The following compounds were used: the NOS inhibitor, NG-nitro-L-arginine methyl ester (L-NAME; Sigma, St Louis, MO, USA); the haeme oxygenase inhibitor, ( ZnPP; Porphyrin Products, Camforth, UK); the dual COX1/COX2 inhibitors, indomethacin and mefenamic acid (Sigma); and the TXA2 analogue 9,11-epithio-11,12-methano-thromboxane A2 (ONO-11113; courtesy of ONO Pharmaceutical, Osaka, Japan). Concentrations of the inhibitors were derived from the literature with the aim of combining efficacy with selectivity.
9,11-Epithio-11,12-methano-thromboxane A2 was dissolved in distilled ethanol (5 mg ml−1), and aliquots (stored at −70 °C) were diluted with Tris buffer (pH 7.4). Indomethacin was also dissolved in ethanol (10 mg ml−1) before preparation of the final solution in the Krebs medium. Likewise, ZnPP was first prepared as a stock solution in 0.1 M NaOH (1 mM) on the day of the experiment. Ethanol in the fluid bathing isolated ductus preparations did not exceed 0.01% (indomethacin) or 0.001% (ONO-11113). Other substances dissolved readily in saline or Krebs medium. ZnPP solutions were protected from light.
Concentrations of all compounds are given in molar units and refer to their final value in the bath. Vehicle alone, without or with ethanol (see above), had no effect on vessel tone.
Analysis of data
Baseline contractile tension, which varied with the preparation (see Results), refers to the net active tension (that is, total tension minus applied tension) developed by the preparation prior to any treatment. Responses to the constrictors were measured by the rise in tension over baseline and were expressed in absolute values.
Data are expressed as the mean±s.e.mean. Comparisons were made using a Student's t-test or ANOVA, followed by the Bonferroni test. Differences are considered significant for P<0.05.
Results
Blood levels of indomethacin
In the nonpregnant animal, indomethacin was measurable in the circulation within 30 min of a single-dose administration (2 mg kg−1) and reached a peak concentration (mean 1.7±0.2 μg ml−1) at about 2 h (Figure 1a). Afterwards, its levels gradually decreased to a value of 0.3±0.1 μg ml−1 at the 24-h mark. When the same treatment was repeated over 4 consecutive days, blood concentrations kept increasing with the first two doses and then attained a plateau just prior to the third dose (Figure 1b). Similar results were obtained with either schedule in the pregnant animal (n=2 and 1, respectively, for acute and chronic treatments) (data not shown). Hence, a three-dose sequence was chosen for chronic treatment in the actual experiment, expecting at the end of the course a stable blood concentration of indomethacin (mean ∼1.2 μg ml−1) for at least 24 h.
mRNA analysis
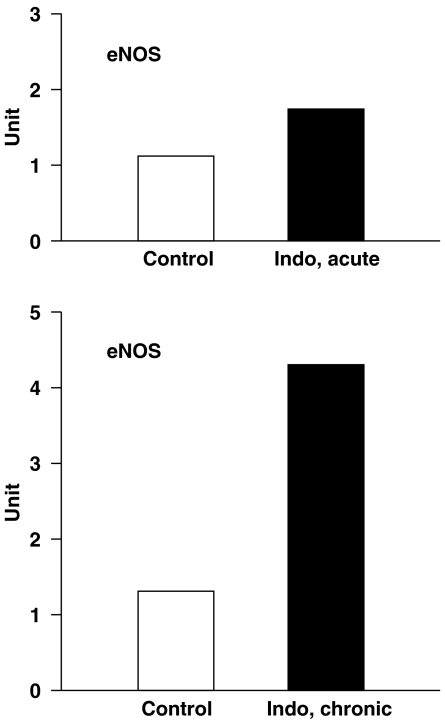
Indomethacin promoted eNOS mRNA expression in the WT ductus dose dependently. As shown in Figure 2, the increase was modest with the acute treatment, while it reached the three- to fourfold mark with the chronic treatment. Hence, eNOS behaved differently from the other NOS isoforms that, upon COX inhibition or deletion, remain barely measurable or are not measurable at all (Baragatti et al., 2003, 2007).
Figure 2.
Quantitative reverse transcription (RT)-PCR on endothelial NOS (eNOS) from wild-type (WT) mouse ductus arteriosus in the absence and presence of indomethacin (indo) pretreatment. Specimens were collected on gestation day 19 after a single dose (indo, acute) or 3 days of treatment (indo, chronic) to the mother (for details, see Methods). With every condition, relative levels of transcript are the mean of two samples (each in triplicate) and are expressed in arbitrary units after normalization against reference cyclophilin. Note that the two controls were obtained from different sets of specimens.
In vitro studies
As shown in Table 1, the excised ductus presented comparable dimensions in WT and eNOS−/− mice, and they were not modified by chronic treatment with indomethacin. The acute treatment, however, caused a narrowing of the vessel (Table 1). All preparations, regardless of their genotype and the prior exposure to indomethacin, developed a similar degree of tension (0.28±0.03 mN mm−1, n=45) during equilibration, which, in most cases, was maintained thereafter. In addition, transient contractions (0.1–0.7 mN mm−1), being superimposed over the baseline with either a slow or a rapid pattern (see Baragatti et al., 2003, 2007), were evident in nearly all preparations across the experimental groups. Likewise, regardless of any intervening treatment with indomethacin, the contraction to the TXA2 analogue ONO-11113 (0.1 μM) had similar magnitude in WT (control, 0.98±0.12, n=6; acute indomethacin, 0.99±0.07, n=5, chronic indomethacin, 1.27±0.08, n=10; all in mN mm−1) and eNOS−/− animals (control, 0.94±0.06, n=7; chronic indomethacin, 1.01±0.08, n=5; all in mN mm−1).
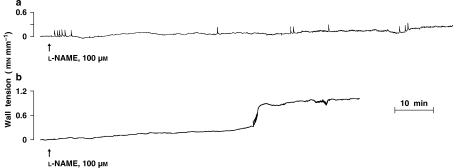
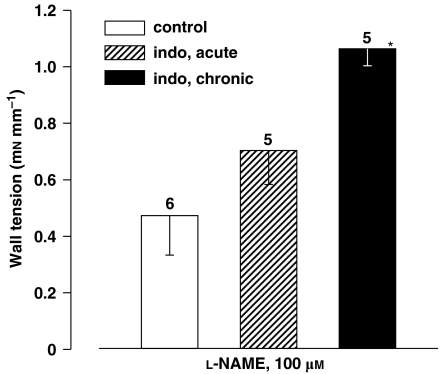
In accord with earlier findings (Baragatti et al., 2003, 2007), L-NAME (100 μM) produced a slow contraction in the WT ductus, starting after some delay (7–70 min, mean 32) and reaching a stable plateau in 46–128 min (mean, 102) (Figure 3a). Its magnitude was smaller than that observed with drugs interfering with either COX (indomethacin 2.8 μM) or haeme oxygenase (ZnPP 10 μM) activity (Figures 4 and 5). However, in those instances in which the mother had been pretreated chronically with indomethacin, the L-NAME contraction began earlier (latency 2–10 min, mean 5) and doubled its magnitude (Figure 4), hence equalling the response of the naive animal to indomethacin and ZnPP (Figure 5). The contraction also changed its pattern and showed a biphasic course, with an initial slow progression giving way at about the 60-min mark to an abrupt rise in tone (Figure 3b). Changes induced by chronic indomethacin mimicked those occurring with the deletion of either COX (Baragatti et al., 2003). A trend for a greater L-NAME effect, although without reaching significance, was also seen after acute indomethacin (Figure 4). Accordingly, the latter response displayed two distinct patterns in its development, with some experiments (n=2) maintaining the slow progression of the control group and others (n=3) presenting instead the characteristics of the chronic indomethacin group.
Figure 3.
Representative response to NG-nitro-L-arginine methyl ester (L-NAME) in the untreated and indomethacin-pretreated (chronic treatment) fetal mouse ductus arteriosus. (a) Control; (b) indomethacin. Length of the vessel is 592 and 570 μm (short side, see Methods) for untreated and treated animal, respectively.
Figure 4.
Isolated ductus arteriosus from wild-type (WT) fetal mouse. Comparison of contractile responses to NG-nitro-L-arginine methyl ester (L-NAME) (100 μM) in control vs indomethacin (indo)-pretreated preparations in vivo (acute and chronic treatments). Baseline wall tension (mN mm−1) was as follows: control, 0; acute indomethacin, 0.04±0.02; chronic indomethacin, 0.01±0.01. For each group, the number of experiments is given above the columns, and a significant difference (P<0.01, ANOVA) between control and chronic indomethacin is indicated with an asterisk.
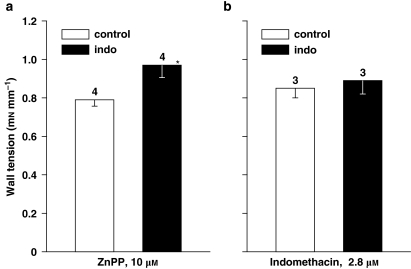
Figure 5.
Isolated ductus arteriosus from wild-type (WT) fetal mouse. Comparison of contractile responses to (a) zinc protoporphyrin (ZnPP) 10 μM and (b) indomethacin 2.8 μM in control vs indomethacin (indo)-pretreated preparations in vivo (chronic treatment). Baseline wall tension (mN mm−1) was as follows: (a) control, 0.39±0.06; indomethacin, 0.03±0.03; (b) control, 0.35±0.08; indomethacin, 0.41±0.07. For each group, the number of experiments is given above the columns. *Vs control, P=0.042 (Student's t-test).
The L-NAME contraction tended to be smaller in the eNOS−/− than the WT ductus (0.31±0.06 vs 0.47±0.14 mN mm−1, n=7 and 6) and was often not sustained (three out of seven experiments). In addition, unlike the WT (Figure 4), this contraction did not increase upon exposure to chronic indomethacin, and the resulting tension (0.30±0.08 mN mm−1, n=8) coincided with that of the untreated preparation. The only sign of treatment was a reduction of the latency from 14–70 min (mean, 35) to 3–38 min (mean, 18) and the presence of a sustained response in all cases.
At variance with the results of COX deletion (Baragatti et al., 2007), ZnPP contraction was enhanced by antenatal exposure to chronic indomethacin (Figure 5a). Such enhancement, however, was modest compared with that seen with L-NAME under the same conditions (see Figure 4). Conversely, no change at all was seen in the indomethacin contraction following the same pretreatment (Figure 5b), implying that COXs had regained their normal function through the equilibration period and baseline recording (in total, about 5 h) despite the low washout rate of the drug (Laneuville et al., 1994). Critical for such restoration is the exceedingly fast turnover of the enzyme (half-life, 5–10 min) with the attendant dynamic adjustment to any new condition (Fagan and Goldberg, 1986). Whether increased or not, responses maintained their original time to peak, which was rapid in the case of indomethacin (about 30 min) and considerably longer with ZnPP (about 2 h).
In vivo studies
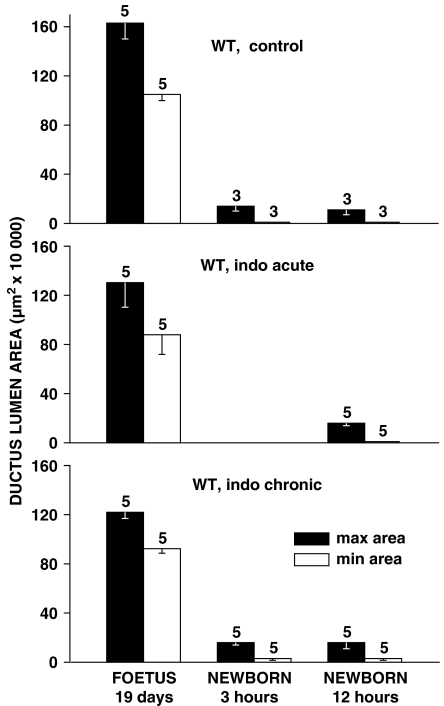
Indomethacin at the dose employed had a minor effect on prenatal patency of the ductus, regardless of the length of treatment. As shown in Figure 6, values for the two dimensions, maximal and minimal, of the cross-sectional area of the vessel only tended to be reduced in the treated compared with the untreated foetuses. Essentially negative findings were also observed in the neonate, with the ductus being in all groups closed, or nearly closed, by the 12-h mark (Figure 6). Furthermore, postnatal changes progressed similarly in the untreated and treated animals, with narrowing of the lumen being either even over the entire length of the vessel or more prominent in its central/centropulmonary portion.
Figure 6.
Ductus arteriosus in wild-type (WT) mouse. Prenatal patency and time course of closure with control vs indomethacin (indo)-pretreated animals (acute and chronic treatments). For each group, the number of experiments is given above the columns. Note that changes in the treated foetuses did not reach significance by ANOVA.
Discussion
This study demonstrates that antenatal exposure to indomethacin, particularly if prolonged in time, enhances the L-NAME contraction and, accordingly, NO activity in the ductus. Significantly, the drug is effective in a therapeutic dosage and the response itself may persist beyond the period of COX inhibition. Indomethacin action is specific inasmuch as it does not cause any evident change in the mechanical characteristics of the vessel (see Methods) and does not modify the contraction to the reference agonist, ONO-11113. Coincidentally, NO upregulation can be linked to eNOS as this particular isoform is upregulated by the treatment, and, moreover, its deletion results into subsidence of the effect. Nevertheless, indomethacin action is not confined to NO as some of its upregulatory influence extends to the CO-based relaxing mechanism. In promoting NO, COX inhibition mimics COX deletion (Baragatti et al., 2003). It differs, however, in that COX deletion does not affect CO function (Baragatti et al., 2007) and also enhances NO function seemingly without increasing eNOS expression (Baragatti et al., 2003). Contrary to the results in vitro, indomethacin action appears rather unremarkable in vivo and is limited to a marginal, yet statistically not significant, narrowing of the ductus lumen. On the basis of this premise, our discussion will address three issues: the meaning of the weak ductus constriction to indomethacin in the living foetus, the mechanism for indomethacin-induced NO upregulation and the implications, both conceptual and practical, deriving from our findings with this NSAID.
Lack of a full-fledged indomethacin constriction in vivo is not entirely surprising. Previous work in the rat, employing doses of inhibitor comparable to ours, has already shown a modest response by the fetal ductus (Momma and Takao, 1987). In fact, somewhat paradoxically the constriction reached its minimum when blood levels of indomethacin were relatively high and also adequate for COX inhibition (Momma and Takao, 1987). Much larger doses of inhibitor, exceeding the therapeutic range, have been utilized for an overt and consistent response in rodents (Sharpe et al., 1974; Momma and Takao, 1987; Loftin et al., 2001; Richard et al., 2004) and in the lamb (Olley et al., 1975). Pertinent in this context are also data from a clinical study in which indomethacin was used for the prevention of premature labour (Moise et al., 1988). Signs of ductus constriction were seen only in 50% of foetuses, and no obvious difference in either gestational age or maternal indomethacin levels could be found between responders and nonresponders. Which is, then, the explanation for these variable, or even negative, results? A methodological factor, inherent to a therapeutic dosage of indomethacin in the mother not being able to yield adequate levels in the foetus, may be ruled out in our as in previous studies. In our case, with a steady indomethacin concentration of about 1.2 μg ml−1 in maternal blood (see Results) and an expected fetal/maternal ratio of 0.4 (Momma and Takao, 1987), we can assume the blood concentration in the foetus (that is, about 0.5 μg ml−1) to be sufficient for a constrictor effect on the ductus (see Takahashi et al., 2000). Indeed, the existence of a suitable concentration is evident from changes in the eNOS transcript and the increased response to L-NAME and ZnPP. Hence, the answer to the question must be sought elsewhere, and, with the available data, it is found most plausibly in the upregulation of relaxing mechanisms upon exposure to indomethacin. In particular, the increased efficiency of NO could counteract a merely adequate constrictor drive originating from the loss of PGE2. In addition, a self-sustaining sequence may take place, whereby repeated administration of indomethacin simply serves to reinforce this relaxing influence. Consistent with our view is one separate test in which the foetus was examined at the peak concentration of indomethacin in blood (that is, 3 h) resulting from a single-dose administration. In that case, the lumen of the ductus was reduced by about 50% (data not shown).
The mechanism by which indomethacin, with its attendant COX inhibition, promotes eNOS function is not clear, but some inference is possible on available data. PGE2 and NO not only operate independently in the regulation of vascular tone, but also interact in a varied manner depending on site and condition. NO, in fact, may function as a messenger for PGE2 (Gobeil et al., 2002; Namkoong et al., 2005; Hristovska et al., 2007) or, conversely, may be engaged in a reciprocal link with PGE2 and thus acquire greater effectiveness with its suppression (Tetsuka et al., 1994; Shimpo et al., 2000; Ribeiro et al., 2004). However, the latter event, outwardly mimicking our situation, has been reported only in the presence of pyrogens and with the involvement of iNOS (inducible NOS) rather than eNOS. Clearly, none of these arrangements accord with the present findings in the ductus where eNOS-derived NO may play a compensatory role in the absence of any special challenge. In actual fact, PGE2 itself is unlikely to be a signalling agent for NO. Previous work has shown NO upregulation in ductus preparations heterozygous for the COX2 mutation, that is under a condition where PGE2 function remains virtually unabated as one can infer from the persistence of a full-fledged contraction to indomethacin (Baragatti et al., 2003). In contrast, this upregulatory drive remains insignificant in the mPGES1 (microsomal prostaglandin E synthase-1)−/− ductus, not withstanding a virtually complete suppression of the indomethacin contraction (B Baragatti, D Sodini, F Coceani, unpublished data). How is, then, COX inhibition translated into NO upregulation? The most reasonable hypothesis is that such interference brings about a rebound upregulation of either, or both, allied pathways in arachidonic acid metabolism (that is, lipoxygenase and monooxygenase pathways). 12S-Lipoxygenase, for example, is well expressed in the ductus (Costa et al., 2006) and may yield a host of potent signalling products (Brash, 1999; Pace-Asciak et al., 1999). Indeed, this particular pathway has already been implicated in the iNOS-based upregulation being elicited by NSAIDs in pyrogen-treated vascular smooth muscle (Shimpo et al., 2000). In our case, one may envisage a dual action of 12-lipoxygenase products, that is, at transcriptional level, as evident from the presence in the eNOS promoter of recognition sites for transcription factors (SP1, AP2) being activated by 12-HETE (12-hydroxyeicosatetraenoic acid) (Nie et al., 2006; see http://www.genomatix.de), and directly onto the enzyme thanks to stimulation of phosphorylating kinases and a rise in free Ca2+ (Bauersachs et al., 1997; Brash, 1999). Relevant to this point is the differential response of the eNOS transcript to the combined COX1/COX2 inhibition (this study) compared with an isoform-selective COX deletion (Baragatti et al., 2003), the increased expression being linked only with the former intervention. Hence, the concept is reinforced of the presence of two signals for NO upregulation, affecting transcriptional and post-transcriptional processes, respectively, along with the notion that both COXs need to be inhibited for transcriptional activation. Further work is warranted to verify these possibilities.
Translated to the clinical situation, this new concept on indomethacin action well accounts for several observations in the management of the prematurely born infant. Not only does it provide a reason for the higher incidence of a persistent duct in infants from pregnancies complicated by NSAID intake (Norton et al., 1993; Suarez et al., 2002), but also allows to interpret the way premature infants often respond to NSAID treatment (Ohlsson et al., 2004; Shah and Ohlsson, 2004). Quite commonly, in fact, patients present a transient constriction of their duct that may not become permanent upon repeated courses of drug. Hence, persistent patency in face of the treatment is linked, at least in its initial stage, to a functional condition rather than a structural change (that is, the inability to initiate the remodelling process) as suggested previously (see Goldbarg et al., 2002). By extension, a better light is shed on the greater efficacy of the combined COX/NOS inhibition over COX inhibition alone in the management of the premature infants (Keller et al., 2005). Indeed, with the inclusion of the NOS inhibitor, the therapeutic intervention may not interfere simply with one among several ductus relaxants, but rather with the prime agent of compensation for any PGE2 loss. In a broader context, however, our data have far-reaching implications that go beyond the subject of the ductus. By showing that NO upregulation is potentially an integral part of the action of indomethacin, and conceivably of other NSAIDs too, it introduces a new dimension in the study and clinical use of these drugs. Depending on the situation, this parallel action may impact, beneficially or adversely, upon the changes deriving from COX inhibition. With the ductus, for example, NO upregulation can be regarded as a positive, protective feature antenatally and, conversely, as an interference for therapy postnatally.
During the completion of this work, a paper has appeared reporting in the same species that antenatal exposure to indomethacin retards closure of the ductus postnatally without seemingly involving NO (Reese et al., 2006). Although no explanation can be provided for the negative finding with NO, which, in fact, runs counter to prior experimental (Seidner et al., 2001) and clinical (Keller et al., 2005) data, persistency of the ductus in that study but not in ours may have a methodological reason. Reese et al. (2006) exposed the newborns to a hyperoxic environment and this procedure, rather than promoting the intended ductus closure, may have activated non-PGE2 dilators (Baragatti et al., 2007) and may also have accelerated the downregulation of myofibrillary proteins (Costa et al., 2006). In addition, their animals were delivered by Caesarean section and this approach may not be exactly comparable to natural birth (this study) with regard to transitional adjustments and their manipulation.
In conclusion, we have shown that enhancement of NO and, in a minor way, CO function is an integral part of indomethacin action on the fetal ductus. This treatment-linked amplification of dilator influences expectedly opposes the constriction resulting from COX inhibition. This new concept has implications for the management of prematurely born infants with persistent duct.
Acknowledgments
This work was supported by grants of the Italian Ministry of Education and Research (FIRB RBNEOIW9 PM; PRIN 2005053227). DS is the recipient of a graduate studentship from the Scuola Superiore Sant' Anna. We gratefully acknowledge the assistance of Vittorio Gattai for the morphological analysis. We are also indebted to Silvia Gonzali for excellent assistance.
Abbreviations
- CO
carbon monoxide
- eNOS
endothelial NOS
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- NSAID
nonsteroidal anti-inflammatory drug
- ONO-11113
9,11-epithio-11,12-methano-thromboxane A2
- PG
prostaglandin
- TX
thromboxane
- WT
wild-type
- ZnPP
zinc protoporphyrin
Conflict of interest
The authors state no conflict of interest.
References
- Baragatti B, Brizzi F, Ackerley C, Barogi S, Ballou LR, Coceani F. Cyclooxygenase-1 and cyclooxygenase-2 in the mouse ductus arteriosus: individual activity and functional coupling with nitric oxide synthase. Br J Pharmacol. 2003;139:1505–1515. doi: 10.1038/sj.bjp.0705391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baragatti B, Brizzi F, Barogi S, Laubach VE, Sodini D, Shesely EG, et al. Interactions between NO, CO and an endothelium-derived hyperpolarizing factor (EDHF) in maintaining patency of the ductus arteriosus in the mouse. Br J Pharmacol. 2007;151:54–62. doi: 10.1038/sj.bjp.0707211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauersachs J, Popp R, Fleming I, Busse R. Nitric oxide and endothelium-derived hyperpolarizing factor: formation and interactions. Prostaglandins Leukot Essent Fatty Acids. 1997;57:439–446. doi: 10.1016/s0952-3278(97)90425-7. [DOI] [PubMed] [Google Scholar]
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–23682. doi: 10.1074/jbc.274.34.23679. [DOI] [PubMed] [Google Scholar]
- Coceani F, Liu Y-A, Seidlitz E, Kelsey L, Kuwaki T, Ackerley C, et al. Endothelin A receptor is necessary for O2 constriction but not closure of ductus arteriosus. Am J Physiol. 1999;277:H1521–H1531. doi: 10.1152/ajpheart.1999.277.4.H1521. [DOI] [PubMed] [Google Scholar]
- Costa M, Barogi S, Socci ND, Angeloni D, Maffei M, Baragatti B, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006;25:250–262. doi: 10.1152/physiolgenomics.00231.2005. [DOI] [PubMed] [Google Scholar]
- Fagan JM, Goldberg AL. Inhibitors of protein and RNA synthesis cause a rapid block in prostaglandin production at the prostaglandin synthase step. Proc Natl Acad Sci USA. 1986;83:2771–2775. doi: 10.1073/pnas.83.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil F, Jr, Dumont I, Marrache AM, Vazquez-Tello A, Bernier SG, Abran D, et al. Regulation of eNOS expression in brain endothelial cells by perinuclear EP3 receptors. Circ Res. 2002;90:682–689. doi: 10.1161/01.res.0000013303.17964.7a. [DOI] [PubMed] [Google Scholar]
- Goldbarg SH, Takahashi Y, Cruz C, Kajino H, Roman C, Liu BM, et al. In utero indomethacin alters O2 delivery to the fetal ductus arteriosus: implications for postnatal patency. Am J Physiol. 2002;282:R184–R190. doi: 10.1152/ajpregu.2002.282.1.R184. [DOI] [PubMed] [Google Scholar]
- Hörnblad PY, Larsson KS. Studies on closure of the ductus arteriosus. I. Whole-body freezing as improvement of fixation procedures. Cardiologia. 1967;51:231–241. [PubMed] [Google Scholar]
- Hristovska A-M, Rasmussen LE, Hansen PB, Nielsen SS, Nüsing RM, Narumiya S, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50:525–530. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- Keller RL, Tacy TA, Fields S, Ofenstein JP, Aranda JV, Clyman RI. Combined treatment with a nonselective nitric oxide synthase inhibitor (L-NMMA) and indomethacin increases ductus constriction in extremely premature newborns. Pediatr Res. 2005;58:1216–1221. doi: 10.1203/01.pdr.0000183659.20335.12. [DOI] [PubMed] [Google Scholar]
- Laneuville O, Breuer DK, Dewitt DL, Hla T, Funk CD, Smith WL. Differential inhibition of human prostaglandin endoperoxide H synthases-1 and -2 by nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 1994;271:927–934. [PubMed] [Google Scholar]
- Leuwerke SM, Kaza AK, Tribble CG, Kron IL, Laubach VE. Inhibition of compensatory lung growth in endothelial nitric oxide synthase-deficient mice. Am J Physiol. 2002;282:L1272–L1278. doi: 10.1152/ajplung.00490.2001. [DOI] [PubMed] [Google Scholar]
- Loftin CD, Trivedi DB, Langenbach R. Cyclooxygenase-1-selective inhibition prolongs gestation in mice without adverse effects on the ductus arteriosus. J Clin Invest. 2002;110:549–557. doi: 10.1172/JCI14924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin CD, Trivedi DB, Tiano HF, Clark JA, Lee CA, Epstein JA, et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci USA. 2001;98:1059–1064. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Eloby-Childress S, Snapp B, Chotinaruemol S, Steen VL, Clark DA. Urinary nitrite excretion in premature infants: effects of transfusion or indomethacin. Acta Paediatr. 1993;82:291–295. doi: 10.1111/j.1651-2227.1993.tb12662.x. [DOI] [PubMed] [Google Scholar]
- Moise KJ, Jr, Huhta JC, Sharif DS, Ou CN, Kirshon B, Wasserstrum N, et al. Indomethacin in the treatment of premature labor. Effects on the fetal ductus arteriosus. N Engl J Med. 1988;319:327–331. doi: 10.1056/NEJM198808113190602. [DOI] [PubMed] [Google Scholar]
- Momma K, Takao A. In vivo constriction of the ductus arteriosus by nonsteroidal antiinflammatory drugs in near-term and preterm fetal rats. Pediatr Res. 1987;22:567–572. doi: 10.1203/00006450-198711000-00018. [DOI] [PubMed] [Google Scholar]
- Momma K, Toyono M. The role of nitric oxide in dilating the fetal ductus arteriosus in rats. Pediatr Res. 1999;46:311–315. doi: 10.1203/00006450-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Namkoong S, Lee S-J, Kim C-K, Kim Y-M, Chung H-T, Lee H, et al. Prostaglandin E2 stimulates angiogenesis by activating the nitric oxide/cGMP pathway in human umbilical vein endothelial cells. Exp Mol Med. 2005;31:588–600. doi: 10.1038/emm.2005.72. [DOI] [PubMed] [Google Scholar]
- Nie D, Krishnamoorthy S, Jin R, Tang K, Chen YC, Qiao Y, et al. Mechanisms regulating tumor angiogenesis by 12-lipoxygenase in prostate cancer cells. J Biol Chem. 2006;281:18601–18609. doi: 10.1074/jbc.M601887200. [DOI] [PubMed] [Google Scholar]
- Norton ME, Merrill J, Cooper B, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Walia R, Shah S.Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants (Cochrane Review) Cochrane Database Syst Rev 2004Wiley: Chichester, UK; Issue 3. [Google Scholar]
- Olley PM, Bodach E, Heaton J, Coceani F. Further evidence implicating E-type prostaglandins in the patency of the lamb ductus arteriosus. Eur J Pharmacol. 1975;34:247–250. doi: 10.1016/0014-2999(75)90248-4. [DOI] [PubMed] [Google Scholar]
- Pace-Asciak CR, Reynaud D, Demin P, Nigam S. The hepoxilins: a review. Adv Exp Med Biol. 1999;447:123–132. [PubMed] [Google Scholar]
- Reese J, Anderson JD, Brown N, Roman C, Clyman RI. Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice. Am J Physiol. 2006;291:R1717–R1723. doi: 10.1152/ajpregu.00259.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Cella M, Farina M, Franchi A. Effects of aminoguanidine and cyclooxygenase inhibitors on nitric oxide and prostaglandin production, and nitric oxide synthase and cyclooxygenase expression induced by lipopolysaccharide in the estrogenized rat uterus. Neuroimmunomodulation. 2004;11:191–198. doi: 10.1159/000076768. [DOI] [PubMed] [Google Scholar]
- Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, et al. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- Sato J, Amizuka T, Niida Y, Umetsu M, Ito K. Simple, rapid and sensitive method for the determination of indomethacin in plasma by high-performance liquid chromatography with ultraviolet detection. J Chromatogr B Biomed Sci Appl. 1997;692:241–244. doi: 10.1016/s0378-4347(96)00463-x. [DOI] [PubMed] [Google Scholar]
- Seidner SR, Chen Y-Q, Oprysko PR, Mauray F, Tse MM, Lin E, et al. Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res. 2001;50:365–373. doi: 10.1203/00006450-200109000-00012. [DOI] [PubMed] [Google Scholar]
- Shah SS, Ohlsson A.Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants (Cochrane Review) Cochrane Database Syst Rev 2004Wiley: Chichester, UK; Issue 3. [Google Scholar]
- Sharpe GL, Thalme B, Larsson KS. Studies on closure of the ductus arteriosus. XI. Ductal closure in utero by a prostaglandin synthesis inhibitor. Prostaglandins. 1974;8:363–368. doi: 10.1016/0090-6980(74)90110-5. [DOI] [PubMed] [Google Scholar]
- Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, et al. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimpo M, Ikeda U, Maeda Y, Ohya K, Murakami Y, Shimada K. Effects of aspirin-like drugs on nitric oxide synthesis in rat vascular smooth muscle cells. Hypertension. 2000;35:1085–1091. doi: 10.1161/01.hyp.35.5.1085. [DOI] [PubMed] [Google Scholar]
- Smith GCS. The pharmacology of the ductus arteriosus. Pharmacol Rev. 1998;50:35–58. [PubMed] [Google Scholar]
- Suarez VR, Thompson LL, Jain V, Olson GL, Hankins GD, Belfort MA, et al. The effect of in utero exposure to indomethacin on the need for surgical closure of a patent ductus arteriosus in the neonate. Am J Obstet Gynecol. 2002;187:886–888. doi: 10.1067/mob.2002.127464. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Roman C, Chemtob S, Tse MM, Lin E, Heymann MA, et al. Cyclooxygenase-2 inhibitors constrict the fetal lamb ductus arteriosus both in vitro and in vivo. Am J Physiol. 2000;278:R1496–R1505. doi: 10.1152/ajpregu.2000.278.6.R1496. [DOI] [PubMed] [Google Scholar]
- Takizawa T, Kihara T, Kamata A. Increased constriction of the ductus arteriosus with combined administration of indomethacin and L-NAME in fetal rats. Biol Neonate. 2001;80:64–67. doi: 10.1159/000047122. [DOI] [PubMed] [Google Scholar]
- Tetsuka T, Daphna-Iken D, Srivastava SK, Baier LD, DuMaine J, Morrison AR. Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc Natl Acad Sci USA. 1994;91:12168–12172. doi: 10.1073/pnas.91.25.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Leffler CW. Compensatory role of NO in cerebral circulation of piglets chronically treated with indomethacin. Am J Physiol. 2002;282:R400–R410. doi: 10.1152/ajpregu.00256.2001. [DOI] [PubMed] [Google Scholar]