Abstract
Background and purpose: Cholecystokinin (CCK) stimulates the release of amylase and lipase from the normal pancreas. However, it is not clear to what extent this occurs in the early stages of pancreatitis induced by biliary tract obstruction in the rat and whether CCK initiates an inflammatory cascade in this condition.
Experimental approach: Selective CCK1 receptor antagonists, JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) and dexloxiglumide, were used to assess the response of plasma amylase and lipase to a CCK analogue, CCK8S, in normal rats and in rats with bile duct ligation.
Key results: Both antagonists suppressed CCK8S-induced elevation of plasma amylase activity in normal rats. JNJ-17156516 was more potent than dexloxiglumide (ED50=8.2 vs >30 μmol kg−1 p.o.) and produced a longer lived inhibition (6 vs 2 h). Plasma amylase and lipase activity were elevated in parallel to CCK plasma concentrations after bile duct ligation and both activities were suppressed in a dose-dependent manner by JNJ-17156516 and dexloxiglumide. JNJ-17156516 was ∼5- to 10-fold more potent than dexloxiglumide. Infusion of CCK8S to naïve rats to achieve levels similar to those observed after bile duct ligation (20 pM) increased plasma amylase activity and activated nuclear factor-κB in the pancreas. These effects were prevented by pretreatment with JNJ-17156516.
Conclusions and implications: The elevation of plasma amylase and lipase activity in the early stages of obstruction-induced pancreatitis is largely driven by elevation of plasma CCK concentration and activation of CCK1 receptors. These data show that CCK is an initiating factor in acute pancreatitis in the rat.
Keywords: cholecystokinin (CCK), CCK1 receptor, pancreatitis, amylase, lipase
Introduction
Pancreatitis is characterized by severe abdominal pain, elevation of the plasma activity of pancreatic amylase and lipase enzymes and is commonly associated with nausea and vomiting. The treatment of acute and chronic pancreatitis remains an unmet medical need and there is little understanding of what initiates pancreatitis. In a few cases, mutations have been identified that are linked to familial pancreatitis, and the association between excessive alcohol consumption and pancreatitis has long been known (Toskes and Greenberger, 2001). Despite these associations, the initiating factors leading to acute pancreatitis remain unknown and it is not clear what pharmacological targets might be pursued to develop a treatment for this condition.
Cholecystokinin (CCK) has been proposed to play a role in the pathogenesis of pancreatitis based on experimental and clinical evidence. The observation that caerulein, an ancestral form of CCK with agonist activity at both CCK1 and CCK2 receptors, elevates plasma amylase activity and causes histological damage to the pancreas was the first observation linking CCK and pancreatitis (reviewed by Beglinger, 1999). Further studies showed that other CCK receptor agonists, such as CCK8S (the sulphated terminal eight amino acids of CCK; Morton et al., 2007), had similar effects and these effects could be blocked by selective, albeit low-affinity, CCK1 receptor antagonists such as proglumide (Niederau et al., 1985, 1986). CCK concentrations are elevated in rat models of pancreatitis such as that induced by bile duct occlusion (Toriumi et al., 1993) and intra-bile duct injection of taurocholic acid (Ohlsson et al., 2000). In addition, administration of exogenous CCK exacerbates pancreatitis in these animal models (Satake et al., 1999). In humans, exogenous administration of CCK stimulates secretion of pancreatic amylase (Wank, 1995). Patients suffering from pancreatitis have elevated serum CCK concentrations despite the fact that food is withheld from these patients from the time of diagnosis until they recover sufficiently (Otsuki, 2000; Toskes and Greenberger, 2001). Indeed, the rationale for fasting these patients lies in part in providing a ‘rest' for the pancreas by reducing the concentration of hormones released by food that stimulate the exocrine pancreas. In addition, patients who suffer from chronic pancreatitis show an exaggerated secretion of CCK in response to a meal (Shirohara and Otsuki, 1997; Otsuki, 2000). These observations led to the exploration of loxiglumide in clinical trials for the treatment of pancreatitis (Ochi et al., 1999; Shiratori et al., 2002). The results of these studies were mixed, potentially because loxiglumide has complex pharmacokinetics (Persiani et al., 2002) and relatively low affinity for the human CCK1 receptor (Morton et al., 2007). Furthermore, these studies included patients who presented with pancreatitis already in its mid- to advanced stages. Thus, despite having conducted clinical trials with a CCK1 receptor antagonist, the role of CCK in the early stages of pancreatitis is not clear.
We sought to use the recently described selective CCK1 receptor antagonist JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) (Morton et al., 2007) to investigate the role of CCK in the initiation of pancreatitis induced by biliary tract obstruction in the rat. JNJ-17156516 exhibits a high affinity for the human (pKI=7.96±0.11) and rat CCK1 receptor (pKI=8.02±0.11) as well as a high bio-availability (100%) and long half-life (t1/2=3.0±0.5 h) in the rat (Morton et al., 2007). The experimental model was selected based on its relevance to two clinical conditions where pancreatitis is known to occur: gall stones and pancreatitis induced by endoscopic retrograde cholangiopancreatography. The potency and duration of action of JNJ-17156516 and the active enantiomer dexloxiglumide (pKI=7.63±0.09 for the rat CCK1 receptor; Morton et al., 2007) were compared for antagonism of CCK1 receptors in the exocrine pancreas of the rat. The role of CCK and CCK1 receptors in initiating a pro-inflammatory cascade was also examined in a rat model.
Materials and methods
Use and care of animals
All procedures and experiments involving animals were performed according to internationally accepted guidelines for the care and use of laboratory animals in research and were approved by the local Institutional Animal Care Committee. Male Sprague–Dawley rats (Harlan, Madison, WI, USA), weighing 270–320 g, were used for all studies. Animals were maintained at 21 °C on a 12 h:12 h light–dark cycle and had free access to standard rat chow and water.
Suppression of CCK8S-induced elevation of plasma amylase
The CCK1 receptor antagonism produced by JNJ-17156516 and dexloxiglumide was evaluated after oral administration to naïve rats by assessing the inhibition of the elevation of plasma amylase activity produced after a subcutaneous dose of CCK8S (Sigma Diagnostics, St Louis, MO, USA). CCK8S was dissolved in dimethyl sulphoxide at a concentration of 1 mM.
Doses of CCK8S between 1 and 30 nmol kg−1 and vehicle control (1 ml kg−1 of 0.3% v v−1 dimethyl sulphoxide in saline) were administered subcutaneously and the plasma amylase activity was measured at various times up to 6 h later. Plasma samples were collected from the tail vein. A dose of 10 nmol kg−1 CCK8S was selected for further studies to assess antagonist potency and duration of action. The response to CCK8S was recorded for each animal by determining the area under the plasma amylase–time up to 6 h curve using commercially available software (WinNonlin, version 3.3). All animals were allowed free access to food and water throughout the study.
JNJ-17156516 was administered over a dose range of 3–30 μmol kg−1, whereas dexloxiglumide was tested only at a single dose of 30 μmol kg−1 due to its limited solubility and relatively low potency. Test compounds were administered via the oral route 2 h before subcutaneous administration of CCK8S, and plasma samples were collected. The duration of action of the two compounds was compared after oral administration at a dose of 30 μmol kg−1 for dexloxiglumide and 20 μmol kg−1 for JNJ-17156516.
Suppression of plasma amylase and lipase elevation after bile duct ligation in the rat
The effects of CCK1 receptor antagonism produced by JNJ-17156516 and dexloxiglumide were examined in a rat model of pancreatitis induced by biliary tract obstruction. The doses to be tested were selected from the previous study to span the range of doses that suppressed the elevations of plasma amylase produced by CCK8S. A dose–response study was conducted administering the antagonists by the oral route before bile duct ligation and the intravenous route of administration was used to examine the effect of administering the antagonist after bile duct ligation.
General surgical procedure
A dose of 30 μg kg−1 of buprenorphine was administered 30 min before the animal was anaesthetized with 1–4% isoflurane carried in clean dry air. The animal's ventral surface was shaved and disinfected with betadine. Thereafter, a mid-line incision was made, the peritoneal cavity was opened and the bile duct was ligated with 5-0 prolene suture. The peritoneum and the skin were sutured closed and animals were allowed to recover from anaesthesia. Plasma samples were collected from the tail vein before administration of the test compound and subsequently pre- and post-bile duct ligation.
Time course for elevation of plasma CCK after bile duct ligation
The bile duct was ligated as described above and blood samples were collected 1, 2, 6 and 24 h after bile duct ligation. A maximal blood volume was collected after killing the animal. The immuno-reactive plasma CCK concentration was assayed using a commercially available radioimmunoassay kit according to the manufacturer's specifications (Alpco Diagnostics, Windham, NH, USA). The antibody used by this kit detects CCK8S and the endogenous form, CCK33 with equal affinity.
Dose–response relationship for JNJ-17156516 and dexloxiglumide in the rat bile duct ligation model of pancreatitis
The test compounds were administered orally 2 h before bile duct ligation to rats that were assigned to one of the following treatment groups in a random and blinded manner: vehicle control, JNJ-17156516 at doses of 2, 6 and 20 μmol kg−1 or dexloxiglumide at doses of 3, 10 and 30 μmol kg−1. Sham occlusion groups were included to assess the effects of each compound in rats in which the surgical procedures were performed but the bile duct was not ligated. Only the highest dose of each compound and a vehicle control were studied. The group size was set to n=5. However, for the highest dose studied, the total number of animals was increased to nine to increase the statistical power of the study. This was also carried out in a randomized and blinded manner after a preliminary analysis of the data. Plasma samples were collected at various times before and up to 6 h after bile duct ligation and were analysed for plasma amylase and lipase activity.
Intravenous administration of JNJ-17156516 and dexloxiglumide after induction of pancreatitis
The effect of the time of administration of CCK1 receptor antagonists was assessed by administering vehicle, 20 μmol kg−1 JNJ-17156516 or 30 μmol kg−1 dexloxiglumide by the intravenous route 30 or 60 min after bile duct ligation. Plasma samples were collected at various time points up to 24 h after bile duct ligation and were analysed for amylase activity.
Determination of plasma amylase and lipase
Plasma amylase activity was determined at room temperature using Infinity amylase reagent (Sigma Diagnostics or Thermo Electron, Louisville, CO, USA) and plasma lipase activity was determined using Lipase-PS (Sigma Diagnostics) according to the manufacturer's protocols with modification to a 96-well microtitre format. Absorbance was measured at 405 or 550 nm.
In vivo assessment of CCK8S-induced activation of NF-κB
CCK8S was infused into the jugular vein of conscious rats and the resulting level of nuclear factor (NF)-κB activation in the pancreas was assessed. Rats were anaesthetized with isoflurane and a cannula was placed in the jugular vein. Buprenorphine 30 μg kg−1 s.c. was administered immediately after completing the surgical procedure and animals were allowed to recover from surgery for 2 days. A heparin lock (1000 U ml−1) was used to ensure that the venous line did not clot during the time between the surgical procedure and the experiment. Animals were pretreated with 20 μmol kg−1 JNJ-17156516 i.v. or vehicle control as an infusion over 5 min before commencing a 30 min infusion of 5 μg kg−1 h−1 of CCK8S or vehicle control (n=4–5). After completing this infusion, the animals were killed with CO2 and plasma was collected for determination of plasma amylase activity and CCK concentration. In addition, a 250–300 mg sample was collected from the splenic area of the pancreas for extraction of nuclear protein and subsequent assay of NF-κB activation.
Preparation of nuclear extracts
Nuclear protein extracts were prepared by using the method described by Maire et al. (1989). The 250–300 mg sample of pancreas tissue was rinsed in ice-cold phosphate-buffered saline and re-suspended in 0.5 ml of homogenization buffer (10 mM HEPES (pH 7.9), 2 M sucrose, 10% glycerol (v v−1), 25 mM KCl, 150 mM spermine, 500 mM spermidine, 2 mM EDTA) to which was added soyabean trypsin inhibitor (Worthington Biochemical, Lakewook, NJ, USA), 1 mM phenylmethylsulphonyl fluoride, 1 mM dithiothreitol and a protease inhibitor cocktail (10 μg ml−1 each of antipain, aprotinin, chymostatin, pepstatin and leupeptin; Roche Diagnostics, Mannheim, Germany). The tissues were then homogenized with a motor-driven pestle for 20 strokes on ice, briefly vortexed and incubated on ice for 15 min. Thereafter, the sample was collected by centrifugation at 14 000 g for 5 min at 4 °C, washed with 1 ml of ice-cold phosphate-buffered saline containing 1 mM EDTA, and then centrifuged again at 14 000 g for another 5 min at 4 °C. The nuclei were re-suspended in 50–100 μl of ice-cold high-salt buffer containing 10 mM HEPES (pH 7.9), 10% glycerol (v v−1), 0.42 M NaCl, 100 mM KCl, 3 mM MgCl2 and 0.1 mM EDTA to which the protease inhibitor cocktail and dithiothreitol were added. The nuclear suspension was incubated on ice for 20 min with intermittent mixing and then centrifuged at 14 000 g for 10 min at 4 °C. The clear supernatant (nuclear extract) was aliquoted and stored at −80 °C until it was used. The protein concentration of the nuclear extract was determined by the Bio-Rad protein assay (Bio-Rad laboratories, Hercules, CA, USA).
Electrophoretic mobility shift assay for NF-κB activation
Aliquots of nuclear extracts with equal amount of protein (10 μg) were mixed in 20 μl reactions with a buffer containing 10 mM HEPES (pH 8), 10% glycerol (v v−1), 1 mM dithiothreitol, 50 mM KCl, 0.1 mM EDTA and 1 μg poly dI-dC (Novagen, Madison, WI, USA). Binding reactions were started by adding 10 000–60 000 c.p.m. of the 22-bp oligonucleotide 5′-AGTTGAGGGGACTTTCCCAGGC-3′ containing the NF-κB consensus sequence (underlined, Santa Cruz Biotechnology, Santa Cruz, CA, USA) that was labelled with [γ-32P]ATP (6000 Ci mmol−1; [γ-32P]ATP was from PerkinElmer Life and Analytical Sciences, Boston, MA, USA) by T4 polynucleotide kinase (Novagen). The reaction was allowed to proceed for 20 min at room temperature. The specificity of the binding was confirmed by two methods. Competition with 200-fold molar excess of unlabelled wild-type or mutated NF-κB oligonucleotide that was added to the reaction together with the labelled probe. In mutated oligonucleotide, the NF-κB motif was changed (lower case) to GGcGACTTTCCC. DNA–protein complexes were resolved by electrophoresis on a 5% non-denaturing polyacrylamide gel in 0.5 × Tris-borate-EDTA buffer (44.5 mM Tris base, 44.5 mM boric acid and 1 mM disodium EDTA, pH 8.3) at 200 V. Gels were vacuum-dried and exposed to Kodak BioMax MS films (Rochester, New York) with intensifying screens at −80 °C. The intensity of bands was quantified by using an image analysis system (Eagle Eye II image analysis system; Stratagene, San Diego, CA, USA).
Statistics
Values are expressed as the mean±s.e.mean. Statistical significance was tested using single factor ANOVA followed by a Tukey test for differences at a significance level of P<0.05 (GraphPad Prism version 4.00 for Windows; GraphPad Software, San Diego, CA, USA, www.graphpad.com). Dose–response data were fitted to a four-parameter logistic equation using the same software.
Drugs
JNJ-17156516 was synthesized as described by Liang et al. (2007), whereas dexloxiglumide was synthesized using known methods. Both compounds were synthesized by Johnson & Johnson Pharmaceutical Research & Development L.L.C. in La Jolla. Dosing solutions were prepared in 20% hydroxypropyl-β-cyclodextrin with the addition of 1 mol equivalent of NaOH to adjust pH to 7. Dosing solution strength was varied to achieve the desired dose upon administration of 1 ml kg−1 for intravenous doses and 2 ml kg−1 for oral doses. Vehicle control groups received the same volume of 20% hydroxypropyl-β-cyclodextrin at pH 7.
Results
Potency and duration of action of JNJ-17156516 and dexloxiglumide in conscious rats
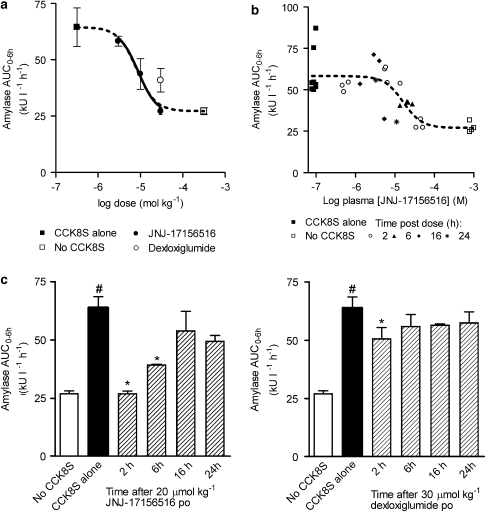
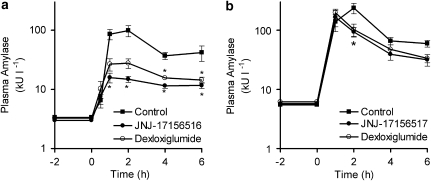
After subcutaneous administration of CCK8S, a characteristic time-dependent elevation of plasma amylase activity was observed. CCK8S doses of 1, 3 and 10 nmol kg−1 caused an increase in plasma amylase activity from the basal level of 3900±425 U l−1 to maximal levels of 8800±1800, 12 000±1600 and 16 500±1200 U l−1, respectively. The maximum level was achieved 1 h after administration of CCK8S at these doses. The effect appeared to reach a maximum between the 10 and 30 nmol kg−1 doses such that the peak plasma amylase activity was actually lower after administration of the 30 nmol kg−1 dose (10 800±2100 U l−1) and at this dose the time to reach this maximum was delayed (3 vs 1 h). From these data, a dose of 10 nmol kg−1 was selected for further studies as it provided a robust increase in plasma amylase activity that was not supra-maximal. Oral administration of JNJ-17156516 2 h before administration of CCK8S produced a dose- and plasma concentration-related reduction in the peak plasma amylase activity as well as the area under the plasma amylase curve integrated with respect to time (Figures 1a and b). The ED50 values for the effect of JNJ-17156516 on peak plasma amylase activity and area under the plasma amylase activity–time curve were 13.2 and 8.2 μmol kg−1 (pED50=4.88±0.10 and 5.09±0.10), respectively. In contrast to JNJ-17156516, dexloxiglumide produced only a small suppression in the peak and area under the plasma amylase activity curve at a dose of 30 μmol kg−1. It was not possible to determine an ED50 for dexloxiglumide from these data.
Figure 1.
Suppression of CCK8S-evoked elevation of plasma amylase activity by JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) and dexloxiglumide in conscious rats. The dose–response curves for JNJ-17156516 and dexloxiglumide (a) as well as plasma concentration–response curve for JNJ-17156516 (b) are shown. All animals treated with JNJ-17156516 or dexloxiglumide also received 10 nmol kg−1 CCK8S s.c. Responses were measured as area under the plasma amylase activity–time curve for the first 6 h (amylase AUC0−6 h) after CCK8S administration. The time course for suppression of the CCK8S response produced by JNJ-171756516 (left) and dexloxiglumide (right) is also shown (c). Values are the mean±s.e.mean, n=4 except for the plasma concentration–response curve where individual values are shown. The plasma amylase AUC0−6 h for the CCK8S and no CCK8S groups are shown at low and high plasma concentrations of JNJ-17156516 in Figure 1b as these data points were used as constraints for curve fitting. No JNJ-17156516 was detected in the plasma of these animals. #P<0.05 compared to the no CCK8S group; *P<0.05 compared to the CCK8S alone group.
The duration of action for JNJ-17156516 and dexloxiglumide were examined in a separate series of experiments. JNJ-17156516 partially suppressed the CCK8S-induced elevation of plasma amylase activity 6 h after oral administration of a 20 μmol kg−1 dose (Figure 1c). However, the effect was not statistically significant 16 and 24 h after oral administration. Dexloxiglumide produced a small effect 2 h after oral administration but no effect at any of the later time points.
To investigate whether the observed suppression of amylase activity was concentration dependent, the plasma concentration of JNJ-17156516 was determined 2 h after administration of the CCK8S challenge. Plotting the plasma concentration of JNJ-17156516 against the response for each animal gave a relationship that could be described by a sigmoidal curve (Figure 1b). The plasma concentration that produced a 50% reduction in the CCK8S-evoked response was 17.8 μM (pEC50=4.75±0.10). It was not possible to determine equivalent parameters for dexloxiglumide due to the small response and limited number of doses investigated.
Suppression of plasma amylase and lipase activity after pancreatitis induced by biliary tract obstruction by CCK1 receptor antagonism
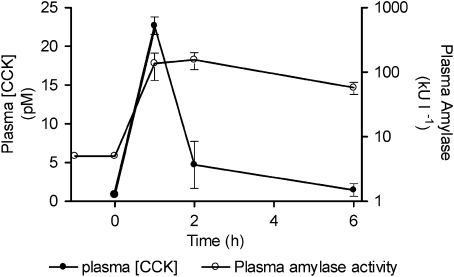
Plasma CCK concentrations and plasma amylase activity were elevated in a bi-phasic manner over the first 6 h after bile duct ligation in the rat (Figure 2). The plasma CCK concentration was rapidly elevated and then fell to a much lower level within 2 h. The initial ∼25-fold elevation of plasma amylase activity followed the plasma CCK concentration closely. However, plasma amylase activity remained elevated and was only reduced modestly at times when the plasma CCK activity had returned to basal values (Figure 2).
Figure 2.
Cholecystokinin (CCK) and plasma amylase activity were elevated in a time-dependent manner after bile duct ligation in the rat. Note that the plasma amylase activity is plotted on a log scale. Values presented are means±s.e.mean, n=3.
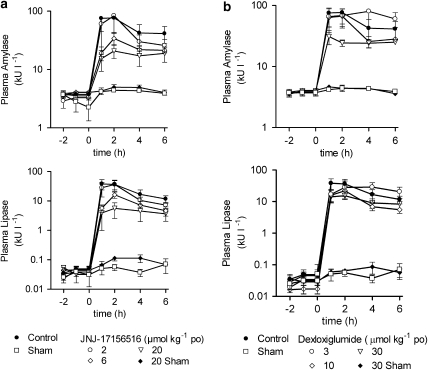
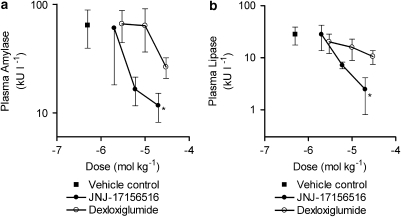
Oral administration of JNJ-17156516 (Figure 3a) and dexloxiglumide (Figure 3b), 2 h before bile duct ligation, produced a dose-related reduction in plasma amylase and lipase activity over the time course of the experiment. This was more pronounced with JNJ-17156516 such that the elevation of plasma amylase activity was suppressed by 20±63, 74±8 and 81±9% 1 h after bile duct ligation by doses of 2, 6 and 20 μmol kg−1 of JNJ-17156516 (vs vehicle control 0±46%). At 1 h after bile ligation, JNJ-17156516 also suppressed the elevation of plasma lipase activity by 27±38, 87±5 and 91±8% relative to vehicle control (0±42%). Dexloxiglumide had a similar profile although the magnitude of the effect was not as large as that produced by JNJ-17156516. At doses of 3, 10 and 30 μmol kg−1, dexloxiglumide reduced plasma amylase activity by 12±29, 16±36 and 60±11%, whereas plasma lipase activity was reduced by 55±19, 58±18 and 63±12% (Figure 3b). A dose–response curve was constructed by plotting the plasma amylase and lipase values 1 h after bile duct ligation. Consistent with its higher CCK1 receptor affinity, longer half-life and higher oral bio-availability, JNJ-17156516 was ∼5- to 10-fold more potent than dexloxiglumide (Figures 4a and b) for effects on plasma amylase activity. Whereas JNJ-17156516 produced a dose-related suppression in plasma lipase activity, dexloxiglumide did not have a statistically significant effect on this variable. These effects were demonstrated to be independent of the effects of anaesthesia or buprenorphine treatment in another study (unpublished observation).
Figure 3.
JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) and dexloxiglumide suppressed the elevation of plasma amylase and lipase activity after bile duct ligation in the rat. The effects of JNJ-17156516 (a) and dexloxiglumide (b) on plasma amylase (top) and lipase activity (bottom) after bile duct ligation are shown as the mean±s.e.mean, n=5–9. A sham bile duct ligation group was included to control for surgical procedure. In these animals, a suture was placed around the bile duct but the bile duct was not ligated. Note that the plasma amylase and lipase activity are plotted on a log scale.
Figure 4.
Dose–response relationship for the effect of JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) and dexloxiglumide on plasma amylase and lipase activity 1 h after bile duct ligation in the rat. Values are shown as the mean plasma amylase (a) and lipase activity (b) ±s.e.mean, (n=5–9). *P<0.05 for control vs bile duct ligation. Note that the plasma amylase and lipase activity are plotted on a log scale.
To determine the effect of the CCK1 receptor antagonists when they were administered after the onset of pancreatitis, 20 μmol kg−1 JNJ-17156516 and 30 μmol kg−1 dexloxiglumide were administrated by the intravenous route 30 or 60 min after bile duct ligation. When administered 30 min after bile duct ligation, JNJ-17156516 reduced plasma amylase activity after bile duct ligation to a similar extent to that observed when the compound was administered orally 2 h before bile duct ligation (Figure 5a). In this study, JNJ-17156516 reduced plasma amylase activity by 81±3%, whereas dexloxiglumide reduced it by 69±7%. The effect of dexloxiglumide was only statistically significant 4 and 6 h after bile duct ligation. Administration of JNJ-17156516 and dexloxiglumide 60 min after bile duct ligation reduced plasma amylase activity by 60±7 and 57±10% (Figure 5b). This effect was only statistically significant 2 h after bile duct ligation (1 h after administration; Figure 5b).
Figure 5.
Effect of intravenous administration of JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid) and dexloxiglumide after bile duct ligation. Doses of 20 μmol kg−1 JNJ-1715616, 30 μmol kg−1 dexloxiglumide or vehicle control were administered 30 (a) and 60 min (b) after bile duct ligation. Values are the mean±s.e.mean, n=6–14. *P<0.05 vs control for bile duct ligation. Note that the plasma amylase activity are plotted on a log scale.
CCK8S-induced activation of NF-κB in the rat pancreas
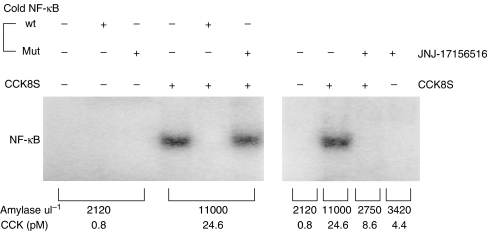
A 30 min infusion of 5 μg kg−1 h−1 CCK8S elevated plasma CCK concentration and this resulted in elevation of plasma amylase activity and activation of NF-κB in the rat pancreas. The plasma CCK concentration was increased from 1.1±0.1 to 22.4±3.6 pM (n=5) in animals that were infused with 5 μg kg−1 h−1 of CCK8S and plasma amylase activity was increased from 2075±35 to 9900±920 U l−1. JNJ-17156516 blocked these effects such that plasma amylase activity after infusion of CCK8S was similar to that found in vehicle controls (3025±190 U l−1), whereas administration of JNJ-17156516 had no effect on basal activity when administered alone (3650±170 U l−1). Translocation of NF-κB to the nucleus was measured using an electrophoretic mobility shift assay from protein isolated from the pancreas of these animals. Figure 6 shows activation of NF-κB after a 30 min infusion of 5 μg kg−1 h−1 CCK8S in control animals. The effect was reproducible in rats infused with CCK8S but was not observed in vehicle controls (n=5, replicate data not shown). Pretreatment with a 20 μmol kg−1 dose of JNJ-17156516 completely prevented the NF-κB activation induced by CCK8S (Figure 6), demonstrating that this effect was mediated via CCK1 receptors. NF-κB activation was not observed in animals that were administered JNJ-17156516 alone (Figure 6) despite an increase in plasma CCK concentration. The specificity of NF-κB activation was demonstrated by including a 200-fold molar excess of the unlabelled wild-type double-stranded oligonucleotide containing the consensus sequence for NF-κB in this assay. Incubation with mutated oligonucleotide had no such effect.
Figure 6.
Antagonism of CCK8S-induced activation of nuclear factor (NF)-κB by JNJ-17156516 ((S)-(3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionic acid). Rats were treated with either vehicle control or 20 μmol kg−1 JNJ-17156516 via a jugular vein 5 min before commencing a 30 min infusion of 5 μg kg−1 h−1 CCK8S. Nuclear extracts were prepared from pancreatic tissue and subjected to electrophoretic mobility shift assay (EMSA) for NF-κB as described in Materials and methods. In competition experiments, 200-fold molar excess of unlabelled wild-type or mutated NF-κB oligonucleotide was added to the reaction together with the labelled probe. All lanes in EMSA contained equal amounts of protein.
Discussion
The present study investigated the role of CCK and CCK1 receptor activation in rat models of acute pancreatitis. JNJ-17156516, a novel and selective CCK1 receptor antagonist, was found to dose-dependently suppress the elevation of plasma amylase and lipase activity occurring after subcutaneous administration of CCK8S and after bile duct ligation in the rat. In the bile duct ligation model of pancreatitis, plasma CCK concentration was elevated ∼25-fold 1 h after the ligation, and infusion of CCK8S to produce similar CCK concentrations resulted in activation of NF-κB in the pancreas. This was demonstrated to be a CCK1 receptor-mediated event as it was prevented by pretreatment with JNJ-17156516. These findings are in agreement with the notion that CCK is an important factor in initiating the release of digestive enzymes in acute pancreatitis. These data also support a link between CCK and inflammation of the pancreas and suggest a novel anti-inflammatory role for CCK1 receptor antagonism.
The increase in plasma amylase activity after subcutaneous administration of CCK8S provided a convenient blood-borne biomarker for CCK1 receptor activation. In this model, JNJ-17156516 was fivefold more potent than dexloxiglumide and had a longer duration of action. The difference between the effects of the two compounds in this model can be attributed to the improved performance of JNJ-17156516 after oral administration and probably relates to the high bio-availability and long half-life of this compound in this species (Morton et al., 2007). For these reasons, JNJ-17156516 proved to be a better tool for examining the role of CCK in acute pancreatitis.
Plasma CCK concentration was elevated ∼20-fold after bile duct ligation within the first hour. This rapid rise in plasma CCK concentration was followed closely by the time-dependent elevation of plasma amylase and lipase activity. The CCK1 receptor antagonists produced a dose-dependent inhibition of plasma amylase and lipase activity over the course of the first 6 h. At the highest dose tested, JNJ-17156516 inhibited the elevation of plasma amylase activity by approximately fivefold, although it did not completely suppress it. The observation that both plasma amylase and plasma lipase activity were elevated after CCK8S administration and bile duct ligation suggests that the same mechanism drives their release in acute pancreatitis and that the driver for this effect is CCK.
It has been argued that the pancreas is refractory to stimulation by CCK in pancreatitis states and this has been taken as evidence against a role of CCK as a mediator of damage in pancreatitis states (Toskes and Greenberger, 2001). It seems possible that the very high concentrations of CCK found in the early stages of pancreatitis may deplete the pancreas of the amylase and lipase available for release by CCK. The finding that the elevation of plasma CCK observed in patients is similar to that observed in the rat bile duct ligation model suggests that CCK does play an important role in the early stages of pancreatitis, particularly that induced by biliary tract obstruction (Otsuki, 2000). CCK is also elevated in patients with chronic pancreatitis, hinting that CCK might play a role in this condition as well (Shirohara and Otsuki, 1997; Otsuki, 2000).
The effects of JNJ-17156516 and dexloxiglumide observed after the ‘onset' of pancreatitis suggest that CCK1 receptor antagonism may provide the maximum possible benefit when administered early after the onset of pancreatitis. Intravenous administration of JNJ-17156516 and dexloxiglumide 30 min after bile duct ligation produced a similar suppression of plasma amylase activity to that found when these compounds were administered before bile duct ligation. Administration of either CCK1 receptor antagonists 1 h after bile duct ligation still produced a significant reduction in plasma amylase but the effect was not as large. It is important to note that the elevation of plasma amylase is near maximal 1 h after bile duct ligation and plasma CCK concentration was 20-fold higher than the physiological level. Thus, CCK appears to be important in the early phases of acute pancreatitis. These data suggest that a CCK1 receptor antagonist can provide benefit but that CCK1 antagonist must be administered quickly after the onset of pancreatitis.
Given that CCK initiated the hallmark features of pancreatitis (that is, elevation of plasma amylase and lipase activity) and this could be partly suppressed by CCK1 receptor antagonism, a link between CCK1 receptor activation and initiation of inflammation was sought. NF-κB is a well-known signalling molecule in the inflammatory cascade and its activation in a variety of cell types leads to the production of pro-inflammatory mediators, such as interleukin-6 and tumour necrosis factor-α (Baeuerle, 1998). Previous studies have demonstrated that CCK receptor agonists, such as CCK8S or caerulein, can activate NF-κB in isolated pancreatic acinar preparations and in the intact pancreas of rodents infused with these agents (Gukovsky et al., 1998; Han and Logsdon, 1999). Despite a number of studies detailing the activation of PKC subtypes (δ and ɛ) involved in the activation of NF-κB (Satoh et al., 2004), it was not clear to date whether the effect is mediated by CCK1 or CCK2 receptors in the rat pancreas and whether the NF-κB can be activated by concentrations of CCK found in the early stages of pancreatitis. In our experiments, pretreatment with JNJ-17156516 prior to infusion of CCK8S prevented the activation of NF-κB. The plasma CCK concentrations achieved via infusion were similar to those found 1 h after bile duct ligation in the rat (∼20 pM). The observation that the CCK concentration was raised very rapidly to this level after bile duct ligation strongly suggests that CCK1 receptor-mediated activation of NF-κB plays an important role in the initiation of inflammation in the pancreas in pancreatitis states. It is also noteworthy that the activation of NF-κB occurs over a similar time course to the release of amylase and lipase into the plasma (30–60 min), consistent with the role of CCK in initiating pancreatitis. The observation that NF-κB activation in the pancreas can be prevented by CCK1 receptor antagonism provides evidence for a novel anti-inflammatory action for this class of molecule.
A number of investigators have previously reported that the CCK1 receptor is expressed only in very low levels or not at all in the human pancreas (see Owyang and Logsdon, 2004). However, auto-digestion while isolating protein and mRNA from this organ complicates interpretation of these data. Newly developed, highly sensitive PCR methodologies demonstrated that the CCK1 receptor is present in the human pancreas (Galindo et al., 2005) and the association of elevated CCK with acute pancreatitis suggests that CCK could be involved in initiation of pancreatitis in humans.
Loxiglumide, the racemate form of dexloxiglumide, has been evaluated in two clinical trials in pancreatitis patients with inconclusive results. In the acute pancreatitis patient population, 500 mg of loxiglumide administered twice a day normalized serum lipase levels more quickly than lower doses but did not have a significant impact on patient outcome. Interpretation of this study is confounded by the fact that a placebo control group was not included in the study. In a trial of chronic pancreatitis patients suffering from an acute flare up of pancreatitis, some positive results were found at the 600 mg dose. However, doses of 300 or 1200 mg were found to be not effective. Further clinical studies are needed to demonstrate the value of a CCK1 receptor antagonist for the treatment of pancreatitis. The present data suggest that patient populations that have elevated CCK concentrations, such as those with gallstone- and endoscopic retrograde cholangiopancreatography-induced pancreatitis may benefit the most from CCK1 receptor antagonist treatment. These data also indicate that administering the CCK1 receptor antagonist as early as possible after the onset of symptoms is most likely to provide benefit.
Acknowledgments
We thank the Johnson & Johnson Pharmaceutical Research & Development, L.L.C. La Jolla Scale-up and Hit-to-lead groups for synthesizing JNJ-17156516 and the Bio-analytical group for determining the JNJ-17156516 plasma concentrations for this study.
Abbreviations
- CCK
cholecystokinin
- NF-κB
nuclear factor-κB
Conflict of interest
The authors state no conflict of interest.
References
- Baeuerle PA. IkappaB–NF-kappaB structures: at the interface of inflammation control. Cell. 1998;11:729–731. doi: 10.1016/s0092-8674(00)81694-3. [DOI] [PubMed] [Google Scholar]
- Beglinger C. Potential role of cholecystokinin in the development of acute pancreatitis. Digestion. 1999;60 Suppl 1:61–63. doi: 10.1159/000051456. [DOI] [PubMed] [Google Scholar]
- Galindo J, Jones N, Powell GL, Hollingsworth SJ, Shankley N. Advanced qRT-PCR technology allows detection of the cholecystokinin 1 receptor (CCK1R) expression in human pancreas. Pancreas. 2005;31:325–331. doi: 10.1097/01.mpa.0000181487.50269.dc. [DOI] [PubMed] [Google Scholar]
- Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-kappaB activation. Am J Physiol. 1999;277:C74–C82. doi: 10.1152/ajpcell.1999.277.1.C74. [DOI] [PubMed] [Google Scholar]
- Liang JT, Mani NS, Jones TK. Design of concise, scalable route to a cholecystokinin 1 (CCK 1) receptor antagonist. J Org Chem. 2007;72:8243–8250. doi: 10.1021/jo071166m. [DOI] [PubMed] [Google Scholar]
- Maire P, Wuarin J, Schibler U. The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science. 1989;244:343–346. doi: 10.1126/science.2711183. [DOI] [PubMed] [Google Scholar]
- Morton MF, Barrett TD, Yan W, Freedman JM, Lagaud G, Prendergast CE, et al. JNJ-17156516 (3-[5-(3,4-dichloro-phenyl)-1-(4-methoxy-phenyl)-1H-pyrazol-3-yl]-2-m-tolyl-propionate) a novel, potent and selective CCK1 receptor antagonist. In vitro and in vivo pharmacological comparison with dexloxiglumide. J Pharmacol Exp Ther. 2007;323:562–569. doi: 10.1124/jpet.107.124578. [DOI] [PubMed] [Google Scholar]
- Niederau C, Grendell JH, Rothman SS. Effects of proglumide on ductal and basolateral secretion of pancreatic digestive enzymes. Am J Physiol. 1985;249:G100–G107. doi: 10.1152/ajpgi.1985.249.1.G100. [DOI] [PubMed] [Google Scholar]
- Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi K, Harada H, Satake K. Clinical evaluation of cholecystokinin-A-receptor antagonist (loxiglumide) for the treatment of acute pancreatitis. A preliminary clinical trial. Study Group of Loxiglumide in Japan. Digestion. 1999;60 Suppl 1:81–85. doi: 10.1159/000051460. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Axelson J, Stenram U, Rehfeld JF, Ihse I. Acute taurodeoxycholate-induced pancreatitis in the rat is associated with hyperCCKemia. Int J Pancreatol. 2000;27:195–201. doi: 10.1385/IJGC:27:3:195. [DOI] [PubMed] [Google Scholar]
- Otsuki M. Pathophysiological role of cholecystokinin in humans. J Gastroenterol Hepatol. 2000;15:D71–D83. doi: 10.1046/j.1440-1746.2000.02178.x. [DOI] [PubMed] [Google Scholar]
- Owyang C, Logsdon CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology. 2004;127:957–969. doi: 10.1053/j.gastro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Persiani S, D'Amato M, Makovec F, Tavares IA, Bishai PM, Rovati LC. Pharmacokinetics of dexloxiglumide after administration of single and repeat oral escalating doses in healthy young males. Int J Clin Pharmacol Ther. 2002;40:198–206. doi: 10.5414/cpp40198. [DOI] [PubMed] [Google Scholar]
- Satake K, Kimura K, Saito T. Therapeutic effects of loxiglumide on experimental acute pancreatitis using various models. Digestion. 1999;60 Suppl 1:64–68. doi: 10.1159/000051457. [DOI] [PubMed] [Google Scholar]
- Satoh A, Gukovskaya AS, Nieto JM, Cheng JH, Gukovsky I, Reeve JR, Jr, et al. PKC-delta and -epsilon regulate NF-kappaB activation induced by cholecystokinin and TNF-alpha in pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G582–G591. doi: 10.1152/ajpgi.00087.2004. [DOI] [PubMed] [Google Scholar]
- Shiratori K, Takeuchi T, Satake K, Matsuno S, Study Group of Loxiglumide in Japan Clinical evaluation of oral administration of a cholecystokinin-A receptor antagonist (loxiglumide) to patients with acute, painful attacks of chronic pancreatitis: a multicenter dose–response study in Japan. Pancreas. 2002;25:e1–e5. doi: 10.1097/00006676-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Shirohara H, Otsuki M. Plasma cholecystokinin levels in acute pancreatitis. Pancreas. 1997;14:249–254. doi: 10.1097/00006676-199704000-00005. [DOI] [PubMed] [Google Scholar]
- Toriumi Y, Samuel I, Wilcockson DP, Turkelson CM, Solomon TE, Joehl RJ. Octreotide and cholecystokinin antagonist reduce edema in obstruction-induced acute pancreatitis. J Lab Clin Med. 1993;112:450–454. [PubMed] [Google Scholar]
- Toskes PP, Greenberger NJ.Disorders of the pancreas Harrison's 15th Edition. Principles of Internal Medicine 2001McGraw-Hill: New York, NY; 1788–1804.In: Braunwald E, Fauci AS, Kasper DL et al. (eds). [Google Scholar]
- Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]