Abstract
Background and purpose: Exposure of pancreatic β-cells to long-chain free fatty acids leads to differential responses according to the chain length and degree of unsaturation. In particular, long-chain saturated molecules such as palmitate (C16:0) cause apoptosis, whereas equivalent mono-unsaturated species (for example, palmitoleate (C16:1)) are not overtly toxic. Moreover, mono-unsaturates exert a powerful cytoprotective response against a range of proapoptotic stimuli. However, the structural requirements that determine cytoprotection have not been determined and form the basis of the present study.
Experimental approach: BRIN-BD11 and INS-1 β-cells were exposed either to the saturated fatty acid palmitate, or to serum withdrawal, to mediate cytotoxicity. The protective effects of a wide range of mono-unsaturated fatty acid derivatives were tested in cytotoxicity assays. Effector caspase activity was also measured and correlated with viability.
Key results: The cytotoxic actions of palmitate were inhibited dose-dependently by long-chain mono-unsaturated fatty acids with a defined potency order C18:1>C16:1≫C14:1. The configuration of the double bond was also important with cis forms being more potent than trans forms. Alkylated mono-unsaturated fatty-acid derivates were also cytoprotective, although their efficacy declined as the alkyl chain length increased. Cytoprotection was achieved rapidly on addition of mono-unsaturates and correlated with a rapid and dramatic inhibition of caspase-3/7 activity in palmitate-treated cells.
Conclusions and implications: The data reveal the structural requirements that dictate the cytoprotective actions of mono-unsaturated fatty acids in pancreatic β-cells. Metabolic activation is not required and the data point at the potential involvement of a fatty acid receptor in mediating cytoprotection.
Keywords: palmitoleate, palmitate, oleate, free fatty acid receptor, lipotoxicity, endocrine pancreas
Introduction
The incidence of type 2 diabetes has increased sharply across the globe in recent years and is strongly correlated with rising obesity (Hull et al., 2005; Kamei et al., 2005). This suggests that the altered metabolic profile associated with obesity may contribute to the development of type 2 diabetes, a disease characterized by peripheral insulin resistance, pancreatic β-cell dysfunction and decreased β-cell mass (Leonardi et al., 2003; Maedler and Donath, 2004; Cnop et al., 2005; Maedler, 2007). In particular, there is mounting evidence that elevated levels of circulating free fatty acids can be detrimental to pancreatic β-cells and that this may contribute to their long-term demise during the progression of type 2 diabetes (Bergman and Ader, 2000; Hardy et al., 2003).
Free fatty acids exert differential effects on pancreatic β-cells according to their chain length, degree of unsaturation and the period of exposure (Newsholme et al., 2007). Thus, when free fatty acids are administered to β-cells acutely, they enhance insulin secretion, whereas chronic treatment can be detrimental to the cells leading ultimately to loss of viability. This phenomenon (recognized as ‘lipotoxicity') can be observed experimentally both in vivo and in vitro and probably recapitulates some of the events associated with the chronic elevation of circulating fatty acids in obesity (Eitel et al., 2003; El-Assaad et al., 2003; Robertson et al., 2004; Haber et al., 2006).
Examination of the chronic effects of saturated fatty acids in vitro has revealed that these molecules are poorly tolerated by β-cells and can induce apoptosis (Moffitt et al., 2005; Newsholme et al., 2007). By contrast, long-chain mono-unsaturates display a much reduced propensity to cause β-cell death. Indeed, at physiological concentrations, certain mono-unsaturates (for example, palmitoleate (C16:1) and oleate (C18:1)) actively promote cell proliferation and viability. Moreover, they potently attenuate the ability of saturated fatty acids to induce β-cell apoptosis (Eitel et al., 2002; Welters et al., 2004; Dimopolous et al., 2006; Karaskov et al., 2006).
The differential responses seen on chronic exposure of β-cells to saturated and unsaturated fatty acids imply that these two molecular species must exert fundamentally different effects within the cells. However, these mechanisms remain to be defined. In particular, the precise structural requirements for cytoprotection by mono-unsaturates have not been established, and it is also unclear whether poorly metabolized derivatives of unsaturated fatty acids are protective. These issues are particularly important in view of the emerging evidence that cells can express receptors for fatty acids (both at the cell surface and intracellularly) whose binding properties are likely to vary according to defined structural parameters (Rayasam et al., 2007). It is not yet known whether the ability of mono-unsaturated fatty acids to promote cell viability reflects their interaction with such a receptor, although this has recently been proposed (Katsuma et al., 2005). Therefore, in the present study, we have assessed the structural requirements that underlie β-cell cytoprotection in response to mono-unsaturated fatty acids as a means to establish the cellular pharmacology of this response.
Materials and methods
Materials
RPMI-1640 medium, penicillin/streptomycin and glutamine were purchased from Invitrogen (Paisley, Scotland). Foetal calf serum was purchased from PAA Laboratories (Somerset, England). Fatty-acid-free BSA, palmitoleate and palmitelaidate were from MP Biomedicals (Oxon, England). Palmitate, oleate, myristoleate, methyl-palmitoleate, methyl oleate, ethyl oleate, butyl oleate, elaidate, pertussis toxin (Ptx), etoposide and β-mercaptoethanol were from Sigma (Dorset, England) and caspase-3/7 luminescence kit was from Promega (Southampton, England).
Cell culture
BRIN-BD11 and INS-1 β-cells were used in the present study. These cell lines were derived from a rat insulinoma by methods reported by Asfari et al. (1992) and McClenaghan et al. (1996). Both were cultured in RPMI-1640 medium containing 11 mM glucose supplemented with 10% foetal bovine serum, 2 mM L-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. The medium used to culture INS-1 cells was also supplemented with 50 μM β-mercaptoethanol. The cells were grown in monolayers at 37 °C and 5% CO2. For individual experiments, cells were seeded into six-well plates at a density of 1 × 105 cells per well for BRIN-BD11 cells and 1.5 × 105 cells per well for INS-1 cells in complete RPMI-1640 media for 24 h. After this period, the medium was removed and replaced by serum-free RPMI-1640 containing appropriate fatty acid-BSA complexes. Controls received BSA and vehicle only.
Preparation of fatty acid-BSA complexes
Stock solutions of palmitate were prepared in 50% ethanol by heating to 70 °C, and stock solutions of all mono-unsaturated fatty acids were prepared in 90% ethanol at room temperature. Fatty acids were bound to 10% fatty-acid-free BSA by incubation at 37 °C for 1 h. The final concentration of BSA was maintained at 1% and that of ethanol was maintained at 0.5%. Control cells received vehicle (BSA plus ethanol) alone.
Quantification of cell death
Routine measurement of cell viability was determined by the ability of the cells to exclude trypan blue. After incubation, all cells (adhered and detached) were collected, centrifuged at 200 g for 5 min and resuspended in complete RPMI-1640 containing trypan blue (0.4% in PBS) in 1:1 ratio. Viable and dead cells were counted using a haemocytometer and the dead cells were expressed as the percentage of the total cells for each condition. Over the course of the present experiments, the extent of cell death recorded in untreated BRIN-BD11 cells averaged 9.8±1.1%.
Caspase-3/7 activity measurement
Caspase-3/7 activity was measured using a commercial luminescence assay kit. Following incubation, cells were counted and 5000 cells per well from each incubation condition were placed in a white-walled 96-well plate. Caspase detection reagent was then added and the cells incubated for a further 30 min at room temperature. Luminescence was measured according to the manufacturer's instructions using a Tecan plate reader.
Statistical analysis
All experiments were performed on at least three separate occasions and either duplicate or triplicate of each condition were used in each experiment. The results are expressed as mean±s.e.m. and the level of significance was calculated using Student's t-test or ANOVA, as appropriate.
Results
The cytoprotective effects of mono-unsaturated fatty acids depend on their chain length and double-bond configuration
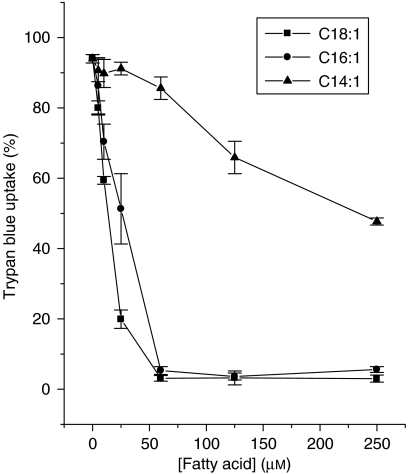
We have previously reported that, in BRIN-BD11 cells, the cytotoxicity mediated by incubation in the presence of the saturated fatty acid, palmitate (C16:0), is ameliorated by the co-presence of long-chain mono-unsaturated fatty acids (Welters et al., 2004; Diakogiannaki et al., 2007). However, the specificity of this response has not been investigated in detail and, therefore, initial experiments were performed with mono-unsaturates of increasing chain length to establish their relative potencies. The results are shown in Figure 1. All three mono-unsaturated fatty acids tested, myristoleate (C14:1), palmitoleate (C16:1) and oleate (C18:1), significantly attenuated the loss of viability caused by palmitate (250 μM), and they also reduced the ‘basal' rate of cell death measured in control cells. However, their potencies were markedly different such that the longer chain molecules displayed the highest potency (oleate EC50 ∼10 μM total concentration; palmitoleate EC50 ∼25 μM) and the greatest efficacy. Myristoleate failed to provide complete protection at concentrations up to 250 μM (Figure 1), although it did reduce the loss of viability by ∼50% at this concentration. These results imply that there is specificity in terms of chain length for the protective actions of mono-unsaturates. We next considered whether the orientation of the double bond is also important.
Figure 1.
Effects of the chain length of mono-unsaturated fatty acids on their protective effects against palmitate-induced toxicity in BRIN-BD11 cells. Cells were treated with 250 μM palmitate in the presence or absence of increasing concentrations of C14:1 (myristoleate), C16:1 (palmitoleate) or C18:1 (oleate) for 18 h. Cell viability was then estimated.
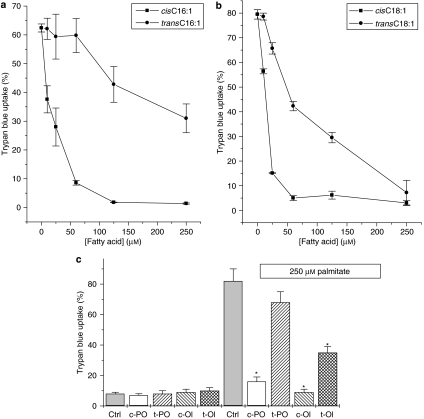
Exposure of BRIN-BD11 cells to palmitate in the presence of increasing concentrations of transC16:1 (palmitelaidate) resulted in an improvement in viability relative to those exposed to palmitate alone. However, the protective response to the trans-fatty acid was reduced in potency compared with that seen with cisC16:1 (palmitoleate; Figure 2a). Similar observations were made when transC18:l (elaidate) and cisC18:l (oleate) were compared (Figure 2b). To ascertain whether these responses were reproduced in a different β-cell line, the effects of the cis- and trans-fatty acids were also examined in INS-1 cells (Figure 2c). As observed in BRIN-BD11 cells, the cis mono-unsaturates were powerfully protective in INS-1 cells, whereas the trans forms were less effective (Figure 2c). Thus, both the chain length and the configuration of the double bond appear to be important in conferring the cytoprotective activity of mono-unsaturated fatty acids in β-cells.
Figure 2.
Effects of the double-bond configuration of mono-unsaturated fatty acids on their protective effect against saturated fatty acid toxicity. (a) BRIN-BD11 cells were exposed to increasing concentrations of cisC16:1 (palmitoleate) or transC16:1 (palmitelaidate) in the presence of 250 μM of palmitate. Viability was assessed following 18 h incubation. (b) BRIN-BD11 cells were incubated with increasing concentrations of cisC18:1 (oleate) or transC18:1 (elaidate) in the presence of 250 μM palmitate. Viability was assessed following 18 h incubation. (c) INS-1 cells were treated with the 250 μM of the cis (c) or trans (t) forms of C16:1 (PO) and C18:1 (Ol) in the presence or absence of 250 μM palmitate. Cell viability was assessed after 48 h incubation. *P<0.01 relative to palmitate alone.
A free carboxylic group is not essential for the protective effects exhibited by mono-unsaturated fatty acids
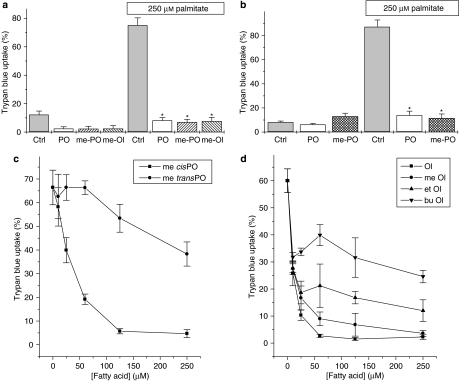
To investigate the importance of the free carboxylic acid moiety in the protective effects of mono-unsaturates, BRIN-BD11 cells were incubated in the presence or absence of palmitate together with the unesterified mono-unsaturated fatty acids, palmitoleate and oleate, or their equivalent alkylated derivatives. The presence of the alkyl group serves to block the functional carboxylate group, thereby rendering the molecules metabolically inert. The results indicated that fatty acid methyl esters were equally effective with the free forms in protecting cells against palmitate-induced toxicity (Figure 3a). Similar results were obtained with INS-1 cells (Figure 3b). Moreover, comparison of the cis and trans forms of methyl-palmitoleate revealed a similar difference in potency to that seen when the non-methylated, parental molecules were employed (compare Figure 3c with Figure 2a). Experiments conducted with ethyl and butyl derivatives of C18:1 revealed a reduced efficacy in comparison with the unesterified fatty acids (Figure 3d), although these derivatives still attenuated the loss of viability promoted by palmitate.
Figure 3.
Effects of methylated derivatives of mono-unsaturated fatty acids on β-cell viability. (a) BRIN-BD11 cells were treated with 250 μM of palmitoleate (PO), oleate (Ol), or their respective methyl derivatives (me-PO or me-Ol) in the presence and absence of 250 μM palmitate. Cell viability was assessed after 18 h incubation. (b) INS-1 cells were incubated with either 250 μM PO or me-PO in the presence and absence of 250 μM palmitate. Cell viability was assessed after 48 h incubation. (c) Dose-dependence of the protective effects of methyl palmitoleate (me-cisPO) and methyl palmitelaidate (me-transPO) against toxicity induced by 250 μM palmitate in BRIN-BD11 cells. Viability was assessed at the end of 18 h incubation. (d) BRIN-BD11 cells were treated with increasing concentrations of oleate (Ol), methyl oleate (me-Ol), ethyl oleate (et-Ol) or butyl oleate (bu-Ol) in the presence of 250 μM palmitate. Cell viability was assessed after 18 h incubation. *P<0.001 relative to palmitate alone.
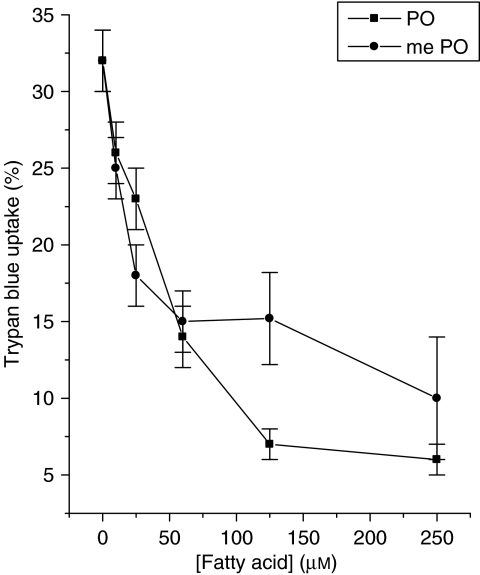
Previously, it was shown that palmitoleate provides protection against the loss of viability seen in BRIN-BD11 cells on serum withdrawal (Welters et al., 2004), and it was considered important to examine whether a similar response is also elicited by its methylated counterpart. Accordingly, BRIN-BD11 cells were incubated with increasing concentrations of palmitoleate or methyl palmitoleate for 30 h in serum-free media. The results revealed that, as in the case of palmitate-induced toxicity, both methyl-palmitoleate and palmitoleate were effective in protecting cells against the loss of viability associated with serum withdrawal (Figure 4).
Figure 4.
BRIN-BD11 cells were treated with increasing concentrations of either palmitoleate (PO) or methyl palmitoleate (me-PO) in the absence of added foetal calf serum as a means to promote loss of viability. Viability was assessed after 30 h incubation.
Time dependency of the protective actions of palmitoleate against palmitate-induced cytotoxicity
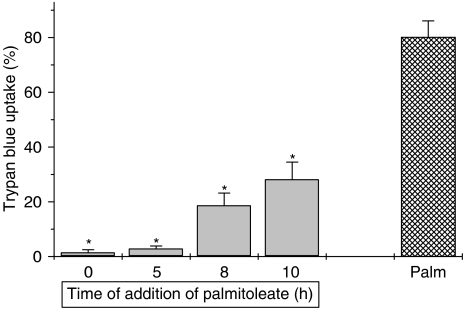
As the results obtained above imply that there are strict structural requirements for induction of the cytoprotective response mediated by mono-unsaturated fatty acids, we next examined whether the protective actions are mediated with rapid onset. To achieve this, BRIN-BD11 cells were initially exposed to palmitate (at t=0) before the introduction of palmitoleate at timed intervals thereafter. Under all conditions, the cells were incubated for a total of 24 h before assessment of their viability (Figure 5). It was observed that when palmitoleate was introduced as late as 10 h after the initial exposure to palmitate, the loss of viability associated with the saturated fatty acid was attenuated significantly. Thus, in an extension of an earlier study (Welters et al., 2004), it was observed that palmitoleate was able to exert a protective influence even when added several hours after the cytotoxic stimulus. To examine these effects further, measurements were made of effector caspase activity as this is usually considered to be one of the end points in the apoptotic pathway in β-cells.
Figure 5.
Time dependency of the protective actions of palmitoleate on palmitate-induced toxicity. BRIN-BD11 were initially exposed to 250 μM palmitate (Palm) at t=0 h and then 250 μM palmitoleate was added at various time points (t=0, t=5, t=8 and t=10 h). The hatched bar represents cells incubated with 250 μM palmitate alone. Cells were incubated for a total of 24 h under all conditions and the viability was assessed at the end of this period. *P<0.001 relative to palmitate alone.
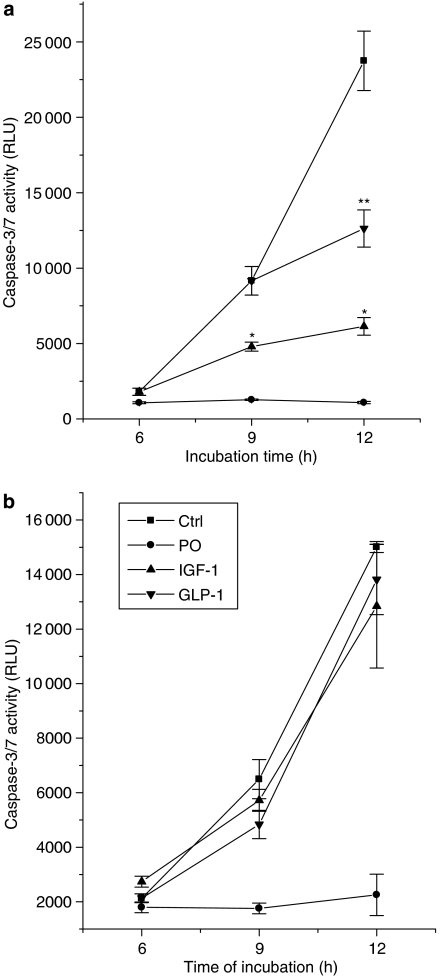
Treatment of cells with 250 μM palmitate caused a marked and time-dependent increase in caspase-3/7 activity (Figure 6a) consistent with the progression of apoptosis previously shown to occur under these conditions (Welters et al., 2006). The increase in caspase-3/7 activity was abolished when cells were also exposed to palmitoleate together with palmitate from t=0. Strikingly, when palmitoleate was added to the cells at later time points (up to 10 h after palmitate), it again prevented any further rise in caspase-3/7 activity beyond that already achieved in response to palmitate at the relevant time point (Figure 6a). Thus, at each time point tested, addition of the mono-unsaturate caused a rapid and dramatic inhibition of caspase activation, which was associated with enhanced cell survival (Figure 5). In additional studies, we examined whether this ability to inhibit the activation of caspase-3/7 induced by palmitate might be common to other agents that also promote cell survival. Accordingly, the effects of insulin-like growth factor-1 (IGF-1) (Figure 6b) and glucagon-like peptide-1 (GLP-1) were studied. However, neither agent attenuated palmitate-induced caspase-3/7 activation when added to cells at the same time as the cytotoxic stimulus (that is, conditions under which palmitoleate was completely protective).
Figure 6.
Caspase-3/7 activity in BRIN-BD11 cells exposed to fatty acids. (a) Cells were treated with 250 μM palmitate at t=0 h and 250 μM palmitoleate was then added at different time points during the incubation (0 h (circles), 8 h (triangles) and 10 h (inverted triangles)). Caspase-3/7 activity was measured after 6, 9 and 12 h of incubation. *P<0.001 relative to palmitate alone at the equivalent time point; **P<0.001 relative to caspase-3/7 activity measured in palmitate-treated cells incubated for 12 h. (b) BRIN-BD11 cells were exposed to 250 μM palmitate alone (Ctrl) or 250 μM palmitate in combination with 250 μM palmitoleate (PO), 10 nM IGF-1 or 1 μM GLP-1, as shown. Caspase-3/7 activity was measured after 6, 9 and 12 h under all conditions.
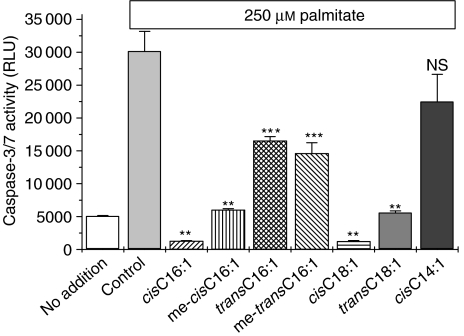
To assess whether the extent of activation of caspase-3/7 in cells exposed to fatty acids correlated with the overall extent of viability seen under these conditions, the effects of chain length, methylation and the configuration of the double bond were examined (Figure 7). A striking correlation was observed between the extent of caspase-3/7 activation and the corresponding cell viability associated with each incubation condition. Thus, methylation of the carboxyl group of either palmitoleate or oleate did not alter the capacity of either fatty acid to inhibit palmitate-induced caspase-3/7 activation (Figure 7), and the trans form of each unsaturated fatty acids was partially effective, but less so than the corresponding cis form. Similarly, the limited attenuation of caspase-3/7 activation by myristoleate (cisC14:l) paralleled its effects on viability under these conditions (Figure 1).
Figure 7.
Caspase-3/7 activity was measured in BRIN-BD11 cells exposed to palmitate in the presence of various mono-unsaturated fatty acid derivatives. Cells were incubated in the presence of 250 μM palmitate in the presence or absence of 250 μM of the various mono-unsaturated fatty acid species, as shown. Caspase-3/7 activity was measured after 12 h incubation. **P<0.001 relative to palmitate alone; ***P<0.01 relative to palmitate alone; NS, not significantly different from palmitate alone.
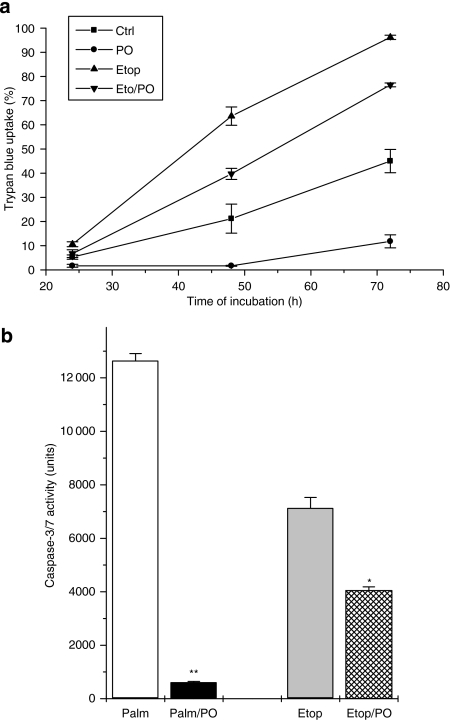
In view of the finding that palmitoleate is able to attenuate caspase-3/7 activity rapidly and completely in palmitate-treated cells, we felt it important to consider whether the mono-unsaturate is also able to inhibit this activity (and promote cell viability) in cells exposed to a different type of proapoptotic stimulus. Therefore, BRIN-BD11 cells were exposed to the topoisomerase inhibitor, etoposide, under serum-free conditions and, as expected, this agent increased caspase-3/7 activity and provoked a time-dependent loss of viability (Figure 8). Palmitoleate did not alter the increased rate of cell death caused by etoposide, although it did lower the absolute number of dead cells under these conditions, reflecting its ability to attenuate the extent of cell death elicited by the removal of serum (as deduced from the controls shown in Figure 8a). In accord with these findings, measurement of caspase-3/7 activity revealed a modest reduction in cells exposed to etoposide and palmitoleate (relative to etoposide alone) but the enzyme activity remained well above control and at a level markedly greater than that seen when palmitate and palmitoleate were combined (Figure 8b).
Figure 8.
Effects of etoposide on the viability of BRIN-BD11 cells and on caspase-3/7 activity. (a) BRIN-BD11 cells were exposed to etoposide (1 μM) in the presence or absence of 250 μM palmitoleate, as shown. Cell viability was assessed at 24, 48 and 72 h incubation. Palmitoleate significantly reduced the loss of viability (P<0.01) at all time points tested both in control cells and those exposed to etoposide (Etop). (b) Caspase-3/7 activity was measured after 12 h exposure of BRIN-BD11 cells to 250 μM palmitate alone (Palm) and in combination with 250 μM palmitoleate (Palm/PO), or 5 μM etoposide alone (Etop) and in combination with 250 μM palmitoleate (Etop/PO). *P<0.01 relative to etoposide alone; **P<0.001 relative to palmitate alone.
Effects of Ptx and prior exposure to agonist on the cytoprotective actions of mono-unsaturated fatty acids
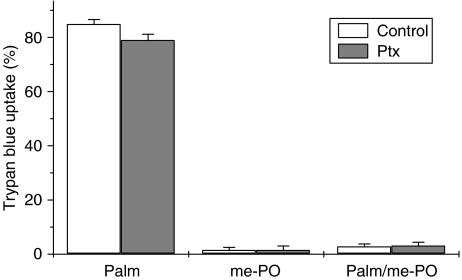
In the next series of experiments, the influence of Ptx on the cytoprotective response to mono-unsaturates was assessed. Pretreatment of BRIN-BD11 cells with Ptx did not modify the ability of palmitate to cause cytotoxicity (Figure 9) and it failed to alter the protection afforded by methyl-palmitoleate.
Figure 9.
Effect of pretreatment of BRIN-BD11 cells with pertussis toxin (Ptx) on the cytoprotective actions of methyl-palmitoleate (me-PO). Cells were incubated with 250 μM palmitate (Palm), 250 μM me-PO or a combination of the two (Palm/me-PO) in the presence and absence of prior incubation (24 h) with 100 ng ml−1 Ptx. Viability was assessed 18 h after the introduction of the fatty acids.
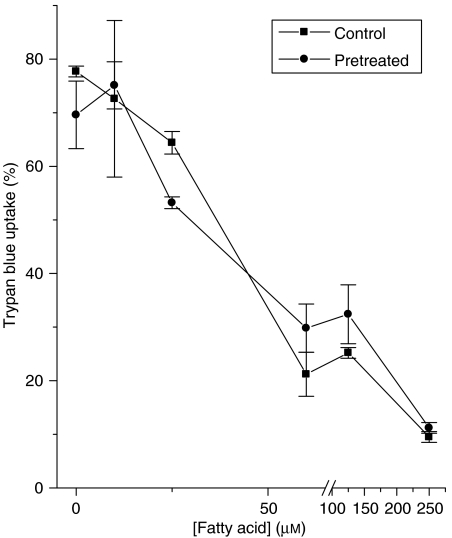
The possibility was also investigated that cells might become desensitized to the protective actions of unsaturated fatty acids during prolonged exposure. Therefore, BRIN-BD11 cells were exposed to a high concentration (250 μM) of methyl-palmitoleate for an initial period of 24 h. After this time, the fatty acid was removed and the culture medium replaced with fresh medium containing palmitate (250 μM) and increasing concentrations of methyl-palmitoleate (Figure 10). The protective action of the unsaturated molecule was then compared with that seen in cells not previously exposed to the agent (Figure 10), but no change in potency was observed.
Figure 10.
Effects of pretreatment of BRIN-BD11 cells with methyl-palmitoleate on their subsequent responsiveness to this fatty acid. Cells were incubated in the presence (pretreated) or absence (control) of 250 μM methyl palmitoleate for 24 h and then washed. They were then exposed to 250 μM palmitate in the presence or absence of increasing concentrations of freshly added methyl-palmitoleate. Viability was measured 18 h after addition of palmitate.
Discussion
Fluctuations in the circulating levels of free fatty acids are probably important for the physiological regulation of insulin secretion in response to nutrients (Stein et al., 1996; Dobbins et al., 2002; Haber et al., 2006), but it is clear that a sustained elevation can be detrimental to pancreatic β-cells (Shimabukuro et al., 1998; Cnop et al., 2005). This response has been observed both in vivo and in vitro and is often termed ‘lipotoxicity' (Newsholme et al., 2007). However, despite its potential importance in type 2 diabetes, the molecular basis of lipotoxicity remains obscure and has been variously attributed to factors ranging from the physical disruption of cells by accumulated triacylglycerol (Moffitt et al., 2005) to the activation of specific proapoptotic pathways by defined subgroups of fatty acids (Diakogiannaki et al., 2007). In this context, others and we have provided evidence that the lipotoxic actions of long-chain free fatty acids in vitro do not arise from nonspecific effects but, rather, the response displays considerable specificity and is dependent on the chain length and saturation of the fatty acid molecules. These observations have spawned the proposition that β-cell lipotoxicity results from the activation of specific proapoptotic pathways that are differentially regulated by saturated and mono-unsaturated molecules. Thus, long-chain saturated fatty acids such as palmitate (C16:0) and stearate (C18:0) are powerfully lipotoxic to pancreatic β-cells, whereas the equivalent mono-unsaturated molecules (palmitoleate (C16:1) and oleate (C18:1)) are not intrinsically toxic. Rather, these molecules are well tolerated by β-cells (at physiological concentrations) and, as confirmed in the present study, they can attenuate the toxicity of their saturated counterparts. This effect is not prevented by etomoxir, an inhibitor of mitochondrial β-oxidation, implying that altered oxidative metabolism is not a prerequisite for this response (Welters et al., 2004). In support of this, we now demonstrate that alkylation of the terminal carboxyl group of the C16:1 mono-unsaturated fatty acid, palmitoleate, or the C18:1 molecule, oleate, does not prevent the cytoprotective actions of either molecule. This manoeuvre is expected to significantly reduce the rate of fatty acyl-CoA formation from these molecules (as confirmed by the failure of methyl-palmitoleate to increase β-cell triacylglycerol formation) and suggests that their actions on cell viability are mediated independent of changes in metabolism (Diakogiannaki et al., 2007).
Until recently, it was widely thought that the regulatory effects of long-chain fatty acids on pancreatic β-cells were mediated solely by alterations in metabolic activity. However, it is now clear that this is an oversimplification and that both cell-surface and intracellular fatty acid receptors exist, which can control cellular functionality by mechanisms that do not depend directly on altered fatty acid metabolism (Rayasam et al., 2007). Thus, the net effect of the addition of a fatty acid to β-cells may result from a combination of factors, including altered signal transduction, changes in metabolic activity and variations in transcriptional activation. In view of these findings, and in the light of our conclusion that changes in metabolism may not be required for cytoprotection, we have considered whether ‘receptor' activation could underlie the improved viability associated with incubation of BRIN-BD11 β-cells with mono-unsaturated fatty acids.
To begin to address this hypothesis, the structure–activity relationships required for the cytoprotective response in BRIN-BD11 cells were explored. The results reveal that both the chain length and the configuration of the double bond are important determinants of the protective actions of mono-unsaturated fatty acids. Molecules with a double bond in the cis configuration and a chain length of 16 or 18 carbon atoms were potent and efficacious in mediating cytoprotection. By contrast, reduction of the chain length to C14 or substitution of a trans double bond in place of the cis configuration was associated with greatly reduced potency.
The total concentration of fatty acids used in the current experiments are within the range that is expected to occur in vivo in patients with type 2 diabetes (Kusunoki et al., 2007), although the true potency of the reported effects is difficult to define due to uncertainties about the absolute free concentration of each fatty acid after complex formation with BSA. In this context, Richieri et al. (1993) and Richieri and Kleinfeld (1995) employed fluorescent methods to determine the free fatty acid concentrations in solutions containing serum albumin under quasi-physiological conditions similar to those employed here and found that these lies within the nanomolar range. Moreover, in other studies where β-cells were exposed to fatty acids complexed to albumin at ratios similar to those used in our experiments, it has been argued that the effective free fatty acid concentration is likely to lie within the nanomolar range (Listenberger et al., 2003; Cnop et al., 2005). Given that the EC50 values for cytoprotection reported in the present work (defined as the total fatty acid concentration in the presence of serum albumin) were ∼25 μM for cis-palmitoleate and ∼10 μM for cis-oleate, it seems likely that these would yield active (free) concentrations within the subnanomolar range. Thus, it is clearly possible that the engagement of a high-affinity receptor might mediate the response.
This possibility is also supported by the results of time-course studies, which revealed that addition of palmitoleate to cells up to 10 h after introduction of the initial cytotoxic stimulus was still effective as a means to promote viability. This implies that the actions of the mono-unsaturate were mediated rapidly as the development of cytotoxicity was already well established under these conditions. This conclusion was verified by direct measurement of caspase-3/7 activity. Caspase-3/7 activation reflects the effector phase of apoptosis in β-cells and was increased dramatically during the progression of cell death in cells exposed to the saturated fatty acid, palmitate. However, addition of palmitoleate to the cells at a time when caspase-3/7 activity was already increasing substantially resulted in a rapid and complete attenuation of the rise. This implies that an early response to the mono-unsaturate is the activation of a signalling pathway that culminates in reduced activation of effector caspases. As the rate of caspase activation was halted at the earliest time point measured (that is, within 1 h of introduction of palmitoleate), we consider it unlikely that alterations in gene transcription mediate this response, as such changes would probably require a longer time period to take complete effect.
In parallel with studies of the regulation of caspase activity by palmitoleate, the effects of both IGF-1 and GLP-1 were also examined. In common with palmitoleate, these agents have been reported to promote cytoprotection and mitogenesis in β-cells (Lingohr et al., 2002; Liu et al., 2002; Geelhoed-Duijvestijn, 2007; Knop et al., 2007) but, unlike palmitoleate, they failed to prevent palmitate-induced caspase-3/7 activation. Thus, it can be deduced that the mechanisms by which mono-unsaturates exert their protective actions are unlikely to be mediated via signalling pathways equivalent to those used by IGF-1 or GLP-1. In support of this, we have been unable to demonstrate any increase in phosphorylation of PKB/Akt in β-cells exposed to palmitoleate (manuscript in preparation), whereas this enzyme is rapidly phosphorylated in response to IGF-1 (Wrede et al., 2002). Moreover, previous work has established that changes in cAMP (which occur in response to GLP-1; Doyle and Egan, 2007) are not required for cytoprotection by palmitoleate (Welters et al., 2006).
To reach the conclusion that palmitoleate mediates a rapid inhibition of caspase-3/7 activity in β-cells, it was important to examine whether this response occurs universally (that is, whether it is independent of the stimulus for caspase activation) or whether it displays selectivity. Accordingly, cells were exposed to the DNA topoisomerase inhibitor, etoposide, which promotes apoptosis by inhibition of DNA replication and culminates in the activation of effector caspases (Karpinich et al., 2006). In cells treated with etoposide, palmitoleate attenuated, but did not abolish, caspase-3/7 activation and it did not prevent the cumulative increase in cell death associated with etoposide treatment (although it did reduce the absolute number of dead cells during etoposide treatment by virtue of its protective action against serum withdrawal seen in control cells). Thus, we conclude that the cytoprotective actions of palmitoleate are stimulus-dependent and this, in turn, implies that its ability to inhibit caspase-3/7 activation is unlikely to be mediated by a nonspecific mechanism, such as direct interaction of the fatty acid with the enzyme.
Taken together, the results obtained in the present study reveal that long-chain mono-unsaturated fatty acids exert their cytoprotective actions in β-cells by a mechanism that is rapidly activated, displays strict structural specificity and does not require their metabolism. As such, it seems possible that interaction with a fatty acid ‘receptor' could be involved. However, any such conclusion must remain tentative as the relevant receptor has not been identified. In this context, it is noteworthy that the response was unaffected by pretreatment of cells with Ptx, which effectively excludes the involvement of a Gi/Go-coupled molecule. Moreover, we were also unable to alter the potency of methyl-palmitoleate by prolonged (24 h) exposure of cells to this fatty acid suggesting that if a receptor is involved, then this is not subject to desensitization in BRIN-BD11 cells.
In raising the possibility that some form of receptor might be involved in mediating the cytoprotective actions of mono-unsaturates, attention inevitably becomes focused on the newly emerging class of G-protein-coupled cell surface receptors for fatty acids. These include GPR40, 41, 43, 119 and 120 (Rayasam et al., 2007). Among these, GPR40 is highly expressed in pancreatic β-cells and has been implicated in the regulation of insulin secretion by fatty acids (Itoh et al., 2003; Shapiro et al., 2005; Briscoe et al., 2006). It has also been proposed that this receptor may be responsible for the cytotoxic actions of saturated fatty acids in at least one β-cell line (Zhang et al., 2007). However, GPR40 is activated efficiently by both saturated and unsaturated molecules and also by medium- and long-chain fatty acids (Rayasam et al., 2007). Thus, the cellular pharmacology of cytoprotection by fatty acids in β-cell does not accord with their reported activities at this molecule. Equivalent considerations can also be applied to GPR41, 43 and 119 as these are selectively responsive to short-chain molecules (GPR41 and 43) or to derivatized fatty acids (GPR119) rather than to long-chain mono-unsaturates (Covington et al., 2006). Thus, we do not consider that these receptors are likely to be responsible for the responses observed in the present work.
In the intestinal L-cell line, STC-1, activation of GPR120 has been reported to underlie the cytoprotective actions of unsaturated fatty acids (Katsuma et al., 2005). Therefore, this receptor might be considered a candidate for mediating the effects of palmitoleate reported here. However, the functional pharmacology of GPR120 has not been established fully, and it is currently unclear whether the observed specificity of fatty acid effects reported here are consistent with the involvement of GPR120 in mediating cytoprotection in β-cells. Nevertheless, the expression of GPR120 can be detected in both BRIN-BD11 and INS-1 β-cells by reverse-transcription PCR (SD and NGM unpublished observations) and, thus, further studies to investigate the functional consequences of GPR120 activation in these cells are warranted.
Acknowledgments
We thank Eleftheria Diakogiannaki for helpful discussions. We are grateful to Dr Alain Ktorza (Institutes Recherches Internationales Servier) for providing PhD student support for SD. We also thank Diabetes UK for financial support and European Foundation for Study of Diabetes for provision of a research fellowship to HJW.
Abbreviations
- GLP-1
glucagon-like peptide-1
- IGF-1
insulin-like growth factor-1
- Ptx
pertussis toxin
Conflict of interest
The authors state no conflict of interest.
References
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- Briscoe CP, Peat AJ, McKeown SC, Corbett DF, Goetz AS, Littleton TR, et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br J Pharmacol. 2006;148:619–628. doi: 10.1038/sj.bjp.0706770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54 Suppl 2:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Covington DK, Briscoe CA, Brown AJ, Jayawickreme CK. The G-protein-coupled receptor 40 family (GPR40–GPR43) and its role in nutrient sensing. Biochem Soc Trans. 2006;34:770–773. doi: 10.1042/BST0340770. [DOI] [PubMed] [Google Scholar]
- Diakogiannaki E, Dhayal S, Childs CE, Calder PC, Welters HJ, Morgan NG. Mechanisms involved in the cytotoxic and cytoprotective actions of saturated versus monounsat. J Endocrinol. 2007;194:283–291. doi: 10.1677/JOE-07-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopolous N, Watson M, Sakamoto K, Hundal HS. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem J. 2006;399:473–481. doi: 10.1042/BJ20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins RL, Szczepaniak LS, Myhill J, Tamura Y, Uchino H, Giacca A, et al. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes. 2002;51:1825–1833. doi: 10.2337/diabetes.51.6.1825. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel K, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Haring HU, et al. Different role of saturated and unsaturated fatty acids in beta-cell apoptosis. Biochem Biophys Res Commun. 2002;299:853–856. doi: 10.1016/s0006-291x(02)02752-3. [DOI] [PubMed] [Google Scholar]
- Eitel K, Staiger H, Brendel MD, Brandhorst D, Bretzel RG, Haring HU, et al. Apoptosis induced by free fatty acids. Med Klin (Munich) 2003;98:248–252. doi: 10.1007/s00063-003-1253-1. [DOI] [PubMed] [Google Scholar]
- El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, Hardy S, et al. Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology. 2003;144:4154–4163. doi: 10.1210/en.2003-0410. [DOI] [PubMed] [Google Scholar]
- Geelhoed-Duijvestijn PH. Incretins: a new treatment option for type 2 diabetes. Neth J Med. 2007;65:60–64. [PubMed] [Google Scholar]
- Haber EP, Procopio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic beta-cell function. Int Rev Cytol. 2006;248:1–41. doi: 10.1016/S0074-7696(06)48001-3. [DOI] [PubMed] [Google Scholar]
- Hardy S, El-Assaad W, Przbytkowski E, Joly E, Prentki M, Langelier Y. Saturated fatty acid-induced apoptosis in MDA-MB-231 breast cancer cells. A role for cardiolipin. J Biol Chem. 2003;278:31861–31870. doi: 10.1074/jbc.M300190200. [DOI] [PubMed] [Google Scholar]
- Hull RL, Kodama K, Utzschneider KM, Carr DB, Prigeon RL, Kahn SE. Dietary-fat-induced obesity in mice results in beta cell hyperplasia but not increased insulin release: evidence for specificity of impaired beta cell adaptation. Diabetologia. 2005;48:1350–1358. doi: 10.1007/s00125-005-1772-9. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fuji R, Fukusumi S, et al. Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40. Nature. 2003;422:173–176. doi: 10.1038/nature01478. [DOI] [PubMed] [Google Scholar]
- Kamei N, Yamane K, Nakanishi S, Ishida K, Ohtaki M, Okubo M, et al. Effects of a westernized lifestyle on the association between fasting serum nonesterified fatty acids and insulin secretion in Japanese men. Metabolism. 2005;54:713–718. doi: 10.1016/j.metabol.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A. Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology. 2006;147:3398–3407. doi: 10.1210/en.2005-1494. [DOI] [PubMed] [Google Scholar]
- Karpinich NO, Tafani M, Schneider T, Russo MA, Farber JL. The course of etoposide-induced apoptosis in Jurkat cells lacking p53 and Bax. J Cell Physiol. 2006;208:55–63. doi: 10.1002/jcp.20638. [DOI] [PubMed] [Google Scholar]
- Katsuma S, Hatae N, Yano T, Ruike Y, Kimura M, Hirasawa A, et al. Free fatty acids inhibit serum deprivation-induced apoptosis through GPR120 in a murine enteroendocrine cell line STC-1. J Biol Chem. 2005;280:19507–19515. doi: 10.1074/jbc.M412385200. [DOI] [PubMed] [Google Scholar]
- Knop FK, Vilsboll T, Hojberg PV, Larsen S, Madsbad S, Holst JJ, et al. The insulinotropic effect of GIP is impaired in patients with chronic pancreatitis and secondary diabetes mellitus as compared to patients with chronic pancreatitis and normal glucose tolerance. Regul Pept. 2007;144:123–130. doi: 10.1016/j.regpep.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Tsutsumi K, Nakayama M, Kurokawa T, Nakamura T, Ogawa H, et al. Relationship between serum concentrations of saturated fatty acids and unsaturated fatty acids and the homeostasis model insulin resistance index in Japanese patients with type 2 diabetes mellitus. J Med Invest. 2007;54:243–247. doi: 10.2152/jmi.54.243. [DOI] [PubMed] [Google Scholar]
- Leonardi O, Mints G, Hussain MA. Beta-cell apoptosis in the pathogenesis of human type 2 diabetes mellitus. Eur J Endocrinol. 2003;149:99–102. doi: 10.1530/eje.0.1490099. [DOI] [PubMed] [Google Scholar]
- Lingohr MK, Buettner R, Rhodes CJ. Pancreatic beta-cell growth and survival—a role in obesity-linked type 2 diabetes. Trends Mol Med. 2002;8:375–384. doi: 10.1016/s1471-4914(02)02377-8. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RJ, Ory DS, et al. Triglyceride accumulation protects against fatty acid induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Chin-Chance C, Lee EJ, Lowe WL. Activation of phosphatidylinositol 3-kinase contributes to insulin-like growth factor I-mediated inhibition of pancreatic beta-cell death. Endocrinology. 2002;143:3802–3812. doi: 10.1210/en.2002-220058. [DOI] [PubMed] [Google Scholar]
- Maedler K, Donath MY. Beta-cells in type 2 diabetes: a loss of function and mass. Horm Res. 2004;62 Suppl 3:67–73. doi: 10.1159/000080503. [DOI] [PubMed] [Google Scholar]
- Maedler K.Beta cells in type 2 diabetes—a crucial contribution to pathogenesis Diabetes Obes Metab 2007 10.111/j.1463-1326.2007.00718.xdoi [DOI] [PubMed]
- McClenaghan NH, Barnett CR, Ah-Sing E, Abdel-Wahab YH, O'Harte FP, Yoon TW, et al. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Keane D, Welters HJ, Morgan NG. Life and death decisions for the pancreatic β-cell: the role of fatty acids. Clin Sci. 2007;112:27–42. doi: 10.1042/CS20060115. [DOI] [PubMed] [Google Scholar]
- Rayasam GV, Tulasi VK, Davis JA, Bansal VS. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin Ther Targets. 2007;11:661–671. doi: 10.1517/14728222.11.5.661. [DOI] [PubMed] [Google Scholar]
- Richieri GV, Kleinfeld AM. Unbound free fatty acid levels in human serum. J Lipid Res. 1995;36:229–240. [PubMed] [Google Scholar]
- Richieri GV, Anel A, Kleinfeld AM. Interactions between long-chain fatty acids and albumin: determination of free fatty acid levels using the fluorescent probe ADIFAB. Biochemistry. 1993;32:7574–7580. doi: 10.1021/bi00080a032. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53 Suppl 1:S119–S124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- Shapiro H, Shachar S, Sekler I, Hershfinkel M, Walker MD. Role of GPR40 in fatty acid action on the beta cell line INS-1E. Biochem Biophys Res Commun. 2005;335:97–104. doi: 10.1016/j.bbrc.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welters HJ, Diakogiannaki E, Mordue JM, Tadayyon M, Smith SA, Morgan NG. Differential protective effects of palmitoleic acid and cAMP on caspase activation and cell viability in pancreatic beta-cells exposed to palmitate. Apoptosis. 2006;11:1231–1238. doi: 10.1007/s10495-006-7450-7. [DOI] [PubMed] [Google Scholar]
- Welters HJ, Tadayyon M, Scarpello JHB, Smith SA, Morgan NG. Mono-unsaturated fatty acids protect against beta-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS Lett. 2004;560:103–108. doi: 10.1016/S0014-5793(04)00079-1. [DOI] [PubMed] [Google Scholar]
- Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1) J Biol Chem. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu M, Zhang S, Yan L, Yang C, Lu W, et al. The role of G protein coupled receptor 40 in lipoapoptosis in mouse beta-cell line NIT-1. J Mol Endocrinol. 2007;38:651–661. doi: 10.1677/JME-06-0048. [DOI] [PubMed] [Google Scholar]