Abstract
Background and purpose: We have previously demonstrated that stimulation of imidazoline receptors in the CNS prevented halothane–adrenaline arrhythmias during halothane anaesthesia and that stimulation of the vagus nerve may be critical to this effect. However, details of the mechanism(s) involved are not yet available. The present study was designed to examine the role of muscarinic receptors, protein kinase C (PKC), ATP-sensitive potassium channels (KATP) and the mitochondrial permeability transition pore (MPTP) in the antiarrhythmic effect of rilmenidine, an imidazoline receptor agonist.
Experimental approach: Rats were anaesthetized with halothane and monitored continuously for arterial blood pressure and premature ventricular contractions. The arrhythmogenic dose of adrenaline was defined as the lowest dose producing three or more premature ventricular contractions within a 15-s period. We confirmed that centrally administered rilmenidine prevented halothane–adrenaline arrhythmias and then examined the antiarrhythmic effect of rilmenidine in the presence of atropine methylnitrate, a muscarinic receptor antagonist, calphostin C, a PKC inhibitor, HMR-1098, a sarcolemmal KATP inhibitor, 5-hydroxydecanoic acid, a mitochondrial KATP inhibitor or atractyloside, an MPTP opener.
Key results: The antiarrhythmic effect of rilmenidine was significantly inhibited by atropine methylnitrate, calphostin C, 5-hydroxydecanoic acid and atractyloside, but the effects of HMR-1098 in our model were not clear.
Conclusions and implications: The present results suggest that muscarinic receptors, PKC, mitochondrial KATP channels and MPTP may be crucial components of the mechanism involved in the antiarrhythmic effect of rilmenidine given into the CNS.
Keywords: halothane, adrenaline, arrhythmias, imidazoline receptors, muscarinic receptors, protein kinase C, ATP-sensitive potassium channel, mitochondrial permeability transition pore
Introduction
It is well known that activation of α2-adrenoceptors, especially the α2A subtype, in the CNS exerts a hypotensive action (Gavras et al., 2001). On the other hand, there is biochemical and pharmacological evidence to show that imidazoline receptors in the CNS are involved in the mechanism of hypotensive action of α2-adrenoceptor agonists, such as clonidine (Bousquet, 2001). The signalling pathway mediated by imidazoline receptors was reported to be different from that mediated by α2-adrenoceptors (Zhang and Abdel-Rahman, 2005; Li et al., 2006) and both receptors may act synergistically to reduce arterial blood pressure (Chan et al., 2005).
Our previous studies demonstrated that activation of imidazoline and α2-adrenoceptors in the CNS prevented adrenaline-induced arrhythmias during halothane anaesthesia (Hayashi et al., 1991; Takada et al., 1997; Kagawa et al., 2005) and that stimulation of the vagus nerve may be critical for this antiarrhythmic effect (Kamibayashi et al., 1995; Mammoto et al., 1996). ACh is the major neurotransmitter in terminals of the vagus nerve, and activation of muscarinic receptors may play a role in this antiarrhythmic effect. However, the detailed mechanism(s) involved in the antiarrhythmic effect of central activation of imidazoline and α2-adrenoceptors are not well known.
In the heart, stimulation of muscarinic receptors with ACh is known to mimic ischaemic preconditioning, resulting in cardioprotection (Opie, 2001; Oldenburg et al., 2002b). Ischaemic preconditioning was first reported by Murry et al. (1986) and the numerous studies of this effect are summarized in several systematic reviews (Oldenburg et al., 2002a; Yellon and Downey, 2003; Halestrap et al., 2007). Although the detailed intracellular mechanism of preconditioning is not fully understood, activation of protein kinase C (PKC), opening of the ATP-sensitive potassium channels (KATP), especially mitochondrial KATP, and inhibition of opening of the mitochondrial permeability transition pores (MPTP) are important components of this phenomenon (Opie, 2001; Yellon and Downey, 2003; Halestrap et al., 2007). Originally, an end point of preconditioning was defined as an anti-infarct effect, but the concept of preconditioning is now generalized to other modes of cardioprotection, including antiarrhythmic effects (Lukas and Botsford, 1997). Several reports have documented that antiarrhythmic preconditioning shares common intracellular mechanism with anti-infarct preconditioning, including PKC activation and KATP opening (Matsumura et al., 2000; Vegh and Parratt, 2002), although an alternative mechanism has been reported to be involved in antiarrhythmic preconditioning (Kita et al., 1998). We have recently found that nicorandil, a KATP opener, prevents halothane–adrenaline arrhythmias (Kawai et al., 2002).
Thus, we hypothesized that the antiarrhythmic effect observed after stimulation of imidazoline receptors in the CNS would involve activation of muscarinic receptors and of several signalling pathways, which facilitated myocardial preconditioning, such as activation of PKC, opening of KATP and inhibition of MPTP. The present study was designed to examine, using atropine methylnitrate, a muscarinic receptor antagonist, calphostin C, a PKC inhibitor, HMR-1098, a sarcolemmal KATP inhibitor, 5-hydroxydecanoic acid (5-HD), a mitochondrial KATP inhibitor and atractyloside, an MPTP opener, whether any of these factors played a role in the antiarrhythmic effect of centrally administered rilmenidine, an imidazoline and α2-adrenoceptor agonist (Yu and Frishman, 1996).
Methods
Experimental preparation
All animal procedures and the study protocol were approved by the Animal Care Committee of Osaka University Faculty of Medicine.
Male Sprague–Dawley rats, weighing 300–430 g, were used and housed in a temperature-controlled environment under 12 h light–12 h dark cycles, with free access to food and water. The animals were anaesthetized with 2.0% halothane in oxygen. After tracheotomy, the lungs were mechanically ventilated with a tidal volume of 12 ml kg−1 at 40–50 breaths min−1 (Rodent Ventilator; Ugo Basile, Vasere, Italy). The ventilation rates were adjusted to maintain PaCO2 at 35–45 mm Hg. The inspired concentration of halothane, 1.5%, was monitored continuously with an anaesthetic gas analyzer (Capnomac Ultima multiple gas monitor; Datex, Helsinki, Finland). Lead II of the ECG and heart rate were monitored continuously by ECG amplifier and pulse counter unit (AC-611G; Nihon Kohden, Tokyo, Japan). Polyethylene catheters (PE-50 and PE-10) were inserted into the carotid artery for blood sampling and pressure monitoring with a pressure transducer unit (AP-641G; Nihon Kohden), and into the jugular vein for administration of drugs. The ECG and arterial blood pressure were recorded continuously with a thermal array recorder (WS-641G; Nihon Kohden). A heating pad was used to maintain rectal temperature at 37.5–38.5 °C. Arterial pH and oxygen tension were maintained at 7.35–7.45 and more than 100 mm Hg, respectively. After completion of the preparation, anaesthesia was maintained for a further 30 min to achieve a steady state.
Determination of arrhythmogenic dose of adrenaline
The arrhythmogenic dose of adrenaline was defined as the dose that produced three or more premature ventricular contractions within 15 s of injection. Adrenaline was injected in logarithmically spaced doses (0.5, 0.71, 1.0, 1.41, 2.0, 2.83, 4.0, 5.67, 8.0, 11.4 μg kg−1, etc.) following an initial dose of 4.0 μg kg−1 (Takada et al., 1993), and the concentration of adrenaline was adjusted to inject a constant volume of 0.2 ml. The 4.0 μg kg−1 dose of adrenaline served as an indicator for the direction of subsequent doses of adrenaline to establish the arrhythmogenic dose, that is, lower or higher dose of adrenaline. This method reduces the number of adrenaline injections necessary to determine the arrhythmogenic dose. A period of 10–30 min was allowed between injections until the arterial blood pressure and heart rate became stable.
When the arrhythmias (three or more premature ventricular contractions within 15 s) occurred, a 2.0 ml arterial blood sample was collected for the measurement of the plasma concentration of adrenaline. The blood samples were put into precooled plastic tubes containing 20 μl of 0.2 M EDTA-2Na and 0.2 M Na2S2O5, which were centrifuged at 1300 g for 10 min at 4 °C to separate the plasma. For analysis of adrenaline, 0.5 ml plasma was acidified by the addition of 0.25 ml of 2.5% perchloric acid to precipitate protein. The samples were stored at −40 °C for no longer than 7 days, until analysis. The plasma concentration of adrenaline was determined in a fully automated HPLC-fluorimetric system (HLC-8030 Catecholamine Analyzer; Tosoh, Tokyo, Japan), by a diphenylethylenediamine condensation method. This assay method has a limit of sensitivity of 10 pg ml−1 for adrenaline, and the inter- and intra-assay variations were less than 3%.
Experimental protocols
Experiment 1: effects of rilmenidine on the halothane–adrenaline-induced arrhythmias (n=32)
We determined the arrhythmogenic dose and plasma concentration of adrenaline in the presence of rilmenidine at 0, 0.01, 0.05 and 0.1 μg kg−1. Rilmenidine or vehicle (rilmenidine=0) was administered intracisternally (i.c.). In each rat, a 30 G stainless steel needle was inserted into the cisterna magna through the atlanto-occipital membrane. The correct position of the cannula was checked by the efflux of clear cerebrospinal fluid. The first administration of adrenaline was started 30 min after the i.c. drug administration. In this study, the doses of rilmenidine were dissolved in a fixed volume of saline (10 μl).
Experiment 2: effects of vagus nerve activity on the antiarrhythmic effect of rilmenidine (n=16)
To determine the role of the vagus nerve and muscarinic receptors mediating the antiarrhythmic effect of centrally administered rilmenidine, we examined the effect of rilmenidine (0.1 μg kg−1, i.c.) in rats either bilaterally vagotomized or given atropine methylnitrate (5.0 mg kg−1, i.v.). Bilateral vagotomy was performed by sectioning both vagus nerves at the level of the fourth cervical vertebra. The dose of atropine methylnitrate we used was reported to block efferent vagal outflow to the heart (Langhans et al., 1985). In this study, atropine methylnitrate was given intravenously 15 min before rilmenidine administration. In our preliminary study, the arrhythmogenic doses of adrenaline in the vagotomized rats and atropine-treated rats were 1.89±0.76 and 1.97±0.58 μg kg−1 (mean±s.d.), respectively, and these data were not significantly different from the arrhythmogenic dose of adrenaline in the control (no rilmenidine treatment) rats (2.35±0.73 μg kg−1).
Experiment 3: the effect of calphostin C on the antiarrhythmic effect of rilmenidine (n=16)
To examine the role of PKC in the intracellular signalling pathway involving the antiarrhythmic effect of central rilmenidine, we examined the effect of calphostin C, an inhibitor of PKC, on the antiarrhythmic effect of central rilmenidine (0.1 μg kg−1, i.c.) or vehicle. Calphostin C (0.1 mg kg−1) in 0.04 ml dimethyl sulphoxide and 0.36 ml saline was given intravenously. At 10 min after the injection of calphostin C, we administered rilmenidine. Here, in our preliminary study we confirmed that the solvent for PKC used in this study (dimethyl sulphoxide in saline) did not affect the arrhythmogenic dose of adrenaline in the control rats. Two previous studies showed that this dose of calphostin C blocked PKC activity in ischaemic and pharmacological preconditioning studies using rats (Li and Kloner, 1995; Weber et al., 2005).
Experiment 4: the effect of KATP channel blockers on the antiarrhythmic effect of rilmenidine (n=27)
To examine the role of sarcolemmal and mitochondrial KATP channels in the antiarrhythmic action of rilmenidine, we administered HMR-1098 (3 mg kg−1) or 5-HD (10 mg kg−1) intravenously and then the arrhythmogenic dose and plasma concentration of adrenaline were determined in the presence of central rilmenidine (0.1 μg kg−1, i.c.) or vehicle. HMR-1098 or 5-HD was administered just before the i.c. administration of rilmenidine or vehicle. Two previous studies demonstrated that HMR-1098 (3 mg kg−1) and 5-HD (10 mg kg−1) were optimal dosages to determine the effectiveness of inhibiting either the sarcolemmal or mitochondrial KATP in rats (Fryer et al., 2000a, 2000b).
Experiment 5: the effect of atractyloside on the antiarrhythmic effect of rilmenidine (n=16)
To determine the role of MPTP in mediating the antiarrhythmic effect of central rilmenidine, we examined the effect of atractyloside (5 mg kg−1), the MPTP opener, on the antiarrhythmic effect of the i.c. rilmenidine (0.1 μg kg−1, i.c.) or vehicle. In this study, atractyloside was given intravenously 15 min before the central administration of rilmenidine or vehicle. The dose of atractyloside was reported to be pharmacologically effective in rats (Zhang et al., 2006; Tosaka et al., 2007).
Data analysis
All data were expressed as mean±s.d. For haemodynamic data (baseline, after pretreatment and after rilmenidine in Table 1 and 2), repeated-measures ANOVA was used to evaluate differences over time between groups. For all other data, one-way ANOVA was used. The multiple comparisons between groups were assessed by Tukey's test. P<0.05 was considered statistically significant.
Table 1.
Mean arterial blood pressure (mm Hg) at baseline (before any intervention), after pretreatment, after rilmenidine and with adrenaline (at the onset of arrhythmias)
| Dose of rilmenidine (μg kg−1, IC) | n | Baseline | After pretreatment | After rilmenidine | With adrenaline |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| 0 | 8 | 71±6 | 74±7 | 123±15 | |
| 0.01 | 8 | 69±10 | 73±11 | 114±31 | |
| 0.05 | 8 | 69±5 | 71±6 | 121±28 | |
| 0.1 | 8 | 70±7 | 71±5 | 126±37 | |
| Experiment 2: rilmenidine+vagotomy | |||||
| 0.1 | 8 | 69±10 | 71±8 | 71±12 | 124±12 |
| Experiment 2: rilmenidine+atropine methylnitrate | |||||
| 0.1 | 8 | 68±6 | 72±7 | 70±4 | 123±16 |
| Experiment 3: rilmenidine+calphostin C | |||||
| 0 | 8 | 74±12 | 74±7 | 72±7 | 116±9 |
| 0.1 | 8 | 71±8 | 72±8 | 70±6 | 121±15 |
| Experiment 4: rilmenidine+5-HD | |||||
| 0 | 7 | 77±11 | 73±9 | 72±5 | 128±12 |
| 0.1 | 7 | 67±10 | 69±9 | 70±10 | 120±14 |
| Experiment 4: rilmenidine+HMR-1098 | |||||
| 0 | 7 | 76±12 | 75±12 | 74±9 | 128±9 |
| 0.1 | 6 | 70±5 | 72±7 | 64±8 | 110±26 |
| Experiment 5: rilmenidine+atractyloside | |||||
| 0 | 8 | 77±9 | 78±15 | 74±15 | 131±8 |
| 0.1 | 8 | 77±13 | 77±11 | 72±12 | 130±11 |
Abbreviation: 5-HD, 5-hydroxydecanoic acid.
Data are mean (±s.d.) values from the number of assays shown.
Table 2.
Heart rate (b.p.m.) at baseline (before any intervention), after pretreatment, after rilmenidine and with adrenaline (at the onset of arrhythmias)
| Dose of rilmenidine (μg kg−1, IC) | n | Baseline | After pretreatment | After rilmenidine | With adrenaline |
|---|---|---|---|---|---|
| Experiment 1 | |||||
| 0 | 8 | 320±17 | 318±31 | 390±46 | |
| 0.01 | 8 | 317±29 | 318±30 | 405±52 | |
| 0.05 | 8 | 303±18 | 303±15 | 391±39 | |
| 0.1 | 8 | 307±43 | 289±43 | 400±42 | |
| Experiment 2:rilmenidine+vagotomy | |||||
| 0.1 | 8 | 327±16 | 354±26*,** | 338±17 | 442±39 |
| Experiment 2: rilmenidine+atropine methylnitrate | |||||
| 0.1 | 8 | 325±22 | 351±11*,** | 342±13 | 415±36 |
| Experiment 3: rilmenidine+calphostin C | |||||
| 0 | 8 | 311±39 | 323±31 | 317±34 | 392±23 |
| 0.1 | 8 | 301±50 | 313±43 | 318±26 | 385±35 |
| Experiment 4: rilmenidine+5-HD | |||||
| 0 | 7 | 312±32 | 319±26 | 324±27 | 399±55 |
| 0.1 | 7 | 325±14 | 320±25 | 319±44 | 372±33 |
| Experiment 4: rilmenidine+HMR-1098 | |||||
| 0 | 7 | 316±33 | 333±33 | 349±38 | 400±62 |
| 0.1 | 6 | 320±14 | 327±21 | 328±20 | 443±48 |
| Experiment 5: rilmenidine+atractyloside | |||||
| 0 | 8 | 312±13 | 313±25 | 318±30 | 386±67 |
| 0.1 | 8 | 290±18 | 307±40 | 297±36 | 348±47 |
Abbreviation: 5-HD, 5-hydroxydecanoic acid.
*P<0.05, compared with the value at baseline.
**P<0.05, compared with corresponding value without rilmenidine.
Data are mean (±s.d.) values from the number of assays shown.
Drugs
HMR-1098 was a kind gift from Aventis Pharmaceuticals Inc. (Frankfurt, Germany). Other chemicals were purchased from the sources indicated; halothane (Takeda Chemical, Osaka, Japan), rilmenidine (Tocris, Bristol, UK), adrenaline (Wako Chemical, Osaka, Japan), atropine methylnitrate, calphostin C, 5-HD and atractyloside (all from Sigma Chemical Co., St Louis, MO, USA).
Results
Haemodynamic data before administration of rilmenidine are shown in Table 1 and 2. Vagotomy and administration of atropine methylnitrate significantly increased heart rate, but not blood pressure and none of the other compounds we tested exerted any significant effect on blood pressure or heart rate following administration (Table 1 and 2).
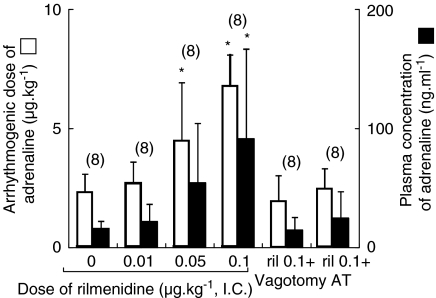
Rilmenidine significantly increased the arrhythmogenic dose and the plasma concentration of adrenaline in a dose-dependent manner (Figure 1). This antiarrhythmic effect of rilmenidine (0.1 μg kg−1, i.c.) was abolished in the vagotomized and atropine methylnitrate-treated rats (Figure 1). The haemodynamic data obtained at the onset of arrhythmias in the presence of central rilmenidine show that rilmenidine did not affect mean arterial pressure or heart rate at the arrhythmias in intact, vagotomized or atropine-methylnitrate-treated rats (Table 1 and 2).
Figure 1.
Arrhythmogenic dose and plasma concentration of adrenaline in the presence of intracisternal (i.c.) rilmenidine (0, 0.01, 0.05 and 0.1 μg kg−1) in the intact, bilaterally vagotomized or atropine methylnitrate (5 mg kg−1, i.v.)-treated rats during halothane anaesthesia. The values are expressed as mean±s.d. and the number of observations is shown in parentheses. *P<0.05, compared with the rilmenidine 0 μg kg−1 value. Ril 0.1+vagotomy: i.c. rilmenidine 0.1 μg kg−1 and bilateral vagotomy. Ril 0.1+AT: i.c. rilmenidine 0.1 μg kg−1 and atropine methylnitrate (5 mg kg−1).
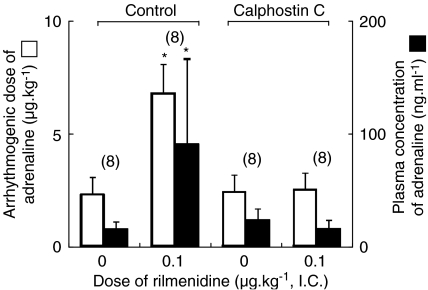
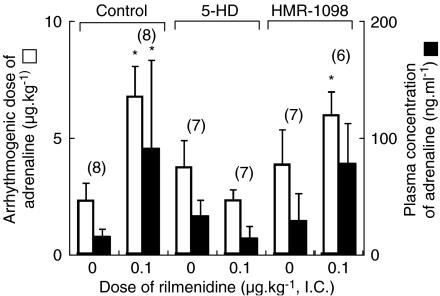
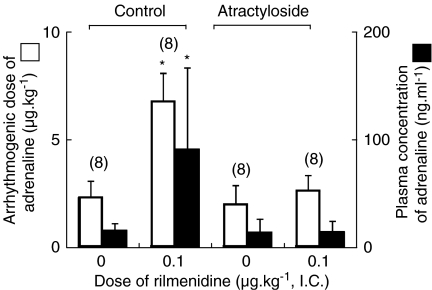
Calphostin C did not significantly alter the arrhythmogenic thresholds of adrenaline during halothane anaesthesia in the absence of rilmenidine treatment (rilmenidine=0), but abolished the antiarrhythmic effect of central rilmenidine (0.1 μg kg−1, i.c.) (Figure 2). Both HMR-1098 and 5-HD did not significantly affect the arrhythmogenic thresholds of adrenaline during halothane anaesthesia, in the absence of rilmenidine treatment (rilmenidine=0), although both HMR-1098 and 5-HD tended to increase the arrhythmogenic dose of adrenaline (P=0.083 and 0.127, respectively) (Figure 3). In the presence of HMR-1098, central administration of rilmenidine significantly increased the arrhythmogenic dose of adrenaline (P=0.012), whereas it did not significantly increase the plasma concentration of adrenaline (P=0.189) (Figure 3). On the other hand, central rilmenidine did not increase the arrhythmogenic dose or plasma concentration of adrenaline in the presence of 5-HD (Figure 3). Pretreatment with atractyloside did not affect the genesis of halothane–adrenaline arrhythmias in the absence of rilmenidine treatment, but the antiarrhythmic action of rilmenidine was abolished in the presence of atractyloside (Figure 4). The haemodynamic data obtained at the onset of arrhythmias in the calphostin C, HMR-1098, 5-HD and atractyloside studies are summarized in Table 1 and 2. There was no significant difference in mean arterial pressure or heart rate during the arrhythmias.
Figure 2.
The effect of calphostin C on arrhythmogenic dose and plasma concentration of adrenaline in the presence of intracisternal (i.c.) rilmenidine (0 and 0.1 μg kg−1) during halothane anaesthesia. The values are expressed as mean±s.d. and the number of observations is shown in parentheses. *P<0.05, compared with the rilmenidine 0 μg kg−1 value in each treatment. Control: no calphostin C pretreatment. The data are quoted from Figure 1. Calphostin C: calphostin C pretreatment.
Figure 3.
The effect of 5-hydroxydecanoic acid (5-HD) and HMR-1098 on arrhythmogenic dose and plasma concentration of adrenaline in the presence of intracisternal (i.c.) rilmenidine (0 and 0.1 μg kg−1) during halothane anaesthesia. The values are expressed as mean±s.d. and the number of observations is shown in parentheses. *P<0.05, compared with the rilmenidine 0 μg kg−1 value in each treatment. Control: no ATP-sensitive potassium channel blocker pretreatment. The data are quoted from Figure 1. 5-HD: 5-HD pretreatment. HMR-1098: HMR-1098 pretreatment.
Figure 4.
The effect of atractyloside on arrhythmogenic dose and plasma concentration of adrenaline in the presence of intracisternal (i.c.) rilmenidine (0 and 0.1 μg kg−1) during halothane anaesthesia. The values are expressed as mean±s.d. and the number of observations is shown in parentheses. *P<0.05, compared with the rilmenidine 0 μg kg−1 value in each treatment. Control: no atractyloside pretreatment. The data are quoted from Figure 1. Atractyloside: atractyloside pretreatment.
Discussion
The results of the present study confirm and extend our previous findings (Hayashi et al., 1991; Kamibayashi et al., 1995; Mammoto et al., 1996; Kagawa et al., 2005), indicating that the antiarrhythmic effect of centrally administered rilmenidine is exerted through a stimulation of the vagus nerve and activation of muscarinic receptors. Intracellular signalling elements involved in the antiarrhythmic effect of central rilmenidine included PKC, mitochondrial KATP and MPTP.
The role of the imidazoline receptors in the cardiovascular regulation of the central nervous system was originally described by Bousquet et al. (1984) who showed that imidazoline receptors were involved in the hypotensive effect of clonidine, which was originally believed to activate central α2-adrenoceptors (Tibirica et al., 1991). It has been suggested that stimulation of imidazoline receptors facilitates a sympatho-inhibitory effect on various organs, including the heart (Gothert and Molderings, 1992; Chan et al., 1996). We and others have previously reported that rilmenidine and moxonidine, imidazoline and α2-adrenoceptor agonists, prevent several types of arrhythmias including halothane–adrenaline arrhythmias through activation of the central imidazoline receptor (Lepran and Papp, 1994; Mest et al., 1995; Mammoto et al., 1996; Yu and Frishman, 1996; Poisson et al., 2000; Kagawa et al., 2005). We have also demonstrated that stimulation of the vagus nerve plays an important role in this mechanism (Mammoto et al., 1996). Many previous investigations have demonstrated that activity of the vagus nerve was protective against some types of arrhythmias, including arrhythmias after coronary ligation and digitalis-induced arrhythmias (Garan et al., 1981; Furey and Levy, 1983; Rardon and Bailey, 1983). Similarly, stimulation of the vagus nerve also suppresses halothane–adrenaline arrhythmias (Zink et al., 1975; Waxman et al., 1989). However, the detailed intracellular signalling pathways that were involved following vagal stimulation were not well elucidated. ACh is the major neurotransmitter found in vagus nerve terminals and its effects on myocardial function are mediated through activation of muscarinic receptors. The present results showed that atropine methylnitrate abolished the antiarrhythmic effect of rilmenidine, indicating that activation of muscarinic receptors are involved in the effect of central rilmenidine.
Several studies have shown that ACh produced a reduction of infarction size after myocardial ischaemia and that this protective mechanism mimicked preconditioning (Yao and Gross, 1993; Cohen et al., 2001). As PKC is one of the key intracellular elements involved in preconditioning (Opie, 2001; Yellon and Downey, 2003), we examined whether PKC was involved in the antiarrhythmic effect of rilmenidine. Calphostin C is a potent and specific inhibitor of PKC and the dose of calphostin C used in the present study was shown to be sufficient to inhibit the PKC activity in previous studies (Li and Kloner, 1995; Weber et al., 2005). In our study, calphostin C did not affect the genesis of halothane–adrenaline arrhythmias, suggesting that PKC was not involved in sensitization of the myocardium to adrenaline by halothane. In contrast, calphostin C significantly attenuated the antiarrhythmic effect of rilmenidine implying an important role for activation of PKC in this effect of rilmenidine.
Another important component of ischaemic and pharmacological preconditioning is the KATP channels and these channels may serve as the end effector (Oldenburg et al., 2002a; Yellon and Downey, 2003; Tanaka et al., 2004), although a recent review questioned the significance of KATP in preconditioning (Halestrap et al., 2007). Both sarcolemmal and mitochondrial KATP channels are considered to be involved in preconditioning and recent reports suggest a predominant contribution from mitochondrial KATP (Fryer et al., 2000a, 2000b; Bouwman et al., 2004). We examined the role of sarcolemmal and mitochondrial KATP in the antiarrhythmic effect of rilmenidine by using HMR-1098 or 5-HD at doses designed to be optimal in inhibiting either the sarcolemmal (HMR-1098) or mitochondrial KATP (5-HD) channels in rats (Fryer et al., 2000a, 2000b). The present results clearly showed that 5-HD abolished antiarrhythmic effect of rilmenidine, but the results of HMR-1098 were somewhat confusing (Figure 3). First, the results of two arrhythmogenic thresholds (arrhythmogenic dose and plasma concentration of adrenaline) were not identical in the presence of HMR-1098. Thus, although rilmenidine still significantly increased the arrhythmogenic dose of adrenaline (P=0.012), the accompanying increase in plasma concentration of adrenaline did not reach statistical significance (P=0.189). It is possible that this discrepancy may be due to wider variation of each value of the plasma concentration of adrenaline. Thus, we may deduce that HMR-1098 may not completely abolish the antiarrhythmic effect of rilmenidine. Second, both 5-HD and HMR-1098 tended to have antiarrhythmic activity in the absence of rilmenidine, although this tendency was not statistically significant. Several ion channel blockers, including K+ channel blockers, generally have potent antiarrhythmic action (Ito et al., 2006), so it is not surprising that KATP blockers could exert some antiarrhythmic effect. However, this tendency in the HMR-1098 study might add to the antiarrhythmic potential of rilmenidine, allowing a significant antiarrhythmic effect without a significant change in the plasma concentration of adrenaline.
The present results did clearly show that 5-HD prevented the antiarrhythmic effect of rilmenidine, suggesting that the mitochondrial KATP was involved in the antiarrhythmic effect of rilmenidine. Here, we have to mention the recent evidence questioning the potential role of KATP in preconditioning and the specificity of 5-HD as a mitochondrial KATP blocker (Halestrap et al., 2007). Thus, we should acknowledge that this interpretation of our data, that is, that the mitochondrial KATP channels were involved in the antiarrhythmic effect of rilmenidine, could be reconsidered in the future.
A recent review on preconditioning describes that prevention of opening of the MPTP is a key mechanism involved in preconditioning (Halestrap et al., 2007). The opening of MPTP has been found to facilitate arrhythmias following reperfusion (García-Rivas et al., 2006). Atractyloside is an MPTP opener and the dose of atractyloside used in the present study was reported to be pharmacologically effective in rats (Zhang et al., 2006; Tosaka et al., 2007). Our findings that atractyloside did not affect the genesis of halothane–adrenaline arrhythmias suggest that MPTP is not involved in the myocardial sensitization to adrenaline by halothane. Nevertheless, atractyloside significantly prevented the antiarrhythmic effect of rilmenidine. Thus, inhibition of opening of MPTP may contribute to the antiarrhythmic effect of rilmenidine.
Haemodynamic variables, including arterial blood pressure and heart rate, are known to be important factors in modulating the genesis of halothane–adrenaline arrhythmias (Atlee and Bosnjak, 1990). Treatment with calphostin C, HMR-1098, 5-HD or atractyloside did not have a significant effect on haemodynamic data before the administration of rilmenidine, although vagotomy and administration of atropine methylnitrate significantly increased heart rate (Table 1 and 2). Thus, calphostin C, HMR-1098, 5-HD or atractyloside may not affect genesis of the adrenaline-induced arrhythmias through modulation of haemodynamic variables. On the other hand, at the onset of arrhythmias, haemodynamic variables in the rilmenidine-treated rats were not significantly different from those of the control rats, despite a larger plasma adrenaline concentration (Table 1 and 2, and Figure 1). Similarly, other treatment groups did not significantly change the haemodynamic data at the arrhythmias (Table 1 and 2). Our previous study using dogs have also shown similar results, that is, intravenous rilmenidine increased the arrhythmogenic dose and plasma concentration of adrenaline at the onset of arrhythmias without significant change of haemodynamic data (Mammoto et al., 1996). This might suggest that rilmenidine inhibited the positive inotropic and chronotropic action of adrenaline by inhibiting sympathetic nerve activity, through imidazoline receptors (Chan et al., 1996).
Imidazoline receptors are predominantly located in the rostral ventrolateral medulla oblongata and the C1 area of this structure is considered to be the site involved in the hypotensive effect of rilmenidine (Gomez et al., 1991; Chan et al., 2005). The vagus nerve and myocardial ACh-activated K+ channels are also involved in this action (Yamada, 2002). The present study also showed that stimulation of the vagus nerve is critical and other intracellular components, including muscarinic receptors, PKC, mitochondrial KATP and MPTP may also contribute importantly to the final antiarrhythmic effect of rilmenidine. It is not clear whether the same intracellular components contribute to the cardiovascular effects of rilmenidine, such as hypotension and bradycardia.
The results of the present study should be interpreted with caution. We have examined the effect of each specific inhibitor on the antiarrhythmic property of rilmenidine to elucidate signal transduction pathways involved in the effect of rilmenidine and have documented several intracellular components involved, including muscarinic receptors, PKC, mitochondrial KATP and MPTP. However, the present data does not demonstrate the precise linking of these components in the signal transduction mechanism of the antiarrhythmic effect of rilmenidine. Yao and Gross (1993) have reported that activation of muscarinic receptors by ACh may mimic a preconditioning state and facilitate cardioprotection. Thus, we deduce that intracellular mechanisms similar to those involved in preconditioning may also be involved in the antiarrhythmic effect of rilmenidine.
The present data, that is activation of PKC, opening of mitochondrial KATP and inhibition of MPTP, contributed to the antiarrhythmic effect of rilmenidine may have clinical relevance. These components are known to facilitate preconditioning to ischaemic insults (Oldenburg et al., 2002a; Yellon and Downey, 2003; Halestrap et al., 2007) And thus, the action of rilmenidine to induce preconditioning-like, as well as antiarrhythmic, effects could be a justification for the use of imidazoline receptor agonists as an adjuvant for clinical anaesthesia.
In conclusion, the present results suggest that muscarinic receptors, PKC, mitochondrial KATP and MPTP may be crucial components of the mechanism involved in the antiarrhythmic effect of centrally administered rilmenidine.
Acknowledgments
This study was supported by grant-in-aid for Scientific Research (B) from the Minister of Education, Science, and Culture in Japan.
Abbreviations
- 5-HD
5-hydroxydecanoic acid
- i.c.
intracisternally
- KATP
ATP-sensitive potassium channel
- MPTP
mitochondrial permeability transition pore
- PKC
protein kinase C
Conflict of interest
The authors state no conflict of interest.
References
- Atlee JL, III, Bosnjak ZJ. Mechanisms for cardiac dysrhythmias during anesthesia. Anesthesiology. 1990;72:347–374. doi: 10.1097/00000542-199002000-00022. [DOI] [PubMed] [Google Scholar]
- Bousquet P. I1 receptors, cardiovascular function, and metabolism. Am J Hypertens. 2001;14:317S–321S. doi: 10.1016/s0895-7061(01)02238-5. [DOI] [PubMed] [Google Scholar]
- Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984;230:232–236. [PubMed] [Google Scholar]
- Bouwman RA, Musters RJ, van Beek-Harmsen BJ, de Lange JJ, Boer C. Reactive oxygen species precede protein kinase C-delta activation independent of adenosine triphosphate-sensitive mitochondrial channel opening in sevoflurane-induced cardioprotection. Anesthesiology. 2004;100:506–514. doi: 10.1097/00000542-200403000-00008. [DOI] [PubMed] [Google Scholar]
- Chan CK, Burke SL, Zhu H, Piletz JE, Head GA. Imidazoline receptors associated with noradrenergic terminals in the rostral ventrolateral medulla mediate the hypotensive responses of moxonidine but not clonidine. Neuroscience. 2005;132:991–1007. doi: 10.1016/j.neuroscience.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Chan CK, Sannajust F, Head GA. Role of imidazoline receptors in the cardiovascular actions of moxonidine, rilmenidine and clonidine in conscious rabbits. J Pharmacol Exp Ther. 1996;276:411–420. [PubMed] [Google Scholar]
- Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Eells JT, Hsu AK, Henry MM, Gross GJ. Ischemic preconditioning in rats: role of mitochondrial K(ATP) channel in preservation of mitochondrial function. Am J Physiol Heart Circ Physiol. 2000a;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Hsu AK, Nagase H, Gross GJ. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J Pharmacol Exp Ther. 2000b;294:451–457. [PubMed] [Google Scholar]
- Furey SA, III, Levy MN. Interactions among heart rate, autonomic activity, and arterial pressure upon the multiple repetitive extrasystole threshold in the dog. Am Heart J. 1983;106:1112–1120. doi: 10.1016/0002-8703(83)90660-9. [DOI] [PubMed] [Google Scholar]
- Garan H, Ruskin JN, Powell WJ., Jr Centrally mediated effect of phenytoin on digoxin-induced ventricular arrhythmias. Am J Physiol. 1981;241:H67–H72. doi: 10.1152/ajpheart.1981.241.1.H67. [DOI] [PubMed] [Google Scholar]
- García-Rivas GJ, Carvajal K, Correa F, Zazueta C. Ru360, a specific mitochondrial calcium uptake inhibitor, improves cardiac post-ischaemic functional recovery in rats in vivo. Br J Pharmacol. 2006;149:829–837. doi: 10.1038/sj.bjp.0706932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavras I, Manolis AJ, Gavras H. The alpha2-adrenergic receptors in hypertension and heart failure: experimental and clinical studies. J Hypertens. 2001;19:2115–2124. doi: 10.1097/00004872-200112000-00001. [DOI] [PubMed] [Google Scholar]
- Gomez RE, Ernsberger P, Feinland G, Reis DJ. Rilmenidine lowers arterial pressure via imidazole receptors in brainstem C1 area. Eur J Pharmacol. 1991;195:181–191. doi: 10.1016/0014-2999(91)90534-w. [DOI] [PubMed] [Google Scholar]
- Gothert M, Molderings GJ. Modulation of norepinephrine release in blood vessels: mediation by presynaptic imidazoline receptors and α[2]-adrenoceptors. J Cardiovasc Pharmacol. 1992;20 Suppl 4:S16–S20. [Google Scholar]
- Halestrap AP, Clarke SJ, Khaliulin CI. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Sumikawa K, Maze M, Yamatodani A, Kamibayashi T, Kuro M, et al. Dexmedetomidine prevents epinephrine-induced arrhythmias through stimulation of central alpha 2 adrenoceptors in halothane-anesthetized dogs. Anesthesiology. 1991;75:113–117. doi: 10.1097/00000542-199107000-00018. [DOI] [PubMed] [Google Scholar]
- Ito I, Hayashi Y, Kawai Y, Iwasaki M, Takada K, Kamibayashi T, et al. Diabetes mellitus reduces the antiarrhythmic effect of ion channel blockers. Anesth Analg. 2006;103:545–550. doi: 10.1213/01.ane.0000229709.29185.88. [DOI] [PubMed] [Google Scholar]
- Kagawa K, Hayashi Y, Itoh I, Iwasaki M, Takada K, Kamibayashi T, et al. Identification of the central imidazoline receptor subtype involved in modulation of halothane–epinephrine arrhythmias in rats. Anesth Analg. 2005;101:1689–1694. doi: 10.1213/01.ANE.0000184185.69471.F6. [DOI] [PubMed] [Google Scholar]
- Kamibayashi T, Hayashi Y, Mammoto T, Yamatodani A, Sumikawa K, Yoshiya I. Role of the vagus nerve in the antidysrhythmic effect of dexmedetomidine on halothane/epinephrine dysrhythmias in dogs. Anesthesiology. 1995;83:992–999. doi: 10.1097/00000542-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Kawai Y, Hayashi Y, Ito I, Kamibayashi T, Takada K, Kagawa K, et al. Nicorandil prevents epinephrine-induced arrhythmias in halothane-anesthetized rats by nitric oxide-dependent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:522–527. doi: 10.1007/s00210-002-0644-9. [DOI] [PubMed] [Google Scholar]
- Kita H, Miura T, Tsuchida A, Hasegawa T, Shimamoto K. Suppression of reperfusion arrhythmias by preconditioning is inhibited by an ATP-sensitive potassium channel blocker, 5-hydroxydecanoate, but not by protein kinase C blockers in the rat. J Cardiovasc Pharmacol. 1998;32:791–797. doi: 10.1097/00005344-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Langhans W, Egli G, Scharrer E. Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J Auton Nerv Syst. 1985;13:255–262. doi: 10.1016/0165-1838(85)90014-1. [DOI] [PubMed] [Google Scholar]
- Lepran I, Papp JG. Effect of moxonidine on arrhythmias induced by coronary artery occlusion and reperfusion. J Cardiovasc Pharmacol. 1994;24 Suppl 1:S9–S15. doi: 10.1097/00005344-199424001-00003. [DOI] [PubMed] [Google Scholar]
- Li F, Wu N, Su RB, Zheng JQ, Xu B, Lu XQ, et al. Involvement of phosphatidylcholine-selective phospholipase C in activation of mitogen-activated protein kinase pathways in imidazoline receptor antisera-selected protein. J Cell Biochem. 2006;98:1615–1628. doi: 10.1002/jcb.20806. [DOI] [PubMed] [Google Scholar]
- Li Y, Kloner RA. Does protein kinase C play a role in ischemic preconditioning in rat hearts. Am J Physiol. 1995;268:H426–H431. doi: 10.1152/ajpheart.1995.268.1.H426. [DOI] [PubMed] [Google Scholar]
- Lukas A, Botsford MW. Cardioprotection induced by ischemic preconditioning in the mammalian heart: effects on arrhythmogenesis. Can J Physiol Pharmacol. 1997;75:316–325. [PubMed] [Google Scholar]
- Mammoto T, Kamibayashi T, Hayashi Y, Yamatodani A, Takada K, Yoshiya I. Antiarrhythmic action of rilmenidine on adrenaline-induced arrhythmia via central imidazoline receptors in halothane-anaesthetized dogs. Br J Pharmacol. 1996;117:1744–1748. doi: 10.1111/j.1476-5381.1996.tb15348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura K, Komori S, Takusagawa M, Osada M, Tanabe F, Itoh M, et al. Protein kinase C is involved in cardioprotective effects of ischemic preconditioning on infarct size and ventricular arrhythmia in rats in vivo. Mol Cell Biochem. 2000;214:39–45. doi: 10.1023/a:1007119622322. [DOI] [PubMed] [Google Scholar]
- Mest HJ, Thomsen P, Raap A. Antiarrhythmic effect of the selective I1-imidazoline receptor modulator moxonidine on ouabain-induced cardiac arrhythmia in guinea pigs. Ann NY Acad Sci. 1995;763:620–633. doi: 10.1111/j.1749-6632.1995.tb32457.x. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;174:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Cohen MV, Yellon DM, Downer JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002a;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Qin Q, Sharma AR, Cohen MV, Downer JM, Benoit JN. Acetylcholine leads to free radical production dependent on K(ATP) channels, G(i) proteins, phosphatidylinositol 3-kinase and tyrosine kinase. Cardiovasc Res. 2002b;55:544–552. doi: 10.1016/s0008-6363(02)00332-2. [DOI] [PubMed] [Google Scholar]
- Opie LH.Mechanisms of cardiac contraction and relaxation Heart Disease 2001WB Saunders Company: Philadelphia; 443–478.In: Braunwald E, Zipes DP, Libby P (eds)6th edn [Google Scholar]
- Poisson D, Christen MO, Sannajust F. Protective effects of I(1)-antihypertensive agent moxonidine against neurogenic cardiac arrhythmias in halothane-anesthetized rabbits. J Pharmacol Exp Ther. 2000;293:929–938. [PubMed] [Google Scholar]
- Rardon DP, Bailey JC. Parasympathetic effects on electrophysiologic properties of cardiac ventricular tissue. J Am Coll Cardiol. 1983;2:1200–1209. doi: 10.1016/s0735-1097(83)80351-9. [DOI] [PubMed] [Google Scholar]
- Takada K, Hayashi Y, Kamibayashi T, Mammoto T, Yamatodani A, Kitamura S, et al. The involvement of pertussis toxin-sensitive G proteins in the post receptor mechanism of central I1-imidazoline receptors. Br J Pharmacol. 1997;120:1575–1581. doi: 10.1038/sj.bjp.0701090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K, Sumikawa K, Kamibayashi T, Hayashi Y, Yamatodani A, Kawabata K, et al. Comparative efficacy of antiarrhythmic agents in preventing halothane–epinephrine arrhythmias in rats. Anesthesiology. 1993;79:563–570. doi: 10.1097/00000542-199309000-00021. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ludwig LM, Kersten JR, Pagel PS, Warltier DC. Mechanisms of cardioprotection by volatile anesthetics. Anesthesiology. 2004;100:707–721. doi: 10.1097/00000542-200403000-00035. [DOI] [PubMed] [Google Scholar]
- Tibirica E, Feldman J, Mermet C, Gonon F, Bousquet P. An imidazoline-specific mechanism for the hypotensive effect of clonidine: a study with yohimbine and idazoxan. J Pharmacol Exp Ther. 1991;256:606–613. [PubMed] [Google Scholar]
- Tosaka S, Makita T, Tosaka R, Maekawa T, Cho S, Hara T, et al. Cardioprotection induced by olprinone, a phosphodiesterase III inhibitor, involved phosphatidylinositol-OH kinase-Akt and a mitochondorial permeability transition pore during early reperfusion. J Anesth. 2007;21:176–180. doi: 10.1007/s00540-006-0485-7. [DOI] [PubMed] [Google Scholar]
- Vegh A, Parratt JR. The role of mitochondrial KATP channels in antiarrhythmic effects of ischemic preconditioning in dogs. Br J Pharmacol. 2002;137:1107–1115. doi: 10.1038/sj.bjp.0704966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman MB, Sharma AD, Asta J, Cameron DA, Wald RW. The protective effect of vagus nerve stimulation on catecholamine–halothane-induced ventricular fibrillation in dogs. Can J Physiol Pharmacol. 1989;67:801–809. doi: 10.1139/y89-127. [DOI] [PubMed] [Google Scholar]
- Weber NC, Toma O, Wolter JI, Obal D, Mullenheim J, Preckel B, et al. The noble gas xenon induces pharmacological preconditioning in the rat heart in vivo via induction of PKC-epsilon and p38 MAPK. Br J Pharmacol. 2005;144:123–132. doi: 10.1038/sj.bjp.0706063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada MJ. The role of muscarinic K(+) channels in the negative chronotropic effect of a muscarinic agonist. J Pharmacol Exp Ther. 2002;300:681–687. doi: 10.1124/jpet.300.2.681. [DOI] [PubMed] [Google Scholar]
- Yao Z, Gross GJ. Role of nitric oxide, muscarinic receptors, and the ATP-sensitive K+ channel in mediating the effects of acetylcholine to mimic preconditioning in dogs. Circ Res. 1993;73:1193–1201. doi: 10.1161/01.res.73.6.1193. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- Yu A, Frishman WH. Imidazoline receptor agonist drugs: a new approach to the treatment of systemic hypertension. J Clin Pharmacol. 1996;36:98–111. doi: 10.1002/j.1552-4604.1996.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Abdel-Rahman AA. Mitogen-activated protein kinase phosphorylation in the rostral ventrolateral medulla plays a key role in imidazoline (i1)-receptor-mediated hypotension. J Pharmacol Exp Ther. 2005;314:945–952. doi: 10.1124/jpet.105.087510. [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH, Bruce IC, et al. κ-Opioid receptors mediate cardioprotection by remote preconditioning. Anesthesilogy. 2006;105:550–556. doi: 10.1097/00000542-200609000-00019. [DOI] [PubMed] [Google Scholar]
- Zink J, Sasyniuk BI, Dresel PE. Halothane–epinephrine-induced cardiac arrhythmias and the role of heart rate. Anesthesiology. 1975;43:548–555. doi: 10.1097/00000542-197511000-00012. [DOI] [PubMed] [Google Scholar]