Abstract
Background and purpose: Antimalarial compounds have been previously shown to inhibit rodent nicotinic acetylcholine (nACh) and 5-HT3 receptors. Here, we extend these studies to include human 5-HT3A, 5-HT3AB, GABAA α1β2, GABAA α1β2γ2 and GABAC ρ1 receptors.
Experimental approach: We examined the effects of quinine, chloroquine and mefloquine on the electrophysiological properties of receptors expressed in Xenopus oocytes.
Key results: 5-HT3A receptor responses were inhibited by mefloquine, quinine and chloroquine with IC50 values of 0.66, 1.06 and 24.3 μM. At 5-HT3AB receptors, the potencies of mefloquine (IC50=2.7 μM) and quinine (15.8 μM), but not chloroquine (23.6 μM), were reduced. Mefloquine, quinine and chloroquine had higher IC50 values at GABAA α1β2 (98.7, 0.40 and 0.46 mM, respectively) and GABAA α1β2γ2 receptors (0.38, 1.69 and 0.67 mM, respectively). No effect was observed at GABAC ρ1 receptors. At all 5-HT3 and GABAA receptors, chloroquine displayed competitive behaviour and mefloquine was non-competitive. Quinine was competitive at 5-HT3A and GABAA receptors, but non-competitive at 5-HT3AB receptors. Homology modelling in combination with automated docking suggested orientations of quinine and chloroquine at the GABAA receptor binding site.
Conclusions and implications: The effects of mefloquine, quinine and chloroquine are distinct at GABAA and GABAC receptors, whereas their effects on 5-HT3AB receptors are broadly similar to those at 5-HT3A receptors. IC50 values for chloroquine and mefloquine at 5-HT3 receptors are close to therapeutic blood concentrations required for malarial treatment, suggesting that their therapeutic use could be extended to include the treatment of 5-HT3 receptor-related disorders.
Keywords: serotonin receptor, GABA, Cys-loop, binding site, ligand docking, malaria, quinine, chloroquine, mefloquine, antagonist
Introduction
For decades, quinine, chloroquine and mefloquine (Lariam) have provided an economical and effective approach for the treatment and prevention of malaria. It is thought that these compounds prevent the growth of parasites within infected erythrocytes, although the biochemistry of their therapeutic actions is not entirely known, and different compounds seem to have different modes of action (Bray et al., 2005; Uhlemann et al., 2005). These compounds have a good clinical record, with relatively few side effects reported. However, there is experimental evidence that they may affect the nervous system, as they have been shown to inhibit neurotransmission between nerves and at the neuromuscular junction (Sieb et al., 1996; Ballestero et al., 2005). In particular, there are detailed pharmacological accounts of competitive and non-competitive behaviour at human nicotinic acetylcholine (nACh) α9α10 and mouse 5-HT3 (type 3 serotonin) receptors (Ballestero et al., 2005; Thompson and Lummis, 2007a). Given the strong structural and functional similarities between these receptors, it is possible that these effects will extend across species and to other members of this receptor family.
5-HT3 and nACh receptors are members of a family of ligand-gated ion channels that are responsible for fast excitatory and inhibitory neurotransmission in the central and peripheral nervous systems. The group is known as the Cys-loop family and also includes GABAA and glycine receptors. These receptors function as pentamers and have a subunit arrangement that can be either homomeric or heteromeric. Five 5-HT3 receptor subunits (A–E) have been identified to date; the A subunit can form functional homomeric receptors, whereas subunits B–E only function as heteromeric receptors in combination with the A subunit (Davies et al., 1999; Niesler et al., 2003, 2007). In the human nervous system, 5-HT3A subunits have been found throughout the adult brain, but are also widely distributed in internal organs and extraneuronal cells such as monocytes, T cells, synovial tissue and primary chondrocytes (Miyake et al., 1995; Fiebich et al., 2004). 5-HT3B receptor subunits are not as widespread, but are still detectable throughout the adult brain, kidney and intestine (Davies et al., 1999; Dubin et al., 1999; Niesler et al., 2003; Tzvetkov et al., 2007). In contrast to the 5-HT3 receptor, GABAA receptor stoichiometry is more complicated and 16 different subunit types can potentially combine to form functional pentameric receptors (Barnard et al., 1998; Akabas, 2004). The most common subunits are α1, β2 and γ2, all of which are abundantly expressed throughout the nervous system (Akabas, 2004). GABAC receptors are classified as a subtype of the GABAA receptors, and GABAC ρ1, ρ2 and ρ3 subunits can combine as homomeric or heteromeric complexes. They are largely restricted to retinal bipolar cells, but have been observed at low densities in various regions of the brain (Cutting et al., 1991; Enz and Cutting, 1999).
The five subunits that constitute a functional receptor of the Cys-loop family are symmetrically arranged around a central ion-conducting pore and each subunit consists of three domains (Thompson and Lummis, 2007b). The N-terminal domain is located at the extracellular side of the membrane and is responsible for ligand binding. Structural details of the extracellular domain of nACh receptors have been revealed from cryo-electron microscopy and a more recent crystal structure of an nACh receptor monomer, but the most significant improvement in our understanding of this domain has come from high-resolution crystallography studies of the acetylcholine binding protein (AChBP) pentamer (Brejc et al., 2001; Unwin, 2005; Dellisanti et al., 2007). AChBP shares structural homology with the extracellular domain of Cys-loop receptors, and has confirmed previous biochemical studies that suggested that the ligand binding site is formed by six loops that converge at the interface of two adjacent subunits (Brejc et al., 2001). Using AChBP crystal structures as templates for computer-generated homology models, a number of researchers have docked ligands into the extracellular domain of a range of Cys-loop receptors, enabling potential receptor–ligand interactions to be identified and experimentally tested (reviewed by Thompson and Lummis, 2006a, 2007b).
In a previous study, we provided a detailed account of the actions of antimalarial compounds at mouse 5-HT3 receptors and performed automated docking of antimalarial compounds into homology models of this receptor (Thompson and Lummis, 2007a). In this paper, we extend these findings by reporting the effects of quinine, chloroquine and mefloquine on human 5-HT3A and 5-HT3AB receptors as well as providing the first direct evidence for their effects on human GABAA receptors.
Methods
Cell culture and oocyte maintenance
Xenopus laevis oocyte-positive females were purchased from NASCO (Fort Atkinson, WI, USA) and maintained according to standard methods (Goldin, 1992). Harvested stage e–f Xenopus oocytes were washed in four changes of ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5), de-folliculated in 1.5 mg ml−1 collagenase Type 1A for approximately 2 h, washed again in four changes of ND96 and stored in ND96 containing 2.5 mM sodium pyruvate, 50 mM gentamycin and 0.7 mM theophylline.
Receptor expression
Human 5-HT3A (accession number P46098), 5-HT3B (AF080582), GABAA α1 (P14867), GABAA β2 (P47870), GABAA γ2 (P18507) and GABAC ρ1 (P24046) subunit cDNA was cloned into pGEMHE for oocyte expression (Liman et al., 1992). cRNA was in vitro transcribed from linearized plasmid cDNA template using the mMessage mMachine T7 Transcription kit (Ambion, Austin, TX, USA). 5-HT3B, GABAA α1, GABAA β2, GABAA γ2 and GABAC ρ1 subunit cDNA was linearized with NheI and 5-HT3A was linearized using SphI. Stage e and f oocytes were injected with 50 nl of 100–700 ng μl−1 cRNA (5–35 ng injected) and currents were recorded 1–4 days after injection. A ratio of 1:3 (A:B) was used for the expression of heteromeric 5-HT3 receptors. GABAA subunits were expressed in the ratio 1:1 (α1:β2) or 1:1:10 (α1:β2:γ2).
Electrophysiology
Using two-electrode voltage clamp, Xenopus oocytes were clamped at −60 mV using an OC-725 amplifier (Warner Instruments, Hamden, CT, USA), Digidata 1322A and the Strathclyde Electrophysiology Software Package (Department of Physiology and Pharmacology, University of Strathclyde, UK; http://www.strath.ac.uk/Departments/PhysPharm/). Currents were filtered at a frequency of 1 kHz and sampled at 350 Hz. Micro-electrodes were fabricated from borosilicate glass (GC120TF-10, Harvard Apparatus, Edenbridge, Kent, UK) using a two-stage horizontal pull (P-87, Sutter Instrument Company, Novato, CA, USA) and filled with 3 M KCl. Pipette resistances ranged from 0.5 to 1.5 MΩ. Oocytes were perfused with saline at a rate of 15 ml min−1. Drug application was via a simple gravity-fed system calibrated to run at the same rate. Extracellular saline contained (in mM) 96 NaCl, 2 KCl, 1 MgCl2 and 5 HEPES (pH 7.4).
Analysis and curve fitting were performed using Prism V3.02 (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com). Concentration–response data for each oocyte were normalized to the maximum current for that oocyte. For inhibition curves, antagonists were routinely co-applied in the presence of agonist or continuously applied for 20 s before and during the administration of 5-HT. For 5-HT3 receptors, a 2 min wash was used between drug applications. For GABAC receptors this was increased to 3 min, and for GABAA receptors it was increased to 6 min. The mean and s.e.mean for a series of oocytes were plotted against agonist or antagonist concentration and iteratively fitted to the following equation:
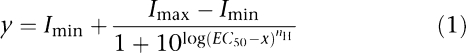 |
where Imin is the baseline current, Imax is the peak current evoked by agonist, EC50 is the concentration of agonist needed to evoke a half-maximal response, x is the ligand concentration and nH is the Hill slope. KB was estimated from IC50 values using the Cheng–Prusoff equation with the modification by Leff and Dougall (1993):
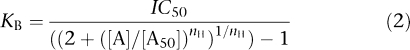 |
where KB is the dissociation constant of the competing drug, IC50 is the concentration of antagonist required to produce half maximal response, [A] is the agonist concentration, [A50] is the agonist EC50 and nH is the Hill slope of the agonist.
Modelling and antagonist docking
The protein sequence of the extracellular domain of the GABAA receptor α1, β2 and γ2 subunits (accession numbers P14867, P47870 and P18507, respectively) were co-aligned with the sequence of AChBP from Lymnaea stagnalis (P58154) using FUGUE, which takes into account secondary structures (Shi et al., 2001). A three-dimensional homology model was generated using MODELLER 6v2 (Sali and Blundell, 1993) based on the crystal structure of AChBP at 2.7 Å resolution (PDB ID 1I9B) and the best fit identified as the model with the lowest energy and steric clashes (http://www-cryst.bioc.cam.ac.uk/~ricardo/).
The three-dimensional structures of quinine and chloroquine were extracted from the Cambridge Structural Database (reference codes KAMDAD and CLQUON01, respectively). Protonated forms of both molecules were constructed in Chem3D Ultra 7.0 (CambridgeSoft, Cambridge, UK) based on the crystal structures and energy-minimized using the MM2 force field.
Docking was performed using methods similar to those previously described (Thompson and Lummis, 2007a). Briefly, docking of the protonated ligands into the β2–α1 interface of the GABAA receptor homology model was carried out using GOLD 3.0 (The Cambridge Crystallographic Data Centre, Cambridge, UK). The binding site centre was defined using the Cɛ2 atom of Y157 or the Cζ atom of Y205, both of which are known to be important binding residues. The binding site radius was defined as 7 Å for quinine and 10 Å for chloroquine. Ten genetic algorithm runs were performed on each docking exercise, giving a total of 20 solutions for each antagonist. The structures were analysed using the implemented GoldScore fitness function.
Materials
All cell culture reagents were obtained from Gibco (Invitrogen Ltd, Paisley, UK), except fetal calf serum, which was from Labtech International (Ringmer, UK). Quinine and chloroquine were from Sigma-Aldrich Co. Ltd (Poole, Dorset, UK). Mefloquine-HCl was kindly provided by CilagAG. All other reagents were of the highest obtainable grade. 5-HT3A and 5-HT3B receptor subunit cDNA was kindly donated by John Peters (University of Dundee), GABAA α1, β2 and γ2 receptor subunit cDNA by Keith Wafford (Merck Sharp & Dohme) and GABAC ρ1 receptor subunit cDNA by David Weiss (University of Texas).
Results
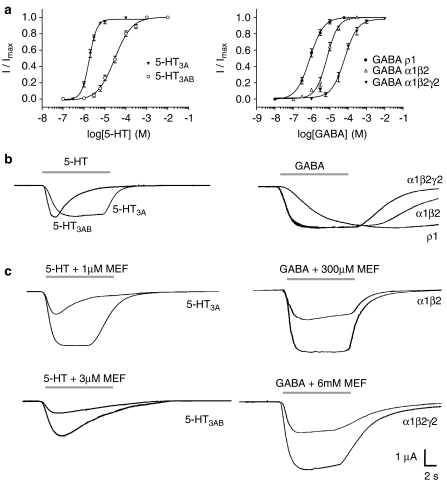
Application of 5-HT or GABA to Xenopus oocytes expressing 5-HT3A, 5-HT3AB, GABAA α1β2 or GABAA α1β2γ2 receptors produced concentration-dependent, rapidly activating, inward currents that desensitized over the time course of the application. GABAC ρ1 receptors also displayed a concentration-dependent fast activation, but did not desensitize during drug application. Plotting current amplitude against a series of 5-HT or GABA concentrations allowed curves to be fitted (Equation (1); Figure 1) and the pEC50 values and Hill slopes determined from these curves are in given in Table 1.
Figure 1.
Concentration–response curves for 5-HT3 and GABA receptors expressed in Xenopus oocytes (a). Parameters derived from these curves are shown in Table 1. Typical agonist EC50 responses (b) and inhibition of these responses by mefloquine (c). Agonist application is indicated by a grey line above the current traces. Mefloquine (MEF) was pre-applied for 20 s and then co-applied at the concentrations shown.
Table 1.
Parameters derived from concentration–response curves (shown in Figure 1) for 5-HT3 receptors expressed in Xenopus oocytes
| Receptor | pEC50 (μM) | EC50 (μM) | nH | n |
|---|---|---|---|---|
| Mouse 5-HT3Aa | 6.12±0.02 | 0.75 | 2.21±0.23 | 12 |
| Human 5-HT3A | 5.76±0.03 | 1.79 | 2.32±0.28 | 6 |
| Human 5-HT3AB | 4.53±0.04 | 29.5 | 1.06±0.09 | 6 |
| GABA α1β2 | 5.15±0.04 | 7.00 | 1.42±0.18 | 4 |
| GABA α1β2γ2 | 4.19±0.05 | 64.8 | 1.23±0.17 | 5 |
| GABA ρ1 | 6.09±0.05 | 0.81 | 1.17±0.14 | 3 |
Values from Thompson and Lummis (2007a) were recorded at the same time as the current work and can be directly compared.
Effects on the homomeric 5-HT3A receptor
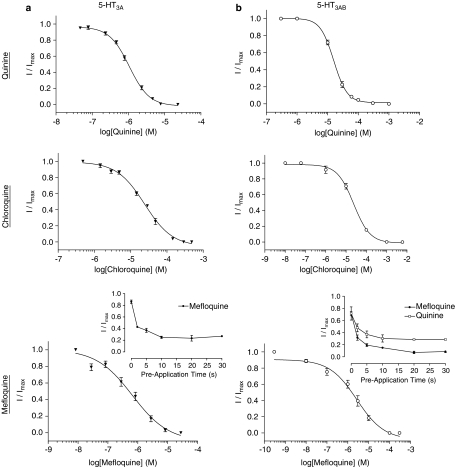
At 5-HT3A receptors, a concentration-dependent inhibition of the 5-HT EC50 response was observed in the presence of quinine, chloroquine or mefloquine and was fully reversible (Figure 2 and Table 2). Application of antagonists alone did not elicit a response. The potency of the inhibition was unaltered by pre-application of quinine or chloroquine, but an increase in potency was seen for mefloquine with no further increase in the level of inhibition after 10 s pre-application (Figure 2a, inset). IC50 values for the three compounds had a rank order of potency of mefloquine>quinine>chloroquine. In previous studies, IC50 values for these compounds were used to calculate binding affinities (KB) using the modified Cheng–Prusoff equation (Equation (2)), and the accuracy of this method was confirmed by comparing these values with binding affinities determined using competition binding, Schild analysis and an analysis of rate constants (Thompson and Lummis, 2007a). Here, binding affinities were similarly estimated from IC50 values, and yielded values of 1.76 and 40.3 μM for quinine and chloroquine. A KB value for mefloquine could not be estimated, as further investigations indicated that this compound was non-competitive (Leff and Dougall, 1993).
Figure 2.
Concentration-dependent inhibition of homomeric (a) and heteromeric (b) 5-HT3 responses. Inhibition was measured in the presence of EC50 concentrations of 5-HT. Values are shown as mean±s.e.mean, and parameters derived from these curves can be seen in Table 2. Inset: Inhibition of the EC50 response versus pre-application time: 5-HT3A by 3 μM mefloquine, 5-HT3AB by 30 μM quinine and 30 μM mefloquine (n=4 for each curve).
Table 2.
Parameters derived from concentration–inhibition curves (shown in Figure 2) in the presence of EC50 concentrations of 5-HT
| Antagonist | pIC50 (μM) | IC50 (μM) | nH | n |
|---|---|---|---|---|
| Mouse 5-HT3A | ||||
| Quininea | 4.00±0.02 | 101.0 | 2.66±0.31 | 7 |
| Chloroquinea | 4.05±0.04 | 89.9 | 2.00±0.39 | 6 |
| Mefloquinea | 5.03±0.02 | 9.36 | 2.08±0.18 | 8 |
| Human 5-HT3A | ||||
| Quinine | 5.98±0.03 | 1.06 | 1.58±0.13 | 14 |
| Chloroquine | 4.61±0.03 | 24.3 | 1.13±0.11 | 13 |
| Mefloquine | 6.18±0.12 | 0.66 | 0.72±0.15 | 7 |
| Human 5-HT3AB | ||||
| Quinine | 4.80±0.01 | 15.8 | 1.98±0.12 | 3 |
| Chloroquine | 4.63±0.06 | 23.6 | 1.08±0.12 | 4 |
| Mefloquine | 5.57±0.17 | 2.70 | 0.66±0.16 | 6 |
Values taken from Thompson and Lummis (2007a) were performed at the same time as the current work and can be directly compared.
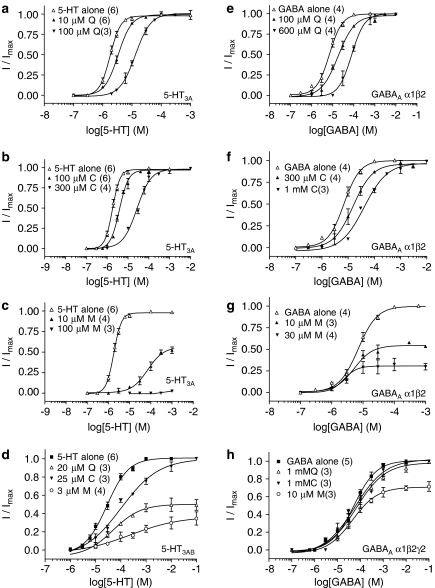
Concentration–response curves were compared in the presence and absence of antagonist. Increasing concentrations of quinine and chloroquine caused parallel rightward shifts in the concentration–response curves with no change in the maximal current, even when applied at concentrations far in excess (that is, 5–100 ×) of the IC50 values for these compounds (Figures 4a and b). In contrast, mefloquine caused an increase in the EC50, a change in the Hill slope and a concomitant reduction in the maximal response at concentrations close to its IC50 value (Figure 4c).
Effects on the heteromeric 5-HT3AB receptor
At 5-HT3AB receptors, a concentration-dependent inhibition of the 5-HT EC50 response was observed in the presence of quinine, chloroquine and mefloquine. Similar to 5-HT3A receptors, antagonists alone did not elicit a response and inhibition was fully reversible after washing (Figure 2b and Table 2). The potency of both quinine and mefloquine was increased by pre-application of these compounds, with no further increase after 10 s (Figure 2b, inset). Similar to 5-HT3A receptors, the rank order of potency was mefloquine>quinine>chloroquine. Concentration–response curves in the presence of antagonist were compared to those in the absence of antagonist and clearly showed that chloroquine caused a rightward shift in the concentration–response curve with no change in the maximal current, whereas quinine and mefloquine caused an increase in the EC50 and a simultaneous reduction in the maximal response (Figure 4d). Ideally, additional curves would have been completed, but at higher concentrations the antimalarial compounds are insoluble. The KB of chloroquine was calculated as 12.7 μM (Equation (2)). Estimates for quinine and mefloquine could not be made, as further investigations indicated that these compounds were non-competitive (Leff and Dougall, 1993).
Effects on GABA receptors
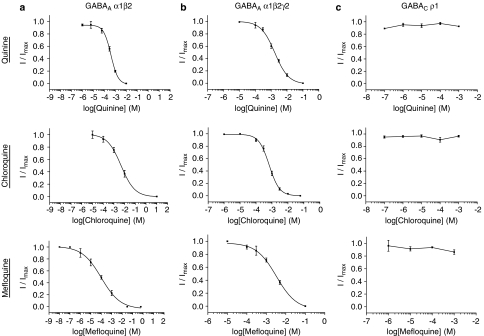
Quinine, chloroquine and mefloquine inhibited the GABAA α1β2 receptor and the GABAA α1β2γ2 receptor EC50 responses in a concentration-dependent manner (Figures 3a and b, and Table 3). In contrast, at a concentration of up to 1 mM, none of the three compounds inhibited the GABAC ρ1 response (Figure 3c and Table 3). Application of antagonists alone did not elicit a response at any of the GABA receptors and inhibition at GABAA α1β2 and α1β2γ2 receptors was fully reversible. The potency of all three antagonists was unaltered by pre-application. IC50 values for all antagonists were higher than those observed at 5-HT3 receptors, indicating that these compounds are less potent at GABA receptors. IC50 values were higher at α1β2γ2 receptors than at α1β2 receptors. The rank order of potency for α1β2 receptors was mefloquine>quinine>chloroquine and at α1β2γ2 receptors it was mefloquine>chloroquine>quinine.
Figure 3.
Concentration-dependent inhibition of GABA responses. Inhibition was measured in the presence of EC50 concentrations of GABA for each receptor. Values are shown as mean±s.e.mean, and parameters derived from these curves can be seen in Table 3.
Table 3.
Parameters derived from concentration–inhibition curves (shown in Figure 3) in the presence of EC50 concentrations of GABA
| Antagonist | pIC50 (μM) | IC50 (μM) | nH | n |
|---|---|---|---|---|
| GABA α1β2 | ||||
| Quinine | 3.32±0.04 | 400 | 1.32±0.17 | 5 |
| Chloroquine | 2.33±0.14 | 465 | 0.68±0.16 | 5 |
| Mefloquine | 4.01±0.17 | 98.7 | 0.48±0.09 | 9 |
| GABA α1β2γ2 | ||||
| Quinine | 2.77±0.05 | 1694 | 1.00±0.10 | 3 |
| Chloroquine | 3.17±0.03 | 670 | 1.35±0.11 | 3 |
| Mefloquine | 2.48±0.11 | 328 | 0.85±0.22 | 4 |
| GABA ρ1 | ||||
| Quinine | NE | NE | NE | 3 |
| Chloroquine | NE | NE | NE | 5 |
| Mefloquine | NE | NE | NE | 7 |
Abbreviation: NE, no effect.
A comparison of concentration–response curves in the presence or absence of antagonist showed that increasing concentrations of quinine and chloroquine resulted in increases in EC50 values with no change in maximal current at GABAA α1β2 receptors (Figures 4e and f), whereas mefloquine caused a significant decrease in the maximal current (Figure 4g). At the GABAA α1β2γ2 receptor, problems of compound solubility prevented us from exploring higher mefloquine concentrations, but it was possible to see a shift in the EC50 and a reduction in the maximal current at 10 μM (a concentration approximately 30-fold lower than its IC50). At concentrations close to the IC50 values, the reduction in maximal response was absent from both the quinine and chloroquine responses (Figure 4h). KB estimates for quinine and chloroquine at the GABAA α1β2 receptor were 0.36 and 0.42 mM respectively, and at the GABAA α1β2γ2 receptor they were 1.18 and 0.46 mM (Equation (2)). The KB of mefloquine could not be determined, as it is a non-competitive antagonist (Leff and Dougall, 1993).
Figure 4.
Inhibition by quinine, chloroquine and mefloquine at 5-HT3A and GABAA receptors. Concentration–response curves were performed in the presence or absence of antagonist. Antagonist concentrations are shown on each graph; Q=quinine, C=chloroquine, M=mefloquine. Values are shown as mean±s.e.mean. n values are shown in parentheses.
Docking studies
Docking quinine and chloroquine into the GABAA receptor binding site (β2–α1 interface) yielded a series of docking solutions that were split into groups based on the orientation of the ligand and GOLD scores (Olsen et al., 2004). Docking of mefloquine was not performed, as this ligand did not display competitive behaviour.
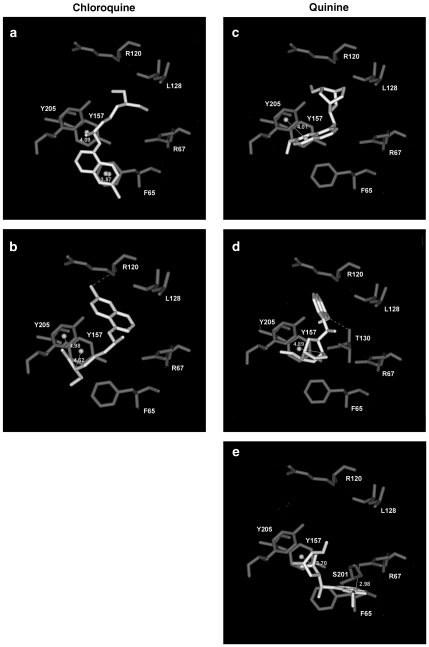
Docking of chloroquine yielded ligand orientations that fell into two distinct groups designated a and b. In model a, the quinoline ring was close to F65 and the tertiary ammonium was orientated towards loop E (Figure 5a). The distance between the centroids of the quinoline ring and the aromatic ring of F65, and the distance between Y157 and the secondary amine of chloroquine were both within 5 Å; these could indicate possible π–π and cation–π interactions, respectively. In model b (Figure 5b), the ligand orientation was reversed, with the tertiary ammonium located between the aromatic rings of Y157 and Y205 and the aromatic rings orientated towards loop E. The distance between the nitrogen atom of the ammonium group and the centroids of the two aromatic rings is ideal for a cation–π interaction. In this orientation, there was also a potential hydrogen bond between the chlorine of the quinoline ring and the backbone amine of R120.
Figure 5.
Chloroquine and quinine docked ligands in the GABAA receptor β2/α1 binding interface showing the orientation of the main residues that define these models. These residues are highlighted in Figure 6. Chloroquine (a, b) and quinine (c–e) are shown in white at the centre of each image.
The results of docking quinine fell into three main groups that we categorized as c, d, e. In all three solutions, either the tertiary ammonium or the quinoline group was located between Y157 and Y205. In solution c, the quinoline ring was located 4–5 Å from the aromatic ring of Y205, indicating a possible π–π interaction, and the tertiary ammonium was orientated towards loop E (Figure 5c). In solution d, the tertiary ammonium was located between the aromatic rings of Y157 and Y205, and the distance between the centroid of Y157 and the tertiary amine of quinine was within 5 Å, suggesting a possible cation–π interaction (Figure 5d). In this orientation, the quinoline ring was positioned towards loop E, which would enable the formation of a hydrogen bond between the hydroxyl group of this ring and the backbone amine of T130. Docking solutions c and d displayed similarities to the orientations of chloroquine in solutions a and b, but solution e was different (Figure 5e). In solution e, the tertiary ammonium was located between Y157 and Y205 and the nitrogen of this ammonium group was within 5 Å of Y157. The quinoline ring was orientated towards loop D, which would allow the formation of a hydrogen bond between the hydroxyl of the quinoline ring and S201 located within loop C.
Discussion
This study describes the effects of the antimalarial compounds quinine, chloroquine and mefloquine on human 5-HT3, GABAA and GABAC receptors. At both 5-HT3A and 5-HT3AB receptors, all three compounds are relatively potent antagonists (IC50=0.66–24.3 μM). GABAA receptors are also inhibited by all three compounds, but at higher concentrations (IC50=98.7–1694 μM). No inhibition was observed at GABAC receptors.
At human 5-HT3A receptors, and at both GABAA α1β2 and α1β2γ2 receptors, quinine and chloroquine displayed effects that were consistent with competitive antagonism, whereas mefloquine was non-competitive. These actions are equivalent to those reported at mouse 5-HT3A receptors (Thompson and Lummis, 2007a). Interestingly, at heteromeric 5-HT3 receptors, chloroquine and mefloquine displayed properties similar to those at homomeric receptors, but quinine had effects that were consistent with non-competitive inhibition. Similarities in the actions of these compounds at 5-HT3A, GABAA and nACh receptors suggest the possibility of conserved sites of action for these compounds (Liu et al., 1991; Sieb et al., 1996; Ballestero et al., 2005; Thompson and Lummis, 2007a). For the compounds that showed competitive behaviour, our ligand docking provides further evidence for this. At the GABAA receptor, we currently favour solution d (Figure 5) for the orientation of quinine and solution a for the orientation of chloroquine; these have the greatest potential to be energetically stable and are comparable to docking solutions for the same compounds in previous 5-HT3A receptor studies (Thompson and Lummis, 2007a). Many of the residues involved in the ligand–receptor interaction are similar to those that have been experimentally identified in studies of other GABAA and 5-HT3 receptor ligands (Boileau et al., 1999; Beene et al., 2002; Lummis et al., 2005; Thompson et al., 2005; Harrison and Lummis, 2006; Thompson and Lummis, 2007b).
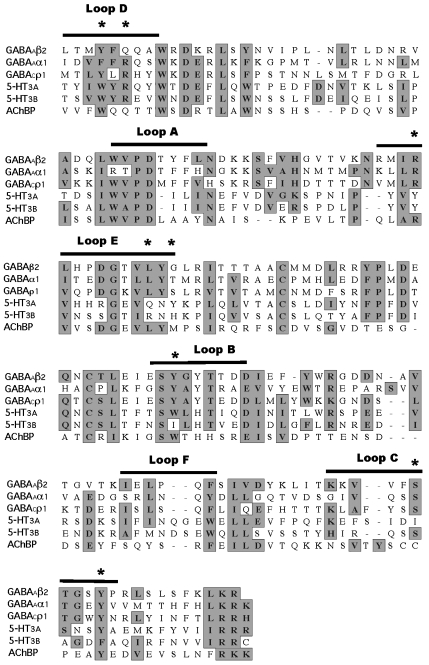
It was surprising that the three compounds could inhibit GABAA but not GABAC receptors, given the strong homology between the binding site residues in these two receptors and, in particular, the similarity of the residues that our model predicts as being close to quinine and chloroquine (Figure 6). However, small changes in binding site residues can have a large impact on binding affinity, and it is possible that changes such as Phe65 (GABAA) with Tyr (GABAC) in loop D, and/or Thr130 for Ser in loop E (Figure 5), might significantly reduce ligand potency. For the GABAA receptors, we have docked quinine and chloroquine into the β2/α1 interface, as they compete with GABA. KB values for the compounds were less than fourfold different in the α1β2 and α1β2γ2 receptors, supporting this hypothesis.
Figure 6.
Sequence alignment of ligand-gated ion channel receptor subunits that have been examined for their susceptibility to antimalarial compounds. Residues with similar chemical properties are shown in grey and the approximate positions of the binding loops (A–F) are indicated with a bar. Residues that are shown in Figure 5 are marked with an asterisk (GABAA β2: Y157, S201, Y205; GABAA α1: F65, R67, L128, R120, T130). Accession numbers for the subunits are GABAA β2 P47870, GABAA α1 P14867, GABAC ρ1 P24046, 5-HT3A P46098, 5-HT3B AF080582 and AChBP P58154.
In contrast to quinine and chloroquine, which showed competitive behaviour, mefloquine was non-competitive at both 5-HT3 and GABAA receptors. A similar action was observed for mouse 5-HT3A receptors, where mefloquine displayed a small voltage dependence, suggesting that it may bind at a shallow position within the channel or channel vestibule (Thompson and Lummis, 2007a). Block by quinine was also found to be slightly voltage-dependent in the nACh α9α10 receptor, suggesting a similarly placed binding site, and it is possible that this site of action is conserved within the family (Ballestero et al., 2005). It is also possible that quinine and mefloquine may have mixed competitive/non-competitive activity at ligand-gated ion channels. At nACh α9α10 receptors, quinine competitively inhibited responses at concentrations up to 100 μM (IC50=0.97 μM), but was non-competitive at higher concentrations (Ballestero et al., 2005). Similarly, at mouse 5-HT3A receptors, Schild analysis has shown that mefloquine is non-competitive, but displacement of a radiolabelled antagonist was recorded at higher concentrations, suggesting both competitive and non-competitive behaviours (Thompson and Lummis, 2007a). As members of the Cys-loop family share strong structural and pharmacological character, a similar mechanism is possible at the receptors described here (Rothlin et al., 1999, 2003). Interestingly, some benzodiazepines (which bind at the γ2–α1 interface of the GABAA receptor) can inhibit the growth of malarial parasite, indicating that some antimalarial compounds and benzodiazepines may also share binding sites (Dzierszinski et al., 2002).
The blood concentration required to be 99% effective at reducing Plasmodium falciparum infection has been reported as 1.57 μM for quinine, 44.4 μM for chloroquine and 2.2 and 4.1 μM for mefloquine (Lobel et al., 1993; Ramharter et al., 2004). These blood concentrations are close to the IC50 values for chloroquine and mefloquine at both mouse and human 5-HT3 receptors, but considerably lower than the IC50 values we found at GABAA receptors. These whole blood values may not be representative of free blood concentrations, as these compounds can bind to plasma proteins; however, as they are able to freely pass the blood–brain barrier and have been found to accumulate in physiologically relevant concentrations in the brain and other tissues, we would expect that they would reach the CNS at concentrations where they could effect receptor responses (Adelusi and Salako, 1982; Baudry et al., 1997; Dow et al., 2003). Consequently, there may be 5-HT3-mediated effects in patients taking these drugs, although reported side effects, such as nausea, are those that might be expected to be ameliorated by 5-HT3 selective antagonists, which are used as antiemetics. However, the neuronal pathways that induce nausea and vomiting are varied, and 5-HT3 antagonists are only effective against post-operative, chemotherapy-induced and radiation-induced nausea and vomiting, but do not inhibit the symptoms elicited by other agents, such as opiate administration, or motion (Bountra et al., 1996; Lynch and Simpson, 2001). It must also be emphasized that for the majority of individuals, all of the drugs studied here rarely display severe adverse reactions when used at the recommended dose (Luzzi and Peto, 1993; Taylor and White, 2004). Indeed, because of their excellent safety record, it is possible that these compounds could have measurable benefits for the treatment of 5-HT3-related disorders, and could thereby circumvent the often slow development and high cost of new therapies, a range of which have already been proposed or are under development (Barann et al., 1997, 2000; Piper et al., 2002; Farber et al., 2007; Thompson and Lummis, 2007b).
In summary, we have shown that the antimalarial compounds quinine, chloroquine and mefloquine antagonize both 5-HT3 and GABAA receptor responses. Inhibition is most potent at 5-HT3 receptors, is only seen at higher concentrations at GABAA receptors and is absent from GABAC receptors. It is not yet clear if the 5-HT3 receptors of patients taking these drugs are inhibited, but blood and tissue concentrations indicate that it is possible. These observations further extend the range of receptors known to be affected by antimalarial agents, and suggest that there may be potential benefits in assessing some of them for the treatment of 5-HT3 receptor-related disorders.
Acknowledgments
We thank the Wellcome Trust for funding. SCRL is a Wellcome Trust Senior Research Fellow in Basic Biomedical Studies. We particularly thank Christian Weh of Cilag AG for mefloquine, John Peters (University of Dundee) for 5-HT3A and 5-HT3B, Keith Wafford (Merck Sharp & Dohme) for GABAA α1, β2 and γ2 subunits and David Weiss (University of Texas) for GABAC ρ1.
Abbreviations
- nACh
nicotinic acetylcholine
- AChBP
acetylcholine binding protein
Conflict of interest
The authors state no conflict of interest.
References
- Adelusi SA, Salako LA. Tissue and blood concentrations of chloroquine following chronic administration in the rat. J Pharmacol. 1982;34:733–735. doi: 10.1111/j.2042-7158.1982.tb06211.x. [DOI] [PubMed] [Google Scholar]
- Akabas MH. GABAA receptor structure–function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. doi: 10.1016/S0074-7742(04)62001-0. [DOI] [PubMed] [Google Scholar]
- Ballestero JA, Plazas PV, Kracun S, Gomez-Casati ME, Taranda J, Rothlin CV, et al. Effects of quinine, quinidine, and chloroquine on α9α10 nicotinic cholinergic receptors. Mol Pharmacol. 2005;68:822–829. doi: 10.1124/mol.105.014431. [DOI] [PubMed] [Google Scholar]
- Barann M, Dilger JP, Bonisch H, Gothert M, Dybek A, Urban BW. Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology. 2000;39:1064–1074. doi: 10.1016/s0028-3908(99)00205-1. [DOI] [PubMed] [Google Scholar]
- Barann M, Gothert M, Bonisch H, Dybek A, Urban BW. 5-HT3 receptors in outside-out patches of N1E-115 neuroblastoma cells: basic properties and effects of pentobarbital. Neuropharmacology. 1997;36:655–664. doi: 10.1016/s0028-3908(97)00059-2. [DOI] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acid A receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Baudry S, Pham YT, Baune B, Vidrequin S, Crevoisier C, Gimenez F, et al. Stereoselective passage of mefloquine through the blood–brain barrier in the rat. J Pharm Pharmacol. 1997;49:1086–1090. doi: 10.1111/j.2042-7158.1997.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Beene DL, Brandt GS, Zhong W, Zacharias NM, Lester HA, Dougherty DA. Cation–pi interactions in ligand recognition by serotonergic (5-HT3A) and nicotinic acetylcholine receptors: the anomalous binding properties of nicotine. Biochemistry. 2002;41:10262–10269. doi: 10.1021/bi020266d. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Evers AR, Davis AF, Czajkowski C. Mapping the agonist binding site of the GABAA receptor: evidence for a beta-strand. J Neurosci. 1999;19:4847–4854. doi: 10.1523/JNEUROSCI.19-12-04847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C, Gale JD, Gardner CJ, Jordan CC, Kilpatrick GJ, Twissell DJ, et al. Towards understanding the aetiology and pathophysiology of the emetic reflex: novel approaches to antiemetic drugs. Oncology. 1996;53:102–109. doi: 10.1159/000227649. [DOI] [PubMed] [Google Scholar]
- Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol Microbiol. 2005;56:323–333. doi: 10.1111/j.1365-2958.2005.04556.x. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, et al. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci USA. 1991;88:2673–2677. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA, Pistis M, Hanna MC, Peters JA, Lambert JJ, Hales TG, et al. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature. 1999;397:359–363. doi: 10.1038/16941. [DOI] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 Å resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Dow GS, Hudson TH, Vahey M, Koenig ML. The acute neurotoxicity of mefloquine may be mediated through a disruption of calcium homeostasis and ER function in vitro. Malar J. 2003;2:14. doi: 10.1186/1475-2875-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin AE, Huvar RD, Andrea MR, Pyati J, Zhu JY, Joy KC, et al. The pharmacological and functional characteristics of the serotonin 5-HT3A receptor are specifically modified by a 5-HT3B receptor subunit. J Biol Chem. 1999;274:30799–30810. doi: 10.1074/jbc.274.43.30799. [DOI] [PubMed] [Google Scholar]
- Dzierszinski F, Coppin A, Mortuaire M, Dewailly E, Slomianny C, Ameisen JC, et al. Ligands of the peripheral benzodiazepine receptor are potent inhibitors of Plasmodium falciparum and Toxoplasma gondiiin vitro. Antimicrob Agents Chemother. 2002;46:3197–3207. doi: 10.1128/AAC.46.10.3197-3207.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enz R, Cutting GR. GABAC receptor rho subunits are heterogeneously expressed in the human CNS and form homo- and heterooligomers with distinct physical properties. Eur J Neurosci. 1999;11:41–50. doi: 10.1046/j.1460-9568.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- Farber L, Drechsler S, Ladenburger S, Gschaidmeier H, Fischer W. The neuronal 5-HT3 receptor network after 20 years of research—evolving concepts in management of pain and inflammation. Eur J Pharmacol. 2007;560:1–8. doi: 10.1016/j.ejphar.2007.01.028. [DOI] [PubMed] [Google Scholar]
- Fiebich BL, Akundi RS, Seidel M, Geyer V, Haus U, Muller W, et al. Expression of 5-HT3A receptors in cells of the immune system. Scand J Rheumatol Suppl. 2004;119:9–11. [PubMed] [Google Scholar]
- Goldin LR.Maintenance of Xenopus laevis and oocyte injection Methods in Enzymology 1992Academic Press: New York; 267–279.In: Bernardo R, Iverson LE (eds)vol. 207 [DOI] [PubMed] [Google Scholar]
- Harrison NJ, Lummis SC. Locating the carboxylate group of GABA in the homomeric rho GABAA receptor ligand-binding pocket. J Biol Chem. 2006;281:24455–24461. doi: 10.1074/jbc.M601775200. [DOI] [PubMed] [Google Scholar]
- Leff P, Dougall IG. Further concerns over Cheng–Prusoff analysis. Trends Pharmacol Sci. 1993;14:110–112. doi: 10.1016/0165-6147(93)90080-4. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Liu L, Katz Y, Weizman R, Rosenberg B, Pasternak GW, Gavish M. Interactions of chloroquine with benzodiazepine, gamma-aminobutyric acid and opiate receptors. Biochem Pharmacol. 1991;41:1534–1536. doi: 10.1016/0006-2952(91)90574-o. [DOI] [PubMed] [Google Scholar]
- Lobel HO, Miani M, Eng T, Bernard KW, Hightower AW, Campbell CC. Long-term malaria prophylaxis with weekly mefloquine. Lancet. 1993;341:848–851. doi: 10.1016/0140-6736(93)93058-9. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Beene DL, Harrison NJ, Lester HA, Dougherty DA. A cation–pi binding interaction with a tyrosine in the binding site of the GABAC receptor. Chem Biol. 2005;12:993–997. doi: 10.1016/j.chembiol.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Luzzi GA, Peto TE. Adverse effects of antimalarials. An update. Drug Saf. 1993;8:295–311. doi: 10.2165/00002018-199308040-00004. [DOI] [PubMed] [Google Scholar]
- Lynch L, Simpson KH.Antiemetic drugs The Royal College of Anaesthetists 2001. bulletin 9 (September)
- Miyake A, Mochizuki S, Takemoto Y, Akuzawa S. Molecular cloning of human 5-hydroxytryptamine3 receptor: heterogeneity in distribution and function among species. Mol Pharmacol. 1995;48:407–416. [PubMed] [Google Scholar]
- Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/s0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- Niesler B, Walstab J, Combrink S, Moeller D, Kapeller J, Rietdorf J, et al. Characterization of the novel human serotonin receptor subunits 5-HT3C, 5- HT3D and 5-HT3E. Mol Pharmacol. 2007;72:8–17. doi: 10.1124/mol.106.032144. [DOI] [PubMed] [Google Scholar]
- Olsen L, Pettersson I, Hemmingsen L, Adolph HW, Jorgensen FS. Docking and scoring of metallo-beta-lactamases inhibitors. J Comput Aided Mol Des. 2004;18:287–302. doi: 10.1023/b:jcam.0000046821.15502.71. [DOI] [PubMed] [Google Scholar]
- Piper SN, Rohm KD, Papsdorf M, Maleck WH, Mattinger P, Boldt J. Dolasetron reduces pain on injection of propofol. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:528–531. doi: 10.1055/s-2002-33767. [DOI] [PubMed] [Google Scholar]
- Ramharter M, Wernsdorfer WH, Kremsner PG. In vitro activity of quinolines against Plasmodium falciparum in Gabon. Acta Trop. 2004;90:55–60. doi: 10.1016/j.actatropica.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Lioudyno MI, Silbering AF, Plaza PV, Casati ME, Katz E, et al. Direct interaction of serotonin type 3 receptor ligands with recombinant and native α9α10-containing nicotinic cholinergic receptors. Mol Pharmacol. 2003;63:1067–1074. doi: 10.1124/mol.63.5.1067. [DOI] [PubMed] [Google Scholar]
- Rothlin CV, Katz E, Verbitsky M, Elgoyhen AB. The α9 nicotinic acetylcholine receptor shares pharmacological properties with type A gamma-aminobutyric acid, glycine, and type 3 serotonin receptors. Mol Pharmacol. 1999;55:248–254. doi: 10.1124/mol.55.2.248. [DOI] [PubMed] [Google Scholar]
- Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- Shi J, Blundell TL, Mizuguchi K. FUGUE: sequence-structure homology recognition using environment-specific substitution tables and structure-dependent gap penalties. J Mol Biol. 2001;310:243–257. doi: 10.1006/jmbi.2001.4762. [DOI] [PubMed] [Google Scholar]
- Sieb JP, Milone M, Engel AG. Effects of the quinoline derivatives quinine, quinidine, and chloroquine on neuromuscular transmission. Brain Res. 1996;712:179–189. doi: 10.1016/0006-8993(95)01349-0. [DOI] [PubMed] [Google Scholar]
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006a;12:3615–3630. doi: 10.2174/138161206778522029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SCR. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007b;11:527–540. doi: 10.1517/14728222.11.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SCRL. The antimalarial drugs quinine, chloroquine and mefloquine are antagonists at 5-HT3 receptors. Br J Pharmacol. 2007a;151:666–667. doi: 10.1038/sj.bjp.0707238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Price KL, Reeves DC, Chan SL, Chau PL, Lummis SC. Locating an antagonist in the 5-HT3 receptor binding site using modeling and radioligand binding. J Biol Chem. 2005;280:20476–20482. doi: 10.1074/jbc.M413610200. [DOI] [PubMed] [Google Scholar]
- Tzvetkov MV, Meineke C, Oetjen E, Hirsch-Ernst K, Brockmoller J. Tissue-specific alternative promoters of the serotonin receptor gene HTR3B in human brain and intestine. Gene. 2007;386:52–62. doi: 10.1016/j.gene.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Uhlemann A, Yuthavong Y, Fidock DA.Mechanisms of antimalarial drug action and resistance Molecular Approaches to Malaria 2005ASM Press: Washington, USA; 429–461.In: Sherman IW (ed) [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]