Abstract
Background and purpose
Flavonoids are known to possess a broad set of pharmacological effects, some of which have been attributed to their antioxidant properties and, more recently, to cell signalling modulation. Nevertheless, flavonoids are extensively metabolized and their metabolites are the potential bioactive forms in vivo. Therefore, a first and crucial step to understand the mechanisms underlying potential health benefits of flavonoids is knowledge of their metabolites and their biological activities.
Experimental approach
To approximate a human dietary pattern of intake of flavonoids, regular rat chow was supplemented with 0.02% quercetin and fed to Sprague–Dawley rats over 3 weeks. Plasma samples were analysed by HPLC and electrospray tandem mass spectrometry, and plasma antioxidant capacity was measured by the 2,2′-azino-bis(3-ethylbenzothiazoline sulphonate) assay.
Key results
Major metabolites were 3′-methylquercetin (isorhamnetin) glucuronide sulphate conjugates, the most plausible conjugation positions being at the 3-, 5- and 7-hydroxyl positions. Isorhamnetin conjugates are methylated at the 3′-OH position, which decreases the high antioxidant activity of quercetin and its metabolites and their contribution to plasma antioxidant potential.
Conclusions and implications
This metabolic pattern differs from that observed after a single high-dose administration, where the major metabolites were quercetin conjugates at 5- and 7-hydroxyl positions and a significantly increased plasma antioxidant activity was observed. These data show altogether that the different metabolic patterns obtained under a prolonged low-dosage regimen or after a single high dose, crucially affected the antioxidant potential of plasma in treated animals. Our data also allow for the establishment of structure–antioxidant activity relationships for quercetin metabolites.
Keywords: quercetin, quercetin metabolites, isorhamnetin, isorhamnetin metabolites, methylation, glucuronidation, sulphation, structure–antioxidant activity
Introduction
The use of dietary phytochemicals for improving human health and for the prevention of chronic and degenerative diseases is a matter of increasing debate. The potential health-promoting properties of flavonoids, in particular, have been highlighted, by the recent publication of various studies suggesting that these compounds may prevent the development of degenerative diseases, such as cardiovascular and cerebrovascular diseases (Hertog et al., 1993a; Keli et al., 1996), some forms of cancer (So et al., 1996) and Parkinson's and Alzheimer's diseases (Ishige et al., 2001; Youdim and Joseph, 2001).
Flavonoids are a large group of polyphenolic compounds that ubiquitously exist in natural products, such as fruits, vegetables and plant extracts, as well as in plant-derived beverages, such as tea, red wine and cocoa (Hertog et al., 1992, 1993b; Arteel and Sies, 1999). These compounds are generally known to possess a broad set of pharmacological effects, many of which have been attributed to their interactions with several key enzymes (Di Carlo et al., 1999), and to their antioxidant properties. The latter properties can be due to the ability of flavonoids to scavenge free radicals (Bors and Saran, 1987; Mira et al., 1994, 1999), to chelate metal ions (Morel et al., 1994; Miller et al., 1996; Moran et al., 1997; Mira et al., 2002) and to act synergistically with other antioxidants (Filipe et al., 2001).
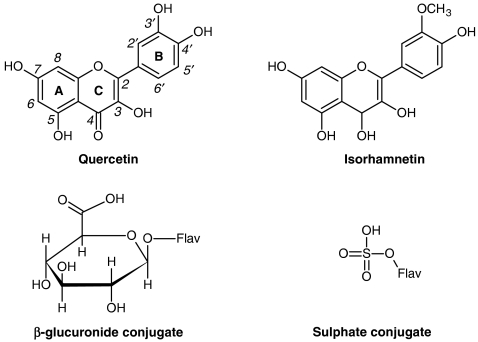
Recently, the biological effects of flavonoids have also been linked to modulatory actions in the cell, through interactions with specific proteins, central to intracellular signalling cascades (Schroeter et al., 2002; Williams et al., 2004). Independently of the mechanism underlying the pharmacological properties of flavonoids, the knowledge about their metabolism is the first step in understanding their actions in vivo. Flavonoids are extensively metabolized, that is, they undergo several chemical modifications in the gastrointestinal tract and in the liver (Hollman and Katan, 1998; Spencer et al., 1999; Kuhnle et al., 2000). These compounds occur essentially as glycosides and, in general, the first stage in metabolism is likely to be deglycosylation, before absorption (Spencer et al., 1999; Day et al., 2000). During transfer across the small intestine and, subsequently, in the liver, flavonoids undergo O-methylation of catechol-containing phenolics, and other conjugation reactions, namely glucuronidation and sulphation. The structures of the flavonol quercetin and of its 3′-O-methylated derivative (isorhamnetin) and of their glucuronide and sulphate conjugated derivatives are presented in Figure 1. Conjugated flavonoids pass into the bile through entero-hepatic circulation, and may reach the colon, where microflora promote extensive modifications, including hydrolysis, ring cleavage and de-hydroxylation, forming lower molecular weight phenolics (Hollman and Katan, 1998; Aura et al., 2002).
Figure 1.
Structures of the flavonols, quercetin and isorhamnetin (3′-methylquercetin) and of their glucuronide and sulphate conjugate derivatives. Conjugate derivatives, resulting from glucuronidation and sulphation reactions can occur at any one of the flavonol hydroxyl groups (Flav-OH).
Importantly, the type and extent of the aforementioned metabolic pathways may depend on the dose, due to possible saturation effects and route of administration, as a result of first passage effects. This point is crucial for the final therapeutic effect, as the modifications on the flavonoid structure can change their biological activity, including their redox potential. Several studies have shown that the flavonol quercetin (3,5,7,3′,4′-pentahydroxyflavone) is metabolized in vivo (Day et al., 2001; Mullen et al., 2002), yielding derivatives that exhibit antioxidant activity (da Silva et al., 1998; Manach et al., 1998; Morand et al., 1998; Yamamoto et al., 1999; Moon et al., 2001). Nevertheless, the structures of the metabolites were not fully determined, that is, the positions of conjugation for many metabolites were not identified, and their influence on the antioxidant activity was not established.
Quercetin (Figure 1) is one of the most potent dietary antioxidants known, and the most common flavonoid in human diet, present in high concentrations in various fruits and vegetables (Hertog et al., 1992). In a previous report (Justino et al., 2004), we studied the in vivo metabolic profile of quercetin after the administration of a single high dose to rats by gavage. The nature of the resulting metabolites, as well as their conjugation positions, was identified, and structure–antioxidant activity relationships were established. Notwithstanding the interest of results obtained for high acute doses, in humans this flavonol is part of the daily diet, although in varying amounts, depending on individual dietary habits. Therefore, a more physiological approach to study in vivo quercetin metabolites is through its administration in small amounts in the diet over a long period of time. This approach may provide new data on the metabolic profile of quercetin during a sustained low oral intake of this flavonol, which may help to understand its contribution to the overall antioxidant activity in vivo.
To accomplish this objective, in the present study, a low but continuous dosage regimen of quercetin was given to rats as a supplement in the regular chow, during 3 weeks, to determine which metabolites are formed and their contribution to the antioxidant activity in plasma. The characterization of the resulting metabolites was carried out using advanced analytical methods, such as HPLC with diode array detection, electrospray ionization mass spectrometry (ESI/MS) and tandem mass spectrometry (MS/MS). The antioxidant activity of plasma was evaluated, through an end point method based on the scavenging of the 2,2′-azino-bis(3-ethylbenzothiazoline sulphonate) (ABTS) radical, enzymically generated (Cano et al., 1998).
The data herein presented highlight the differences in the pattern of metabolites obtained from quercetin under sustained low-dosage regimen, when compared with a single high dose (Justino et al., 2004). The nature of the resulting metabolites, the antioxidant activity of which differs significantly, allows the establishment of structure–antioxidant activity relationships.
Methods
Animals and quercetin dosage regimen
All animal experiments were carried out with the permission of the local animal ethical committee, and in accordance with the Declaration of Helsinki. Adult male Sprague–Dawley rats (3–4 months old) were obtained from Instituto de Investigação Científica Bento da Rocha Cabral (Lisboa, Portugal). Rats were housed two per cage at ambient temperature of 22 °C, humidity between 40 and 60%, and 12 h:12 h light–dark cycle. Access to water and regular chow (60 g day−1) was given ad libitum.
Twelve animals weighing approximately 450 g were randomly divided into two groups, test animals and control animals. The regular chow of test animals was supplemented with 20 mg of quercetin per 100 mg of animal food (0.02% quercetin diet). The food intake was measured daily and an average intake of 4.2 mg day−1of quercetin was estimated. After 3 weeks, rats were anaesthetized with ethyl ether, and blood was withdrawn by cardiac puncture into heparinized tubes and stored on ice. These procedures occurred always between 0900 and 1000 hours.
Preparation of plasma samples
Blood plasma was separated by centrifugation at 800 g for 10 min at 4 °C and plasma samples from either test or control animals were combined. Each pool was acidified with 0.1 volume of 0.58 M acetic acid (final pH approximately 5), and supplemented with 100 μM diethylenetriaminepentaacetic acid to prevent the decomposition of flavonol metabolites. Samples were stored at −80 °C for further studies.
Qualitative and quantitative analysis of plasma quercetin metabolites by HPLC
The analysis of quercetin metabolites in plasma was performed as previously described (Justino et al., 2004). A surveyor HPLC system with spectrophotometric diode array detection from Thermo Fisher Scientific Inc. (Waltham, MA, USA) was used for the experiments. Separations were carried out with a LiChrosphere RP-18 (5 μm) column from Merck (Darmstadt, Germany; 250 mm × 4 mm i.d.). A binary gradient of 0.5% (v v−1) aqueous orthophosphoric acid (eluent A) and acetonitrile (eluent B), was used with a flow rate of 1.0 ml min−1 with the following profile: 15% B (0–2 min); 15–40% B (2–22 min); 40% B (22–24 min) and 40–15% B (24–32 min). The chromatograms were recorded at 370 nm.
For the study of quercetin metabolites, acidified plasma aliquots of 750 μl were incubated at 37 °C for 120 min, with 50 μl of either β-glucuronidase or/and sulphatase and without hydrolytic enzymes, as follows: (a) with 3860 U β-glucuronidase ml−1 (to determine quercetin glucuronides), (b) with 89 U sulphatase ml−1 (to determine quercetin sulphates), (c) with both enzymes (to determine quercetin sulphoglucuronides) and (d) without hydrolytic enzymes (to determine non-conjugated quercetin). In the assay with sulphatase, D-saccharic acid 1,4-lactone (4 mg ml−1) was added to inhibit the β-glucuronidase contaminant activity present in the sulphatase preparation. After the incubation period, morin in ethanol (internal standard) was added to a final concentration of 10 μM. Morin flavonol (3,5,7,2′,4′-pentahydroxyflavone) was used as an internal standard, as its hydroxylation pattern is similar to that of quercetin (3,5,7,3′,4′-pentahydroxyflavone) and its peak did not overlap with other peaks from the sample. Flavonols were then extracted with 7.5 volumes of acetone and the mixture was centrifuged for 30 min at 16 000 g. The supernatant was concentrated to half of the initial volume of plasma, approximately, under a stream of nitrogen, at room temperature. After this, 1 volume of ethanol and 2 volumes of n-hexane were added to the extract. This mixture was shaken on a vortex for approximately 1 min and then centrifuged for 10 min at 2500 g to remove lipids. The ethanol phase was concentrated sevenfold, under a stream of nitrogen. Samples were stored at −80 °C for further analysis by HPLC.
The metabolites were identified comparing the chromatograms corresponding to the assays performed in the presence and absence of hydrolytic enzymes. We tried to synthesize enzymically, standards of ishorhamnetin conjugates, which correspond to the major plasma metabolites detected in our study, but these experiments were unsuccessful. Then we performed successive plasma extractions with acetone and the extracts were further analysed by HPLC to evaluate the recovery of the metabolites. We observed, however, that a single extraction ensured an almost complete recovery of metabolites, >95%, from the samples without hydrolytic enzymes. Nevertheless, some undetectable losses may have occurred.
To calculate the areas of quercetin and isorhamnetin and their derivatives, the detector response factor of quercetin, with respect to isorhamnetin, was taken into account. From the calibration curves plotted for quercetin and isorhamnetin, the u.v./visible diode array detector response factor for quercetin relative to isorhamnetin was calculated and a value of 1.6 was found, that is, the molar absorptivity coefficient (ɛ) for quercetin is 1.6 times higher than that for isorhamnetin. Similar ɛ values were considered for quercetin and their derivatives, and for isorhamnetin and their derivatives, as the effects of conjugation on u.v./visible spectra are not significant (Williamson et al., 2000). The percentage of each metabolite and of unmetabolized quercetin was calculated from the metabolite and the quercetin areas, respectively, in the chromatogram of the plasma sample incubated without hydrolytic enzymes relative to the sum of all peak areas obtained in the same chromatogram. The concentrations (μM) of each metabolite and of unmetabolized quercetin were calculated from the corresponding peak areas (in the chromatogram of the plasma sample incubated without hydrolytic enzymes), which were further corrected taking into account the correction factor calculated relative to the internal standard, morin. This correction factor was obtained by comparing the morin peak area in the sample chromatogram with the average area obtained from the peaks resulting from several injections of a morin solution, in the same experimental conditions. From the HPLC calibration curves plotted for quercetin and isorhamnetin, the concentrations of quercetin and isorhamnetin conjugates were obtained.
MS studies
The mass spectrometric experiments were carried out on a LCQ Duo quadrupole ion trap mass spectrometer from Thermo Finnigan (San Jose, CA, USA), equipped with an ESI source. With the LCQ Duo, the flow rate through the electrospray interface was 5 μl min−1 and the instrumental parameters (sheath gas flow rate (20 AU), ionspray voltage (4.50 kV), capillary temperature (200 °C), capillary voltage (28.00 V), lens and octapole voltage) were optimized for maximum abundance of the ions of interest. The pressure, measured with the convectron gauge during electrospray experiments, was normally 0.88 Torr and the ion trap base pressure (with helium) was typically 11.2 μTorr. All mass spectrometric data were acquired in the positive ionization mode.
Full-scan mass spectra were measured using 50 ms for collection of the ions in the trap and three microscans were summed. The data for mass spectra were based on 10–100 scans. Tandem mass (MS/MS) experiments were performed to obtain fragment ion patterns. Helium was used as collision gas, and the collision energy was gradually increased until both the precursor and product ions could be observed. MS/MS spectra were measured using 200 ms for collection of the ions in the trap, and three microscans were averaged. The mass spectra data were based on 10–100 scans.
Evaluation of antioxidant activity of the non-protein fraction of rat plasma
To evaluate modifications of the plasma antioxidant capacity conferred by low molecular mass compounds, such as quercetin metabolites, a protein- and lipid-free fraction of whole rat plasma was prepared. Its antioxidant activity was estimated through the ABTS assay, expressed as Trolox equivalents per volume of plasma, as performed previously (Justino et al., 2004), and essentially as described by Cano et al. (1998). This end point method, in which ABTS radicals are pre-generated through the ABTS/H2O2/peroxidase system, has several advantages, which include enzyme availability, higher sensitivity and reproducibility. Although this method, as well as other methods used to evaluate the total antioxidant activity, lacks specificity, it is adequate to evaluate the antioxidant activity conferred by all hydrogen atom donor compounds, including quercetin metabolites. Therefore, the differences between the antioxidant activity of the non-protein fraction of plasma from test and control animals can only be attributed to quercetin metabolites.
Sample preparations
The non-protein fraction was prepared by acetone extraction (Cao et al., 1995). The plasma samples of at least six animals were pooled and diluted with 3 volumes of acetone, kept at room temperature for 20 min, with vortex mixing every 2 min, and centrifuged at 10 000 g at 4 °C for 15 min. The supernatant was concentrated, under a stream of nitrogen, and lipids were removed with 1 volume of ethanol and 2 volumes of n-hexane, as previously described. The ethanolic phase was concentrated up to a volume of one-seventh of the initial plasma volume, under a stream of nitrogen and used for the ABTS assay.
ABTS assay
The reaction mixture contained 1.7 mM ABTS, 25 μM hydrogen peroxide and 5 nM horseradish peroxidase in 50 mM sodium phosphate buffer (pH 7.4) in a total volume of 3 ml. ABTS, horseradish peroxidase and H2O2 solutions were prepared immediately before use and protected from light. The assay was maintained at 25 °C with continuous stirring, and the reaction was monitored at 730 nm, using a UNICAM u.v./visible spectrophotometer, until a stable absorbance, due to the ABTS radical formation, was obtained. Different amounts of protein- and lipid-free extract, or Trolox, were subsequently added and the decrease in absorbance, after each addition, was determined. From the calibration curve, correlating the decrease of absorbance with the Trolox concentration, the antioxidant activity (absorbance decrease) observed for each sample volume was expressed as Trolox equivalents (nmol). The slope of the plot of Trolox equivalents, against plasma extract volumes, gives the antioxidant activity per volume of the non-protein fraction of plasma. In addition, for comparative purposes, the antioxidant activity of the compounds quercetin and isorhamnetin was also evaluated as Trolox equivalents.
Statistical analysis
Data are expressed as mean and s.d. The level of significance chosen was P<0.05. To test whether the differences between the mean values were significant, the Student's t-test was used.
Chemicals
All the reagents were of the highest quality available and were used as supplied. Flavonols quercetin, and morin, D-saccharic acid 1,4-lactone, ABTS in the crystallized diammonium salt form, β-glucuronidase (EC 3.2.1.31 from Escherichia coli), H-1 sulphatase (EC 3.1.6.1 from Helix pomatia), horseradish peroxidase and diethylenetriaminepentaacetic acid were obtained from Sigma Chemical Company (St Louis, MO, USA). Flavonol 3′-methylquercetin (isorhamnetin) was from Extraynthese (Genay, France). Acetone, ethanol, methanol and o-phosphoric acid were from Panreac (Barcelona, Spain). Hydrogen peroxide (30% v v−1) and acetonitrile, HPLC grade, were from Riedel-de Häen (Hanover, Germany). Heparin was from B Braun (Queluz de Baixo, Portugal) and 2-carboxyl-2,5,7,8-tetramethyl-6-cromanol (Trolox) was from Aldrich (Steinheim, Germany). All other chemicals were from Merck (Darmstadt, Germany).
Results
Analysis of plasma quercetin metabolites by HPLC
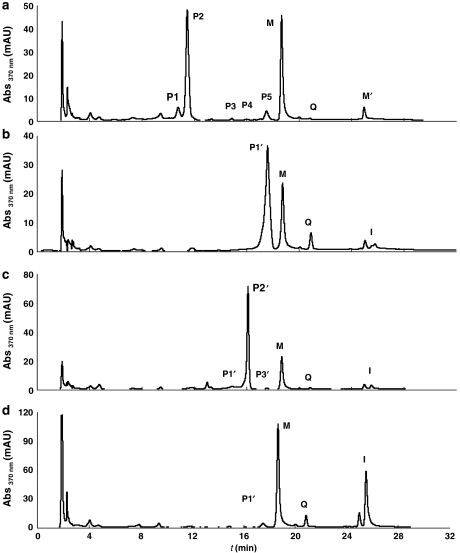
To study the in vivo metabolism of quercetin, rats were treated with a low-dose regimen of this flavonol over 3 weeks. After this period of time, plasma samples were taken and further analysed by HPLC after enzymic hydrolysis. The comparison of the chromatographic profiles of samples not incubated with enzymes with those obtained from samples incubated with hydrolytic enzymes allows the qualitative and quantitative analysis of major circulating quercetin metabolites. Figures 2a–d show representative chromatograms of plasma from test animals incubated without hydrolytic enzymes, with β-glucuronidase, with sulphatase and with β-glucuronidase and sulphatase. The analysis of plasma samples from control rats (animals receiving regular chow not supplemented with quercetin) did not show any trace of quercetin metabolites.
Figure 2.
Analysis of plasma quercetin metabolites. Representative HPLC chromatograms show the effect of deconjugating enzymes on the quercetin metabolite profile of plasma samples from test animals, incubated in the absence (a) or presence of hydrolytic enzymes β-glucuronidase (b), sulphatase (c) and β-glucuronidase and sulphatase (d). Q, quercetin; I, isorhamnetin; M, morin (internal standard); M′, contaminant present in the morin reagent; P1–P5, conjugated metabolites. Samples were analysed as described in Methods.
Chromatogram a, resulting from the HPLC analysis of a plasma sample without hydrolytic enzymes, shows five peaks consistent with species more polar than quercetin (P1–P5); these five peaks correspond to five different quercetin metabolites and Q represents the non-metabolized quercetin. In chromatograms b–d, a peak corresponding to isorhamnetin (I), the 3′-methoxy derivative of quercetin, appears with a retention time (tr) of 25.50 min.
Peaks present in chromatogram a but absent in b–d (peaks P1, tr=10.75 min, and P2, tr=11.45 min) most likely correspond to quercetin/isorhamnetin glucuronide sulphate conjugates. This is in agreement with the increase in the P1′ (chromatogram b) and P2′′ (chromatogram c) peak areas, corresponding to sulphate and glucuronide conjugates, respectively. In chromatogram d, the peak corresponding to isorhamnetin is significantly higher than the one observed for quercetin, showing that quercetin is mainly metabolized to isorhamnetin derivatives. P1 and P2 are, therefore, attributed to glucuronide sulphate conjugates of quercetin and isorhamnetin, respectively.
The overlapping peaks in chromatograms a and c, and absent in b and d, correspond to quercetin/isorhamnetin glucuronides. This applies to peaks P3 and P4 in a, that overlap peaks P1′′ and P2′′ in c, with relative retention times (t′r) of 0.79 and 0.86, respectively. P3 and P4 may therefore correspond to quercetin and isorhamnetin glucuronides, respectively.
The overlapping peaks in chromatograms a and b, and absent in c and d, correspond to quercetin/isorhamnetin sulphates. The only peak in these conditions is the peak P5 (tr=17.50 min), which overlaps peak P1′ (b) corresponding to the isorhamnetin sulphate (resulting from the hydrolysis of the isorhamnetin glucuronide sulphate by β-glucuronidase). Previous in vitro studies of quercetin and isorhamnetin sulphation showed that quercetin and isorhamnetin sulphates correspond to peaks with very close retention times (Justino et al., 2004). Therefore, peak P5 can also be attributed to a quercetin sulphate. This premise is in agreement with the fact that a peak corresponding to quercetin appears in chromatogram c, the relative area of which is greater than the one in a. Chromatograms c and d also exhibit small peaks, P3′′ (tr=17.53 min) and P1′′′ (tr=17.51 min), both corresponding to quercetin sulphates, which are due to the low hydrolytic activity of the sulphatase enzyme (Justino et al., 2004).
In summary, and taking into account the approaches described in the Methods for the quantitative analysis, it can be established that most of the quercetin (99.4%) was metabolized and only a small amount of free quercetin was present in plasma. Quercetin was mainly metabolized to isorhamnetin (88%), of which 87% corresponded to glucuronide sulphate conjugate(s), the major quercetin metabolite(s) in plasma (4.2±0.24 μM).
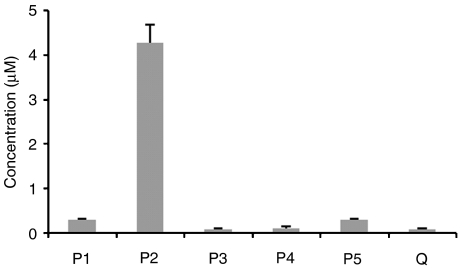
In addition, an isorhamnetin glucuronide and a quercetin glucuronide are also formed, in very small amounts. Sulphate (0.26±0.01 μM) and glucuronide sulphate (0.24±0.02 μM) conjugates of quercetin are other minor quercetin derivatives. Figure 3 shows the circulating metabolites in rat plasma after a 3-week period, during which quercetin intake was estimated to be 4.2 mg day−1.
Figure 3.
Circulating concentrations of quercetin metabolites formed after administration of quercetin to rats during 3 weeks. Q, quercetin; P1–P5, conjugate metabolites (P1, quercetin glucuronide sulphate conjugate(s); P2, isorhamnetin glucuronide sulphate conjugate(s); P3, quercetin glucuronide; P4, isorhamnetin glucuronide; P5, quercetin sulphate). The quantitative analysis of quercetin metabolites was performed based on the chromatograms obtained from the HPLC analysis of a pool of, at least, six test plasma samples (without hydrolytic enzymes) and using the flavonol morin as an internal standard. The data presented are the means±s.d. of four independent experiments.
Analysis of plasma isorhamnetin glucuronide and sulphate conjugate(s) by MS techniques
Electrospray MS spectra of plasma samples from control and test animals exhibit mainly very intense matrix peaks (not shown). Nevertheless, low-intensity peaks, corresponding to protonated molecules of quercetin and ishoramnetin metabolites, could be detected in the spectra of the plasma samples of test animals. These peaks were observed at m/z 925, 829, 669 and 653 for isorhamnetin conjugates, namely isorhamnetin triglucuronide sulphate (IG3S), isorhamnetin diglucuronide disulphate (IG2S2), isorhamnetin diglucuronide (IG2) and isorhamnetin glucuronide disulphate (IGS2), respectively. The peaks observed at m/z 1007, 911, 831 and 655 can be ascribed to quercetin conjugates, that is, quercetin tetraglucuronide (QG4), quercetin triglucuronide sulphate (QG3S), quercetin triglucuronide (QG3), quercetin diglucuronide (QG2) and quercetin trisulphate (QS3), respectively.
HPLC and MS data suggest that isorhamnetin and quercetin derivatives may exist as such in solution. Isorhamnetin conjugates are the major quercetin metabolites (88%). The HPLC chromatogram peak P2 (glucuronide sulphate conjugate(s) of ishoramnetin) corresponds to 87% and the peak P4 (isorhamnetin glucuronide) corresponds to 1% of the total metabolites present in the plasma of rats to which quercetin was administrated for 3 weeks. ESI/MS/MS studies were therefore focused on the peaks observed at m/z 925, 829, 669 and 653 ascribed to protonated molecules of isorhamnetin metabolites, IG3S, IG2S2, IG2 and IGS2, respectively, to obtain further structural information.
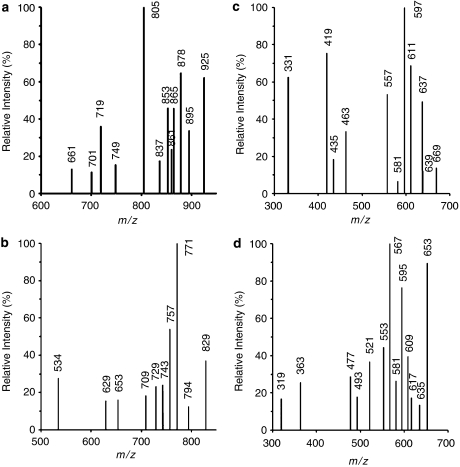
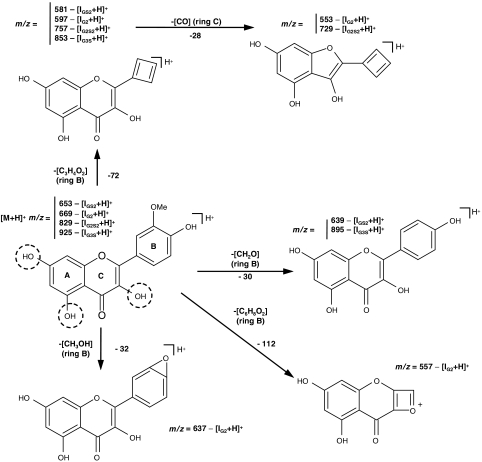
In the MS/MS spectrum (Figure 4a) of the protonated isorhamnetin derivative, IG3S (m/z 925), the peaks at m/z 895 and 853 are ascribed to B-ring fragmentations, that is, elimination of CH2O and C3H4O2, respectively, suggesting that conjugation should not occur at 4′-OH. The other peaks in the MS/MS spectrum are ascribed to losses from glucuronide residues. The main fragmentation patterns proposed for the protonated isorhamnetin derivative, IG3S (m/z 925), are depicted in Scheme 1. Losses from one glucuronide residue, indicated between parentheses, correspond to the peaks at m/z 879 (CH2O2), 865 (C2H4O2), 861 (CH2O2+H2O), 837 (C3H4O3) and 805 (C4H8O4), the latter peak being the most intense in the spectrum, leading to a very stable fragment ion, and m/z 749 (an entire glucuronide group). The peaks at 719, 701 and 661 are ascribed to C7H10O7, C7H12O8 and C9H12O9 losses, respectively, from two glucuronide residues, and suggest that the two glucuronides are linked to each other. As it is from the fragmentation patterns that conjugation positions can be inferred, for simplicity, the glucuronide (G) and sulphate (S) residues, which can replace the free hydroxyl groups of isorhamnetin, are not represented in the fragmentation in Scheme 1.
Figure 4.
Electrospray ionization tandem mass spectrometry (ESI/MS/MS) spectra from protonated isorhamnetin metabolite [M+H]+ ions. (a) Protonated isorhamnetin triglucuronide sulphate [IG3S+H] at m/z=925; (b) protonated isorhamnetin diglucuronide disulphate [IG2S2+H] at m/z 829; (c) protonated isorhamnetin diglucuronide [IG2+H] at m/z 669 and (d) protonated isorhamnetin glucuronide disulphate [IGS2+H] at m/z 653.
Scheme 1.
Main fragmentation patterns proposed for protonated isorhamnetin metabolites obtained by ESI/MS/MS. Protonated isorhamnetin triglucuronide sulphate [IG3S+H] at m/z=925; protonated isorhamnetin diglucuronide disulphate [IG2S2+H] at m/z=829; protonated isorhamnetin diglucuronide [IG2+H] at m/z=669 and protonated isorhamnetin glucuronide disulphate [IGS2+H] at m/z=653. For simplicity, the glucuronyl and sulphate residues, conjugated to the hydroxyl groups of quercetin, are not represented, and the most plausible conjugation positions are marked with dashed circles.
The MS/MS spectrum (Figure 4b) of the protonated isorhamnetin derivative, IG2S2 (m/z 829), exhibits peaks at m/z 757 and 729 that are ascribed to ring fragmentations. The former peak corresponds to a C3H4O2 loss from the B-ring, whereas the latter peak may result from the loss of C3H4O2 from the B-ring together with CO loss from the C-ring (Scheme 1). The losses from B-ring again suggest that 4′-OH group is not a preferred position for conjugation. The other peaks in the MS/MS spectrum are ascribed to losses from glucuronide and/or sulphate residues. Losses from one glucuronide residue, indicated between parentheses, correspond to the peaks at m/z 794 (H2O+OH) and 771 (C2H2O2), the latter being the most intense peak in the spectrum leading to a very stable fragment ion, m/z 743 (C3H2O3), 709 (C4H8O4) and 653 (one entire glucuronyl unit). The peaks at m/z 629 and 534 are ascribed to losses from both glucuronide and sulphate residues and from two glucuronide residues, respectively, that is, C4H8O4+SO3 and C10H15O10, respectively. These observations suggest, similarly to what has been observed for IG3S, that the two glucuronide groups are attached to each other and, moreover, that they are not linked to the sulphate.
In the MS/MS spectrum (Figure 4c) of the protonated isorhamnetin derivative, IG2 (m/z 669), the peaks at m/z 639, 637, 597 and 557 are ascribed to B-ring fragmentations, that is, elimination of CH2O, CH3OH, C3H4O2 and C6H8O2, respectively, suggesting that conjugation should occur at either 5-OH or 7-OH groups. The peak at m/z 597 is the most intense in the MS/MS spectrum (Scheme 1). The other peaks in the spectrum are ascribed to losses from glucuronide residues. Losses from one glucuronide residue correspond to the peaks at m/z 611 (C2H2O2) and 581 (C3H4O3). The peaks at m/z 463, 435, 419 and 331 ascribed to C7H10O7, C7H10O7+CO, C7H10O7+CO2 and C11H14O12 losses, respectively, from two glucuronide residues, again suggest that the two glucuronides are attached to each other and can be conjugated at either 5-OH or 7-OH groups.
The MS/MS spectrum (Figure 4d) of the protonated isorhamnetin derivative, IGS2 (m/z 653), exhibits peaks at m/z 581 and 553, ascribed to ring fragmentations. The former peak corresponds to a C3H4O2 loss from the B-ring, whereas the latter may result from the loss of C3H4O2 from the B-ring together with the loss of CO from the C-ring (Scheme 1). The losses from B-ring again suggest that 4′-OH group is not a preferred position for conjugation. The other peaks in the MS/MS spectrum are ascribed to losses from glucuronide and/or sulphate residues. Losses from the glucuronide residue, indicated between parentheses, correspond to the peaks at m/z 635 (H2O), 617 (2H2O), 609 (CO2), 595 (C2H2O2) and 567 (C3H2O3), the latter two being the most intense peaks in the spectrum, leading to very stable fragment ions, m/z 521 (C4H4O5) and 477 (one entire glucuronyl unit). The peak at m/z 493, ascribed to the loss of 2SO3, suggests that two sulphate groups are attached to each other, through an anhydride bond. The peaks at m/z 363 and 319 are ascribed to losses from glucuronide and sulphate residues together with aglycone moieties, that is, entire glucuronide and sulphate residues and either 2OH or OH+CHOCH3 from the aglycone, respectively. These observations suggest that the two sulphate groups are attached to each other and, moreover, that they are not linked to the glucuronide residue. This assumption is in accordance with what has been postulated for the glucuronide residues.
The data obtained further suggest that the most plausible conjugation positions are at 3-OH of C-ring and 5-OH and 7-OH of A-ring, as glucuronation and sulphation cannot occur at 4′-OH group, as it was deduced from the fragmentation pattern proposed for the protonated isorhamnetin derivative IG2 (Scheme 1).
Contribution of metabolites to the antioxidant activity of plasma
The antioxidant properties of isorhamnetin- and quercetin-conjugated derivatives present in the plasma were studied using a protein- and lipid-free fraction of whole plasma collected from rats treated with quercetin. The protein- and lipid-free extracts enable a better evaluation of the changes in antioxidant capacity that may be attributed to low molecular mass antioxidants (Cao et al., 1995), such as quercetin metabolites, which are hydrogen atom donors.
The plasma antioxidant activities from test and control rats, expressed as Trolox equivalents, as well as that of quercetin and isorhamnetin, are represented in Figure 5. The antioxidant status of the plasma collected from test animals (58±6 nmol of Trolox equiv ml−1 of plasma) was not significantly higher (P>0.1) than that of control animals (49±4 nmol of Trolox equiv ml−1 of plasma). This result shows that the circulating metabolites of quercetin exhibited very little antioxidant activity and may be explained by the extensive metabolism of quercetin into isorhamnetin (88%). As shown in Figure 5b, the methylation of the 3′-OH of the o-catechol group, giving rise to isorhamnetin, markedly decreased its antioxidant activity (2.60±0.18 nmol Trolox equiv nmol−1 of isorhamnetin) in relation to quercetin (5.16± 0.16 nmol Trolox equiv nmol−1 of quercetin). Additionally, isorhamnetin was further metabolized to glucuronide sulphate conjugates (87%), the major plasma metabolites. Quercetin conjugates were only a minor proportion of the quercetin derivatives present in plasma, and only a very small amount of free quercetin (0.6%) was circulating in plasma.
Figure 5.
Antioxidant activity of the non-protein fraction of plasma from test and control animals (a) and of quercetin and isorhamnetin compounds (b), estimated by the 2,2′-azino-bis(3-ethylbenzothiazoline sulphonate) (ABTS) assay and expressed as Trolox equivalents. The assays were performed as described in Methods. The slope of the plot of Trolox equivalents, against plasma extract volumes, gives the antioxidant activity per millilitre of the non-protein fraction of plasma. The antioxidant status of the non-protein fraction of plasma from test animals and from control animals was 58±6 and 49±4 nmol of Trolox equiv ml−1 of plasma, respectively. The values shown are means±s.d. from two independent experiments performed in triplicate, and for each experiment pools of six plasma samples from test and control animals were used.
Discussion
The HPLC analysis of metabolites in plasma of Sprague–Dawley rats, fed for 3 weeks with a diet supplemented with quercetin with an estimated average intake of 4.2 mg day−1, shows that the major metabolites of quercetin present in plasma (87%) are glucuronide sulphate conjugates of isorhamnetin (4.2±0.24 μM). From the ESI/MS/MS data, it can be inferred that isorhamnetin derivatives exist as IG3S, IG2S2, IGS2 and IG2 and that the most plausible positions for glucuronidation and sulphation are the 3-OH, 5-OH and 7-OH hydroxyl groups. These isorhamnetin conjugates are quercetin metabolites where the 3′-OH of the o-catechol group is methylated and the 3-OH hydroxyl group is involved in conjugation reactions with either glucuronic acid and/or sulphate. For flavonoids, the o-catechol group (3′,4′-OH) in the B-ring is the main structural feature for conferring a high radical scavenging activity, but the presence of both the 2,3-double bond and the 3-OH group is also important for a higher reactivity (Bors and Saran, 1987; van Acker et al., 1996; Silva et al., 2002). Therefore, in relation to the parent quercetin, the antioxidant activity of isorhamnetin derivatives is significantly decreased and these metabolites contribute very little to the total antioxidant potential of plasma. In addition, quercetin conjugates are minor quercetin derivatives, and only a very small amount of free quercetin (0.6%, 0.03±0.01 μM) is circulating in plasma. These data explain the unchanged antioxidant status of the plasma collected from test animals.
The results obtained in the present study, where a small amount of quercetin was given over 3 weeks, are noticeably in contrast with our previous study, where a bolus of this flavonol was given, by gavage (10 mg quercetin per 200 g of body weight), to rats fasted for 16 h weighing approximately 400 g (Justino et al., 2004). The concentrations of plasma metabolites herein described are smaller than the ones found in our previous study or those in other published studies (Morand et al., 1998; Manach et al., 1999). This may be attributed to the low-dosage regimen used here. The present study, however, provides a much more realistic study, in terms of the average intake of quercetin (4.2 mg day−1) supplied in the diet (0.02%), which is 10 times smaller than other amounts reported in the literature for studies with rats (Morand et al., 1998; Manach et al., 1999). In humans, several studies have been performed to estimate the daily intake of flavonoids in several countries. Large differences in consumption were observed. For flavonols in particular, the average daily consumption is the lowest in Finland (∼4 mg day−1) and generally similar for the populations studied in Denmark, Japan, Holland and USA (16–31 mg day−1) (Beecher, 1993). Few studies have been carried out to identify the plasma quercetin metabolites in humans, and quercetin intake is generally estimated from the consumption of just one meal of either fried onions (Day et al., 2001; Wittig et al., 2001) or a complex meal rich in plant products (Manach et al., 1998). Therefore, it is difficult to compare the metabolic profile obtained in this study with rats, under a prolonged low-dosage regimen, with the few data obtained from studies in humans. Nevertheless, it seems that the methylation process is less important in humans than in rats (Manach et al., 1998).
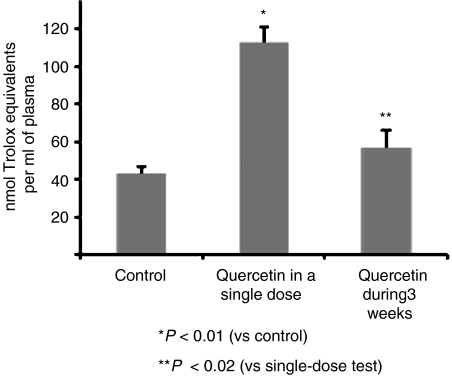
It is also noteworthy that the metabolic pattern indicated by the results of the present study contrast with the one we proposed when quercetin was given intragastrically in a single high dose (Justino et al., 2004). In that study, the resulting plasma metabolites were primarily quercetin glucuronides (37%) and quercetin glucuronide sulphate conjugates (37%) at the 5-OH and 7-OH positions. In addition, it was observed that those metabolites could significantly contribute to the antioxidant activity of plasma. Indeed, in the present study the antioxidant activity of plasma from test animals (58±6 nmol of Trolox equiv ml−1 of plasma) is significantly smaller (P<0.02) than the one obtained in test plasma of the single-dose study (113±7 nmol of Trolox equiv ml−1 of plasma) (Justino et al., 2004). These data can be clearly observed in Figure 6. Although the concentration of metabolites in the single-dose study was found to be higher (∼14 μM) than the one in the present work (∼5 μM), the increase in antioxidant activity, with respect to the control groups, is approximately 4.9 and 1.8 nmol of Trolox equiv nmol−1 of metabolite, for the single high-dose and the sustained low-dose studies, respectively. These results can be easily understood as, in case of the single dose administration of quercetin, in the majority of the resulting metabolites, the o-catechol group did not undergo conjugation reactions, and, therefore, a high contribution to plasma antioxidant activity is expected.
Figure 6.
Comparison of the antioxidant activity presented by the non-protein fractions of test plasma, obtained after quercetin administration in a single dose and after supplementation during 3 weeks, in relation to control plasma. The single dose of quercetin, dissolved in propylene glycol, was given by gavage (10 mg quercetin per 200 g of body weight) to rats weighing approximately 450 g (Justino et al., 2004). The supplemented diet (0.02% quercetin diet) was given for a 3-week period, during which the average quercetin intake was estimated to be 4.2 mg day−1. For the quercetin single-dose study, the antioxidant status of the non-protein fraction of plasma from test animals was markedly higher (P<0.01) than that from control animals. For the 3-week study, the values in control and treated animals were not significantly different (P>0.1). However, the difference between the antioxidant status for test animals in the two studies (high dose vs low dose) is significantly different (P<0.02). The values shown are means±s.d. from two independent experiments performed in triplicate, and for each experiment pools of six plasma samples from test and control animals were used. In both studies, the antioxidant status of the non-protein fractions of plasma samples was determined as described in Figure 5.
Several studies have been performed to identify plasma quercetin metabolites but only a few test their antioxidant properties. Some of these studies report that quercetin metabolites contribute to the overall antioxidant capacity of the plasma (Manach et al., 1998; Morand et al., 1998; da Silva et al., 1998), but the positions of conjugation for many metabolites were not described. Therefore, structure–antioxidant activity relationships could not be established. In a more recent study, quercetin-3-rutinoside metabolites were identified in urine, but not in plasma, due to analytical difficulties, and it was concluded that these metabolites had lower antioxidant activity (Olthof et al., 2003). Taking into account that metabolites are the potential bioactive forms in vivo, more and more studies have been carried out on quercetin metabolites regarding their antioxidant properties in vitro (Yamamoto et al., 1999; Moon et al., 2001; Janisch et al., 2004; Pollard et al., 2006). Nevertheless, the potential health-promoting properties of flavonoids, based on their antioxidant effects, have recently been challenged, due to the very low plasma concentrations achieved after dietary flavonoid intake. More likely, the protective effects of flavonoids are linked to the modulation of intracellular signalling pathways, vital to cellular function (Spencer et al., 2003; Williams et al., 2004; Scalbert et al., 2005; Bao and Lou, 2006; Angeloni et al., 2007).
It is worth mentioning that, independently of the mechanisms underlying the action of flavonoids, the biological effects of quercetin, like those of other flavonoids, rely on the activity of their metabolites due to rapid and extensive biotransformation. Information regarding which metabolites appear in plasma and in what amounts is therefore essential to the proper evaluation of their potential against pathogenesis of several diseases, such as some cardiovascular diseases, which are secondary to the condition of atherosclerosis. In this context, a recent report showed that isorhamnetin has protective effects on endothelial cell damage induced by oxidized low-density lipoproteins (Bao and Lou, 2006). These effects were obtained via activation of p38-mitogen-activated protein kinase and the antioxidant activity of isorhamnetin.
In conclusion, comparison of the data obtained in the present study with the data from our previous study shows how the mode of administration of quercetin critically affects the nature of the resulting metabolites, the antioxidant activity of which decreases significantly when the o-catechol group undergoes conjugation reactions. From these results, we can conclude that a first and crucial step for understanding the mechanisms of action of flavonoids, either as antioxidants, or modulators of cell signalling, is the knowledge of their metabolism and the influence of the metabolites on flavonoid biological effects. Nevertheless, further studies are required to evaluate the potential benefits of flavonoids, in particular to analyse their uptake from circulation by different cell types and to determine if they are metabolized intracellularly.
Acknowledgments
MR Santos and GC Justino acknowledge a PhD grant (FCT, Portugal) and MJ Rodríguez-Gómez acknowledges a Post-Doc grant (Junta de Extremadura, Consejería de Educación, Ciencia y Tecnología y el Fondo Social Europeo, Spain). We are also grateful to Professor Eduarda Fernandes for helpful discussion.
Abbreviations
- ABTS
2,2′-azino-bis(3-ethylbenzothiazoline sulphonate)
- ESI/MS
electrospray ionization mass spectrometry
- MS/MS
tandem mass spectrometry
- tr
retention time
- t′r
relative retention time
Conflict of interest
The authors state no conflict of interest.
References
- Angeloni C, Spencer JP, Leoncini E, Biagi PL, Hrelia S. Role of quercetin and its in vivo metabolites in protecting H9c2 cells against oxidative stress. Biochimie. 2007;89:73–82. doi: 10.1016/j.biochi.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Arteel GE, Sies H. Protection against peroxynitrite by cocoa polyphenol oligomers. FEBS Lett. 1999;462:167–170. doi: 10.1016/s0014-5793(99)01498-2. [DOI] [PubMed] [Google Scholar]
- Aura AM, O'Leary KA, Williamson G, Ojala M, Bailey M, Puupponen-Pimia R, et al. Quercetin derivatives are deconjugated and converted to hydroxyphenylacetic acids but not methylated by human fecal flora in vitro. J Agric Food Chem. 2002;50:1725–1730. doi: 10.1021/jf0108056. [DOI] [PubMed] [Google Scholar]
- Bao M, Lou Y. Isorhamnetin prevent endothelial cell injuries from oxidized LDL via activation of p38MAPK. Eur J Pharmacol. 2006;547:22–30. doi: 10.1016/j.ejphar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 1993;133:3248S–3254S. doi: 10.1093/jn/133.10.3248S. [DOI] [PubMed] [Google Scholar]
- Bors W, Saran M. Radical scavenging by flavonoid antioxidants. Free Radic Res Commun. 1987;2:289–294. doi: 10.3109/10715768709065294. [DOI] [PubMed] [Google Scholar]
- Cano A, Hernández-Ruíz J, García-Cánovas F, Acosta M. An end-point method for estimation of the total antioxidant activity in plant material. Phytochem Anal. 1998;9:196–202. [Google Scholar]
- Cao G, Verdon CP, Wu AH, Wang H, Prior RL. Automated assay of oxygen radical absorbance capacity with the COBAS FARA II. Clin Chem. 1995;41:1738–1744. [PubMed] [Google Scholar]
- Da Silva EL, Piskula MK, Yamamoto N, Moon JH, Terao J. Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Lett. 1998;430:405–408. doi: 10.1016/s0014-5793(98)00709-1. [DOI] [PubMed] [Google Scholar]
- Day AJ, Canada FJ, Diaz JC, Kroon PA, Mclauchlan R, Faulds CB, et al. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000;468:166–170. doi: 10.1016/s0014-5793(00)01211-4. [DOI] [PubMed] [Google Scholar]
- Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65:337–353. doi: 10.1016/s0024-3205(99)00120-4. [DOI] [PubMed] [Google Scholar]
- Filipe P, Lança V, Silva JN, Morliere P, Santus R, Fernandes A. Flavonoids and urate antioxidant interplay in plasma oxidative stress. Mol Cell Biochem. 2001;221:79–87. doi: 10.1023/a:1010944919952. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J Agric Food Chem. 1992;40:2379–2383. [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993a;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Hollman PCH, van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J Agric Food Chem. 1993b;41:1242–1246. [Google Scholar]
- Hollman PCH, Katan MB.Polyphenols absorption and metabolism Flavonoids in Health and Disease 1998Marcel Dekker: New York; 483–522.In: Rice-Evans C, Packer L (eds). [Google Scholar]
- Ishige K, Schubert D, Sagara Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: modulation of LDL oxidation and binding to human serum albumin. Free Radic Res. 2004;38:877–884. doi: 10.1080/10715760410001728415. [DOI] [PubMed] [Google Scholar]
- Justino GC, Santos MR, Canario S, Borges C, Florencio MH, Mira L. Plasma quercetin metabolites: structure–antioxidant activity relationships. Arch Biochem Biophys. 2004;432:109–121. doi: 10.1016/j.abb.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- Kuhnle G, Spencer JP, Schroeter H, Shenoy B, Debnam ES, Srai SK, et al. Epicatechin and catechin are O-methylated and glucuronidated in the small intestine. Biochem Biophys Res Commun. 2000;277:507–512. doi: 10.1006/bbrc.2000.3701. [DOI] [PubMed] [Google Scholar]
- Manach C, Morand C, Crespy V, Demigne C, Texier O, Regerat F, et al. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- Manach C, Texier O, Morand C, Crespy V, Regerat F, Demigne C, et al. Comparison of the bioavailability of quercetin and catechin in rats. Free Radic Biol Med. 1999;27:1259–1266. doi: 10.1016/s0891-5849(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters—radical scavengers or metal chelators. FEBS Lett. 1996;392:40–44. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- Mira L, Fernandez MT, Santos M, Rocha R, Florencio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- Mira L, Silva M, Manso CF. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem Pharmacol. 1994;48:753–759. doi: 10.1016/0006-2952(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Mira L, Silva M, Rocha R, Manso CF. Measurement of relative antioxidant activity of compounds: a methodological note. Redox Rep. 1999;4:69–74. doi: 10.1179/135100099101534666. [DOI] [PubMed] [Google Scholar]
- Moon JH, Tsushida T, Nakahara K, Terao J. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic Biol Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- Moran JF, Klucas RV, Grayer RJ, Abian J, Becana M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: prooxidant and antioxidant properties. Free Radic Biol Med. 1997;22:861–870. doi: 10.1016/s0891-5849(96)00426-1. [DOI] [PubMed] [Google Scholar]
- Morand C, Crespy V, Manach C, Besson C, Demigne C, Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am J Physiol. 1998;275:R212–R219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- Morel I, Lescoat G, Cillard P, Cillard J. Role of flavonoids and iron chelation in antioxidant action. Methods Enzymol. 1994;234:437–443. doi: 10.1016/0076-6879(94)34114-1. [DOI] [PubMed] [Google Scholar]
- Mullen W, Graf BA, Caldwell ST, Hartley RC, Duthie GG, Edwards CA, et al. Determination of flavonol metabolites in plasma and tissues of rats by HPLC-radiocounting and tandem mass spectrometry following oral ingestion of [2-(14)C]quercetin-4′-glucoside. J Agric Food Chem. 2002;50:6902–6909. doi: 10.1021/jf020598p. [DOI] [PubMed] [Google Scholar]
- Olthof MR, Hollman PC, Buijsman MN, van Amelsvoort JM, Katan MB. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J Nutr. 2003;133:1806–1814. doi: 10.1093/jn/133.6.1806. [DOI] [PubMed] [Google Scholar]
- Pollard SE, Kuhnle GG, Vauzour D, Vafeiadou K, Tzounis X, Whiteman M, et al. The reaction of flavonoid metabolites with peroxynitrite. Biochem Biophys Res Commun. 2006;350:960–968. doi: 10.1016/j.bbrc.2006.09.131. [DOI] [PubMed] [Google Scholar]
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81:215S–217S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Silva MM, Santos MR, Caroço G, Rocha R, Justino G, Mira L. Structure–antioxidant activity relationships of flavonoids: a re-examination. Free Radic Res. 2002;36:1219–1227. doi: 10.1080/198-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Boyd C, Spencer JP, Williams RJ, Cadenas E, Rice-Evans C. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging. 2002;23:861–880. doi: 10.1016/s0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr Cancer. 1996;26:167–181. doi: 10.1080/01635589609514473. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Chowrimootoo G, Choudhury R, Debnam ES, Srai SK, Rice-Evans C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999;458:224–230. doi: 10.1016/s0014-5793(99)01160-6. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Rice-Evans C, Williams RJ. Modulation of pro-survival Akt/protein kinase B and ERK1/2 signaling cascades by quercetin and its in vivo metabolites underlie their action on neuronal viability. J Biol Chem. 2003;278:34783–34793. doi: 10.1074/jbc.M305063200. [DOI] [PubMed] [Google Scholar]
- Van Acker SA, van den Berg DJ, Tromp MNJL, Griffioen DH, van Bennekom WP, van der Vijgh WJ. Structural aspects of antioxidant activity of flavonoids. Free Radic Biol Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules. Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Williamson G, Day AJ, Plumb GM, Couteau D. Human metabolic pathways of dietary flavonoids and cinnamates. Biochem Soc Trans. 2000;28:16–22. doi: 10.1042/bst0280016. [DOI] [PubMed] [Google Scholar]
- Wittig J, Herderich M, Graefe EU, Veit M. Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2001;753:237–243. doi: 10.1016/s0378-4347(00)00549-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Moon JH, Tsushida T, Nagao A, Terao J. Inhibitory effect of quercetin metabolites and their related derivatives on copper ion-induced lipid peroxidation in human low-density lipoprotein. Arch Biochem Biophys. 1999;372:347–354. doi: 10.1006/abbi.1999.1516. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free Radic Biol Med. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]