Abstract
Background and purpose:
The Na+/H+ exchange (NHE) inhibitor cariporide is known to ameliorate ischaemia/reperfusion (I/R) injury by reduction of cytosolic Ca2+ overload. Leukocyte activation and infiltration also mediates I/R injury but whether cariporide reduces I/R injury by affecting leukocyte activation is unknown. We studied the effect of cariporide on thrombin and I/R induced leukocyte activation and infiltration models and examined P-selectin expression as a potential mechanism for any identified effects.
Experimental approach:
An in vivo rat mesenteric microcirculation microscopy model was used with stimulation by thrombin (0.5 μ ml−1) superfusion or ischaemia (by haemorrhagic shock for 60 min) and reperfusion (90 min).
Key results:
Treatment with cariporide (10 mg kg−1 i.v.) significantly reduced leukocyte rolling, adhesion and extravasation after thrombin exposure. Similarly, cariporide reduced leukocyte rolling (54±6.2 to 2.4±1.0 cells min−1, P<0.01), adherence (6.3±1.9 to 1.2±0.4 cells 100 μm−1, P<0.01) and extravasation (9.1±2.1 to 2.4±1.1 cells per 20 × 100 μm perivascular space, P<0.05), following haemorrhagic shock induced systemic ischaemia and reperfusion. The cell adhesion molecule P-selectin showed a profound decrease in endothelial expression following cariporide administration in both thrombin and I/R stimulated groups (35.4±3.2 vs 14.2±4.1% P-selectin positive cells per tissue section, P<0.01).
Conclusions and implications:
The NHE inhibitor cariporide is known to limit reperfusion injury by controlling Ca2+ overload but these data are novel evidence for a vasculoprotective effect of NHE inhibition at all levels of leukocyte activation, an effect which is likely to be mediated at least in part by a reduction of P-selectin expression.
Keywords: sodium hydrogen exchange, cariporide, leukocyte activation, P-selectin, cell adhesion molecules, ischaemia/reperfusion, inflammation, haemorrhagic shock
Introduction
Although early reperfusion of ischaemic tissue is a desirable therapeutic goal, reperfusion itself contributes to additional injury. ‘Reperfusion injury' has been partly attributed to neutrophil infiltration and subsequent release of proteases and oxygen-derived radicals, resulting in severe tissue injury (Menown and Adgey, 2001).
One of the major mechanisms causing tissue injury after reperfusion is an intracellular Ca2+ overload (Przyklenk et al., 1999) resulting in myocardial contracture and irreversible tissue injury (necrosis) and apoptosis. Two transmembrane proteins regulate intracellular pH: the Na+/H+ and the Na+/HCO3− transporters. Ischaemia and reperfusion lead to intracellular acidification and both transporters are activated. While the intracellular protons are exchanged for extracellular Na+ via the Na+/H+-exchanger (NHE), the rising intracellular Na+ produces an increase in intracellular Ca2+ via the NHE. Thus, intracellular Ca2+ overload ensues (Baartscheer et al., 2003). This Ca2+ excess leads to the generation of superoxide anions by converting xanthine dehydrogenase to xanthine oxidase, and these reactive oxygen species, as well as the intracellular Ca2+ increase itself, can be responsible for severe cellular injury.
In addition to direct injury, the increasing intracellular Ca2+ also acts as second messenger. It contributes to the generation and release of proinflammatory cytokines (for example, interleukin 6 and tumour necrosis factor-α) following nuclear transcription factor-κB translocation, and acts as a messenger for the expression of adhesion molecules such as intracellular adhesion molecule 1 (Niessen et al., 2002), L-selectin (Kao and Millette, 2007) and P-selectin (Huck et al., 2007). Not only does this calcium flux induce adhesion molecule expression, but thrombin, a coagulation and inflammatory cascade mediator, is also known to drive P-selectin expression (Cleator et al., 2006).
Adhesion molecule expression results in profound changes in endothelial and leukocyte function, resulting ultimately in leukocyte infiltration. P-selectin is a key molecule and is involved in the early stages of the leukocyte–endothelium adhesion cascade, and promotes leukocyte rolling, which enables adherence to the endothelium and subsequent trans-endothelial migration. This recruitment of leukocytes into injured tissue contributes further to tissue injury in inflammatory states (Hayward et al., 1999). Previous studies (Redlin et al., 2001; Wang et al., 2006) have shown an interaction between NHE and the leukocyte activation/accumulation cascade (including influencing other adhesion molecules; Wang et al., 2006), but none has examined the link between calcium and thrombin-induced P-selectin expression with leukocyte transmigration and control of these events by inhibition of NHEs.
Na+/H+-exchangers are membrane-bound proteins that transport H+ and Na+ in an electro-neutral manner, utilizing the trans-membrane Na+ gradient as their driving force. Six mammalian isoforms of NHE (NHEs 1–6) have been identified (Avkiran and Marber, 2002). NHEs 1 and 6 are ubiquitous in tissues with high mitochondrial content, whereas NHEs 2–5 are expressed to a lesser extent. The Na+/H+ exchange inhibitor, cariporide (4-isopropyl-3-methylsulphonyl-bensonyl-guanidine-methanesulphonate), inhibits the most abundant subtype-1 isoform of NHE and has been shown to produce cardioprotection in both animals and humans (Buerke et al., 1999; Rupprecht et al., 2000).
The aim of this study was to evaluate whether the Na+/H+ exchange inhibitor, cariporide, could influence leukocyte–endothelial cell interactions and P-selectin expression in the rat mesenteric microvasculature after inflammatory activation with either thrombin superfusion or haemorrhage and re-infusion. This model of inflammatory activation has similarities with a wide spectrum of inflammatory states in man, from ischaemia/reperfusion (I/R) to haemorrhagic shock, and systemic inflammatory response states such as disseminated intravascular coagulation.
Methods
Intravital microscopy of the rat mesentery
Sixty-eight male Sprague–Dawley rats (weight range 250–275 g) were anaesthetized with sodium pentobarbital (60 mg kg−1) injected intraperitoneally. Assessment of anaesthesia was made using vital signs, absence of spontaneous ventilation and neuromotor indicators. A tracheotomy was performed to maintain a patent airway throughout the experiment. A polyethylene catheter was inserted into the left carotid artery. Mean arterial blood pressure (MABP) was continuously recorded on a Hellige Servomed blood pressure and heart rate recorder using a Medex pressure transducer. All experiments were approved by the State and University Animal Care Committee.
A loop of ileal mesentery was exteriorized through a midline incision and placed in a temperature-controlled, fluid-filled plexiglas chamber for observation of the mesenteric microcirculation by intravital microscopy (Borders and Granger, 1984). The ileum and mesentery were superfused throughout the experiment with a buffer–saline solution (containing (mmol l−1): 140 Na+, 4 K+, 2.5 Ca2+, 1 Mg2+, 106 Cl−, 45 HCO3−) warmed to 37 °C and bubbled with 95% N2 and 5% CO2. A microscope with a × 40 water-immersion lens was used to visualize the mesenteric microcirculation and the mesenteric tissue. The image was projected by a CCD video camera onto a high-resolution monitor, and the images were recorded with a videocassette recorder. All images were then analysed using computerized imaging software. Red blood cell velocity was determined online using an optical Doppler velocimetre. This method gives an average red blood cell velocity, which is digitally displayed on a metre, and allows the calculation of shear rates. Red blood cell velocity (V) and venular diameter (D) were used to calculate venular shear rate (g), employing the formula g=8 (Vmean/D), where (Vmean=V/1.6) (Borders and Granger, 1984).
The rats were allowed to stabilize for 20–30 min following surgery. Thereafter, a 30–50 μm diameter post-capillary venule was chosen for observation. The number of rolling and adherent leukocytes was determined off-line by analysing the videocassette recording. Leukocytes (principally neutrophils) were considered to be rolling if they were moving at a velocity significantly slower than the red blood cells. Leukocyte rolling is expressed as the number of cells moving past a designated point per minute (that is, leukocyte flux). A leukocyte was judged to be adherent if it remained stationary for >30 s. Adherence is expressed as the number of leukocytes adhering to the endothelium in 100 μm of vessel length. In order to quantify the number of transmigrated leukocytes, the tissue area adjacent to the chosen 100-μm length of post-capillary venule up to a distance of 20 μm from the vessel wall was analysed. The number of extravasated leukocytes was counted and normalized with respect to this area.
Thrombin superfusion protocol
Rats were randomly divided into four groups as follows: (1) control group (mesenteries superfused with buffer alone±cariporide; 5 or 10 mg kg−1, intravenously (i.v.)); (2) rats treated with vehicle (1 ml NaCl 0.9%, i.v.) before thrombin activation; (3) rats treated with 5 mg kg−1 cariporide, i.v., before thrombin activation; (4) rats treated with 10 mg kg−1 cariporide, i.v., before thrombin activation. In groups 2 through 4, activation of the inflammatory state was achieved by the addition of thrombin (0.5 μ ml−1) to the mesenteric buffer superperfusate. A baseline recording was made to establish basal values for leukocyte rolling, adherence and transmigration (time 0) before the addition of thrombin. Video recordings were made initially, 30, 60, 90 and 120 min after initiation of perfusion, for quantification of leukocyte rolling, adherence and transmigration.
Haemorrhagic shock (I/R) protocol
Rats were subjected to global ischaemia by haemorrhagic shock through withdrawal of blood to produce an MABP of 40 mm Hg for 60 min. The blood was collected in a syringe containing heparin and kept at 37 °C until re-infusion. After 60 min, rats were resuscitated by re-infusion of the shed blood. This procedure reliably produces I/R. Rats were randomly assigned to one of the following four experimental groups: (1) sham-operated rats (that is, undergoing an identical surgical mesenteric externalization procedure, but without I/R) treated with 10 mg kg−1 cariporide; (2) I/R rats treated with vehicle (1 ml 0.9% NaCl 5 min before reperfusion); (3) I/R rats treated with 5 mg kg−1 cariporide 5 min prior to reperfusion; (4) I/R rats treated with 10 mg kg−1 cariporide, i.v., 5 min before reperfusion. Video recordings were made initially, 30, 60, 90, 120 and 180 min after initiation of perfusion, for quantification of leukocyte rolling, adherence and transmigration.
Immunolocalization of P-selectin in the microvasculature
Immunohistochemical localization of P-selectin was performed in ileal samples after intravital microscopy was completed. This assay was performed in three broad groups (each with five animals): (i) in buffer-only or sham-operated rats treated with 10 mg kg−1 cariporide, (ii) in thrombin- or I/R-activated rats treated with vehicle and (iii) in thrombin- or I/R-activated rats treated with 10 mg kg−1 cariporide. A segment of ileum was isolated from the intestine and fixed in 4.5% paraformaldehyde in phosphate-buffered saline (pH 7). After dehydration, the sections were embedded in paraffin and cut into 5-μm tissue sections. P-selectin was identified by the avidin/biotin immunoperoxidase technique, using a monoclonal antibody against P-selectin exposed on the endothelial cell surface. Venules were analysed per tissue section of five sections per rat examined. The proportion of venules positive for P-selectin staining is expressed as a percentage of the total number of venules.
Data analysis
All data on leukocyte rolling, adherence, transmigration and P-selectin expression, as well as arterial blood pressure and shear rates, are presented as means±s.e.mean. Data were compared by analysis of variance using post hoc analysis with Fisher's corrected protected least significant difference test, incorporating repeated measurements. Probabilities of 0.05 or less were considered statistically significant.
Materials used
The Hellige Servomed blood pressure and heart rate recorder was obtained from Hellige (Freiburg, Germany); Medex pressure transducer was from Medex Inc. (Klein-Winternheim, Germany); the microscope with a × 40 water-immersion lens was from Seiss (Goettingen, Germany); the optical Doppler velocimetre was from the Microcirculation Research Institute (College Station, TX, USA); the avidin/biotin immunoperoxidase system was from Vectasin ABC Reagent (Vector Laboratories, Burlingame, CA, USA); the monoclonal antibody against P-selectin was from Pharmingen (Hamburg, Germany). Cariporide was obtained from Aventis Pharmaceuticals (Frankfurt, Germany).
Results
Haemodynamic effects of cariporide following thrombin-induced leukocyte–endothelial cell interaction
There was no difference in the initial MABP between any of the groups following surgical procedures to expose ileal mesentery. MABPs ranged between 120 to 140 mm Hg. Neither cariporide nor thrombin (alone or in combination) produced any significant change in haemodynamic measurements of heart rate, MABP and venular shear rates throughout the 120-min observation period. Venular diameters ranged from 35 to 42 μm in all groups.
Effect of cariporide on thrombin-induced leukocyte–endothelial cell interaction
Superfusion of control rat mesenteries with buffer alone for 120 min consistently resulted in a low number of rolling (12.5±4 cells min−1), adhering (0.8–1.2 cells per 100 μm vessel length) and transmigrated leukocytes (1.8±0.8 cells per 20 × 100 μm perivascular space). Treatment of control rats with cariporide, either 5 or 10 mg kg−1 (Figure 1 , 2 and 3), did not produce any significant change in baseline parameters of leukocyte activation compared with control.
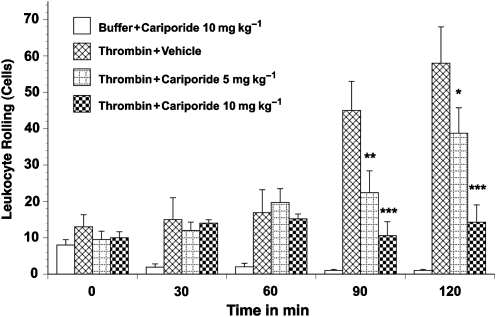
Figure 1.
Leukocyte rolling in thrombin-activated rat mesenteric venules. Superfusion of the mesentery with buffer containing 0.5 μ ml−1 thrombin significantly increased leukocyte rolling. Leukocyte rolling was significantly inhibited by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. Asterisks indicate a significant difference from the time-matched thrombin plus vehicle group, where *P<0.05, **P<0.01 and ***P<0.001. There were 5–9 rats in each group.
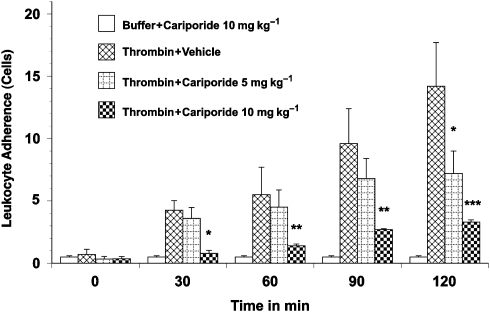
Figure 2.
Leukocyte adherence in thrombin-activated rat mesenteric venules. Superfusion of the mesentery with buffer containing 0.5 μ ml−1 thrombin significantly increased leukocyte adherence. Leukocyte adherence was significantly inhibited by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. Asterisks indicate a significant difference from the time-matched thrombin plus vehicle group, where *P<0.05, **P<0.01 and ***P<0.001. There were 5–9 rats in each group.
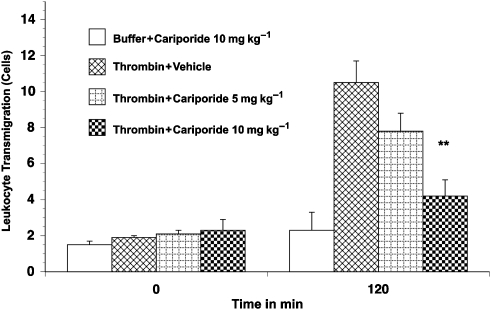
Figure 3.
Leukocyte transmigration in thrombin-activated rat mesenteric venules. Superfusion of the mesentery with buffer containing thrombin 0.5 μ ml−1 for 120 min significantly increased the number of transmigrated leukocytes. Transmigration was significantly reduced by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. ** Indicate a significant difference from the time-matched thrombin plus vehicle group (P<0.01). There were 5–9 rats in each group.
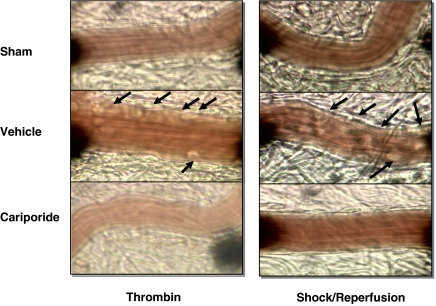
Superfusion of the rat mesentery with 0.5 μ kg−1 thrombin over 120 min resulted in a significant time-dependent increase in leukocyte rolling (18±4 to 55±8 cells min−1; Figure 1) and leukocyte adherence (Figure 2). Similarly, transmigration of leukocytes into the extravascular space (to a distance of 20 μm from the vessel wall) increased sixfold with thrombin superfusion (Figure 3). This thrombin-induced leukocyte rolling, adhesion and transmigration was significantly and substantially reduced by cariporide pretreatment. Furthermore, this cariporide-mediated reduction in leukocyte activation was dose-dependent (Figure 1, 2 and 3). Figure 4 shows videomicroscopy stills captured from the sham, stimulated and cariporide-treated groups in both thrombin- and haemorrhagic shock-activated models. Microvascular flow appearances were normal in sham animals. Following thrombin and simulated I/R activation, marked slowing of cells at the periphery of the vessel due to leukocyte rolling and adherence was seen. The rolling and adherence of leukocytes was abolished by treatment with cariporide in thrombin and simulated ischaemia groups.
Figure 4.
Representative videomicroscopy stills showing the mesenteric microcirculation from sham (top panels), activated (middle panels) and activated, cariporide-treated (bottom panels) groups. The images on the left are from the thrombin-activated series and those on the right are from the haemorrhagic series of experiments. Vascular flow is normal in the sham group. Thrombin exposure or haemorrhagic shock activates leukocytes and endothelium, causing rolling and adherence of these cells to the vessel wall (arrows). The observed rolling and adherence of leukocytes to endothelium was abolished by cariporide treatment.
Haemodynamic effects of cariporide in haemorrhagic shock
All groups of rats exhibited similar initial MABP values in the range of 119 to 130 mm Hg. In sham-operated rats, MABP did not significantly change over the 180-min experimental period. Among the I/R rats, MABP did not differ significantly. Heart rates increased during the haemorrhage phase of the I/R experiments, but returned to baseline upon reperfusion (data not shown).
Initial venular shear rates (analogous to mesenteric blood supply) were similar in the four experimental groups. After haemorrhage, shear rates in mesenteric venules abruptly decreased to less than 5% of the observed initial values and returned to initial values upon re-infusion of shed blood. Therefore, this haemorrhagic shock model is characterized by a marked hypoperfusion of the splanchnic microvasculature during the oligaemic phase and reperfusion of the splanchnic microvasculature following re-infusion of the shed blood.
Effect of cariporide on I/R-induced leukocyte–endothelial cell interaction
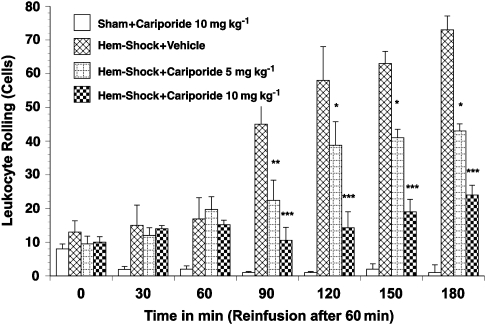
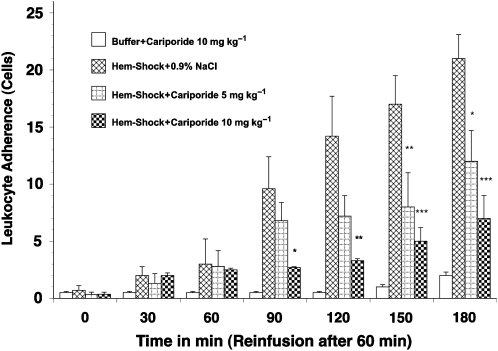
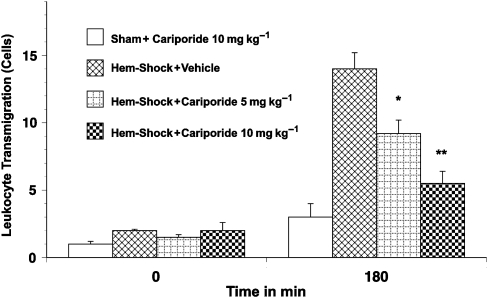
A low baseline number of rolling (5–10 cells min−1; Figure 5) and adherent cells (0.3–1.0 cells per 100 μm; Figure 6) were observed in the mesenteric microvasculature in all groups. However, the number of rolling and adherent leukocytes in I/R rats treated with vehicle exhibited a greater than fivefold increase (up to 54±6.2 rolling cells per minute and 6.3±1.9 adhering cells per 100 μm, respectively, P<0.01) after re-infusion of the shed blood (Figure 5 and 6). Similarly the number of extravasated leukocytes in the surrounding tissue was significantly increased in I/R rats given vehicle-only treatment (2.1±0.8 to 9.1±2.1 cells per 20 × 100 μm perivascular space, P<0.01; Figure 7). The administration of cariporide significantly inhibited the number of rolling (70% reduction) and adherent (60% reduction) leukocytes along the mesenteric endothelium after reperfusion (Figure 5 and 6). Similarly, cariporide (at 5 and 10 mg kg−1) significantly attenuated the number of extravasated leukocytes. At the 10 mg kg−1 dose, the degree of attenuation was from 9.1±2.1 to 2.4±1.1 cells per 20 × 100 μm perivascular space (P<0.05; Figure 7). This effect was dose-dependent since 5 mg kg−1 cariporide resulted only in less inhibition. Notably, no significant change in the total number of circulating white blood cells was observed in any of the experimental groups, hence changes in leukocyte activation could not be attributed to systemic leukopaenic effects following cariporide.
Figure 5.
Leukocyte rolling in haemorrhagic shock-induced, I/R-activated rat mesenteric venules. Rats subjected to I/R showed increased leukocyte rolling in the mesenteric microvasculature. Leukocyte rolling was significantly inhibited by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. Asterisks indicate a significant difference from the time-matched I/R plus vehicle group, where *P<0.05, **P<0.01 and ***P<0.001. There were 6–10 rats in each group. I/R, ischaemia/reperfusion.
Figure 6.
Leukocyte adherence in haemorrhagic shock-induced, I/R-activated rat mesenteric venules. Rats subjected to I/R showed increased leukocyte adherence in the mesenteric microvasculature. Leukocyte adherence was significantly inhibited by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. Asterisks indicate a significant difference from the time-matched I/R plus vehicle group, where *P<0.05, **P<0.01 and ***P<0.001. There were 6–10 rats in each group. I/R, ischaemia/reperfusion.
Figure 7.
Leukocyte transmigration in haemorrhagic shock-induced, I/R-activated rat mesenteric venules. Rats subjected to I/R showed increased leukocyte transmigration in the mesenteric microvasculature. Transmigration was significantly inhibited by pretreatment with 10 mg kg−1 cariporide. Values are means±s.e.mean. Asterisks indicate a significant difference from the time-matched I/R plus vehicle group, where *P<0.05 and **P<0.01. There were 6–10 rats in each group. I/R, ischaemia/reperfusion.
Immunolocalization of P-selectin in the rat mesenteric microvasculature
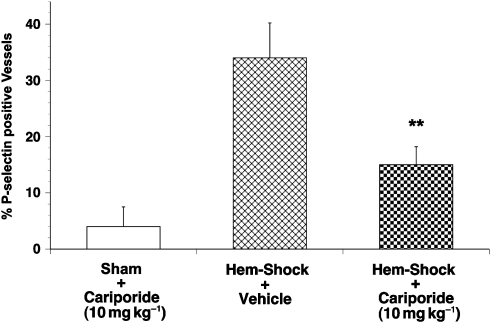
Figure 8 illustrates the immunohistochemical data for P-selectin expression from the I/R series. The percentage of venules staining positively for P-selectin in rats subjected to a sham surgical procedure was consistently low (that is, <2%). In contrast, P-selectin expression on the venular endothelium was significantly increased by I/R stimulation (up to 36.6±3.9% P-selectin-positive cells, P<0.05). Treatment with cariporide (10 mg kg−1) caused a significant reduction (by over half) of this P-selectin expression by the endothelium to 15.8±2.9% of cells compared with vehicle (P<0.01).
Figure 8.
P-selectin expression in haemorrhagic shock-induced, I/R-activated rat mesenteric venules. Columns show the percentage of positively staining venules expressed as mean±s.e.mean. Vehicle-treated animals had a substantial rise in expression from control levels. Treatment with cariporide significantly reduced expression compared with vehicle-treated animals (**P<0.01). Five rats were studied in each group and 20 sections were studied in each rat. I/R, ischaemia/reperfusion.
The thrombin stimulation model yielded similar results. Buffer-perfused (control) animals had low levels of P-selectin expression (<2%). Thrombin activation produced a profound increase in P-selectin expression to 25.3±1.9% of cells (P<0.05). Again, cariporide treatment significantly reduced the thrombin-induced P-selectin expression by approximately half to 13.4±2.7% cells (P<0.01).
Discussion and conclusions
Cariporide is known to limit reperfusion injury by controlling intracellular Ca2+ overload (ten Hove et al., 2003). Leukocyte infiltration is a recognized feature of reperfusion injury (Repo and Harlan, 1999) and we speculated that cariporide may exert some of its beneficial effects by affecting the leukocyte–endothelial cell interaction via a mechanism involving changes in P-selectin expression.
Our data show that NHE 1 inhibition with cariporide results in a significant and substantial reduction in all stages of leukocyte–endothelial cell interaction (that is, rolling, adhesion and transmigration) in an in vivo model eliciting activation using thrombin stimulation or a haemorrhagic shock/reperfusion model. The use of intravital microscopy allowed a direct determination of the separate steps of the leukocyte–endothelial cell interaction. We observed that the attenuation of leukocyte activation by cariporide is dose-dependant. Furthermore, we showed that the effect of cariporide on leukocyte activation is associated with suppression of P-selectin expression on the endothelial surface. Importantly, we found that the effects of cariporide occurred without any significant alterations in local microvascular flow changes (such as shear rates) or systemic changes (such as altered haemodynamic state or leukopaenia).
The cellular mechanisms underlying ischaemia reperfusion injury have received much attention (Avkiran and Marber, 2002). Hypoxia leads to ATP depletion and cytosolic acidosis. Cells attempt to deal with the acidosis using the NHE, which results in increasing intracellular sodium. The Na+/Ca2+ antiporter (which normally exports Ca2+) is recruited in a reversed direction to remove sodium, but this results in cytosolic Ca2+ overload, which is central to reperfusion injury. Interestingly, thrombin stimulation (such as employed in the present study) is known to produce a similar sequence of intracellular Ca2+ overload and subsequent tissue injury (Lorant et al., 1991). Following reperfusion, the Ca2+ leads to contracture injury and a cascade of protease-driven redox reactions, resulting in hypoxanthine production and formation of superoxide anion, each of which mediates the deleterious effects of reperfusion injury. NHE inhibition with agents such as cariporide is known to limit or abolish this Ca2+ overload (Lee et al., 2005), and it is via this mechanism that cariporide is assumed to have conferred cardioprotection in numerous animal studies (Castella et al., 2003; Kristo et al., 2004; Davies et al., 2005; Fantinelli et al., 2006). Evidence for NHE inhibition mediating cardioprotective effects in human studies (Rupprecht et al., 2000; Theroux et al., 2000) has been conflicting and is at odds with the animal data, a fact that is somewhat surprising given the relative consistency with which the cellular apparatus that mediates reperfusion injury is conserved between species (Pan et al., 1998). Some of the observed differences between the positive animal studies and negative human studies may relate to the point of NHE inhibition in the ischaemia reperfusion timescale.
In addition to this ‘classical' mechanism of injury, the importance of leukocyte activation and migration into the ischaemic tissue as a contributor to reperfusion injury is being realized (Marshall and Haskard, 2002). Leukocyte migration through the endothelium is a key step in the inflammatory response known to occur in various pathological states like trauma, I/R and shock (McIntyre et al., 2003). Having followed a chemotactic gradient, migrated leukocytes unleash a further cascade of injurious effects within the reperfused tissue. Systemic neutropoenia has long been recognized as a potent cause of cardioprotection in various pathological states (Romson et al., 1983). The carefully choreographed sequence of leukocyte activation involving capture, rolling, adhesion and transmigration, and this sequence, are dependent upon the de novo expression or release of previously synthesized cell adhesion molecules such as selectins and integrins. Selectins mediate leukocyte capture and rolling, whereas integrins facilitate firm adhesion and transmigration through the endothelium (Lefer, 2000). Of the selectins, P-selectin seems to be an early and important mediator in this process. Both I/R and thrombin exposure are known to enhance P-selectin expression on endothelial cell surfaces (Lorant et al., 1991). In fact, I/R and thrombin both produce intracellular hypercalcaemia (Baartscheer et al., 2003; Cleator et al., 2006), and previous data show that the Ca2+ overload behind these insults provide the signalling for calcium-induced adhesion molecule recruitment. Thus, I/R and thrombin both cause intracellular calcium overload, which provides signal for the expression of adhesion molecule, including changes in intracellular adhesion molecule 1 synthesis (may be mediated by nuclear transcription factor-κB) (Sun et al., 2001) and, similarly, increased P-selectin expression by its inclusion into Weibel–Palade bodies and subsequent transport to the cell surface for expression. These events explain one way in which tissue stress can lead to leukocyte infiltration and hence injury.
Evidence shows that NHE inhibition affects cell–cell and cell–endothelial call interactions. Activation of cellular components such as polymorphonuclear leukocytes (Gewirtz et al., 1998), monocytes (Kaloyianni et al., 2005) and platelets (Lee et al., 2006), as well as the endothelial component (Gumina et al., 2001) of cell migration, are inhibited by cariporide. The effect of the cariporide inhibition of these various components is to inhibit leukocyte migration following reperfusion in many tissue types, an effect that has been shown in several studies (Gumina et al., 2000).
Although an effect of cariporide on other cell adhesion molecules has been shown, its impact on P-selectin was hitherto unknown. Previous studies have demonstrated an effect of cariporide on L-selectin (Redlin et al., 2001) and intracellular adhesion molecule 1 (Hattori et al., 2001; Wang et al., 2006) expression following inflammatory stimulation. Other groups have used specific pharmacological inhibitors of these and other adhesion molecules to produce cardioprotection (Fukushima et al., 2006). Thus, previous data clearly show that I/R and thrombin cause calcium signalled adhesion molecule expression and, hence, leukocyte infiltration with tissue injury, and that these effects can be reduced by NHE inhibition (Huck et al., 2007). However, the present study is the first to show both ischaemic and inflammatory (thrombin) insults generate P-selectin expression together with all subsequent steps of leukocyte infiltration, and that this entire sequence can be inhibited (at all stages) by NHE inhibition with cariporide. Given the previous data, we propose that the effect of cariporide is, via NHE inhibition, to suppress calcium-induced P-selectin expression and hence endothelial cell–leukocyte activation. Since P-selectin activation occurs early in the leukocyte infiltration sequence, blockade of P-selectin activation may allow to control of all subsequent steps of adhesion molecule and hence leukocyte activation in inflammatory states.
In conclusion, in the past it has been shown that intracellular calcium overload results from ischaemic and proinflammatory insults (such as thrombin exposure). This calcium flux is a trigger for the expression of adhesion molecules. NHE inhibition is known to control such calcium and pH fluxes and may thereby limit selectin expression. We provide novel data linking NHE inhibition and P-selectin expression. This was shown to limit the initial ‘rolling' stage of leukocyte–endothelial call interaction and also all subsequent stages of this interaction, culminating in a reduction in leukocyte tissue accumulation and tissue injury.
This may be an important additional mechanism by which cariporide inhibits leukocyte–endothelial cell interaction in inflammatory states. Evidence is accumulating that inhibition of the NHE represents a potent tool to modulate leukocyte-induced endothelial dysfunction. In this way, leukocyte accumulation and leukocyte-mediated injury may be reduced in a wide variety of tissues (such as hepatic, pulmonary, myocardial) and disease processes (such as I/R, thrombosis and other systemic inflammatory states).
Acknowledgments
This study was supported in part by grants from the Robert Mueller-Foundation (to DP and MB), the Roux-Program and the Deutsche Forschungsgemeinschaft (Grant Bu 319/3-1). UB was supported by a state grant of Saxony-Anhalt, Germany. JMC was supported by the South Cleveland Heart Fund, UK.
Abbreviations
- I/R
ischaemia/reperfusion
- MABP
mean arterial blood pressure
- NHE
Na+/H+ exchanger
Conflict of interest
The authors state no conflict of interest.
References
- Avkiran M, Marber MS. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- Baartscheer A, Schumacher CA, van Borren MM, Belterman CN, Coronel R, Fiolet JW. Increased Na+/H+-exchange activity is the cause of increased [Na+]i and underlies disturbed calcium handling in the rabbit pressure and volume overload heart failure model. Cardiovasc Res. 2003;57:1015–1024. doi: 10.1016/s0008-6363(02)00809-x. [DOI] [PubMed] [Google Scholar]
- Borders JL, Granger HJ. An optical Doppler intravital velocimeter. Microvasc Res. 1984;27:117–127. doi: 10.1016/0026-2862(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Buerke M, Rupprecht HJ, vom Dahl J, Terres W, Seyfarth M, Schultheiss HP, et al. Sodium–hydrogen exchange inhibition: novel strategy to prevent myocardial injury following ischemia and reperfusion. Am J Cardiol. 1999;83:19G–22G. doi: 10.1016/s0002-9149(99)00316-1. [DOI] [PubMed] [Google Scholar]
- Castella M, Buckberg GD, Saleh S, Tan Z, Ignarro LJ. A new role for cardioplegic buffering: should acidosis or calcium accumulation be counteracted to salvage jeopardized hearts. J Thorac Cardiovasc Surg. 2003;126:1442–1448. doi: 10.1016/s0022-5223(03)00599-3. [DOI] [PubMed] [Google Scholar]
- Cleator JH, Zhu WQ, Vaughan DE, Hamm HE. Differential regulation of endothelial exocytosis of P-selectin and von Willebrand factor by protease-activated receptors and cAMP. Blood. 2006;107:2736–2744. doi: 10.1182/blood-2004-07-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Digerness SB, Killingsworth CR, Zaragoza C, Katholi CR, Justice RK, et al. Multiple treatment approach to limit cardiac ischemia–reperfusion injury. Ann Thorac Surg. 2005;80:1408–1416. doi: 10.1016/j.athoracsur.2005.04.022. [DOI] [PubMed] [Google Scholar]
- Fantinelli JC, Cingolani HE, Mosca SM. Na+/H+ exchanger inhibition at the onset of reperfusion decreases myocardial infarct size: role of reactive oxygen species. Cardiovasc Pathol. 2006;15:179–184. doi: 10.1016/j.carpath.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Fukushima S, Coppen SR, Varela-Carver A, Yamahara K, Sarathchandra P, Smolenski RT, et al. A novel strategy for myocardial protection by combined antibody therapy inhibiting both P-selectin and intercellular adhesion molecule-1 via retrograde intracoronary route. Circulation. 2006;114:I251–I256. doi: 10.1161/CIRCULATIONAHA.105.000794. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Seetoo KF, Simons ER. Neutrophil degranulation and phospholipase D activation are enhanced if the Na+/H+ antiport is blocked. J Leukoc Biol. 1998;64:98–103. doi: 10.1002/jlb.64.1.98. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Auchampach J, Wang R, Buerger E, Eickmeier C, Moore J, et al. Na(+)/H(+) exchange inhibition-induced cardioprotection in dogs: effects on neutrophils versus cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1563–H1570. doi: 10.1152/ajpheart.2000.279.4.H1563. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Moore J, Schelling P, Beier N, Gross GJ. Na(+)/H(+) exchange inhibition prevents endothelial dysfunction after I/R injury. Am J Physiol Heart Circ Physiol. 2001;281:H1260–H1266. doi: 10.1152/ajpheart.2001.281.3.H1260. [DOI] [PubMed] [Google Scholar]
- Hattori R, Otani H, Moriguchi Y, Matsubara H, Yamamura T, Nakao Y, et al. NHE and ICAM-1 expression in hypoxic/reoxygenated coronary microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2001;280:H2796–H2803. doi: 10.1152/ajpheart.2001.280.6.H2796. [DOI] [PubMed] [Google Scholar]
- Hayward R, Campbell B, Shin YK, Scalia R, Lefer AM. Recombinant soluble P-selectin glycoprotein ligand-1 protects against myocardial ischemic reperfusion injury in cats. Cardiovasc Res. 1999;41:65–76. doi: 10.1016/s0008-6363(98)00266-1. [DOI] [PubMed] [Google Scholar]
- Huck V, Niemeyer A, Goerge T, Schnaeker EM, Ossig R, Rogge P, et al. Delay of acute intracellular pH recovery after acidosis decreases endothelial cell activation. J Cell Physiol. 2007;211:399–409. doi: 10.1002/jcp.20947. [DOI] [PubMed] [Google Scholar]
- Kaloyianni M, Zolota Z, Paletas K, Tsapas A, Koliakos G. Cariporide counteracts atherosclerosis-related functions in monocytes from obese and normal individuals. Obes Res. 2005;13:1588–1595. doi: 10.1038/oby.2005.195. [DOI] [PubMed] [Google Scholar]
- Kao TJ, Millette CF. L-type voltage-operated Ca(+2) channels modulate transient Ca(+2) influx triggered by activation of Sertoli cell surface L-selectin. J Cell Biochem. 2007;101:1023–1037. doi: 10.1002/jcb.21135. [DOI] [PubMed] [Google Scholar]
- Kristo G, Yoshimura Y, Ferraris SP, Jahania SA, Mentzer RM, Jr, Lasley RD. The preischemic combination of the sodium-hydrogen exchanger inhibitor cariporide and the adenosine agonist AMP579 acts additively to reduce porcine myocardial infarct size. J Am Coll Surg. 2004;199:586–594. doi: 10.1016/j.jamcollsurg.2004.05.274. [DOI] [PubMed] [Google Scholar]
- Lee C, Dhalla NS, Hryshko LV. Therapeutic potential of novel Na+–Ca2+ exchange inhibitors in attenuating ischemia–reperfusion injury. Can J Cardiol. 2005;21:509–516. [PubMed] [Google Scholar]
- Lee KS, Jin YR, Lee JJ, Lim Y, Son DJ, Lee CK, et al. Anti-platelet activity of KR-32560, a novel sodium/hydrogen exchanger-1 inhibitor. Pharmacol Res. 2006;53:265–270. doi: 10.1016/j.phrs.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Lefer DJ. Pharmacology of selectin inhibitors in ischemia/reperfusion states. Annu Rev Pharmacol Toxicol. 2000;40:283–294. doi: 10.1146/annurev.pharmtox.40.1.283. [DOI] [PubMed] [Google Scholar]
- Lorant DE, Patel KD, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D, Haskard DO. Clinical overview of leukocyte adhesion and migration: where are we now. Semin Immunol. 2002;14:133–140. doi: 10.1006/smim.2001.0350. [DOI] [PubMed] [Google Scholar]
- McIntyre TM, Prescott SM, Weyrich AS, Zimmerman GA. Cell–cell interactions: leukocyte–endothelial interactions. Curr Opin Hematol. 2003;10:150–158. doi: 10.1097/00062752-200303000-00009. [DOI] [PubMed] [Google Scholar]
- Menown IB, Adgey AA. Cardioprotective therapy and sodium–hydrogen exchange inhibition: current concepts and future goals. J Am Coll Cardiol. 2001;38:1651–1653. doi: 10.1016/s0735-1097(01)01607-2. [DOI] [PubMed] [Google Scholar]
- Niessen HW, Krijnen PA, Visser CA, Meijer CJ, Hack CE. Intercellular adhesion molecule-1 in the heart. Ann N Y Acad Sci. 2002;973:573–585. doi: 10.1111/j.1749-6632.2002.tb04703.x. [DOI] [PubMed] [Google Scholar]
- Pan J, Xia L, McEver RP. Comparison of promoters for the murine and human P-selectin genes suggests species-specific and conserved mechanisms for transcriptional regulation in endothelial cells. J Biol Chem. 1998;273:10058–10067. doi: 10.1074/jbc.273.16.10058. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Simkhovich BZ, Bauer B, Hata K, Zhao L, Elliott GT, et al. Cellular mechanisms of infarct size reduction with ischemic preconditioning. Role of calcium. Ann N Y Acad Sci. 1999;874:192–210. doi: 10.1111/j.1749-6632.1999.tb09236.x. [DOI] [PubMed] [Google Scholar]
- Redlin M, Werner J, Habazettl H, Griethe W, Kuppe H, Pries AR.Cariporide (HOE 642) attenuates leukocyte activation in ischemia and reperfusion Anesth Analg 2001931472–1479.table of contents [DOI] [PubMed] [Google Scholar]
- Repo H, Harlan JM. Mechanisms and consequences of phagocyte adhesion to endothelium. Ann Med. 1999;31:156–165. doi: 10.3109/07853899909115974. [DOI] [PubMed] [Google Scholar]
- Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rupprecht HJ, vom Dahl J, Terres W, Seyfarth KM, Richardt G, Schultheibeta HP, et al. Cardioprotective effects of the Na(+)/H(+) exchange inhibitor cariporide in patients with acute anterior myocardial infarction undergoing direct PTCA. Circulation. 2000;101:2902–2908. doi: 10.1161/01.cir.101.25.2902. [DOI] [PubMed] [Google Scholar]
- Sun B, Fan H, Honda T, Fujimaki R, Lafond-Walker A, Masui Y, et al. Activation of NF kappa B and expression of ICAM-1 in ischemic-reperfused canine myocardium. J Mol Cell Cardiol. 2001;33:109–119. doi: 10.1006/jmcc.2000.1280. [DOI] [PubMed] [Google Scholar]
- ten Hove M, van Emous JG, van Echteld CJ. Na+ overload during ischemia and reperfusion in rat hearts: comparison of the Na+/H+ exchange blockers EIPA, cariporide and eniporide. Mol Cell Biochem. 2003;250:47–54. doi: 10.1023/a:1024985931797. [DOI] [PubMed] [Google Scholar]
- Theroux P, Chaitman BR, Danchin N, Erhardt L, Meinertz T, Schroeder JS, et al. Inhibition of the sodium–hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations. Main results of the GUARDIAN trial. Guard during ischemia against necrosis (GUARDIAN) investigators. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- Wang SX, Sun XY, Zhang XH, Chen SX, Liu YH, Liu LY. Cariporide inhibits high glucose-mediated adhesion of monocyte–endothelial cell and expression of intercellular adhesion molecule-1. Life Sci. 2006;79:1399–1404. doi: 10.1016/j.lfs.2006.04.008. [DOI] [PubMed] [Google Scholar]