Abstract
Background and purpose: TRPM4 and TRPM5 are calcium-activated non-selective cation channels with almost identical characteristics. TRPM4 is detected in several tissues including heart, kidney, brainstem, cerebral artery and immune system whereas TRPM5 expression is more restricted. Determination of their roles in physiological processes requires specific pharmacological tools. TRPM4 is inhibited by glibenclamide, a modulator of ATP binding cassette proteins (ABC transporters), such as the cystic fibrosis transmembrane conductance regulator (CFTR). We took advantage of this similarity to investigate the effect of hydroxytricyclic compounds shown to modulate ABC transporters, on TRPM4 and TRPM5.
Experimental approach: Experiments were conducted using HEK-293 cells permanently transfected to express human TRPM4 or TRPM5. Currents were recorded using the whole-cell and inside-out variants of the patch-clamp technique.
Key results: The CFTR channel activator benzo[c]quinolizinium MPB-104 inhibited TRPM4 current with an IC50 in the range of 2 × 10−5 M, with no effect on single-channel conductance. In addition, 9-phenanthrol, lacking the chemical groups necessary for CFTR activation, also reversibly inhibited TRPM4 with a similar IC50. Channel inhibition was voltage independent. The IC50 determined in the whole-cell and inside-out experiments were similar, suggesting a direct effect of the molecule. However, 9-phenanthrol was ineffective on TRPM5, the most closely related channel within the TRP protein family.
Conclusions and implications: We identify 9-phenanthrol as a TRPM4 inhibitor, without effects on TRPM5. It could be valuable in investigating the physiological functions of TRPM4, as distinct from those of TRPM5.
Keywords: TRPM4, TRPM5, cation channel, benzo[c]quinolizinium, 9-phenanthrol, CFTR
Introduction
The recent discovery of a novel family of cation channels, the ‘transient receptor potential' (TRP) protein family, has revived interest in non-selective cation (NSC) channels (Nilius et al., 2007). In particular, transient receptor potential melastatin 4 (TRPM4) and 5 (TRPM5), two closely related members of the family, provide molecular correlates for a variety of Ca2+-activated NSC channels. Both channels display a linear current/voltage (I/V) relationship with a conductance of 25 pS (Launay et al., 2002; Hofmann et al., 2003; Prawitt et al., 2003). These channels are activated by membrane depolarization, an increase in [Ca2+]i and phosphatidylinositol-4,5-bisphosphate (Liu and Liman, 2003; Nilius et al., 2003; Prawitt et al., 2003; Zhang et al., 2005). They are equally permeable to Na+ and K+, but in contrast to all other TRPs, they are not permeable to Ca2+. Intracellular ATP blocks TRPM4, a phenomenon potentiated by decavanadate (Nilius et al., 2004). TRPM4 mRNA is expressed in a variety of mammalian cells with the highest expression in the heart and the kidney (Launay et al., 2002; Nilius et al., 2003). TRPM5 expression is more limited to specific tissues such as taste receptors, pancreatic β-cells and brainstem (Perez et al., 2002; Prawitt et al., 2003; Crowder et al., 2007). Taken together, the TRPM4 and TRPM5 fingerprints match the properties of Ca2+-activated non-selective cation currents described in a large variety of tissues (Teulon, 2000).
Because of its wide distribution, TRPM4 has been implicated in a variety of physiological mechanisms (see Nilius et al., 2007). It is involved in the immune response (Launay et al., 2004; Vennekens et al., 2007), the regulation of myogenic constriction by PKC in small arteries (Earley et al., 2004, 2007) and insulin secretion in pancreatic β-cells (Cheng et al., 2007). We showed that TRPM4 is functionally expressed in several cardiac cell preparations where it is expected to participate in after depolarizations at the onset of arrhythmias (see Guinamard and Bois, 2007 for review). On the other hand, TRPM5 is mainly involved in taste transduction (Perez et al., 2002; Liu and Liman, 2003).
Because of the almost identical electrophysiological properties of TRPM4 and TRPM5 and because they can be expressed in the same tissues, it is difficult to assess the involvement of each of these channels in physiological processes, separately. The identification of pharmacological tools with effects specific to one channel, would help to address this difficulty.
Several compounds have been shown to modulate TRPM4 and TRPM5. Flufenamic acid, a non-steroidal anti-inflammatory drug, has been reported to block NSC channels in several preparations including cardiac cells (Gogelein et al., 1990; Guinamard et al., 2006) and was shown to be effective on TRPM4 and TRPM5 (Ullrich et al., 2005). Also, both channels are inhibited by spermine (Ullrich et al., 2005). In T cells, TRPM4 was shown to be activated by N-[4-3,5-bis(trifluromethyl)pyrazol-1-yl]-4-methyl-1,2,3-thiadiazole-5-carboxamide (YM-58483), an immunosuppressive compound (Takezawa et al., 2006).
Interestingly, we have described previously the inhibition of TRPM4 current by the sulphonylurea, glibenclamide (Demion et al., 2007). This compound is known to be effective on ATP-binding cassette proteins (ABC proteins), such as the cystic fibrosis transmembrane conductance regulator (CFTR) and the sulphonylurea receptor (Sheppard and Welsh, 1992; Frelet and Klein, 2006). The TRPM4 protein also contains two ABC transporter signature-like motifs (Nilius et al., 2004), which may explain the effect of glibenclamide. This ABC signature is unique to TRPM4 within the TRP protein family and, particularly, it is not present in TRPM5 (Ullrich et al., 2005).
We took advantage of the similarities between TRPM4 and ABC proteins to investigate the effect of ABC modulators on TRPM4 channel activity. We focused on the hydroxytricyclic family because several of these heterocyclic compounds activate the CFTR channel (Becq et al., 1999; Marivingt-Mounir et al., 2004). Here, we report the inhibitory effects of two different hydroxytricyclic derivatives, the benzo[c]quinolizinium agent 5-butyl-7-chloro-6-hydroxybenzo[c]quinolizinium chloride (MPB-104) and the 9-hydroxyphenanthrene (9-phenanthrol), on TRPM4 heterologously expressed in human embryonic kidney (HEK)-293. We also observed that 9-phenanthrol had no effect on TRPM5.
Methods
Cell culture
Tetracycline-inducible HEK-293 Flag-TRPM4-expressing cells were obtained as previously described, by inserting the human TRPM4 cDNA into a modified version of the pCDNA4/TO vector (Invitrogen, Cergy Pontoise, France) (Launay et al., 2002). Tetracycline-inducible HEK-293 Flag-TRPM5-expressing cells were obtained as follows. Full-length human TRPM5 cDNA was inserted into a modified version of the pCDNA4/TO vector (Invitrogen) with an N-terminal Flag epitope tag. The Flag-TRPM5 cDNA in pCDNA4/T0 was electroporated into HEK-293 cells previously transfected with the pCDNA6/TR construct for Tet-repressor expression. Cells were placed under zeocin selection, and zeocin-resistant clones were screened for tetracycline-inducible expression of the Flag-tagged TRPM5 protein (immunoprecipitation and immunoblot with anti-Flag antibody). To verify functional expression of TRPM5, positive clones were tested by the patch-clamp technique.
Both TRPM4- and TRPM5-transfected HEK-293 cells were cultured at 37 °C/5% CO2 in DMEM (Dulbecco's Modified Eagle Medium; Cambrex Bioscience, Verviers, Belgium) supplemented with 10% fetal bovine serum and 2 mM glutamine. The medium was supplemented with S-blasticidin (5 μg ml−1; Invitrogen) and zeocin (0.4 mg ml−1; Invitrogen). For all experiments, cells were resuspended in media containing 1 μg ml−1 tetracycline (Invitrogen) 18–22 h before experiments.
Solutions and chemicals
For patch-clamp experiments in inside-out conditions, cells were bathed in a solution containing (in mM) 140 NaCl; 4.8 KCl; 1.2 MgCl2; 0.1 CaCl2; 10 glucose; and 10 HEPES. Pipette and solutions perfused at the inside of the membrane contained (in mM) 145 NaCl; 1.2 MgCl2; 10 glucose; and 10 HEPES. Pipette and perfused solutions contained 10−3 and 10−6 M CaCl2, respectively, except when specified in the text. [Ca2+]i was determined with a combination of CaCl2 and Ca-EGTA buffers or addition of EGTA (Guinamard et al., 2002). External solutions (pipette and bath) were adjusted to pH 7.4 with NaOH. The pH of perfused solutions was adjusted to 7.2.
For patch-clamp experiments in whole-cell conditions, pipette solutions contained (in mM) 156 CsCl, 1 MgCl2 and 10 HEPES. pH was adjusted to 7.2 with CsOH and [Ca2+] to 10−6 M. Bath and perfused solutions contained (in mM) 156 NaCl, 5 CaCl2, 10 glucose and 10 HEPES. pH was adjusted to 7.4 with NaOH.
5-Butyl-7-chloro-6-hydroxybenzo[c]quinolizinium chloride, 9-phenanthrol and glibenclamide were dissolved in dimethyl sulfoxide to a maximal final dimethyl sulfoxide concentration of 0.1% that had no effect on channel activity and such concentrations were added in the control solutions.
Patch-clamp measurements
Single-channel currents were recorded from membrane patches of HEK-293-transfected cells held under voltage clamp with an RK400 (Biologic, Claix, France) patch-clamp amplifier, using the inside-out configuration of the patch-clamp technique (Hamill et al., 1981; Guinamard et al., 2002). Experiments were conducted at room temperature (20–25 °C).
Whole-cell currents were recorded under voltage clamp with an Axopatch 200A (Molecular Devices Corporation, Sunnyvale, CA, USA) patch-clamp amplifier interfaced to a personal computer and driven by PClamp 8 software (Molecular Devices Corporation). TRPM4 and TRPM5 currents were investigated using both a ramp protocol and a step protocol. The holding potential was 0 mV. The 400 ms increasing ramp from −100 to +100 mV ends with a 20 ms step at +100 mV. A new ramp was performed every 2 s. In the step protocol, a 100 ms pulse was performed every 300 ms with increasing steps of 10 mV from −100 to +100 mV (holding potential 0 mV).
Iodide efflux
Iodide efflux experiments with [125I] NaI were performed in Chinese hamster ovary cells (CHO) stably expressing CFTR and analysed as previously described (Marivingt-Mounir et al., 2004).
Data analysis
Single-channel signals were analysed with Bio-patch software (version 3.30; Biologic, Claix, France). Amplitude histograms were generated to construct I/V curves and estimate open probability (Po). When the number of channels was too high to clearly identify single-channel openings, data were compared according to the mean current. Zero current level was determined using a low Ca2+ solution (10−9 M) that causes a total inhibition of TRPM4 and TRPM5 channels (Launay et al., 2002; Guinamard et al., 2006).
Whole-cell currents were analysed using Clampfit 8 (Molecular Devices Corporation). Zero current level was determined using flufenamic acid (10−4 M) that induced a total inhibition of the TRPM4 channel (Ullrich et al., 2005; Guinamard et al., 2006). When specified in the text, the amplitude of the whole-cell current was normalized to cell capacitance and reported as pA pF−1.
Significance was tested by Student's t-test when parameters were compared on the same cells and by ANOVA when compared on different membrane patches (P<0.05 for significance). Pooled data are given as mean±s.e.mean of n cells, excepted for the iodide efflux experiments where results are expressed as mean±s.e.mean of n cell populations.
Materials
All chemicals were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France), except for MPB 104, which was synthesized as described by Marivingt-Mounir et al. (2004). [125I] NaI was from PerkinElmer Life Sciences, Courtaboueuf, France.
Results
Biophysical properties of TRPM4 in HEK-293 cells
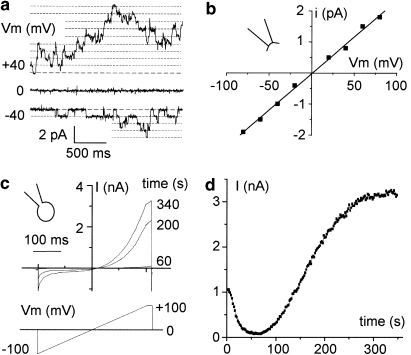
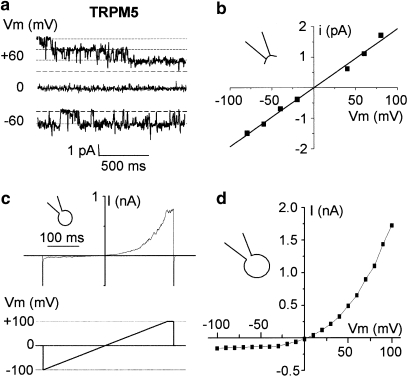
In the permanently TRPM4-transfected HEK-293 cells, we recorded the classical properties of TRPM4 currents (that is, voltage dependence and linear single-channel I/V relationship with a conductance of 25 pS) (Figure 1). In inside-out configuration, the maximal number of open channels was estimated at 40.6±7 (n=16) at Vm=+40 mV but 10±1.9 (n=21) at Vm=−80 mV, indicating channel regulation by voltage, responsible for the strong whole-cell outwardly rectifying current (Figure 1c). Figure 1d shows the time course of changes in TRPM4 current (measured at +100 mV) elicited by the ramp protocol represented in Figure 1c. In agreement with the observations of Ullrich et al. (2005), a transient decline of the TRPM4 current was first observed for 1 min after membrane break, then the whole-cell current developed to stabilize within 434±26 s (n=10). The maximal whole-cell current amplitude measured at +100 mV after stability (using the ramp protocol) was estimated at 2.4±0.2 nA (106.1±21.3 pA pF−1, n=45) in TRPM4-transfected HEK-293 cells versus 0.04±0.04 nA (1.6±1.7 pA pF−1, n=9) in non-transfected HEK-293 cells.
Figure 1.
Biophysical properties of TRPM4 in HEK-293 cells. (a) Single-channel tracings recorded at various voltages from an inside-out patch from a TRPM4-transfected HEK-293 cell. Pipette and bath contained 145 mM NaCl solution (Ca2+: bath, 10−6 M; pipette, 10−3 M). Vm corresponds to the membrane potential. Bold dotted lines indicate the current level of closed channels. Channel activity is higher at +40 mV than at −40 mV. (b) Current–voltage relationship determined from the patch-clamp recording presented in (a). Data points were fitted by linear regression, yielding a value for slope conductance (g) of 25 pS. (c) Current tracing recorded in the whole-cell condition using the ramp protocol from −100 to +100 mV, as shown under the trace. Pipette contains mainly 156 mM CsCl and bath mainly 156 mM NaCl (see Methods). Current tracings were recorded at several time points after membrane break. (d) Time course of the maximum whole-cell current after membrane break from the cell shown in (c). Maximal current is determined as the mean current recorded during the ending step of 20 ms at +100 mV of the ramp protocol. Note the current rundown followed by a strong rise and a stabilization within few minutes. These properties are consistent with a TRPM4 current. HEK, human embryonic kidney; TRPM4, transient receptor potential melastatin 4.
Next, we tested pharmacological agents on TRPM4 current after stabilization. In the following experiments, the zero current was estimated by using either a solution with [Ca2+]i=10−9 M (inside-out recordings) or 10−4 M flufenamic acid (whole-cell recordings) that were shown to produce a total inhibition of the channel (Guinamard et al., 2006).
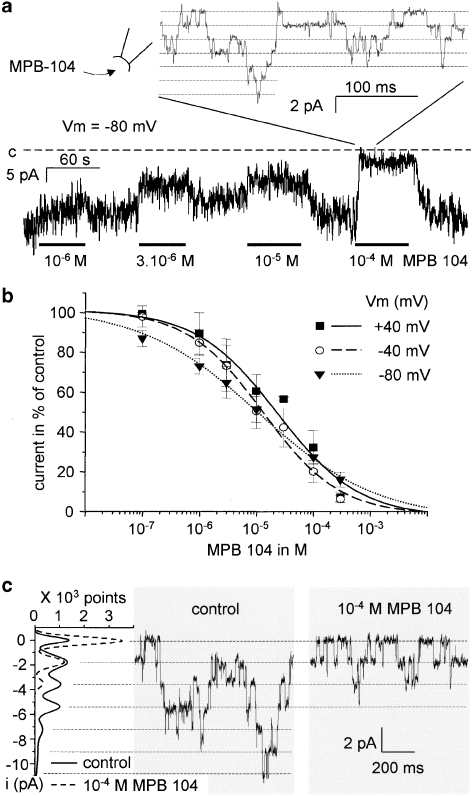
TRPM4 inhibition by MPB-104
The effect of MPB-104 was investigated during inside-out recordings. MPB-104 perfused at the inside of the membrane produced a rapid and reversible channel inhibition (Figure 2a). Seven concentrations were tested from 10−7 to 3.10−4 M, allowing the construction of concentration/response curves. To study the voltage dependence of channel inhibition, the concentration/response curves were measured at three different holding membrane potentials, that is +40, −40 and −80 mV, and are presented in Figure 2b. Concentrations obtained for half maximal inhibition (IC50) and Hill coefficients were similar for the three voltages (IC50=23.7±6.6; 13.2±2.4; 11.3±2 × 10−6 M and Hill coefficient=0.65±0.07; 0.61±0.1; 0.48±0.1 for Vm=+40, −40 and −80 mV, respectively), suggesting that the drug effect was independent of membrane potential.
Figure 2.
TRPM4 inhibition by MPB-104. (a) Current recorded from an inside-out patch from a TRPM4-transfected HEK-293 cell showing the reversible inhibition of channel activity by several concentrations of MPB-104 (Vm=−80 mV). Magnification allows observing single-channel currents. Label ‘c' indicates the current level corresponding to the closed state of all channels. (b) Averaged currents (in % of control) in the presence of several concentrations of MPB-104 at Vm=+40, −40 and −80 mV (n=4–9 for each point). Error bars indicate s.e.mean. Data points were fitted to a Hill equation providing similar IC50 in the three conditions. (c) Single-channel currents in control conditions and in the presence of 10−4 M MPB-104 (Vm=−80 mV) on the same membrane patch. Current traces and corresponding amplitude histograms provided on the left of the panel indicate a decrease in channel activity but not in single-channel current amplitude. HEK, human embryonic kidney; MPB-104, 5-butyl-7-chloro-6-hydroxybenzo[c]quinolizinium chloride; TRPM4, transient receptor potential melastatin 4.
Also, single-channel conductance was not significantly affected by MPB-104 (Figure 2c). The value of the unitary conductance, estimated at −80 mV, was 25.8±1.3 pS (n=7) in control conditions and 26.2±1.6 pS (n=7) in the presence of 10−4 M MPB-104.
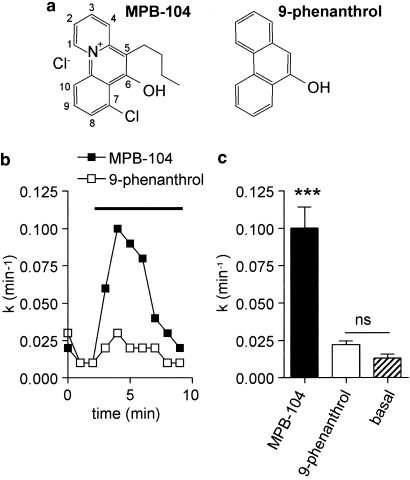
Comparative effects of MPB-104 and 9-phenanthrol on CFTR
Among a variety of benzo[c]quinolizinium compounds, MPB-104 (Figure 3a) was shown to be the more potent product for CFTR activation. This effect would be due to the presence of a hydroxyl group at position 6 of the benzo[c]quinolizinium skeleton, together with a chlorine atom at position 7 and an alkyl chain at position 5 (Marivingt-Mounir et al., 2004). Also, similar molecules lacking the quaternary ammonium are inactive on CFTR (Becq et al., 1999). To discriminate between CFTR and TRPM4, we tested the effect on these currents of 9-phenanthrol, a benzoquinolozinium derivative devoid of the quaternary ammonium, the alkyl chain and the chlorine atom (Figure 3a).
Figure 3.
Effect of MPB-104 and 9-phenanthrol on iodide efflux in CHO cells stably expressing CFTR chloride channels. (a) Chemical structure of MPB-104 and 9-phenanthrol. (b) Representative curves of iodide efflux as function of time for cells treated with MPB-104 or 9-phenanthrol (2.5 × 10−4 M each) during the period indicated by the horizontal line. (c) Histograms showing rate of iodide efflux (mean±s.e.mean, n=4 for each condition) for cells stimulated by MPB-104 and 9-phenanthrol compared with non-stimulated cells (noted basal). ***P<0.001. CFTR, cystic fibrosis transmembrane conductance regulator; CHO, Chinese hamster ovary; MPB-104, 5-butyl-7-chloro-6-hydroxybenzo[c]quinolizinium chloride; ns, nonsignificant difference.
The effects of MPB-104 and 9-phenanthrol on CFTR activity were investigated using iodide efflux experiments, as previously described (Marivingt-Mounir et al., 2004). Figure 3 shows a significant activation of CFTR-dependent efflux by MPB-104, but not by 9-phenanthrol, both at a concentration of 2.5 × 10−4 M.
Inhibition of TRPM4 by 9-phenanthrol
The effect of 9-phenanthrol on TRPM4 activity was investigated using the HEK-293 cells, as for MPB-104. The first series of experiments was performed in the inside-out configuration. The perfusion at the inside of the membrane of cumulative doses of 9-phenanthrol (3 × 10−6–10−4 M) produced an inhibition similar to that seen with MPB-104 (Figure 4). In the representative example illustrated in Figure 4a, the inhibition was dose dependent and reversible. In this experiment, the zero current was estimated by perfusing a solution with [Ca2+]i=10−9 M. It is worth noting that 9-phenanthrol (at 10−4 M) almost totally inhibited the channel current, an effect comparable to that of lowering the internal Ca2+ concentration to 10−9 M. Seven concentrations of 9-phenanthrol from 10−8 to 10−4 M were tested at membrane potentials of +40, −40 and −80 mV (Figure 4b). The fitting of the dose/response curves in Figure 4b with the Hill equation yielded an IC50 in the range of 2 × 10−5 M, comparable to that of MPB-104, and showed no voltage dependence of the drug effect (Table 1).
Figure 4.
TRPM4 inhibition by 9-phenanthrol in inside-out conditions. (a) Current recorded from an inside-out patch from a TRPM4-transfected HEK-293 cell showing the reversible inhibition of channel activity by several concentrations of 9-phenanthrol (Vm=+40 mV). The zero current level was determined by lowering [Ca2+]i to a level estimated at 10−9 M that produced a total inhibition of the channel. (b) Averaged currents (in % of control) in the presence of several concentrations of 9-phenanthrol at Vm=+40, −40 and −80 mV (n=4–9 for each point). Data points were fitted to a Hill equation providing an IC50 in the range of 2 × 10−5 M and a Hill coefficient close to 1 in the three conditions (see Table 1). HEK, human embryonic kidney; TRPM4, transient receptor potential melastatin 4.
Table 1.
Inhibition of TRPM4 by 9-phenanthrol under different conditions
| Vm (mV) | IC50 (× 10−5 M) | Hill coefficient |
|---|---|---|
| +40 (inside-out) | 1.91±0.33 | 1.08±0.19 |
| −40 (inside-out) | 1.71±0.45 | 1.07±0.27 |
| −80 (inside-out) | 2.28±0.75 | 0.82±0.21 |
| +100 (whole-cell) | 1.67±0.45 | 0.68±0.34 |
Abbreviation: TRPM4, transient receptor potential melastatin 4.
The IC50 and Hill coefficient values for the inhibition of TRPM4 current by 9-phenanthrol were determined at various membrane potentials (Vm) in inside-out and whole-cell conditions.
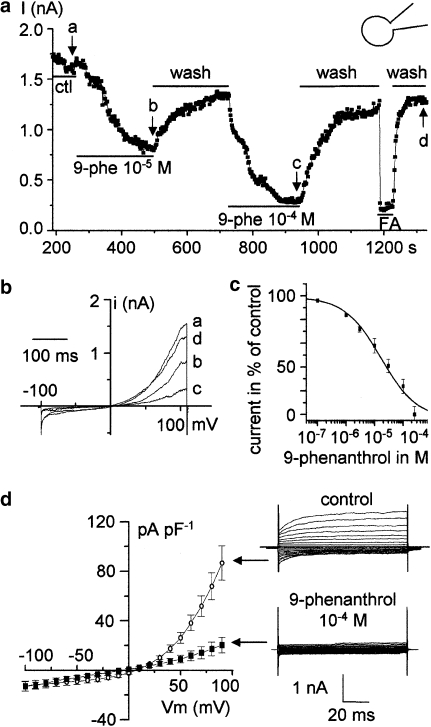
In a second experimental approach, the effect of 9-phenanthrol on TRPM4 current was investigated in the whole-cell configuration either with ramp or with pulse protocol. Figure 5a shows the time course of changes in TRPM4 current (measured at +100 mV) elicited by the ramp protocol described in Figure 1c. Whole-cell currents at points designated by the letters in Figure 5a are shown in Figure 5b. As illustrated in Figure 5, 9-phenanthrol reversibly inhibited the whole-cell current. The addition of 10−5 and 10−4 M of 9-phenanthrol to the bath solution induced a decrease in TRPM4 amplitude estimated at 54 and 94% respectively (Figure 5a). In this experiment, the current level corresponding to the closed state of all TRPM4 channels was determined by perfusion of 10−4 M flufenamic acid. Seven concentrations of 9-phenanthrol were tested from 10−7 to 2.5 × 10−4 M (Figure 5c). The concentration/response curve yielded an IC50 of 16.7±4.5 × 10−6 M that was not statistically different from values obtained in the cell-free configuration (Table 1). Comparable inhibition was observed using a pulse protocol. Figure 5d shows the mean I/V relationships obtained in control conditions and in the presence of 10−4 M 9-phenanthrol. Owing to the current rectification, the inhibition is hardly detectable at negative voltages but is statistically significant in voltages higher than +30 mV.
Figure 5.
TRPM4 inhibition by 9-phenanthrol in whole-cell conditions. (a and b) Time course after membrane break of the maximal whole-cell current recorded from a TRPM4-transfected HEK-293 cell showing the reversible inhibition of the current by 9-phenanthrol (9-phe) (a). Maximal current was determined using the ramp protocol as indicated in legends of Figure 1d. Zero current level was determined by perfusion of 10 μM flufenamic acid (FA). Labels ‘a–d' indicate the values corresponding to the current recordings provided in (b). (c) Dose–response curve of 9-phenanthrol in the whole-cell condition determined using the ramp protocol (n=4–6 for each point). Data points were fitted to a Hill equation providing an IC50 of 1.67±0.45 × 10−5 M. (d) Current/voltage (I/V) relationship of the whole-cell TRPM4 current determined using a step protocol of 100 ms pulse performed every 300 ms with increasing steps of 10 mV from −100 to +100 mV (holding potential 0 mV). I/V were performed in control condition (n=4) and in the presence of 9-phenanthrol at 100 μM (n=4). Note that to construct I/V curves, the current was expressed as current density (current/cell capacitance; pA pF−1). Current tracings on the right provide an example of currents recorded using the step protocol with or without 9-phenanthrol, on the same cell. HEK, human embryonic kidney; TRPM4, transient receptor potential melastatin 4.
TRPM5 lacks sensitivity to 9-phenanthrol
Sensitivity to 9-phenanthrol was evaluated for TRPM5, the most closely related member of the TRP protein family. Experiments were performed using HEK-293 cells expressing TRPM5. The permanently TRPM5-transfected HEK-293 cells exhibited a typical TRPM5 current. Figure 6 illustrates its biophysical properties, displaying a linear single-channel conductance of 21.7±1.1 pS (n=7) and channel activation by depolarizing voltages (Figures 6a and b). The voltage dependence observed in single-channel recordings explains the strong outwardly rectifying current recorded in the whole-cell configuration (Figures 6c and d). The whole-cell current was estimated at 1.14±0.11 nA (n=23, Vm=+100 mV), corresponding to a current density of 48.1±4.7 pA pF−1. These biophysical properties are in agreement with previous results describing TRPM5 currents expressed in HEK-293 cells (Prawitt et al., 2003; Ullrich et al., 2005).
Figure 6.
Biophysical properties of TRPM5 in HEK-293 cells. (a) Single-channel tracings recorded at various voltages from an inside-out patch from a TRPM5-transfected HEK-293 cell. Pipette and bath contained 145 mM NaCl solution (Ca2+: bath, 10−6 M; pipette, 10−3 M). (b) Current–voltage relationship determined from the patch-clamp recording presented in (a). Data points were fitted by linear regression, yielding a value for slope conductance (g) of 20.3 pS. (c) Current tracing recorded in the whole-cell condition using the ramp protocol from −100 to +100 mV as shown under the trace (same ionic conditions as in Figure 1c). Note the characteristic outward rectification (d) I/V determined using the step protocol as described in Figure 5d from a whole-cell recording of a TRPM5-transfected cell. Outward rectification is similar as the one observed in (c). These properties are consistent with a TRPM5 current. HEK, human embryonic kidney; TRPM5, transient receptor potential melastatin 5.
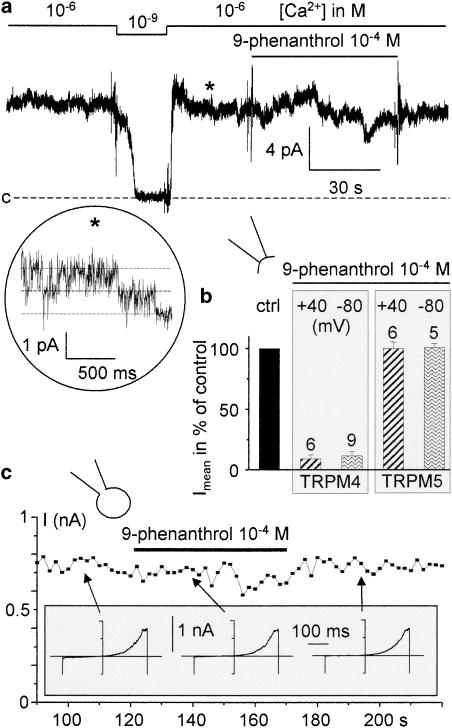
Pharmacological tests were done in the same ionic conditions as for TRPM4. 9-phenanthrol was applied in inside-out conditions at 10−4 M but had no significant effect on TRPM5 current either at Vm=+40 mV or at −80 mV (n=5 and 6 respectively). Figure 7a illustrates a representative TRPM5 current trace recorded in the inside-out configuration, in the absence and in the presence of 10−4 M 9-phenanthrol. Mean currents in the presence of 9-phenanthrol were normalized to control conditions and compared to the inhibition induced by the molecule on TRPM4 (histograms in Figure 7b). Contrary to the effects observed on TRPM4, TRPM5 current was not significantly altered by 9-phenanthrol. Similarly, the TRPM5 current was not affected by the perfusion of 9-phenanthrol (10−4 M) in the whole-cell recording conditions (n=10; Figure 7c).
Figure 7.
Lack of effect of 9-phenanthrol on TRPM5 current. (a) Current tracing recorded from an inside-out patch from a TRPM5-transfected HEK-293 cell (Vm=+40 mV). Although reducing [Ca2+]i to 10−9 M totally inhibits channel activity, perfusing 9-phenanthrol at 10−4 M has no significant effect. The magnification in the inset shows single-channel currents and corresponds to the period indicated by an asterisk in the whole trace. (b) Mean current (Imean) in the presence of 9-phenanthrol at 10−4 M normalized to mean current in control (ctrl) conditions at Vm=+40 and −80 mV for TRPM4- and TRPM5-transfected cells. Values are from inside-out recordings. Numbers above bars indicate the number of trials. (c) Time course of the maximal current recorded in the whole-cell conditions using the ramp protocol (see legend of Figure 1d) from a TRPM5-transfected HEK-293 cell. Perfusion of 9-phenanthrol had no effect. The inset shows the current traces used to determine the points indicated by arrows. HEK, human embryonic kidney; TRPM4, transient receptor potential melastatin 4; TRPM5, transient receptor potential melastatin 5.
Discussion
Because of the growing evidence that TRPM4 is implicated in physiological as well as pathological processes, it is considered as a relevant pharmaceutical target. Thus, the development of modulators of this Ca2+-activated NSC channel is of key importance for our understanding of its physiological role in renal, cardiac and immune tissues where TRPM4 is highly expressed.
By investigating several hydroxytricyclic compounds, we showed for the first time their reversible inhibitory effect on TRPM4 current. Among this family, MPB-104 was shown to be one of the most effective benzo[c]quinolizinium agent acting on the ABC protein CFTR chloride channel (Marivingt-Mounir et al., 2004). It is conceivable that the ABC signature motifs could be the target for hydroxytricyclic compounds, as the molecular similarities between TRPM4 and CFTR are expressed only as the presence of ABC signature-like motifs and Walker-B ATP-binding sites (Nilius et al., 2005). Interestingly, MPB-104 produces an activation of CFTR current, whereas it induces an inhibition of TRPM4 current, suggesting a different mechanism of action on the two channels.
Another pharmacological difference between CFTR and TRPM4 was the effect of 9-phenanthrol. It was reported that the modulation of CFTR by hydroxytricyclic compounds required the presence of a quaternary ammonium, a hydroxyl group at position 6 of the benzo[c]quinolizinium skeleton associated with a chlorine atom at position 10 or 7 and an alkyl chain at position 5 (Marivingt-Mounir et al., 2004). This was confirmed by our results indicating that 9-phenanthrol, which is devoid of the quaternary ammonium, the chlorine atom and the alkyl chain, was not effective on CFTR. However, 9-phenanthrol produced a rapid and reversible inhibition of TRPM4 current. The effect of 9-phenanthrol on whole-cell TRPM4 current may have several explanations, including direct action on the channel, indirect effect on channel modulators (Ca2+ or ATP) and/or modulation of the channel trafficking. This last possibility was described for the CFTR channel whose trafficking is facilitated by benzoquinoliziniums (Dormer et al., 2001). The effects on intracellular Ca2+ or ATP levels are less probable. Indeed, it has been shown that benzoquinolizinium derivatives do not modify the intracellular ATP content, either in rat submandibular acinar cells or in CHO cells where CFTR has been expressed (Becq et al., 1999). Furthermore, the inhibitory effect that we reported in the inside-out configuration would support a direct interaction with the channel, as also reported for CFTR (Becq et al., 1999).
The inhibitory effect of hydroxytricyclic derivatives on TRPM4 should be taken into account when investigating the effect of these compounds on CFTR currents in some tissues. Indeed, both proteins are expressed in some tissues in common, not only in epithelial tissues such as intestine, kidney and lung but also in pancreas, testis and immune cells (Chen et al., 1989; Riordan et al., 1989; Launay et al., 2002). In these tissues, CFTR mediates cAMP-dependent chloride transepithelial transport. Defects in this transport induced by point mutations in the CFTR gene are responsible for the genetic disease cystic fibrosis characterized by airway obstruction and infection, pancreatic failure and male infertility (Riordan et al., 1989). During the last 6 years, high-throughput screening assays have been developed to identify novel molecules to correct these defects, including benzoquinolizinium derivatives (see Becq, 2006). The effect of the new compounds, such as MPB-104, on TRPM4 would modify the membrane potential and thus the driving force for all ions including chloride, inducing a modification in transepithelial ionic fluxes. A comparison of results obtained using 9-phenanthrol that is ineffective on CFTR, but effective on TRPM4, may be useful to estimate the contribution of TRPM4 to these transepithelial ionic fluxes.
Another major point of the present work is the lack of inhibition of TRPM5 current by 9-phenanthrol. This difference with TRPM4 may be useful to discriminate between currents that exhibit very similar electrophysiological and regulatory properties. Indeed, voltage-sensitive and Ca2+-activated NSC currents were reported in a wide variety of tissues (Teulon, 2000) but, without specific tools, the identification of the proteins involved is difficult to address. It has to be noted that most of these NSC currents may be mediated by TRPM4, which is ubiquitously expressed (Launay et al., 2002), as opposed to TRPM5, which is more restricted to specific tissues such as taste receptor cells, cerebral arteries, pancreatic β-cells and brainstem (Perez et al., 2002; Prawitt et al., 2003; Earley et al., 2004; Crowder et al., 2007). In tissues where both molecular signatures (for TRPM4 and 5) were detected, 9-phenanthrol may open new perspectives. As an example, it was recently reported that inspiratory bursts in the prebotzinger complex depend on a Ca2+-activated non-selective cationic current attributed to TRPM4 or TRPM5 (Crowder et al., 2007; Pace et al., 2007). Also, in pancreatic β-cells where both channels are present, it is not yet clear which one contributes predominantly to the membrane depolarization involved in insulin secretion (Cheng et al., 2007).
In conclusion, we report here the discovery of two hydroxytricyclic compounds as novel scaffold inhibitors of the TRPM4 cationic channel. These drugs could be used as pharmacologic tools in experiments using whole-cell and single patch-clamp recordings. Moreover, hydroxytricyclic compounds are potentially useful drugs because they show an apparent low cellular toxicity (Becq et al., 1999; Marivingt-Mounir et al., 2004).
At this point, the specificity of these compounds, in particular 9-phenanthrol, should be further assessed by performing experiments on other ionic currents, including additional members of the TRP family. For 9-phenanthrol, the lack of effect on TRPM5 and CFTR currents is a promising start in the search for channel-specific pharmacological tools. However, 9-phenanthrol also inhibits bovine cAMP-dependent protein kinase (Wang et al., 1994) and such actions will have to be taken into consideration when investigating the effects of this compound at the tissue level. Thus, screening or design of additional benzoquinolizinium derivatives may identify more selective TRPM4 inhibitors. Providing these tools in the TRPM4 field will not only help to elucidate its function in the many tissues in which it is expressed but may also lead to clinical applications by modulating the immune response or preventing cardiac arrhythmias.
Acknowledgments
We thank Dirk Prawitt (University of Mainz, Germany) for providing the cDNA encoding human TRPM5 and Christophe Magaud (Université de Poitiers, France) for technical assistance. Frederic Becq, Caroline Norez and Yvette Mettey researches are supported by specific grants from Vaincre La Mucoviscidose (Paris, France).
Abbreviations
- TRP
transient receptor potential
- TRPM4
transient receptor potential melastatin 4
- TRPM5
transient receptor potential melastatin 5
- CFTR
cystic fibrosis transmembrane conductance regulator
- HEK
human embryonic kidney
- ABC
ATP-binding cassette
- NSC
non-selective cation (channel)
- MPB-104
5-butyl-7-chloro-6-hydroxybenzo[c]quinolizinium chloride
- MPB
benzo[c]quinolizinium compound
- CHO
Chinese hamster ovary
Conflict of interest
The authors state no conflict of interest.
References
- Becq F. On the discovery and development of CFTR chloride channel activators. Curr Pharm Des. 2006;12:471–484. doi: 10.2174/138161206775474459. [DOI] [PubMed] [Google Scholar]
- Becq F, Mettey Y, Gray MA, Galietta LJ, Dormer RL, Merten M, et al. Development of substituted Benzo[c]quinolizinium compounds as novel activators of the cystic fibrosis chloride channel. J Biol Chem. 1999;274:27415–27425. doi: 10.1074/jbc.274.39.27415. [DOI] [PubMed] [Google Scholar]
- Chen JH, Schulman H, Gardner P. A cAMP-regulated chloride channel in lymphocytes that is affected in cystic fibrosis. Science. 1989;243:657–660. doi: 10.1126/science.2464852. [DOI] [PubMed] [Google Scholar]
- Cheng H, Beck A, Launay P, Gross SA, Stokes AJ, Kinet JP, et al. TRPM4 controls insulin secretion in pancreatic beta-cells. Cell Calcium. 2007;41:51–61. doi: 10.1016/j.ceca.2006.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBötzinger complex. J Physiol. 2007;582:1047–1058. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demion M, Bois P, Launay P, Guinamard R. TRPM4, a Ca2+-activated non-selective cation channel in mouse sino-atrial node cells. Cardiovasc Res. 2007;73:531–538. doi: 10.1016/j.cardiores.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Dormer RL, Derand R, McNeilly CM, Mettey Y, Bulteau-Pignoux L, Metaye T, et al. Correction of delF508-CFTR activity with benzo(c)quinolizinium compounds through facilitation of its processing in cystic fibrosis airway cells. J Cell Sci. 2001;114:4073–4081. doi: 10.1242/jcs.114.22.4073. [DOI] [PubMed] [Google Scholar]
- Earley S, Straub SV, Brayden J. Protein kinase C regulates vascular myogenic tone through activation of TRPM4. Am J Physiol Heart Circ Physiol. 2007;292:H2613–H2622. doi: 10.1152/ajpheart.01286.2006. [DOI] [PubMed] [Google Scholar]
- Earley S, Waldron BJ, Brayden JE. Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circ Res. 2004;95:922–929. doi: 10.1161/01.RES.0000147311.54833.03. [DOI] [PubMed] [Google Scholar]
- Frelet A, Klein M. Insight in eukaryotic ABC transporter function by mutation analysis. FEBS Lett. 2006;580:1064–1084. doi: 10.1016/j.febslet.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Gogelein H, Dahlem D, Englert HC, Lang HJ. Flufenamic acid, mefenamic acid and niflumic acid inhibit single non-selective cation channels in the rat exocrine pancreas. FEBS Lett. 1990;268:79–82. doi: 10.1016/0014-5793(90)80977-q. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Bois P.Involvement of transient receptor potential proteins in cardiac hypertrophy Biochim Biophys Acta 2007. doi:10.1016/j.bbadis.2007.02.007 [DOI] [PubMed]
- Guinamard R, Demion M, Magaud C, Potreau D, Bois P. Functional expression of the TRPM4 cationic current in ventricular cardiomyocytes from spontaneously hypertensive rats. Hypertension. 2006;48:587–594. doi: 10.1161/01.HYP.0000237864.65019.a5. [DOI] [PubMed] [Google Scholar]
- Guinamard R, Rahmati M, Lenfant J, Bois P. Characterization of a Ca2+-activated non-selective cation channel during dedifferentiation of cultured rat ventricular cardiomyocytes. J membr Biol. 2002;188:127–135. doi: 10.1007/s00232-001-0180-4. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Chubanov V, Gudermann T, Montell C. TRPM5 is a voltage-modulated and Ca(2+)-activated monovalent selective cation channel. Curr Biol. 2003;13:1153–1158. doi: 10.1016/s0960-9822(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science. 2004;306:1374–1377. doi: 10.1126/science.1098845. [DOI] [PubMed] [Google Scholar]
- Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP. TRPM4 is a Ca2+-activated non-selective cation channel mediating cell membrane depolarization. Cell. 2002;109:397–407. doi: 10.1016/s0092-8674(02)00719-5. [DOI] [PubMed] [Google Scholar]
- Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci USA. 2003;100:15160–15165. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivingt-Mounir C, Norez C, Derand R, Bulteau-Pignoux L, Nguyen-Huy D, Viossat B, et al. Synthesis, SAR, crystal structure, and biological evaluation of benzoquinoliziniums as activators of wild-type and mutant cystic fibrosis transmembrane conductance regulator channels. J Med Chem. 2004;47:962–972. doi: 10.1021/jm0308848. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, et al. Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem. 2003;278:30813–30820. doi: 10.1074/jbc.M305127200. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Janssens A, Voets T, Droogmans G. Decavanadate modulates gating of TRPM4 cation channels. J Physiol. 2004;560:753–765. doi: 10.1113/jphysiol.2004.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, et al. Regulation of the Ca2+ sensitivity of the non-selective cation channel TRPM4. J Biol Chem. 2005;280:6423–6433. doi: 10.1074/jbc.M411089200. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, et al. A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci. 2002;5:1169–1176. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- Prawitt D, Monteilh-Zoller MK, Brixel L, Spangenberg C, Zabel B, Fleig A, et al. TRPM5 is a transient Ca2+-activated cation channel responding to rapid changes in [Ca2+]i. Proc Natl Acad Sci USA. 2003;100:15166–15171. doi: 10.1073/pnas.2334624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Sheppard DN, Welsh MJ. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992;100:573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, et al. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69:1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- Teulon J.Ca2+-activated non-selective cation channels Pharmacology of Ionic Channel Function: Activators and Inhibitors 2000Springer-Verlag: Berlin; 625–649.In: Endo M, Kurachi Y, Mishina M (eds) [Google Scholar]
- Ullrich ND, Voets T, Prenen J, Vennekens R, Talavera K, Droogmans G, et al. Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium. 2005;37:267–278. doi: 10.1016/j.ceca.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, et al. Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated non-selective cation channel TRPM4. Nat Immunol. 2007;8:312–320. doi: 10.1038/ni1441. [DOI] [PubMed] [Google Scholar]
- Wang BH, Ternai B, Polya GM. Specific inhibition of cyclic AMP-dependent protein kinase by the antimalarial halofantrine and by related phenanthrenes. Biol Chem Hoppe Seyler. 1994;375:527–535. doi: 10.1515/bchm3.1994.375.8.527. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Okawa H, Wang Y, Liman ER. Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 2005;280:39185–39192. doi: 10.1074/jbc.M506965200. [DOI] [PubMed] [Google Scholar]