Abstract
Background and purpose: Thymol, a major component of thyme and oregano, has medical uses in oral care products as an astringent and antibiotic. Its distinctive sharp odour and pungent flavour are considered aversive properties. The molecular basis of these aversive properties is not well understood.
Experimental approach: The ability of thymol to activate human transient receptor potential channel A1 (hTRPA1) expressed in stably transfected human embryonic kidney 293 (HEK293) cells was measured by membrane potential and calcium-sensitive dyes in a fluorescence-imaging plate reader (FLIPR) assay. Direct activation of hTRPA1 currents was measured by whole-cell voltage clamp recording. Intracellular calcium changes were measured using fura-2 dye. The FLIPR assay was also used to measure membrane potential changes elicited by thymol after pretreatment with camphor, a known TRPA1 inhibitor. The ability of related alkyl phenols to activate hTRPA1 was also determined.
Key results: Thymol potently activated a membrane potential response and intracellular calcium increase in hTRPA1-expressing HEK293 cells in a concentration-dependent manner. Activation by thymol desensitized hTRPA1 to further exposure to thymol or the known ligand allyl isothiocyanate (AITC). The related phenols 2-tert-butyl-5-methylphenol, 2,6-diisopropylphenol (propofol) and carvacrol also activated hTRPA1. Phenols with less bulky carbon substitutions and lower logP values were less potent in general. The response to thymol was blocked by camphor.
Conclusions and implications: These results suggest a role for hTRPA1 activation in the reported pungent and aversive properties of some of these pharmaceutically important phenols.
Keywords: TRPA1, thymol, alkyl-substituted phenols, cinnamaldehyde, FLIPR assay, whole-cell electrophysiology, calcium imaging, logP
Introduction
Thymol, a phenolic compound, is one of the predominant components of oil derived from the thyme plant, Thymus vulgari, and from oregano, Origanum vulgare (Hazzit et al., 2006). Its antimicrobial (Lambert et al., 2001), anti-inflammatory (Braga et al., 2006a) and anti-oxidant properties (Braga et al., 2006b; for review, see Burt, 2004) make it a common addition to many consumer products such as cosmetics, pharmaceutical preparations and oral rinses. However, thymol also has an unpleasantly pungent flavour on its own, often described as medicinal. This strong taste limits consumer acceptance of thymol-containing products, in particular those that might have a taste sensory component. Other flavourings often have to be added to mask its taste, and identifying the proper formulation can be difficult.
Although the molecular basis of thymol's pungency is not yet known, thymol is known to activate various receptors. For example, thymol has been shown to activate transient receptor potential (TRP)V3 (Xu et al., 2006). Thymol is also a direct agonist of the GABAA ion channel (Mohammadi et al., 2001). In addition, thymol may partially block voltage-gated sodium channels, which could be a potential molecular mechanism for its pain-relieving properties (Haeseler et al., 2002). Although the temperature-sensitive TRPV3 channel is expressed in the tongue (Xu et al., 2002), its role in taste, if any, is not yet known. Likewise, neither the GABAA ion channel nor the voltage-gated sodium channels are known to be important in taste. Thus, the receptor responsible for mediating thymol's pungent taste remains to be discovered.
We are interested in identifying the ability of small molecules involved in taste to activate members of the TRP ion channel family. TRP channels have been identified as mediators for the taste sensations of several spicy molecules, including capsaicin (TRPV1, Caterina et al., 1997), menthol (TRPM8, Peier et al., 2002) and cinnamaldehyde (TRPA1, Bandell et al., 2004), as well as transducing signals from G-protein-coupled receptors that mediate the bitter, sweet and umami taste modalities (TRPM5, Margolskee, 2002; Zhang et al., 2003). It is already known that thymol activates TRPV3 (Xu et al., 2006) and TRPM8 (Vogt-Eisele et al., 2007). This suggests that thymol could activate other TRP channels as well. In the process of evaluating a panel of tastants, we found that thymol is also a potent activator of human transient receptor potential channel A1 (hTRPA1).
TRPA1, originally designated as ANKTM1, was identified as a channel that responds to noxious cold (Story et al., 2003). In addition to its role in cold detection, it is also activated by a number of natural ligands, many of which are characterized by having pungent, strong flavours or by causing pain sensations. Some of these pungent compounds include allyl isothiocyanate (AITC) or mustard oil (Jordt et al., 2004); cinnamaldehyde, found in cinnamon oil (Bandell et al., 2004); and carvacrol, found in oregano (Xu et al., 2006). Allicin, a component of the pungent flavour of raw garlic (Bautista et al., 2005; Macpherson et al., 2005) also activates TRPA1, although TRPV1 appears to be important as well (Park et al., 2007). TRPA1 appears to have multiple activation mechanisms, ranging from mechanical (Hill and Schaefer, 2007), chemical via the covalent modification of cysteine residues on the intracellular domains of TRPA1 (Hinman et al., 2006; Macpherson et al., 2007) and direct intracellular activation by calcium ions via the EF-domain binding (Doerner et al., 2007; Zurborg et al., 2007). Blockers of hTRPA1 have been identified as well. Activation of TRPA1 is blocked by camphor (Xu et al., 2005). Menthol blocks TRPA1 at high (1 mM) concentrations (Macpherson et al., 2006), although it displays agonist properties at lower concentrations (Karashima et al., 2007).
In this study, we have shown that hTRPA1 is activated by thymol in a concentration-dependent manner. While this paper was under review, Karashima et al. (2007) also demonstrated the activation of TRPA1 by thymol. In addition, we found that this activation of hTRPA1 by thymol is blocked by camphor. We also found that related alkyl-substituted phenols activate hTRPA1 and that some structure-activity relationships (SARs) exist. Thus, we suggest that hTRPA1 stimulation is responsible in part for the aversive properties of the thymol taste.
Methods
Cloning of hTRPA1
Human transient receptor potential channel A1 cDNA was generated from a human lung polyA+ RNA library using the Thermoscript RT-PCR system. The full-length hTRPA1 cDNA was amplified by PCR. Recognition sites for restriction enzymes Kpn1 and Xho1 were introduced at the 3′ and 5′ ends, respectively, with GC Melt. The PCR product was purified using the PureLink PCR purification kit and then subcloned into the pENTR3C vector. Four mutations identified by sequencing were corrected with the QuikChange multisite-directed mutagenesis kit. The sequence was then inserted into vector pcDNA 3.2/v5-DEST using the LR recombination reaction kit.
Development of hTRPA1-HEK293 stable cell line
To create a stably transfected cell line expressing hTRPA1, 1.0 × 106 human embryonic kidney 293 (HEK293) cells were seeded in 35 mm tissue culture dishes and grown overnight in a 37 °C and 5% CO2 incubator, in culture medium consisting of DMEM, 10% foetal bovine serum and penicillin with streptomycin. On the next day, the cells were transfected using 4 μg of pcDNA 3.2-hTRPA1 with 7 μl of Lipofectamine 2000 following the manufacturer's protocol. After 2 days in culture, the cells were replated at 1:10 and 1:100 in the presence of 1 mg ml−1 Geneticin. Once stably expressing clones were identified, the concentration of Geneticin was reduced to 0.25 mg ml−1 for expansion and maintenance. Clones were selected on the basis of their response to cinnamaldehyde and AITC in the fluorescence-imaging plate reader (FLIPR) assay using the membrane potential assay kit RED.
FLIPR assay
Human transient receptor potential channel A1-HEK293 or HEK293 cells were seeded overnight in poly-D-lysine coated 384-well plates at 15 000 cells per well in 20 μl of media. The assay was performed using a fluorometric-imaging plate reader (FLIPR-Tetra). To monitor changes in membrane potential, the cells were loaded with 20 μl of membrane potential assay kit RED dye per well and were incubated at 37 °C and 5% CO2 for 1 h. To measure intracellular calcium changes, the calcium 3 dye, supplemented with 5 mM of probenecid, was used. The plates were equilibrated to room temperature for 15 min before the start of the assay. The plates were read on the FLIPR for a total of 3 min, including an initial 10 s reading window to determine baseline fluorescence levels before the application of any compound. After the compounds were applied by the FLIPR, the plates were read for an additional 2 min and 50 s. Membrane potential experiments were read using the excitation 510–545 nm and emission 565–625 nm filter set. Calcium experiments were read using the excitation 474–495 nm and emission 515–575 nm filter set. Results are presented as relative fluorescence units (RFU).
The test compounds camphor, carvacrol, o-cresol, 2,6-diisopropylphenol, 2,5-dimethylphenol, 2,6-dimethylphenol, 3,4-dimethylphenol, phenol, 2-tert-butyl-5-methylphenol and thymol were prepared in dimethyl sulphoxide. Because the final concentration of dimethyl sulphoxide was 1% in the assay, vehicle controls of 1% dimethyl sulphoxide were also included in each assay plate.
Electrophysiology
Whole-cell recordings of TRP channel currents were obtained from freshly trypsinized, stably transfected cells. The cells were plated on the glass coverslip bottom of the recording chamber and then allowed to attach for 5–10 min before the start of the experiment. The bath solution was Hank's balanced salt solution (HBSS), composed of (mM): 1.2 CaCl2, 0.5 MgCl2, 0.4 MgSO4, 5.3 KCl, 0.4 KH2PO4, 137.9 NaCl, 0.3 Na2HPO4 and 5.5 d-glucose, supplemented with 20 mM HEPES, pH 7.4 (NaOH). The internal pipette solution contained, in mM: 135 caesium glutamate, 8 NaCl, 5.1 CaCl2, 10 HEPES, 6 EGTA and 2 ATP-Mg, pH 7.2 (NaOH). Recording pipettes were pulled from fire-polished borosilicate glass to a resistance of approximately 2 MΩ using a Flaming/Brown micropipette puller. Serially diluted dimethyl sulphoxide compound stocks were diluted in bath solution and applied to the cells during the recording by perfusion using a multi-barrel applicator (SF-72).
Voltage clamp recordings were obtained in whole-cell mode using MultiClamp 700B amplifier and Digidata 1322A converter running on Clampex 9.2 software (Molecular Devices, Sunnyvale, CA, USA). Recordings were performed at room temperature at a holding potential of −80 mV. Series resistance (2–6 MΩ) and capacitance (10–19 pF) were measured and automatically compensated (to ∼75%) immediately after the break-in, and the resulting capacitance measurements were used for current density calculations. Data were filtered at 1 kHz and sampled at 5 kHz.
Calcium imaging
For these experiments, hTRPA1-HEK293 or HEK293 parental cells were trypsinized, allowed to recover at room temperature and then plated on the glass coverslip floor of the imaging chamber. The cells were then loaded with 4 μM fura-2 AM dye for 30 min. The cells were viewed through a 40 × Plan Fluor magnification objective using a TE2000S inverted microscope. Images were acquired with a Pixel Fly CCD camera. A xenon lamp (175 W) controlled by the Lambda 10 shutter controller was used to excite cells at 340 and 380 nm. Ratiometric images were obtained every 2 s with the use of InCyt 2 imaging software (Intracellular Imaging Inc., Cincinnati, OH, USA). The calcium ionophore ionomycin (10 μM) was applied at the end of some experiments as a positive control.
Data analysis
Results from electrophysiological recordings were analysed using Origin software to fit the concentration–response curves. The results from the FLIPR assays were analysed using Prism software. For ΔRFU measurements, the baseline fluorescence signal (RFUmin) was subtracted from the peak fluorescence signal (RFUmax) at each compound concentration (RFUmax−RFUmin). The results shown are from one experiment, n=3, that is representative of a total of two to four independent FLIPR experiments. To calculate EC50 values for compounds displaying dual agonist–antagonist properties, as originally shown for thymol by others (Karashima et al., 2007), compound concentrations exhibiting antagonism were excluded from the curve-fit analysis. Curves were fitted using a sigmoidal dose–response equation with variable slope, and EC50 values calculated from that equation are shown with 95% confidence intervals. Error bars for each data point represent s.d.
Chemical property calculations
The logP values were calculated using the Molecular Operating Environment 2006.8 software system (Chemical Computing Group, Montreal, QC, Canada) with the Wildman and Crippen function. The pKa values for thymol and trinitrophenol were obtained from the PhysProp Database (http://www.syrres.com/esc/physdemo.htm, Syracuse Research Corporation, Syracuse, NY, USA).
Materials
Human lung polyA+ RNA library (BD Biosciences, San Jose, CA, USA); Thermoscript RT-PCR system, PureLink PCR purification kit, pENTR3C vector and LR recombination reaction kit (Invitrogen, Carlsbad, CA, USA); GC Melt (BD Biosciences); QuikChange multi site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). HEK293 cells (ATCC, Manassas, VA, USA); 35 mm tissue culture dishes (Falcon, BD Biosciences, Bedford, MA, USA); Lipofectamine 2000 and Geneticin (Invitrogen); membrane potential assay kit RED, calcium 3 dye and FLIPR-Tetra (Molecular Devices). Camphor, carvacrol, o-cresol, 2,6-diisopropylphenol, 2,5-dimethylphenol, 2,6-dimethylphenol, 3,4-dimethylphenol, phenol, 2-tert-butyl-5-methylphenol and thymol were purchased from Sigma-Aldrich (St Louis, MO, USA). Hank's balanced salt solution and HEPES were obtained from Invitrogen. Flaming/Brown micropipette puller (Sutter Instruments, Novato, CA, USA); multi-barrel applicator (SF-72, Warner, Hamden, CT, USA); fura-2 AM dye (Molecular Probes, Invitrogen). Plan Fluor magnification objective and TE2000S inverted microscope (Nikon, Japan); Pixel Fly CCD camera (Cooke Corporation, Romulus, MI, USA); xenon lamp (175W; Intracellular Imaging Inc.); Lambda 10 shutter controller (Sutter Instruments).
Results
Time course of hTRPA1 activation by thymol versus activation by cinnamaldehyde
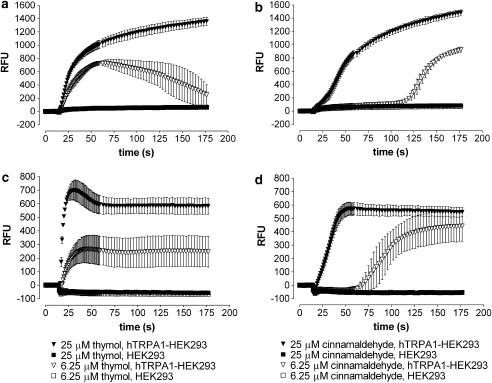
A panel of small molecules known for their pungency, astringency or strong taste was evaluated for the ability to activate hTRPA1 in a FLIPR-based assay. In the membrane potential assay, thymol depolarized hTRPA1-HEK293 cells (Figure 1a) as shown by a strong increase in dye fluorescence for 6.25 and 25 μM thymol. As a positive control, cinnamaldehyde was tested at 6.25 and 25 μM and also depolarized hTRPA1-HEK293 cells (Figure 1b) to a similar extent. Neither compound had an effect on the parental HEK cells, which do not express hTRPA1 (Figures 1a and b).
Figure 1.
Thymol activates hTRPA1 more rapidly than cinnamaldehyde. Both thymol (a, c) and cinnamaldehyde (b, d) activated hTRPA1, but the time course of activation by thymol was more rapid than that by cinnamaldehyde, as shown for two concentrations of agonist by membrane potential response (a, b) and internal calcium changes (c, d). The difference was more marked with 6.25 μM thymol. Parental cells, which do not express hTRPA1, were not activated by either thymol or cinnamaldehyde. Each compound concentration was assayed in triplicate, and the average RFU ±s.d. is plotted versus time. The compounds were added 15 s after the start of the experiment. hTRPA1, human transient receptor potential channel A1; RFU, relative fluorescent units.
It is known that hTRPA1 is a calcium-permeable channel (Story et al., 2003). Activation of hTRPA1 should therefore lead to an increase in intracellular calcium. To confirm the activation of hTRPA1 by thymol, we performed the FLIPR assay using a calcium-sensitive dye and measured changes in intracellular calcium concentration following compound application. Thymol, applied at 6.25 and 25 μM, increased intracellular calcium (Figure 1c), further suggesting that it can activate the hTRPA1 receptor. Cinnamaldehyde also increased the intracellular calcium concentration to a similar extent (Figure 1d). No activation of parental cells was detected for either compound (Figures 1c and d). Interestingly, the time course of thymol activation was more rapid than the time course of cinnamaldehyde activation. In the membrane potential assay, it took 31 and 28 s to reach half of maximal activation for 25 and 6.25 μM thymol, respectively, compared to 52 and 132 s for 25 and 6.25 μM cinnamaldehyde, respectively (Figures 1a and b). The differences in the lag time for the calcium assay were comparable. A single experiment is shown, but results from a total of four independent experiments, performed in triplicate, displayed a similar trend.
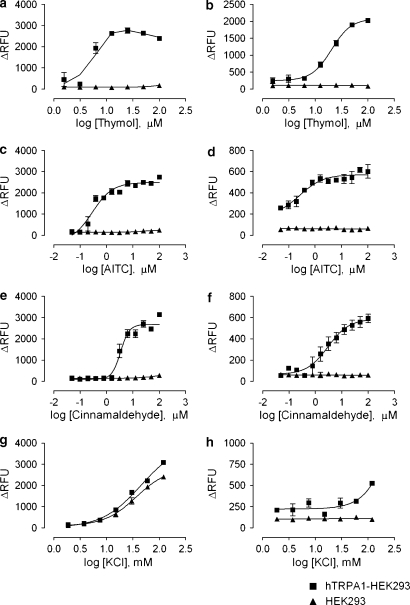
Concentration-dependent activation of hTRPA1 by thymol
Thymol was found to depolarize hTRPA1-HEK293 cells, in a concentration-dependent manner, with an EC50 value of 6 μM (Figure 2a). Thymol application also increased intracellular calcium (Figure 2b) with an EC50 of 20 μM. As a control, the cells were treated with the known TRPA1 ligands AITC and cinnamaldehyde. Both AITC and cinnamaldehyde depolarized hTRPA1-HEK293 cells (Figures 2c and e) and led to intracellular calcium increases (Figures 2d and f) with EC50 values of 0.3 and 3 μM, respectively. The parental HEK293 cell line was not activated by thymol, AITC or cinnamaldehyde, indicating that the increase in membrane potential in hTRPA1-HEK293 cells was due to activation of the hTRPA1 channel and not of the endogenous channels expressed by the cell. Non-receptor-mediated depolarization by KCl gave similar membrane potential dye responses in TRPA1-transfected and parental cells (Figure 2g). Depolarization by KCl, up to a concentration of 100 mM, did not lead to substantial increases in intracellular calcium (Figure 2h), indicating that depolarization in the absence of TRPA1 activation is not sufficient to increase intracellular calcium levels.
Figure 2.
Concentration–response curves of hTRPA1 ligands. The concentration-dependent effects of thymol (a, b), AITC (c, d), cinnamaldehyde (e, f) and KCl-mediated depolarization (g, h) on hTRPA1-HEK293 cells were evaluated by FLIPR assay. Membrane potential responses (left-hand column) and intracellular calcium changes (right-hand column) were measured. Each data point represents n=3, and the graphs are representative of 2–4 experiments. AITC, allyl isothiocyanate; FLIPR, fluorescence-imaging plate reader; HEK293, human embryonic kidney 293; hTRPA1, human transient receptor potential channel A1.
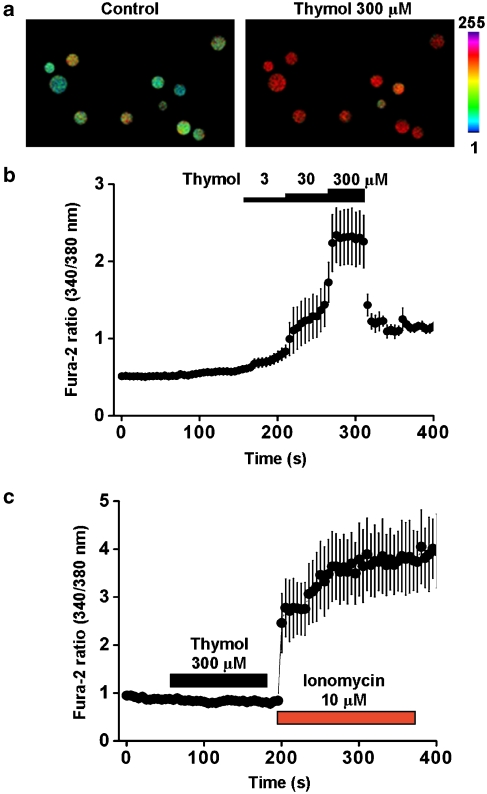
Intracellular calcium increase following hTRPA1 activation by thymol
To confirm that thymol induced changes in intracellular calcium, single-cell calcium imaging experiments were performed with hTRPA1-HEK293 cells loaded with fura-2 dye. Fluorescence ratios (F340/F380) were calculated before and after thymol addition. Bath application of 300 μM thymol resulted in a significant increase in the fura-2 ratio in hTRPA1-HEK293 cells (Figure 3a). This activation was concentration-dependent (Figure 3b) with an average EC50 value of 64 μM (n=6). Parental HEK cells did not respond to an initial concentration of 300 μM thymol (Figure 3c). As a positive control, 10 μM ionomycin applied at the end of this recording increased intracellular calcium levels (Figure 3c).
Figure 3.
Thymol activates hTRPA1-dependent calcium transients. (a) Ratiometric calcium imaging from hTRPA1-HEK293 cells loaded with fura-2 AM dye before and after addition of 300 μM thymol. (b) Ratiometric responses of six cells before and during perfusion with increasing concentrations of thymol. (c) Addition of 300 μM thymol to parental HEK cells was without effect, whereas 10 μM ionomycin produced a robust increase in fluorescence ratio. Data shown in (b) and (c) represent mean±s.e.mean. HEK293, human embryonic kidney 293; hTRPA1, human transient receptor potential channel A1.
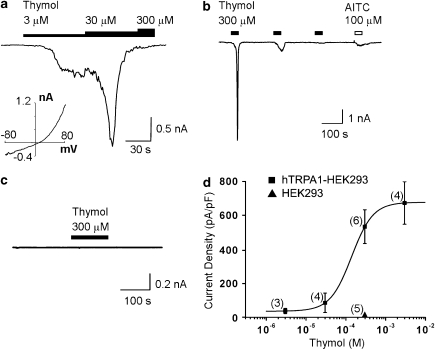
Whole-cell electrophysiological recordings
Whole-cell electrophysiological recordings were obtained to measure the effect of thymol on hTRPA1 currents directly. The hTRPA1-HEK293 cells were recorded during bath application of thymol or AITC. Thymol activated currents in these cells (Figure 4a). Initially, we tried to examine the concentration-dependence of thymol activation of hTRPA1. However, repeated applications of thymol on hTRPA1-HEK293 cells caused the channel to desensitize, eliminating the concentration-dependent effect (Figures 4a and b). The activation of hTRPA1 currents by an initial application of thymol was comparable in magnitude to the activation of hTRPA1 by AITC (data not shown). Desensitization of hTRPA1 by thymol reduced its response to AITC, suggesting that the two compounds were acting on the same channel (Figure 4b). Parental HEK cells, did not respond to an initial concentration of 300 μM thymol (n=3), excluding the possibility of nonspecific effects on the cells (Figure 4c). To calculate an EC50 value, a concentration–response curve could be compiled by measuring only the response to the initial application of thymol to minimize the effects of desensitization on the calculation. This method revealed a concentration-dependent activating effect of thymol on hTRPA1, with a calculated EC50 value of 127 μM (Figure 4d).
Figure 4.
Thymol activates hTRPA1 current. (a) Whole-cell voltage clamp recordings from an isolated hTRPA1-expressing HEK293 cell held at −80 mV. hTRPA1 current was activated by 3 μM thymol. At 30 μM, the activation was followed by desensitization that prevented a response to a higher thymol concentration. The inset shows the current response to a depolarizing ramp from −80 to +80 mV. (b) The robust current evoked by an initial application of 300 μM thymol in a different cell was greatly decreased in subsequent applications. Thymol application also reduced the response to AITC. (c) Sample trace showing that in an isolated non-transfected HEK293 cell, thymol (300 μM) produced no effect. (d) The concentration–response curve for initial application of thymol gave an EC50 value of 127 μM. Error bars show s.e.mean, and n for each data point is shown in parentheses. AITC, allyl isothiocyanate; HEK293, human embryonic kidney 293; hTRPA1, human transient receptor potential channel A1.
SAR of the hTRPA1 activation
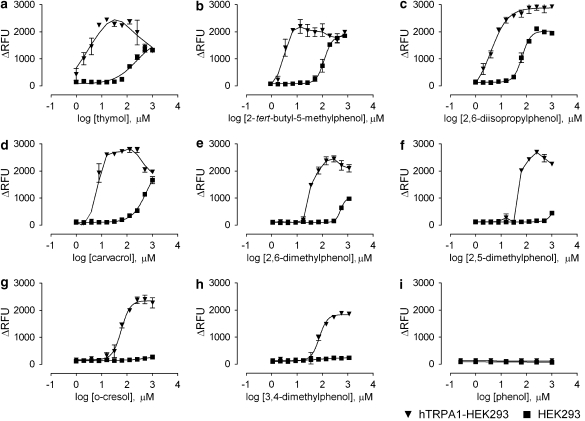
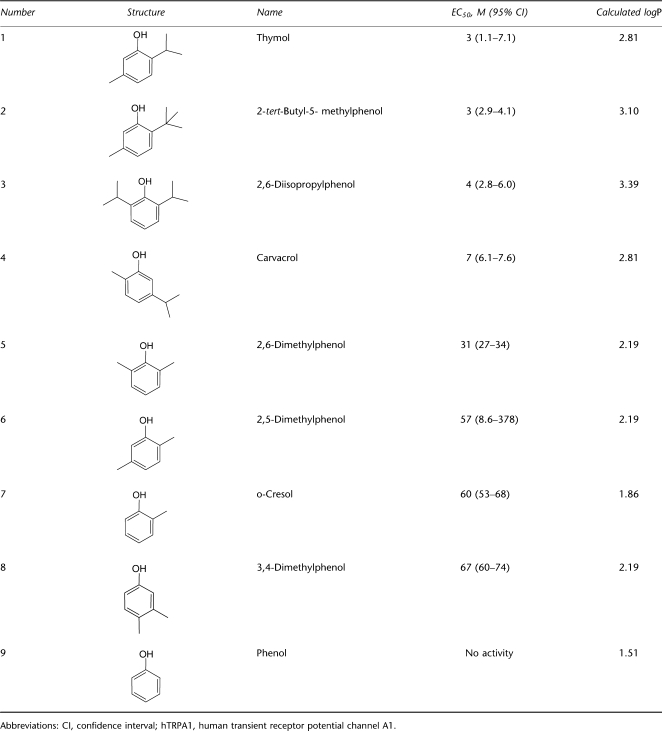
To continue exploring the activation of hTRPA1 by thymol, we performed a SAR analysis. The ability of eight commercially available alkyl-substituted phenols to activate hTRPA1 was evaluated in our membrane potential assay (Figure 5). Many of these compounds, in particular thymol (Figure 5a), 2-tert-butyl-5-methylphenol (Figure 5b), carvacrol (Figure 5d), 2,6-dimethylphenol (Figure 5e) and 2,5-dimethylphenol (Figure 5f), showed a bimodal activity pattern similar to that observed by Karashima et al. (2007). Table 1 shows the structures and calculated EC50 values for this panel of compounds. Although the specific EC50 values for each compound varied between trials, the rank order of potency remained consistent across four independent experiments. The calculated logP value for each compound is also shown in Table 1. We observed that for TRPA1 activation, the potency of these alkyl-substituted phenols generally declined as the logP value decreased.
Figure 5.
Compounds that are structurally similar to thymol can activate the hTRPA1 channel. The EC50 values for these related alkyl phenols are shown in Table 1. Results shown are from one representative experiment, n=3, out of four independent experiments. hTRPA1, human transient receptor potential channel A1; HEK293, human embryonic kidney 293.
Table 1.
Structure, hTRPA1 activity and calculated logP values of substituted alkyl phenols
Effect of an hTRPA1 antagonist on the thymol response
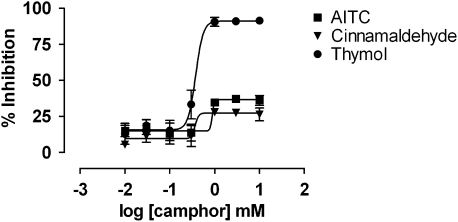
Taken together, our data suggest that thymol is a potent activator of the hTRPA1 channel. If thymol is directly activating the hTRPA1 channel, its effect should be blocked by pretreatment with hTRPA1 inhibitors. To confirm the specificity of hTRPA1 activation, we measured the membrane potential change after application of 30 μM thymol, 100 μM cinnamaldehyde or 30 μM AITC in the presence of racemic camphor, a known TRPA1 blocker (Xu et al., 2005) (Figure 6). The data are presented as a percentage of inhibition of membrane potential increase compared to control and show the results of one experiment (n=3), which was representative of two independent experiments. Camphor blocked the thymol activation of hTRPA1 in a concentration-dependent manner with an IC50 value of 400 μM. In comparison, camphor only partially blocked hTRPA1 activation by cinnamaldehyde and AITC even at the highest concentration, 10 mM, used in the assay.
Figure 6.
Thymol stimulation of hTRPA1 was selectively blocked by the hTRPA1 inhibitor camphor. hTRPA1-HEK293 cells were pretreated with camphor at various concentrations and then treated with 30 μM thymol, 100 μM cinnamaldehyde or 30 μM AITC. Camphor blocked hTRPA1 activation by thymol with an IC50 value of 400 μM. AITC, allyl isothiocyanate; HEK293, human embryonic kidney 293; hTRPA1, human transient receptor potential channel A1.
Discussion
Thymol is a useful antimicrobial component of consumer health products, in particular of oral care products such as oral rinses. However, its pungent, medicinal and unpleasant taste limits its use in formulations where taste qualities are important. The molecular basis of this strong and unpleasant flavour is not yet known, and its identification would be useful for the design of blockers, which could be used in combination with thymol to develop more pleasant formulations of oral care products. Thymol has been shown to act as a positive allosteric modulator of a subset of ionotropic GABA receptors (Priestley et al., 2003), to activate the TRPV3 receptor (Xu et al., 2006) and to have direct agonist activity on the GABAA chloride channel (Mohammadi et al., 2001). However, the taste qualities of these channels are largely unknown. Thymol is also known to activate TRPM8 (Vogt-Eisele et al., 2007), but this interaction is not likely to account for all of its pungent taste.
Here, we show that thymol directly activates hTRPA1. This member of the TRP channel family is activated by other compounds that are known to have an aversive pungent taste or to cause pain and inflammation. For example, allicin and diallyl disulphide, two of the pungent components found in garlic, activated hTRPA1 heterologously expressed in HEK293t cells and Xenopus oocytes and endogenously expressed in nocioceptive sensory neurons. Although these sensory neurons also expressed hTRPV1, the pungency of these compounds was attributed to hTRPA1 activation (Bautista et al., 2005). hTRPA1 is also activated by isothiocyanate compounds, such as AITC, all of which have very strong aversive sensory qualities (Jordt et al., 2004). In addition, hTRPA1 is known to be expressed in the rat in cells of the geniculate ganglion, which is mainly composed of primary sensory neurons involved in taste (Katsura et al., 2006). Using FLIPR-based membrane potential and calcium influx assays, whole-cell electrophysiology and calcium imaging, we show here that thymol activates hTRPA1 directly and potently and that its activation can be blocked by the known hTRPA1 antagonist camphor.
The mechanism of action of thymol on hTRPA1 is still unclear. In examining the time course traces from the FLIPR assay, we noted that the action of thymol on membrane potential in hTRPA1-HEK293 cells is two- to fourfold faster than the action of cinnamaldehyde (Figure 1). Other groups studying TRP channels have shown that differences in time lag of agonist response result from a difference in mechanisms of activation. For example, menthol evokes TRPM8 current immediately, whereas icilin evokes a current only after a delay of several seconds. These two agonists have been shown to act on TRPM8 via different mechanisms based on the observation that they are differentially modulated by pH (Andersson et al., 2004). Our differences in activation time may indicate that thymol and cinnamaldehyde act on hTRPA1 via different binding sites or mechanisms. Hinman et al. (2006) and Macpherson et al. (2007) showed that AITC and cinnamaldehyde activate hTRPA1 via covalent modification of cysteine residues located in the cytoplasmic N-terminal domain, which could explain the delay we observed in activation by these compounds. Other structurally unrelated electrophilic compounds have also been found to bind TRPA1, presumably via mechanisms similar to AITC and cinnamaldehyde (McNamara et al., 2007; Taylor-Clark et al., 2008). However, on the basis of their structures, thymol and related simple alkyl phenols would not be expected to act as electrophiles and are therefore unlikely to act on TRPA1 via a covalent mechanism. The amphipathic molecule trinitrophenol has been shown to activate TRPA1 by a mechanosensitive mechanism involving membrane deformation (Hill and Schaefer, 2007). The phenolic group of thymol is much less acidic than that of trinitrophenol (pKa 10.6 and 0.4, respectively; see Methods section), which suggests that thymol would not have the amphipathic properties needed to trigger membrane deformation.
During the preparation of the paper, Karashima et al. (2007) demonstrated the bimodal activation of TRPA1 by thymol and menthol. We observed similar bimodal behaviour with thymol and some of the other alkyl phenols as well. The mechanism of the bimodal behaviour of these ligands is unclear. One explanation could be the overlapping effects of activation and desensitization at high concentrations. Interestingly, this bimodal behaviour was not observed with cinnamaldehyde or AITC, further supporting the hypothesis that these two ligands activate hTRPA1 by a different mechanism from that of thymol.
Within the group of alkyl phenols tested for TRPA1 activation (Table 1), we found a positive correlation between logP and potency. The more highly substituted phenols (Table 1, 1–4), with calculated logP values of 2.81 or higher, were more potent than the lesser substituted phenols with calculated logP values of 2.19 or lower (5–9). We also find evidence for an SAR, indicating that the presence of bulkier alkyl groups, such as isopropyl (1) or tert-butyl (2), increase the potency of the compound when they are adjacent to the phenolic hydroxyl (1–3). The dimethyl phenol 2,6-dimethlyphenol (5) was more active than 2,5-dimethylphenol (6) or 3,4-dimethylphenol (8), further supporting the idea that alkyl substitution around the hydroxyl moiety improves potency. We noted, however, that carvacrol (4), which was originally shown to activate the rat isoform of TRPA1 by Xu et al. (2006), has a single methyl group adjacent to the hydroxyl but is nearly as active as thymol. Its relatively high-calculated logP value may be important for its activity. Phenol (9) had no activity at any concentration tested in the FLIPR assay up to 1 mM. Other groups have found that related structures are able to activate hTRPA1. Methyl-p-hydroxybenzoate, for example, activates TRPA1 with an EC50 value in the millimolar range (Fujita et al., 2007), which corresponds to our SAR findings regarding the requirement for alkyl substitution around the hydroxyl moiety to have a potency less than 100 μM. Taken together, the logP values and alkyl substituent pattern suggest that thymol and related alkyl phenols might bind in a hydrophobic pocket to activate TRPA1.
Previously, Xu et al. (2005) showed a complete blockade of TRPA1 activation with 10 mM camphor. In their experiments, the TRPA1 current was activated by 200 μM AITC. In contrast, we only observed a partial block of the TRPA1 current by 10 mM camphor when AITC or cinnamaldehyde was used to activate the channel. We have in the past observed differences between the magnitudes of EC50 values calculated from FLIPR assay data versus electrophysiology data. This could provide one explanation for the difference between our data and previously published results. Alternatively, there may be functional differences between the rat homologue of TRPA1 used by Xu et al. and the human homologue of TRPA1 used in our experiments. Sequence differences between the two homologues may result in variations in the structure of binding sites of both agonists and antagonists for the channel, potentially modifying the magnitude of their effect. In addition, because we expect that the mechanism of binding to TRPA1 for thymol is different from that for AITC or cinnamaldehyde, it is not unreasonable to also expect that an antagonist such as camphor would also exhibit different potency against them. The mechanism of camphor inhibition is not yet known. It should be noted that camphor acts as an agonist of other channels such as TRPV1 (Xu et al., 2005).
There is no clear explanation for the discrepancies observed in EC50 values calculated from data obtained by different techniques. In the present study, we obtained EC50 values for thymol of 6 μM in the FLIPR-based membrane potential assay and 127 μM from the whole-cell patch clamp experiments. In addition, the EC50 value was 20 μM in the FLIPR-based calcium influx assay and 64 μM from calcium imaging. These differences in our own experiments could be caused by the differences in preparation; whereas calcium imaging measurements and whole-cell electrophysiological recordings collected data from individual freshly trypsinized and dissociated cells, experiments using the FLIPR platform collected population data from a monolayer of cells grown overnight in 384-well plates. Other laboratories have also observed similar differences. For example, the EC50 of capsaicin for TRPV1 by whole cell recording in Xenopus oocytes was originally found by one laboratory to be 712 nM (Caterina et al., 1997). A second laboratory obtained an EC50=34 nM (Jerman et al., 2000) for capsaicin activation of rTRPV1 in FLIPR-based experiments but obtained an EC50 of 497 nM by electrophysiological recording (Gunthorpe et al., 2000). A third laboratory found an EC50=2 nM in a FLIPR assay (Valenzano et al., 2003). In another example, EC50 values for AITC activation of TRPA1 were found to be 2–5 μM when calculated by a calcium imaging assay (McNamara et al., 2007), but 64 μM in Xenopus oocyte recordings from the same laboratory (Hinman et al., 2006). We observed similar differences between the FLIPR and the electrophysiology assays for capsaicin activation of the hTRPV1 channel and for AITC activation of hTRPA1 (data not shown). However, these differences do not detract from the observation that thymol potently activates hTRPA1 in a specific and concentration-dependent manner.
In conclusion, the present study demonstrates that thymol and other alkyl-substituted phenols can activate the ion channel hTRPA1. Because hTRPA1 is known to be activated by compounds with pungent or unpleasant tastes, hTRPA1 activation may be responsible for the aversive taste qualities of thymol. In this regard, an hTRPA1 antagonist could improve the taste and acceptance of thymol-containing oral care products. The new data we provide on SAR, together with recent results presented in the literature regarding the multiple mechanisms of activation of hTRPA1, could be useful in the design of such an antagonist.
Acknowledgments
We thank Ray Salemme, Kyle Palmer and Phil Stein for helpful comments and discussion. This work was originally presented as Abstract no. 396 at the 2007 annual meeting of the Association for Chemoreception Sciences.
Abbreviations
- AITC
allyl isothiocyanate
- FLIPR
fluorescence-imaging plate reader
- HEK293
human embryonic kidney 293
- hTRPA1
human transient receptor potential subfamily A member 1
- RFU
relative fluorescence unit
Conflict of interest
The authors state no conflict of interest.
References
- Andersson DA, Chase HW, Bevan S. TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH. J Neurosci. 2004;24:5364–5369. doi: 10.1523/JNEUROSCI.0890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, et al. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA. 2005;102:12248–12252. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga PC, Dal Sasso M, Culici M, Bianchi T, Bordoni L, Marabini L. Anti-inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006a;77:130–136. doi: 10.1159/000093790. [DOI] [PubMed] [Google Scholar]
- Braga PC, Dal Sasso M, Culici M, Galastri L, Marceca MT, Guffanti EE. Antioxidant potential of thymol determined by chemiluminescence inhibition in human neutrophils and cell-free systems. Pharmacology. 2006b;76:61–68. doi: 10.1159/000089719. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Fujita F, Moriyama T, Higashi T, Shima A, Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthorpe MJ, Harries MH, Prinjha RK, Davis JB, Randall A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J Physiol. 2000;525 Part 3:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseler G, Maue D, Grosskreutz J, Bufler J, Nentwig B, Piepenbrock S, et al. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur J Anaesthesiol. 2002;19:571–579. doi: 10.1017/s0265021502000923. [DOI] [PubMed] [Google Scholar]
- Hazzit M, Baaliouamer A, Faleiro ML, Miguel MG. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J Agric Food Chem. 2006;54:6314–6321. doi: 10.1021/jf0606104. [DOI] [PubMed] [Google Scholar]
- Hill K, Schaefer M. TRPA1 is differentially modulated by the amphipathic molecules trinitrophenol and chlorpromazine. J Biol Chem. 2007;282:7145–7153. doi: 10.1074/jbc.M609600200. [DOI] [PubMed] [Google Scholar]
- Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci USA. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerman JC, Brough SJ, Prinjha R, Harries MH, Davis JB, Smart D. Characterization using FLIPR of rat vanilloid receptor (rVR1) pharmacology. Br J Pharmacol. 2000;130:916–922. doi: 10.1038/sj.bjp.0703390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsura H, Tsuzuki K, Noguchi K, Sakagami M. Differential expression of capsaicin-, menthol-, and mustard oil-sensitive receptors in naive rat geniculate ganglion neurons. Chem Senses. 2006;31:681–688. doi: 10.1093/chemse/bjl009. [DOI] [PubMed] [Google Scholar]
- Lambert RJ, Skandamis PN, Coote PJ, Nychas GJ. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, et al. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol. 2005;15:929–934. doi: 10.1016/j.cub.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Margolskee RF. Molecular mechanisms of bitter and sweet taste transduction. J Biol Chem. 2002;277:1–4. doi: 10.1074/jbc.R100054200. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci UA. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi B, Haeseler G, Leuwer M, Dengler R, Krampfl K, Bufler J. Structural requirements of phenol derivatives for direct activation of chloride currents via GABA(A) receptors. Eur J Pharmacol. 2001;421:85–91. doi: 10.1016/s0014-2999(01)01033-0. [DOI] [PubMed] [Google Scholar]
- Park JJ, Lee J, Kim MA, Back SK, Hong SK, Na HS. Induction of total insensitivity to capsaicin and hypersensitivity to garlic extract in human by decreased expression of TRPV1. Neurosci Lett. 2007;411:87–91. doi: 10.1016/j.neulet.2006.10.046. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Priestley CM, Williamson EM, Wafford KA, Sattelle DB. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABA(A) receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol. 2003;140:1363–1372. doi: 10.1038/sj.bjp.0705542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, Macglashan DW, Jr, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Grant ER, Wu G, Hachicha M, Schmid L, Tafesse L, et al. N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J Pharmacol Exp Ther. 2003;306:377–386. doi: 10.1124/jpet.102.045674. [DOI] [PubMed] [Google Scholar]
- Vogt-Eisele AK, Weber K, Sherkheli MA, Vielhaber G, Panten J, Gisselmann G, et al. Monoterpenoid agonists of TRPV3. Br J Pharmacol. 2007;151:530–540. doi: 10.1038/sj.bjp.0707245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Blair NT, Clapham DE. Camphor activates and strongly desensitizes the transient receptor potential vanilloid subtype 1 channel in a vanilloid-independent mechanism. J Neurosci. 2005;25:8924–8937. doi: 10.1523/JNEUROSCI.2574-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]