Abstract
The epidermal growth factor receptor (EGFR) network, including its seven ligands and four related receptors, represents one of the most complex signaling systems in biology. In many tissues, including the skin and its appendages (notoriously the hair follicles), its correct function is necessary for proper development and tissue homeostasis, and its deregulation rapidly results in defects in cellular proliferation and differentiation. The consequences are impaired wound healing, development of psoriasis-like lesions, structural and functional defects of the hair follicles, and tumorigenesis. In addition to in vitro experiments and data from clinical studies, several genetically modified mouse models displaying alterations in the interfollicular skin and hair follicles attributable to mutations in components of the EGFR system have been reported. These animals, in many cases representing bona fide models of known human diseases, have been seminal in the study of the role of EGFR and its ligands in the skin and its appendages. In this review, we take the multiple phenotypes of these animal models as a basis to summarize and discuss the effects elicited by members of the EGFR system in diverse aspects of skin biology and pathology, including cellular proliferation and differentiation, wound healing, hair follicle morphogenesis, and tumorigenesis.
The epidermal growth factor receptor (EGFR) and its ligands represent one of the most powerful and complex signaling networks in higher vertebrates. In this pleiotropic system that exerts an unusually wide array of diverse bioregulatory functions, several peptide growth factors promote the homo- or heterodimerization and subsequent autophosphorylation of a family of tyrosine kinase receptors. Consequently, adaptor proteins and enzymes initiate signaling cascades, culminating in biological outcomes ranging from cell division to cell death, differentiation, or malignant transformation.
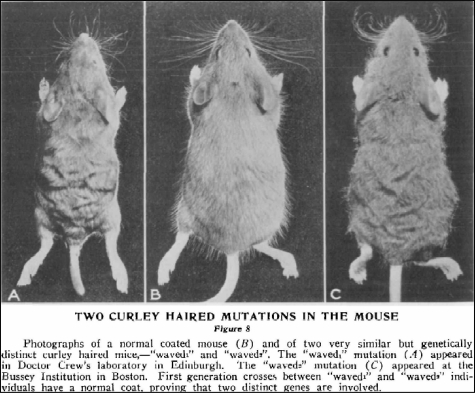
Retrospectively, the first indications that EGFR-mediated signaling plays a central role in skin biology and pathology, can be traced back to 1933, when Francis A.E. Crew published a report describing mice with “coats which looked exactly as though the animals had been to the hairdresser and had had a permanent wave treatment.”1 This pleasantly written account (considering the sobriety of today’s scientific literature style) is probably the first description of the phenotypic consequences of a perturbation in the activity of the epidermal growth factor receptor. The mouse line described, carrying a point mutation in the gene encoding transforming growth factor-α (TGF-α), is known today as waved-1 (wa-1). Only 2 years after Crew’s description of the wa-1 mutation, mice with a similar phenotype were described by Clyde Keeler2 and named waved-2. Keeler2 reported (without missing the obligatory reference to a hairdresser) that wa-2 mice, as compared with wa-1, showed a more marked phenotype of “marcelled” hairs and curled vibrissae (Figure 1). Sixty years later, the identification of a point mutation in the egfr gene as the genetic basis of the wa-2 phenotype3,4 confirmed the correctness of his observations: a mutation in the receptor is expected to result in a more severe phenotype than a mutation affecting only one of its ligands. Both mouse lines are still being used by researchers for examining the effects of reduced levels of these proteins in different organs.
Figure 1.
Photographs published by Clyde E. Keeler2 in 1935 comparing a wa-1 (A), a wa-2 (C), and a normal coated mouse (B). Reproduced from The Journal of Heredity, 1935, Vol 26, pp 189–191, with permission from Oxford University Press.
Since the initial description of wa-1 mice, evidence has accumulated from numerous molecular, cellular, and whole-organism studies that, collectively, indicate a central role for the EGFR family and its ligands in cutaneous biology and pathology: 1) EGFR ligands are autocrine- and paracrine-acting growth factors for keratinocytes, playing a central role in controlling proliferation of these cells5,6,7,8,9,10; 2) EGFR ligands also activate mesenchymal cells and stimulate fibroblast proliferation and angiogenesis11,12; 3) diverse EGFR ligands were detected in wound fluid13,14,15 and the expression of EGFRs transiently increases after wounding,16 indicating a role for this network in healing of skin wounds; 4) overexpression of multiple EGFR ligands is a hallmark of psoriatic epidermis9,17,18,19; and 5) epithelial squamous cell carcinomas overexpress EGFR,20,21 and substantial evidence implicates EGFR signaling as a major component in the pathogenesis of melanoma22 and nonmelanoma skin cancer.23,24,25,26
Numerous genetically engineered mouse models displaying alterations in the skin and hair follicles attributable to changes in the activity of members of the EGFR family (Table 1) or their ligands (Table 2) have been reported during the last decades. Their phenotypes, ranging from alopecia and psoriasis-like lesions to skin tumors, highlight the exceptionally wide range of effects elicited by the EGFR signaling system in the skin. Here, we summarize and critically discuss the most relevant genetic mouse models with altered activity of these molecules and, based on their phenotypes, examine the role of the EGFR system in the physiology and pathology of the skin and its appendages.
Table 1.
Summary of the Skin Phenotype of Genetically Modified Mouse Models of EGFR and ERBB2
| Receptor | Mutation | Phenotype | References |
|---|---|---|---|
| EGFR (ERBB1) | KO | Open eyelids at birth, impaired epidermal as well as hair follicle differentiation; minor differences and severity depend strongly on the genetic background | 27,28,29 |
| Delayed hair development and multiple hair shaft abnormalities; EGFR-deficient skin grafts respond aberrantly to the wound environment | 30 | ||
| The growth of squamous papillomas produced by grafting EGFR-deficient, v-ras-transformed keratinocytes onto nude mice is strongly impaired | 31 | ||
| EGFR is essential for the development of skin tumors in K5-SOS transgenic mice | 23 | ||
| EGFR is essential for maintaining the proliferative population in the basal cell compartment of papillomas | 24 | ||
| Humanized (KI) | Curly whiskers, altered morphology, and distribution of hair follicles; progressive degeneration with loss of most follicles over time | 32 | |
| TG (K5-EGFR-DN) | Waved hairs, curly whiskers, progressive hair degeneration, and alopecia | 33 | |
| ERBB2 | TG (K5-ERBB2*) | Thickened skin, patchy hair growth, severe follicular hyperplasia, and spontaneous papilloma; lethal | 34 |
| Transgenic (K5-ERBB2) | Alopecia, follicular hyperplasia, and sebaceous gland enlargement as well as spontaneous skin tumor development | 35 | |
| Transgenic (K14-ERBB2*) | Extensive skin phenotype; epidermal hyperplasia, preneoplasia, papilloma, hyperkeratosis, and dyskeratosis; disruption in hair follicle morphogenesis; lethal | 36 | |
| Transgenic (K14-ERBB2*-I) | Conditional overexpression causes reversible hyperproliferation of epidermal basal cells and hyperplasia of the skin and other stratified epithelia | 37 |
KO, knockout; KI, knockin; K5, keratin 5 promoter; K14, keratin 14 promoter; DN, dominant-negative;
, constitutively active form; I, doxycycline-inducible.
Table 2.
Summary of the Skin Phenotypes of Genetically Modified Mouse Models of EGFR Ligands
| Ligand | Mutation | Phenotype | References |
|---|---|---|---|
| AREG | TG (K14-AREG) | Psoriasis-like phenotype; erythematous skin with alopecia, occasional papillomatous growth; dermal and epidermal lymphocytic and neutrophilic infiltration | 38 |
| TG (INV-AREG) | Similar to K14-AREG mice; reduced E-cadherin levels in psoriatic lesions | 39, 40 | |
| BTC | TG (CBA-BTC) | Delayed hair cycle induction; increased angiogenesis at the wound site | 41 |
| EGF | TG (CMV-EGF) | Hyperproliferation of basal layer cells, arrest of hair follicle development | 42 |
| EREG | KO | Chronic dermatitis | 43 |
| HBEGF | KO | Impaired wound healing | 44 |
| TGF-α | KO | Wavy hair, curly whiskers, defective ORS, and altered hair follicle structure; impaired early wound epithelialization | 45,46,47 |
| MT-TGF-α | Hyperplastic skin, papillomas, sebaceous adenomas, and more rarely, sebaceous and squamous cell carcinomas after DMBA treatment; synergy with TPA promotion | 48, 49 | |
| TG (K14-TGF-α) | Epidermal thickening and stunted hair growth; spontaneous papillomas; psoriasis-like lesions; tumor formation without an initiator agent | 50, 51 | |
| TG (K1-TGF-α) | Epidermal hyperproliferation and hyperkeratosis; spontaneous papilloma formation with high sensitivity to tumor promotion by TPA | 52, 53 | |
| TGF-α/v-fosdouble-transgenic mice: aberrant keratinocyte differentiation; accelerated papillomatogenesis and malignant conversion after TPA promotion | 54, 55 | ||
| TGF-α/v-Ha-ras double-transgenic mice: increased epidermal hyperproliferation and tumorigenesis and malignant conversion | 56, 57 |
TG, transgenic; KO, knockout; K14, keratin 14 promoter; K1, keratin 1 promoter; INV, involucrin promoter; CBA, chicken β-actin promoter; CMV, cytomegalovirus promoter; MT, metallothionein promoter.
The EGFR Network
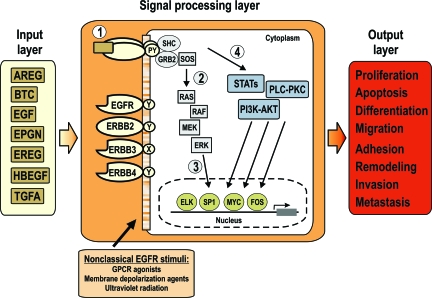
Together with their ligands, EGFR and its related receptors (see below) are essential for normal embryonic development and adult tissue homeostasis, and their deregulation has been associated with many human diseases, including cancer.58 Because they are interesting therapeutic targets, the role of components of this system in tumorigenesis has attracted much attention during the past few years. In cancer cells, constitutive activation of EGFR is achieved by several mechanisms, including increased production of ligands, elevated levels of the receptor, mutations in EGFR extracellular or intracellular domains, defective down-regulation of EGFR or extensive cross talk with other systems like G-protein-coupled receptors, other tyrosine kinase receptors, or with cell adhesion molecules.59 Overexpression of ligands and receptors appears to play a major role in many tumor entities, including non-small-cell lung cancers, ovarian, breast, gastric, and bladder cancer, and their co-expression is often associated with poor survival.58 A useful way to describe the EGFR system is as a multilayered signal-computing network comprising an input layer, a signal-processing layer, and an output layer.60,61
Input Layer
The uppermost level comprises the seven ligands amphiregulin (AREG), betacellulin (BTC), heparin-binding EGF-like growth factor (HBEGF), TGF-α, epiregulin (EREG), epigen (EPGN), and EGF itself (Figure 2). The existence of additional ligands is unlikely because a genome-wide search using algorithms based on genomic and cDNA structures failed to identify further potential EGFR ligands.62 EGFR ligands are initially synthesized as membrane-bound precursors consisting of an EGF motif flanked by an N-terminal extension and a C-terminal membrane-anchoring region. The EGF-like domain, characterized by a consensus sequence composed of six conserved cysteines forming three intramolecular disulfide bonds, can be cleaved (shedded) to release the mature, circulating form.63 The released ligand can activate EGFRs on the cell of its origin (autocrine mode of action), on neighboring cells (paracrine mode, for example, the activation of epithelial cells by mesenchyme-derived factors), or on distant cells after systemic distribution (endocrine mode). In addition, a juxtacrine mechanism, in which the precursor form exerts biological activity by stimulating adjacent cells via cell-cell contacts, has been described for AREG, HBEGF, and TGF-α.64 Interestingly, juxtacrine signaling can possibly induce different biological responses as compared with the effects of the shedded growth factor.
Figure 2.
The EGFR network can be viewed as a multilayered system. The input layer comprises the seven EGFR ligands. In the signal processing layer, the four receptors form 10 possible dimeric combinations after ligand binding. Note that ERBB2 and ERBB3 homodimers are nonfunctional because of a lack of a ligand or to a dead kinase, respectively. Once activated by a ligand (1), molecules with adaptor or enzymatic function are recruited to the receptors and activate downstream signaling pathways including the RAS-MAPK cascade (2), which is shown in more detail because of its relevance for keratinocyte proliferation and survival. Finally, activated downstream components change the activity of multiple nuclear transcription factors, altering the cellular transcriptional program (3). Signaling via the STAT, PLC-PKC, and PI3K-AKT pathways are also important in mediating EGFR activity (4). EGFR signaling is also activated by EGFR-independent signaling pathways (nonclassical use) including ligand-free receptor activation. The output layer is the result of the altered cellular transcriptional program and represents the cellular effects elicited by the combined activity of the previous layers.
Signal-Processing Layer
This layer includes the ERBB receptors and a myriad of downstream signaling molecules including phosphotyrosine-binding proteins, adaptor molecules, and transcription factors (Figure 2). EGFR (ERBB1; HER1) is the prototype of a family of four tyrosine kinase receptors, which also includes ERBB2 (neu; HER2), ERBB3 (HER3), and ERBB4 (HER4). The four ERBBs share an overall structure with an extracellular ligand-binding domain, a single hydrophobic transmembrane domain and an intracellular kinase domain flanked by a carboxy-terminal tail with tyrosine autophosphorylation sites. Ligand binding induces the formation of homo- or heterodimers and subsequent activation of the kinase domains. Next, tyrosine phosphorylation on residues within the carboxy-terminal tail enables the recruitment and activation of effectors containing Src homology 2 and phosphotyrosine binding domains.58,60,61 The sites that undergo autophosphorylation and the identity and relative strength of the activated signaling cascades are determined by the individual ligand as well as by the heterodimerization partner. On ligand binding, multiple signaling cascades are activated simultaneously, including the mitogen-activated protein kinase pathway (MAPK), the phosphoinositide-3-kinase pathway, protein kinase C, and signal transducers and activators of transcription (STATs). This leads to changes in the cellular transcriptional program, mediated by the proto-oncogene products c-Fos, c-Jun, and c-Myc, by zinc-finger-containing transcription factors such as Sp1 and Egr1, and others.60 The signaling cascade RAS-MAPK is the major pathway mediating keratinocyte survival and proliferation65 and it is shown in more detail in Figure 2. It is, however, beyond the scope of this review to summarize the knowledge on EGFR signaling in detail, and the authors recommend recently published, excellent overviews.58,61,66,67 Signal attenuation is reached predominantly by the internalization of receptor-ligand units through clathrin-coated invaginations of the plasma membrane. Subsequent sorting steps direct the receptors either back to the cell surface or to lysosomes for degradation.68
Although all seven ligands can bind and activate EGFR, some of them (BTC, EREG, HBEGF, EPGN) can additionally bind to ERBB4. Because ERBB2 has no known ligand69 and because ERBB3 carries a defective kinase activity because of substitutions in critical residues,70 homodimers of these two receptors are believed to be inactive. However, both receptors are able to generate potent cellular signals by forming heterodimeric complexes with ERBB1, ERBB4, or with each other.71 A further and important aspect concerns the integration of heterologous signals by the signal-processing layer (Figure 2). These nonclassical stimuli include ligands of other transmembrane receptors, membrane depolarization agents, and stress inducers.72 The interconnection to other signaling modules supports the integration and coordination of cellular responses to extracellular stimuli.
Output Layer
This level embraces essentially every imaginable aspect of cellular and organismic biology, ranging from cell division, migration, to differentiation, and apoptosis (Figure 2). Reflecting its essential roles in mammalian development, complete loss of EGFR activity in knockout mice results in death either at an embryonic stage, in the perinatal period, or after a few weeks of postnatal life, depending on the genetic background.27,28,29 Knockout mice lacking ERBB2, -3, or -4 die inevitably during embryonic development.73,74,75 The specific output depends on several factors. Ligands and receptors are present at different levels in various tissues, and, as impressively demonstrated by their overexpression in tumors,58,59 quantitative changes are important. The type of ERBB dimer formed is obviously also decisive. Interestingly, homodimeric receptor combinations have been shown to be less mitogenic and transforming than the heterodimeric complexes, with heterodimers containing ERBB2 being most potent.60 Biochemical properties of the ligands, such as heparin binding, their presence in a predominantly soluble or membrane-bound (precursor) form, or differential binding strength and trafficking of receptor-ligand complexes, can possibly also alter their activity. However, the exact mechanisms responsible for distinct biological activities after the activation of the same receptor dimer by different ligands are unknown.
EGFR and ERBB2: Gatekeepers of Skin Homeostasis
EGFR (ERBB1)
EGFR is most strongly expressed in the proliferation-competent basal cells of the rodent30,76 and human77 epidermis. The number of receptors decreases as keratinocytes enter the program of terminal differentiation and migrate to the suprabasal layers of the epidermis.30,78,79 EGFR transcripts have been localized along the entire length of the outer root sheath of vibrissal and pelage hair follicles.3 Although no obvious skin phenotype was noted in egfr+/− mice, further decreases in EGFR activity result in the appearance of the waved phenotype. This is seen in transgenic mice expressing a dominant-negative EGFR mutant in the epidermis,33 in mice carrying a humanized (and hypomorphic) EGFR,32 and in mice carrying the antimorphic alleles wa580 or velvet.81 Surprisingly, constitutive activation of this receptor, as observed in the mutant mouse line Dsk5,82 results in very similar phenotypic manifestations, including wavy hairs and curly whiskers. Although the reason for this contradictory observation is not clear, it indicates that the wavy coat is a general response to altered (in either direction) EGFR signaling. Surviving EGFR knockout mice display a severe phenotype of epidermal atrophy and extremely low rates of keratinocyte proliferation.27,28,29 Further studies (including graft-based experiments) revealed that EGFR-deficient hair follicles are highly proliferative, but undergo premature differentiation and are unable to progress normally from anagen via catagen to telogen.30 Instead, cell proliferation in the interfollicular epidermis (but not in the hair follicle epithelium) is strongly reduced in the absence of EGFR. Interestingly, multiple EGFR-null grafts were consumed by an inflammatory reaction.30,83 This suggests that EGFR may play a role in protecting the hair follicle from immunological reactions.
Although apparently not essential for tumor initiation per se, EGFR was shown to facilitate tumor development. Squamous papillomas produced by grafting EGFR-null, v-rasHa-transformed keratinocytes onto nude mice were strongly reduced in size as compared with v-rasHa-transformed keratinocytes with intact EGFR.31 Further studies revealed that EGFR is necessary to maintain the proliferative population in the basal cell compartment of papillomas.24 EGFR deficiency results in the migration of proliferating cells into the suprabasal layer and premature differentiation in association with cell cycle arrest.24 This concept is supported by experiments showing that the tumor formation seen in transgenic mice expressing a dominant form of the signaling protein son of sevenless (SOS) in the epidermis under the control of the keratin 5 promoter is impaired in the absence of a functional EGFR. This was attributable to increased apoptosis of EGFR-deficient tumor cells.23 Finally, UV irradiation was shown to enhance EGFR signaling (via different mechanisms, including blocking phosphatase deactivation, altering receptor internalization and degradation, and increasing the expression of EGFR ligands), leading to keratinocyte proliferation, reduced apoptosis, and epidermal hyperplasia.25 The phenotype of EGFR-deficient hair follicles and squamous papillomas collectively indicates that the main function of EGFR is to delay commitment to differentiation and to maintain keratinocytes in a proliferative state. Thus, EGFR can be considered as a survival factor for tumor cells, rendering this signaling system an interesting target for therapeutic intervention.
ERBB2
The expression pattern of ERBB2 is similar to that of EGFR. ERBB2 is predominantly expressed in the basal cell layer of the epidermis, in the epithelial cells of the sebaceous glands,35,84,85 and in the outer root sheath of hair follicles.86 ERBB2 was shown to be activated in EGF-treated epidermal keratinocytes, after treatment of the epidermis with the phorbol ester and tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA), and in the skin of transgenic mice expressing TGF-α in the epidermis,87 raising the hypothesis that activation of this receptor plays an important role in tumor promotion.
To study the role of ERBB2 in epidermal homeostasis and skin carcinogenesis, diverse transgenic mouse lines were generated. Constitutive expression of the activated form of ERBB2 (neu*) in epidermal basal cells under the control of the keratin 5 (K5)34 or K1436 promoter resulted in a dramatic phenotype characterized by epithelial hyperplasia, which was particularly severe in the hair follicles. Early mortality because of the severity of the phenotype precluded further analysis and forced the development of additional lines. Doxycycline-inducible, conditional expression of activated ERBB237 in adult animals resulted in hyperplasia of the epidermis and hair follicles; prenatal expression caused perinatal death.
More informative results were obtained by the overexpression of wild-type ERBB2 under the control of the K5 promoter.35 These animals show a milder skin phenotype (nevertheless including alopecia, follicular and interfollicular epidermal hyperplasia, and enlarged sebaceous glands) and have a longer life span. Analysis of proliferation and differentiation markers indicated an increase in epidermal proliferation and a delay in differentiation. Importantly, spontaneous papillomas, which sometimes converted to squamous cell carcinomas, appeared in homozygous animals as early as 6 weeks of age. K5-ERBB2 transgenic mice were also more sensitive to TPA treatment and to two-stage carcinogenesis. The results of this study indicate that ERBB2 overexpression provides both an initiating and promoting stimulus and demonstrates an important role for this receptor in tumorigenesis of the skin. Finally, similar to EGFR, ERBB2 appears to play an important role in UV light-induced pathologies such as skin cancer.88
ERBB3
This receptor is expressed in all layers of the epidermis (with highest levels in the suprabasal and spinous layers), in hair follicles, but not in sebaceous glands.35,89 Its localization to the upper strata of the epidermis, in contrast to the predominantly basal localization of EGFR and ERBB2, suggests a role in epidermal maturation and differentiation. Little is known, however, about its actions in the epidermis. In a study involving transfection of partial thickness porcine wounds with adenoviral particles containing an ERBB3 expression cassette, a positive influence on wound repair was observed.90
ERBB4
Expression of this receptor is undetectable in the mouse epidermis35,79,87 and its possible role in cutaneous biology is unknown.
Summary
In summary, EGFR and ERBB2 play central roles in many aspects of cutaneous biology and pathology. This signaling system is important for normal hair follicle morphogenesis and cycling, and may serve to protect the follicle from immunological reactions. Furthermore, it regulates proliferation and differentiation of follicular and interfollicular keratinocytes and strongly influences the outcome of oncogenic transformation. In the next section, we will discuss how this broad spectrum of potential actions is initiated and modulated by individual EGFR ligands.
The EGFR Ligands: Individual and Overlapping Effects
AREG
Although AREG is present at relatively low levels in the normal epidermis, its expression increases in cutaneous squamous cell carcinomas,26 after topical retinoic acid treatment,91 in psoriatic lesions,92 and in several other hyperproliferative skin diseases.17 Although no skin phenotype has been reported for knockout mice lacking AREG,93 targeted expression of AREG in the basal38 or suprabasal39 layers of the epidermis of transgenic mice triggered the development of a severe, highly vascularized inflammatory-proliferative psoriasis-like cutaneous pathology. In the latter model, transgenic mice also showed synovitis, a precursor to psoriatic arthritis. Thus, AREG is remarkable in its ability to induce a complete psoriasis-like response, including, in addition to epidermal hyperproliferation, skin and joint inflammation. Recently, an anti-AREG antibody was shown to reduce the epidermal thickness of transplanted psoriatic skin,94 suggesting that inhibiting AREG activity may be an efficient strategy for the treatment of psoriasis.
BTC
Although BTC is expressed in the skin, its role in skin biology is largely unknown. In normal human skin, BTC expression appears to be restricted to suprabasal keratinocytes, in particular to the granular cell layer.89,91 BTC expression is down-regulated in psoriatic skin,89 after retinoid-induced cell hyperplasia91 and in basal and squamous cell carcinomas.26 Interestingly, AREG and many other EGFR ligands are up-regulated under these situations (see below). Mice with targeted inactivation of the Btc gene are viable and show no obvious phenotype,95 possibly because of functional redundancy among EGF family members. However, overexpression of BTC in transgenic mice resulted in increased keratinocyte proliferation, delayed hair follicle morphogenesis, and delayed hair cycle induction.41 In addition, although wound closure was normal, angiogenesis at the wound site was significantly increased in BTC-overexpressing mice.
EGF
The founding member of the EGF family of ligands was soon recognized as an important factor for the normal development of the skin and hair follicles, because its administration to neonatal mice delayed the development of hair follicles and reduced hair diameter.96 In sheep, EGF administration caused a weakness in the hair (wool), allowing the fleece to be sheared by hand.97 Expression of this ligand has been reported in the upper layers of both adult mouse and fetal sheep epidermis as well as in the outer root sheath and the differentiating cells of the sebaceous glands in developing and mature sheep follicles.42,98,99
As for AREG and BTC, no skin abnormalities have been detected in mice lacking EGF.93 Transgenic mice overexpressing EGF showed an increase in the proliferation rate of basal keratinocytes and a delay in epidermal differentiation, resulting in a thicker epidermis as compared with control animals.42 Expression of endogenous EGF in hair follicles from control mice is normally turned off once the telogen phase is reached. In the transgenic mice, the continuous expression of EGF in hair follicles arrested follicular development at the final stage of morphogenesis.42 According to a model proposed by these authors, EGF could function as a biological switch regulating the entry to and the exit from the anagen phase. However, arrest of EGF overexpression in late-stage morphogenesis would rather suggest an inhibition of entry into hair follicle cycling (ie, first catagen induction around P17); this, however, is confusing, because EGF is a powerful catagen inducer in sheep and human hair follicles.97,100 Therefore, the exact function of EGF in the control of murine and human hair follicle morphogenesis and cycling under physiological conditions remains to be clarified.
Epigen
The newest EGFR ligand was cloned from a mouse keratinocyte cDNA library.101 EPGN is expressed in the inner and outer root sheath of hair follicles in newborn mouse skin, but not in the hair shaft.62 Further characterization of potential actions of this EGFR ligand in skin biology will require the generation of knockout or transgenic mouse models.
Epiregulin
Levels of this protein in skin are relatively low.91 EREG was shown to act as an autocrine growth factor for normal human keratinocytes in vitro.10 Although the targeted elimination of EREG did not significantly affect keratinocyte growth or wound healing, the loss of this EGFR ligand was associated with a late-onset (5 months at the earliest) chronic dermatitis affecting ear and face with gradual involvement of the neck.43 Histologically, the lesions were characterized by increased thickness of epithelial layers and fibrosis with infiltration of mast cells, eosinophils, and other inflammatory cells. This study also revealed distinct functions of the secreted and membrane-anchored forms of EREG: whereas the former is critical for the regulation of IL-18 in keratinocytes, the latter is essential for proper cytokine production by tissue resident macrophages. It remains to be determined why an independently generated EREG knockout mouse line did not show any overt abnormal phenotype.102 Possibilities include the deletion of different gene regions, different genetic backgrounds, or maintenance conditions.
HBEGF
This EGFR ligand is predominantly found in the suprabasal layer of the epidermis and is also up-regulated in squamous cell carcinomas26 and by retinoic acid treatment.91 HBEGF is a major component of the mix of growth factors present in wound fluid14 and its expression was detected in the advancing epithelial margin, in islands of regenerating epithelium within burn wounds, and in eccrine sweat glands,103 suggesting a role in wound healing. This idea was further supported by the report of rapid and robust induction of Hbegf mRNA expression after scrape-wounding of epithelial cell monolayers.44,104 HBEGF was also detected in hair follicle epithelial cells and keratinocytes at the wound edge, with particularly high levels during maximal, anagen-associated keratinocyte proliferation.105 Retinoid-induced keratinocyte proliferation appears to depend on the induction of HBEGF and subsequent activation of EGFR,19,91,106,107 indicating a major role for this EGFR ligand in keratinocyte biology.
The targeted deletion of the hbegf gene108 or its replacement by an uncleavable form109 results in early postnatal lethality because of defects in cardiac chamber dilation and valve malformations. Replacement of the hbegf gene by a transmembrane truncated mutant form, resulting in higher than normal levels of the shedded, soluble growth factor, was associated with epidermal hyperplasia and perturbed differentiation of keratinocytes.109 Unfortunately, mutant mice were short-lived, precluding further studies. This problem was overcome by the generation of keratinocyte-specific HBEGF-deficient mice.44 These mice, although otherwise apparently normal, showed a marked impairment in wound closure. Although keratinocyte proliferation was unaffected, cell migration was impaired at the wound site. Hbegf mRNA was up-regulated at the migrating epidermal wound edge. It should be considered, however, that (subtle) hair follicle abnormalities, which could contribute to the wound healing phenotype, were not rigorously excluded.44 Taken together, these findings indicate that HBEGF is the major, and essential, EGFR ligand involved in re-epithelialization of skin wounds.
TGF-α
This ligand is expressed in the basal, spinous, and granular layers of the epidermis and in the inner root sheath of hair follicles, limited longitudinally to a specific region above the bulb.45,110 Tgfa knockout mice show an obvious epithelial phenotype with wavy hairs, curly whiskers, and altered hair follicle structure, including septulation of the hair medulla and reduced dermal adipose tissue.45,46 This phenotype is remarkably similar to the phenotype associated with the spontaneous mutant mouse wa-1, and the mutations are allelic. The wound healing process was shown to occur normally in tgfa knockout mice, although an increased variability in the rate of wound closure was reported by one group.45 However, in an ear wound model, in which healing is mainly achieved by re-epithelialization, a delay of this process was seen in tgfa knockout mice.47
The most important actions of TGF-α, however, are related to its role in tumorigenesis. TGF-α expression is up-regulated by the oncogene v-rasHa in keratinocytes,111 in papillomas elicited by chemical carcinogenesis,112 and in human cutaneous squamous cell carcinomas.26 TGF-α overexpression under the control of a ubiquitously active promoter did not result in spontaneous skin lesions, probably attributable to relatively low expression in this tissue. However, the increased TGF-α levels were sufficient to cause the development of hyperplasia, papillomas, adenomas, and even, although less frequent, squamous cell carcinomas after a single dose of the mutagenic agent 7,12-dimethylbenzanthracene.48 Furthermore, arising tumors could be separated in two mutually exclusive genetic classes: tumors harboring ha-ras mutations displayed low transgene-derived TGF-α expression, whereas tumors harboring only wild-type ha-ras genes showed highly increased TGF-α expression. This indicates that TGF-α can act as an autonomous tumor promoter and can functionally substitute for ha-ras mutational activation in skin tumorigenesis.
This concept is further supported by the epidermal hyperplasia and spontaneous papilloma formation seen on targeted overexpression of TGF-α in the epidermis via K1450 or K152 promoters. In these transgenic mice, tumors arise without the need for an initiator (eg, 7,12-dimethylbenzanthracene), while the severity of the phenotype is enhanced by TPA treatment (tumors were negative for activating ha-ras mutations).51,53 The interaction of TGF-α with other oncogene products in tumor initiation, promotion, and progression was further analyzed using K1-TGF-α transgenic mice.54,55,56,57,113
Conclusion and Perspectives
The existence of seven EGFR ligands with specific affinities to members of a family of four receptors allows numerous combinatorial possibilities of signaling. The complexity of this multifaceted, multipurpose signaling system appears to reflect the need for sophisticated pathways that regulate the intricate interactions between adjacent and spatially distant cell populations of different tissues, including the skin, in higher vertebrates. In addition to their ability to initiate distinct signaling cascades after receptor binding, the EGFR ligands exert specific effects because of distinct biochemical properties (like heparin binding), to differential expression of ligand precursor forms and their activating proteinases, and as a result of regulated shedding.
As reflected by the mostly mild phenotypes of knockout mice lacking single EGFR ligands, redundancy appears to be the evolutionary strategy that assures correct development and tissue homeostasis even in case expression of a single EGFR ligand is lost (with the notable exception of HBGEF, which is indispensable for correct heart development and normal re-epithelialization after wounding). Nevertheless, more subtle skin/hair phenotypes may have been overlooked in knockout mice lacking AREG, BTC, or EGF, and functional studies such as experimental tumorigenesis, wounding, or the application of other stressors, may be necessary to reveal them. Transgenic and knockout mice with altered expression of individual components of the EGFR network have been very useful tools for obtaining insight into the roles of these molecules in skin biology and pathology. For instance, although multiple EGFR ligands are overexpressed in psoriatic epidermis,9,17,18,19 only AREG induced a corresponding disease when overexpressed in transgenic mice. Furthermore, multiple EGFR ligands are present in wound fluid, but a clear phenotype of impaired wound healing is only present in HBEGF-deficient mice.44 Along the same lines, expression of multiple EGFR ligands is induced by v-rasHa in keratinocytes,111 but neoplastic lesions arise only after TGF-α overexpression. Notably, TGF-α is dispensable for skin tumorigenesis,111,114 demonstrating the fine balance between overlapping and specific actions of EGFR ligands.
There are several examples of EGFR ligands acting both individually and as a collective to orchestrate essential processes in tissues other than skin. In each situation, different ligands take on the major role. For instance, although primarily AREG and HBEGF prepare the uterine epithelium for interacting with the embryo during blastocyst implantation, at least three additional EGFR ligands are expressed in the uterus around implantation time.115 Not unexpectedly, implantation defects have not been reported in mice lacking individual EGFR ligands. Further examples are the induction of AREG, BTC, and EREG by luteinizing hormone in ovary granulosa cells116 or the induction of AREG, TGF-α, and HBEGF by parathyroid hormone in osteoblasts.117
An emerging aspect in the biology of the EGFR network is the importance of feedback inhibition. Loss of the negative feedback regulator of ERBBs, RALT/MIG6 adaptor protein, resulted in hyperactivation of EGFR signaling, increased proliferation, and reduced differentiation of keratinocytes, as well as higher susceptibility to neoplastic transformation.118 In contrast, skin-targeted expression of the molecule caused a waved-like phenotype typical for situations of reduced EGFR activity.119 Because of its remarkable sensitivity to reduced or increased EGFR-mediated activity, the skin will certainly continue to serve as an excellent readout system for the dissection of basic mechanisms of EGFR signaling.
In addition, the clinical relevance of mammalian skin’s exquisite sensitivity to altered EGFR activity has become acutely evident in recent years with the increasing clinical use of different EGFR tyrosine kinase inhibitors and anti-EGFR monoclonal antibodies for treating patients with various types of cancer. A common, severe, and often therapy-limiting undesired treatment effect of these, oncologically very attractive, new agents is the development of extensive inflammatory rashes, often in association with prominent, disseminated (sterile) folliculitis. In many cases, there is a consistent positive correlation between the presence of cutaneous drug eruptions and tumor regression or even survival.120 This suggests that the skin offers an excellent clinical read-out system for evaluating the efficacy of EGFR-antagonistic agents, and raises the question whether the cutaneous adverse effects even award (as yet undefined) clinical anti-tumor benefits.120
Future research aimed at elucidating the pleiotropic functions of the EGFR network in the skin and its multiple layers of regulation should place more emphasis on as yet insufficiently explored aspects like nonclassical activation of EGFR, possible EGFR-independent actions of EGFR ligands, and the regulation of their shedding (as well as its consequences for ligand binding). A better understanding of this powerful signaling system should allow the development of innovative therapeutic strategies for diseases of the skin and its appendages.
Footnotes
Address reprint requests to Dr. Marlon R. Schneider, Institute of Molecular Animal Breeding and Biotechnology, Gene Center, LMU Munich, Feodor-Lynen-Str. 25, D-81377 Munich, Germany. E-mail: schnder@lmb.uni-muenchen.de.
Writing of this review was made possible in part by grants from the Deutsche Forschungsgemeinschaft (GRK 1029, to M.R.S. and E.W.; and Pa 345/12-1, to R.P.) and from the Swiss National Science Foundation (grant 3100A9-109340/1, to S.W.).
References
- Crew FAE. Waved: an autosomal recessive coat form character in the mouse. J Genet. 1933;27:95–96. [Google Scholar]
- Keeler CE. A second rexoid coat character in the house mouse. J Hered. 1935;26:189–191. [Google Scholar]
- Luetteke NC, Phillips HK, Qiu TH, Copeland NG, Earp HS, Jenkins NA, Lee DC. The mouse waved-2 phenotype results from a point mutation in the EGF receptor tyrosine kinase. Genes Dev. 1994;8:399–413. doi: 10.1101/gad.8.4.399. [DOI] [PubMed] [Google Scholar]
- Fowler KJ, Walker F, Alexander W, Hibbs ML, Nice EC, Bohmer RM, Mann GB, Thumwood C, Maglitto R, Danks JA, Chetty R, Burgess AW, Dunn AR. A mutation in the epidermal growth factor receptor in waved-2 mice has a profound effect on receptor biochemistry that results in impaired lactation. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Cook PW, Mattox PA, Keeble WW, Pittelkow MR, Plowman GD, Shoyab M, Adelman JP, Shipley GD. A heparin sulfate-regulated human keratinocyte autocrine factor is similar or identical to amphiregulin. Mol Cell Biol. 1991;11:2547–2557. doi: 10.1128/mcb.11.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Higashiyama S, Asada H, Hashimura E, Kobayashi T, Sudo K, Nakagawa T, Damm D, Yoshikawa K, Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994;269:20060–20066. [PubMed] [Google Scholar]
- Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation in skin organ culture. J Clin Invest. 1997;100:1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M, Pittelkow MR, Cook PW. Autocrine regulation of keratinocytes: the emerging role of heparin-binding, epidermal growth factor-related growth factors. J Invest Dermatol. 1998;111:715–721. doi: 10.1046/j.1523-1747.1998.00390.x. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Komurasaki T, Toyoda H, Hanakawa Y, Yamasaki K, Tokumaru S, Sayama K, Hashimoto K. Epiregulin, a novel member of the epidermal growth factor family, is an autocrine growth factor in normal human keratinocytes. J Biol Chem. 2000;275:5748–5753. doi: 10.1074/jbc.275.8.5748. [DOI] [PubMed] [Google Scholar]
- Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Grosios K, Lane HA, McSheehy P, Mestan J, Meyer T, Tang C, Wartmann M, Wood J, Caravatti G. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64:4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Soma Y, Takehara K, Charette M. EGF and TGF-alpha are potent chemoattractants for endothelial cells and EGF-like peptides are present at sites of tissue regeneration. J Cell Physiol. 1989;139:617–623. doi: 10.1002/jcp.1041390323. [DOI] [PubMed] [Google Scholar]
- Marikovsky M, Breuing K, Liu PY, Eriksson E, Higashiyama S, Farber P, Abraham J, Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci USA. 1993;90:3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono I, Gunji H, Zhang JZ, Maruyama K, Kaneko F. Studies on cytokines related to wound healing in donor site wound fluid. J Dermatol Sci. 1995;10:241–245. doi: 10.1016/0923-1811(95)00454-z. [DOI] [PubMed] [Google Scholar]
- Stoscheck CM, Nanney LB, King LE., Jr Quantitative determination of EGF-R during epidermal wound healing. J Invest Dermatol. 1992;99:645–649. doi: 10.1111/1523-1747.ep12668143. [DOI] [PubMed] [Google Scholar]
- Piepkorn M. Overexpression of amphiregulin, a major autocrine growth factor for cultured human keratinocytes, in hyperproliferative skin diseases. Am J Dermatopathol. 1996;18:165–171. doi: 10.1097/00000372-199604000-00010. [DOI] [PubMed] [Google Scholar]
- Elder JT, Fisher GJ, Lindquist PB, Bennett GL, Pittelkow MR, Coffey RJ, Jr, Ellingsworth L, Derynck R, Voorhees JJ. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989;243:811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Exp Dermatol. 1998;7:391–397. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Kamata N, Kawano H, Shimizu S, Kuroki T, Toyoshima K, Rikimaru K, Nomura N, Ishizaki R, Pastan I, Gamou S, Shimizu N. High incidence of amplification of the epidermal growth factor receptor gene in human squamous carcinoma cell lines. Cancer Res. 1986;46:414–416. [PubMed] [Google Scholar]
- Derynck R, Goeddel DV, Ullrich A, Gutterman JU, Williams RD, Bringman TS, Berger WH. Synthesis of messenger RNAs for transforming growth factors alpha and beta and the epidermal growth factor receptor by human tumors. Cancer Res. 1987;47:707–712. [PubMed] [Google Scholar]
- Gordon-Thomson C, Jones J, Mason RS, Moore GP. ErbB receptors mediate both migratory and proliferative activities in human melanocytes and melanoma cells. Melanoma Res. 2005;15:21–28. doi: 10.1097/00008390-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Sibilia M, Fleischmann A, Behrens A, Stingl L, Carroll J, Watt FM, Schlessinger J, Wagner EF. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell. 2000;102:211–220. doi: 10.1016/s0092-8674(00)00026-x. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Woodson RL, Holbus S, Strain K, Lo YC, Yuspa SH. The epidermal growth factor receptor is required to maintain the proliferative population in the basal compartment of epidermal tumors. Cancer Res. 2000;60:3328–3332. [PubMed] [Google Scholar]
- El Abaseri TB, Putta S, Hansen LA. Ultraviolet irradiation induces keratinocyte proliferation and epidermal hyperplasia through the activation of the epidermal growth factor receptor. Carcinogenesis. 2006;27:225–231. doi: 10.1093/carcin/bgi220. [DOI] [PubMed] [Google Scholar]
- Rittié L, Kansra S, Stoll SW, Li Y, Gudjonsson JE, Shao Y, Michael LE, Fisher GJ, Johnson TM, Elder JT. Differential ErbB1 signaling in squamous cell versus basal cell carcinoma of the skin. Am J Pathol. 2007;170:2089–2099. doi: 10.2353/ajpath.2007.060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibilia M, Wagner EF. Strain-dependent epithelial defects in mice lacking the EGF receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Dlugosz AA, Hansen LA, Tennenbaum T, Lichti U, Yee D, LaMantia C, Mourton T, Herrup K, Harris RC, Barnard JA, Yuspa SH, Coffey RJ, Magnuson T. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Berger JE, Meneses J, Phung Y, Pedersen RA, Werb Z, Derynck R. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–341. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- Hansen LA, Alexander N, Hogan ME, Sundberg JP, Dlugosz A, Threadgill DW, Magnuson T, Yuspa SH. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am J Pathol. 1997;150:1959–1975. [PMC free article] [PubMed] [Google Scholar]
- Dlugosz AA, Hansen L, Cheng C, Alexander N, Denning MF, Threadgill DW, Magnuson T, Coffey RJ, Jr, Yuspa SH. Targeted disruption of the epidermal growth factor receptor impairs growth of squamous papillomas expressing the v-ras(Ha) oncogene but does not block in vitro keratinocyte responses to oncogenic ras. Cancer Res. 1997;57:3180–3188. [PubMed] [Google Scholar]
- Sibilia M, Wagner B, Hoebertz A, Elliott C, Marino S, Jochum W, Wagner EF. Mice humanised for the EGF receptor display hypomorphic phenotypes in skin, bone and heart. Development. 2003;130:4515–4525. doi: 10.1242/dev.00664. [DOI] [PubMed] [Google Scholar]
- Murillas R, Larcher F, Conti CJ, Santos M, Ullrich A, Jorcano JL. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J. 1995;14:5216–5223. doi: 10.1002/j.1460-2075.1995.tb00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bol D, Kiguchi K, Beltran L, Rupp T, Moats S, Gimenez-Conti I, Jorcano J, Digiovanni J. Severe follicular hyperplasia and spontaneous papilloma formation in transgenic mice expressing the neu oncogene under the control of the bovine keratin 5 promoter. Mol Carcinog. 1998;21:2–12. [PubMed] [Google Scholar]
- Kiguchi K, Bol D, Carbajal S, Beltran L, Moats S, Chan K, Jorcano J, Digiovanni J. Constitutive expression of erbB2 in epidermis of transgenic mice results in epidermal hyperproliferation and spontaneous skin tumor development. Oncogene. 2000;19:4243–4254. doi: 10.1038/sj.onc.1203778. [DOI] [PubMed] [Google Scholar]
- Xie W, Wu X, Chow LT, Chin E, Paterson AJ, Kudlow JE. Targeted expression of activated erbB-2 to the epidermis of transgenic mice elicits striking developmental abnormalities in the epidermis and hair follicles. Cell Growth Differ. 1998;9:313–325. [PubMed] [Google Scholar]
- Xie W, Chow LT, Paterson AJ, Chin E, Kudlow JE. Conditional expression of the ErbB2 oncogene elicits reversible hyperplasia in stratified epithelia and up-regulation of TGFalpha expression in transgenic mice. Oncogene. 1999;18:3593–3607. doi: 10.1038/sj.onc.1202673. [DOI] [PubMed] [Google Scholar]
- Cook PW, Piepkorn M, Clegg CH, Plowman GD, DeMay JM, Brown JR, Pittelkow MR. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J Clin Invest. 1997;100:2286–2294. doi: 10.1172/JCI119766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook PW, Brown JR, Cornell KA, Pittelkow MR. Suprabasal expression of human amphiregulin in the epidermis of transgenic mice induces a severe, early-onset, psoriasis-like skin pathology: expression of amphiregulin in the basal epidermis is also associated with synovitis. Exp Dermatol. 2004;13:347–356. doi: 10.1111/j.0906-6705.2004.00183.x. [DOI] [PubMed] [Google Scholar]
- Chung E, Cook PW, Parkos CA, Park YK, Pittelkow MR, Coffey RJ. Amphiregulin causes functional downregulation of adherens junctions in psoriasis. J Invest Dermatol. 2005;124:1134–1140. doi: 10.1111/j.0022-202X.2005.23762.x. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Antsiferova M, Feldmeyer L, Dahlhoff M, Bugnon P, Hasse S, Paus R, Wolf E, Werner S. Betacellulin regulates hair follicle development and hair cycle induction and enhances angiogenesis in wounded skin. J Invest Dermatol. 2008;128:1256–1265. doi: 10.1038/sj.jid.5701135. [DOI] [PubMed] [Google Scholar]
- Mak KK, Chan SY. Epidermal growth factor as a biologic switch in hair growth cycle. J Biol Chem. 2003;278:26120–26126. doi: 10.1074/jbc.M212082200. [DOI] [PubMed] [Google Scholar]
- Shirasawa S, Sugiyama S, Baba I, Inokuchi J, Sekine S, Ogino K, Kawamura Y, Dohi T, Fujimoto M, Sasazuki T. Dermatitis due to epiregulin deficiency and a critical role of epiregulin in immune-related responses of keratinocyte and macrophage. Proc Natl Acad Sci USA. 2004;101:13921–13926. doi: 10.1073/pnas.0404217101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, Higashiyama S, Hashimoto K. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mann GB, Fowler KJ, Gabriel A, Nice EC, Williams RL, Dunn AR. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73:249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Kim I, Mogford JE, Chao JD, Mustoe TA. Wound epithelialization deficits in the transforming growth factor-alpha knockout mouse. Wound Repair Regen. 2001;9:386–390. doi: 10.1046/j.1524-475x.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- Jhappan C, Takayama H, Dickson RB, Merlino G. Transgenic mice provide genetic evidence that transforming growth factor alpha promotes skin tumorigenesis via H-ras-dependent and H-ras-independent pathways. Cell Growth Differ. 1994;5:385–394. [PubMed] [Google Scholar]
- Shibata MA, Ward JM, Green JE, Merlino G. Enhanced sensitivity to tumor growth and development in multistage skin carcinogenesis by transforming growth factor-alpha-induced epidermal growth factor receptor activation but not p53 inactivation. Mol Carcinog. 1997;18:160–170. doi: 10.1002/(sici)1098-2744(199703)18:3<160::aid-mc5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Vassar R, Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Vassar R, Hutton ME, Fuchs E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol Cell Biol. 1992;12:4643–4653. doi: 10.1128/mcb.12.10.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey AM, Wang XJ, King LE, Jr, Nanney LB, Gagne TA, Sellheyer K, Bundman DS, Longley MA, Rothnagel JA, Greenhalgh DA. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 1993;4:1071–1082. [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Eckhardt JN, Rothnagel JA, Roop DR. Epidermal expression of transforming growth factor-alpha in transgenic mice: induction of spontaneous and 12-O-tetradecanoylphorbol-13-acetate-induced papillomas via a mechanism independent of Ha-ras activation or overexpression. Mol Carcinog. 1994;10:15–22. doi: 10.1002/mc.2940100104. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Lu XR, Bickenbach JR, Roop DR. TGF alpha and v-fos cooperation in transgenic mouse epidermis induces aberrant keratinocyte differentiation and stable, autonomous papillomas. Oncogene. 1995;10:279–289. [PubMed] [Google Scholar]
- Wang XJ, Liefer KM, Greenhalgh DA, Roop DR. 12-O-tetradecanoylphorbol-13-acetate promotion of transgenic mouse epidermis coexpressing transforming growth factor-alpha and v-fos: acceleration of autonomous papilloma formation and malignant conversion via c-Ha-ras activation. Mol Carcinog. 1999;26:305–311. [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Roop DR. Transgenic coexpression of v-Ha-ras and transforming growth factor alpha increases epidermal hyperproliferation and tumorigenesis and predisposes to malignant conversion via endogenous c-Ha-ras activation. Mol Carcinog. 2000;27:200–209. [PubMed] [Google Scholar]
- Wang XJ, Greenhalgh DA, Donehower LA, Roop DR. Cooperation between Ha-ras and fos or transforming growth factor alpha overcomes a paradoxic tumor-inhibitory effect of p53 loss in transgenic mouse epidermis. Mol Carcinog. 2000;29:67–75. doi: 10.1002/1098-2744(200010)29:2<67::aid-mc3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–2023. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- Kochupurakkal BS, Harari D, Di Segni A, Maik-Rachline G, Lyass L, Gur G, Kerber G, Citri A, Lavi S, Eilam R, Chalifa-Caspi V, Eshhar Z, Pikarsky E, Pinkas-Kramarski R, Bacus SS, Yarden Y. Epigen, the last ligand of ErbB receptors, reveals intricate relationships between affinity and mitogenicity. J Biol Chem. 2005;280:8503–8512. doi: 10.1074/jbc.M413919200. [DOI] [PubMed] [Google Scholar]
- Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- Singh AB, Harris RC. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 2005;17:1183–1193. doi: 10.1016/j.cellsig.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131:1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Waterman H, Yarden Y. Molecular mechanisms underlying endocytosis and sorting of ErbB receptor tyrosine kinases. FEBS Lett. 2001;490:142–152. doi: 10.1016/s0014-5793(01)02117-2. [DOI] [PubMed] [Google Scholar]
- Klapper LN, Glathe S, Vaisman N, Hynes NE, Andrews GC, Sela M, Yarden Y. The ErbB-2/HER2 oncoprotein of human carcinomas may function solely as a shared coreceptor for multiple stroma-derived growth factors. Proc Natl Acad Sci USA. 1999;96:4995–5000. doi: 10.1073/pnas.96.9.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., III Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc Natl Acad Sci USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Exp Cell Res. 2003;284:54–65. doi: 10.1016/s0014-4827(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Carpenter G. Employment of the epidermal growth factor receptor in growth factor-independent signaling pathways. J Cell Biol. 1999;146:697–702. doi: 10.1083/jcb.146.4.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Green MR, Basketter DA, Couchman JR, Rees DA. Distribution and number of epidermal growth factor receptors in skin is related to epithelial cell growth. Dev Biol. 1983;100:506–512. doi: 10.1016/0012-1606(83)90243-9. [DOI] [PubMed] [Google Scholar]
- Nanney LB, Magid M, Stoscheck CM, King LE., Jr Comparison of epidermal growth factor binding and receptor distribution in normal human epidermis and epidermal appendages. J Invest Dermatol. 1984;83:385–393. doi: 10.1111/1523-1747.ep12264708. [DOI] [PubMed] [Google Scholar]
- Peus D, Hamacher L, Pittelkow MR. EGF-receptor tyrosine kinase inhibition induces keratinocyte growth arrest and terminal differentiation. J Invest Dermatol. 1997;109:751–756. doi: 10.1111/1523-1747.ep12340759. [DOI] [PubMed] [Google Scholar]
- Stoll SW, Kansra S, Peshick S, Fry DW, Leopold WR, Wiesen JF, Sibilia M, Zhang T, Werb Z, Derynck R, Wagner EF, Elder JT. Differential utilization and localization of ErbB receptor tyrosine kinases in skin compared to normal and malignant keratinocytes. Neoplasia. 2001;3:339–350. doi: 10.1038/sj.neo.7900170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Cross SH, Strunk KE, Morgan JE, Bailey CL, Jackson IJ, Threadgill DW. Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm Genome. 2004;15:525–536. doi: 10.1007/s00335-004-2384-2. [DOI] [PubMed] [Google Scholar]
- Du X, Tabeta K, Hoebe K, Liu H, Mann N, Mudd S, Crozat K, Sovath S, Gong X, Beutler B. Velvet, a dominant Egfr mutation that causes wavy hair and defective eyelid development in mice. Genetics. 2004;166:331–340. doi: 10.1534/genetics.166.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch KR, McGowan KA, van Raamsdonk CD, Fuchs H, Lee D, Puech A, Herault Y, Threadgill DW, Hrabe DA, Barsh GS. Genetics of dark skin in mice. Genes Dev. 2003;17:214–228. doi: 10.1101/gad.1023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen LA, Lichti U, Tennenbaum T, Dlugosz A, Threadgill DW, Magnuson T, Yuspa SH. Altered hair follicle morphogenesis in epidermal growth factor receptor deficient mice. Van Neste DJJ, Randall VA, editors. Amsterdam: Elsevier,; Hair research for the next millennium. 1996:pp 425–431. [Google Scholar]
- Kokai Y, Cohen JA, Drebin JA, Greene MI. Stage- and tissue-specific expression of the neu oncogene in rat development. Proc Natl Acad Sci USA. 1987;84:8498–8501. doi: 10.1073/pnas.84.23.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirke P, Pickles A, Tuzi NL, Mohamdee O, Gullick WJ. Pattern of expression of c-erbB-2 oncoprotein in human fetuses. Br J Cancer. 1989;60:64–69. doi: 10.1038/bjc.1989.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire HC, Jr, Jaworsky C, Cohen JA, Hellman M, Weiner DB, Greene MI. Distribution of neu (c-erbB-2) protein in human skin. J Invest Dermatol. 1989;92:786–790. doi: 10.1111/1523-1747.ep12696796. [DOI] [PubMed] [Google Scholar]
- Xian W, Rosenberg MP, Digiovanni J. Activation of erbB2 and c-src in phorbol ester-treated mouse epidermis: possible role in mouse skin tumor promotion. Oncogene. 1997;14:1435–1444. doi: 10.1038/sj.onc.1200980. [DOI] [PubMed] [Google Scholar]
- Madson JG, Lynch DT, Tinkum KL, Putta SK, Hansen LA. Erbb2 regulates inflammation and proliferation in the skin after ultraviolet irradiation. Am J Pathol. 2006;169:1402–1414. doi: 10.2353/ajpath.2006.060082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepkorn M, Predd H, Underwood R, Cook P. Proliferation-differentiation relationships in the expression of heparin-binding epidermal growth factor-related factors and erbB receptors by normal and psoriatic human keratinocytes. Arch Dermatol Res. 2003;295:93–101. doi: 10.1007/s00403-003-0391-x. [DOI] [PubMed] [Google Scholar]
- Okwueze MI, Cardwell NL, Pollins AC, Nanney LB. Modulation of porcine wound repair with a transfected ErbB3 gene and relevant EGF-like ligands. J Invest Dermatol. 2007;127:1030–1041. doi: 10.1038/sj.jid.5700637. [DOI] [PubMed] [Google Scholar]
- Rittie L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732–739. doi: 10.1038/sj.jid.5700202. [DOI] [PubMed] [Google Scholar]
- Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Jr, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Res. 1992;52:3224–3227. [PubMed] [Google Scholar]
- Luetteke NC, Qiu TH, Fenton SE, Troyer KL, Riedel RF, Chang A, Lee DC. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. doi: 10.1242/dev.126.12.2739. [DOI] [PubMed] [Google Scholar]
- Bhagavathula N, Nerusu KC, Fisher GJ, Liu G, Thakur AB, Gemmell L, Kumar S, Xu ZH, Hinton P, Tsurushita N, Landolfi NF, Voorhees JJ, Varani J. Amphiregulin and epidermal hyperplasia: amphiregulin is required to maintain the psoriatic phenotype of human skin grafts on severe combined immunodeficient mice. Am J Pathol. 2005;166:1009–1016. doi: 10.1016/S0002-9440(10)62322-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, Lee DC. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. EMBO J. 2003;22:2704–2716. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GP, Panaretto BA, Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983;205:47–55. doi: 10.1002/ar.1092050107. [DOI] [PubMed] [Google Scholar]
- Moore GP, Panaretto BA, Robertson D. Inhibition of wool growth in merino sheep following administration of mouse epidermal growth factor and a derivative. Aust J Biol Sci. 1982;35:163–172. doi: 10.1071/bi9820163. [DOI] [PubMed] [Google Scholar]
- du Cros DL, Isaacs K, Moore GP. Localization of epidermal growth factor immunoreactivity in sheep skin during wool follicle development. J Invest Dermatol. 1992;98:109–115. doi: 10.1111/1523-1747.ep12496010. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Nelson KG, Snedeker S, Bossert NL, Walker MP, McLachlan J, DiAugustine RP. Expression of epidermal growth factor in suprabasal cells of stratified squamous epithelia: implications for a role in differentiation. Cell Growth Differ. 1994;5:527–535. [PubMed] [Google Scholar]
- Philpott MP, Kealey T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J Invest Dermatol. 1994;102:186–191. doi: 10.1111/1523-1747.ep12371760. [DOI] [PubMed] [Google Scholar]
- Strachan L, Murison JG, Prestidge RL, Sleeman MA, Watson JD, Kumble KD. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J Biol Chem. 2001;276:18265–18271. doi: 10.1074/jbc.M006935200. [DOI] [PubMed] [Google Scholar]
- Lee D, Pearsall RS, Das S, Dey SK, Godfrey VL, Threadgill DW. Epiregulin is not essential for development of intestinal tumors but is required for protection from intestinal damage. Mol Cell Biol. 2004;24:8907–8916. doi: 10.1128/MCB.24.20.8907-8916.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DW, Downing MT, Brigstock DR, Luquette MH, Brown KD, Abad MS, Besner GE. Production of heparin-binding epidermal growth factor-like growth factor (HB-EGF) at sites of thermal injury in pediatric patients. J Invest Dermatol. 1996;106:49–56. doi: 10.1111/1523-1747.ep12327214. [DOI] [PubMed] [Google Scholar]
- Ellis PD, Hadfield KM, Pascall JC, Brown KD. Heparin-binding epidermal-growth-factor-like growth factor gene expression is induced by scrape-wounding epithelial cell monolayers: involvement of mitogen-activated protein kinase cascades. Biochem J. 2001;354:99–106. doi: 10.1042/0264-6021:3540099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribbs RK, Harding PA, Luquette MH, Besner GE. Endogenous production of heparin-binding EGF-like growth factor during murine partial-thickness burn wound healing. J Burn Care Rehabil. 2002;23:116–125. doi: 10.1097/00004630-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Xiao JH, Feng X, Di W, Peng ZH, Li LA, Chambon P, Voorhees JJ. Identification of heparin-binding EGF-like growth factor as a target in intercellular regulation of epidermal basal cell growth by suprabasal retinoic acid receptors. EMBO J. 1999;18:1539–1548. doi: 10.1093/emboj/18.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J, Zeigler M, Dame MK, Kang S, Fisher GJ, Voorhees JJ, Stoll SW, Elder JT. Heparin-binding epidermal-growth-factor-like growth factor activation of keratinocyte ErbB receptors mediates epidermal hyperplasia, a prominent side-effect of retinoid therapy. J Invest Dermatol. 2001;117:1335–1341. doi: 10.1046/j.0022-202x.2001.01564.x. [DOI] [PubMed] [Google Scholar]
- Iwamoto R, Yamazaki S, Asakura M, Takashima S, Hasuwa H, Miyado K, Adachi S, Kitakaze M, Hashimoto K, Raab G, Nanba D, Higashiyama S, Hori M, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor and ErbB signaling is essential for heart function. Proc Natl Acad Sci USA. 2003;100:3221–3226. doi: 10.1073/pnas.0537588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Iwamoto R, Saeki K, Asakura M, Takashima S, Yamazaki A, Kimura R, Mizushima H, Moribe H, Higashiyama S, Endoh M, Kaneda Y, Takagi S, Itami S, Takeda N, Yamada G, Mekada E. Mice with defects in HB-EGF ectodomain shedding show severe developmental abnormalities. J Cell Biol. 2003;163:469–475. doi: 10.1083/jcb.200307035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi E, Harkins R, Horn T. TGF-alpha is widely expressed in differentiated as well as hyperproliferative skin epithelium. J Invest Dermatol. 1991;96:328–332. doi: 10.1111/1523-1747.ep12465223. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Cheng C, Williams EK, Darwiche N, Dempsey PJ, Mann B, Dunn AR, Coffey RJ, Jr, Yuspa SH. Autocrine transforming growth factor alpha is dispensable for v-rasHa-induced epidermal neoplasia: potential involvement of alternate epidermal growth factor receptor ligands. Cancer Res. 1995;55:1883–1893. [PubMed] [Google Scholar]
- Imamoto A, Beltran LM, Digiovanni J. Evidence for autocrine/paracrine growth stimulation by transforming growth factor-alpha during the process of skin tumor promotion. Mol Carcinog. 1991;4:52–60. doi: 10.1002/mc.2940040109. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DA, Wang XJ, Donehower LA, Roop DR. Paradoxical tumor inhibitory effect of p53 loss in transgenic mice expressing epidermal-targeted v-rasHa, v-fos, or human transforming growth factor alpha. Cancer Res. 1996;56:4413–4423. [PubMed] [Google Scholar]
- Humble MC, Szczesniak CJ, Luetteke NC, Spalding JW, Cannon RE, Hansen LA, Lee DC, Tennant RW. TGF alpha is dispensable for skin tumorigenesis in Tg.AC mice. Toxicol Pathol. 1998;26:562–569. doi: 10.1177/019262339802600413. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Wolf E. The epidermal growth factor receptor and its ligands in female reproduction: insights from rodent models. Cytokine Growth Factor Rev. 2008;19:173–181. doi: 10.1016/j.cytogfr.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- Qin L, Tamasi J, Raggatt L, Li X, Feyen JH, Lee DC, Dicicco-Bloom E, Partridge NC. Amphiregulin is a novel growth factor involved in normal bone development and in the cellular response to parathyroid hormone stimulation. J Biol Chem. 2005;280:3974–3981. doi: 10.1074/jbc.M409807200. [DOI] [PubMed] [Google Scholar]
- Ferby I, Reschke M, Kudlacek O, Knyazev P, Pante G, Amann K, Sommergruber W, Kraut N, Ullrich A, Fassler R, Klein R. Mig6 is a negative regulator of EGF receptor-mediated skin morphogenesis and tumor formation. Nat Med. 2006;12:568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- Ballarò C, Ceccarelli S, Tiveron C, Tatangelo L, Salvatore AM, Segatto O, Alema S. Targeted expression of RALT in mouse skin inhibits epidermal growth factor receptor signalling and generates a Waved-like phenotype. EMBO Rep. 2005;6:755–761. doi: 10.1038/sj.embor.7400458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peréz-Soler R, Saltz L. Cutaneous adverse effects with HER1/EGFR-targeted agents: is there a silver lining? J Clin Oncol. 2005;23:5235–5246. doi: 10.1200/JCO.2005.00.6916. [DOI] [PubMed] [Google Scholar]