Abstract
Renal inflammation, characterized by the influx of inflammatory cells, is believed to play a critical role in the initiation and progression of a wide range of chronic kidney diseases. Here, we show that hepatocyte growth factor (HGF) inhibited renal inflammation and proinflammatory chemokine expression by disrupting nuclear factor (NF)-κB signaling. In vivo, HGF gene delivery inhibited interstitial infiltration of inflammatory T cells and macrophages, and suppressed expression of both RANTES (regulated on activation, normal T cell expressed and secreted) and monocyte chemoattractant protein-1 in a mouse model of obstructive nephropathy. In vitro, HGF abolished RANTES induction in human kidney epithelial cells, which was dependent on NF-κB signaling. HGF did not significantly affect the phosphorylation or degradation of IκBα; it also did not influence the phosphorylation or nuclear translocation of p65 NF-κB. However, HGF prevented p65 NF-κB binding to its cognate cis-acting element in the RANTES promoter. HGF action was dependent on the activation of the phosphoinositide 3-kinase/Akt pathway, which led to the phosphorylation and inactivation of glycogen synthase kinase (GSK)-3β. Suppression of GSK-3β activity mimicked HGF and abolished RANTES expression, whereas ectopic expression of GSK-3β restored RANTES induction. HGF also induced renal GSK-3β phosphorylation and inactivation after obstructive injury in vivo. These observations suggest that HGF is a potent anti-inflammatory cytokine that inhibits renal inflammation by disrupting NF-κB signaling and may be a promising therapeutic agent for progressive renal diseases.
Renal inflammation, characterized by the influx of inflammatory cells, is one of the major pathological findings in a variety of chronic kidney diseases (CKD).1,2,3 Although infiltration of the T cells and monocytes is a tissue-wounding response after injury that may be beneficial in combating various injurious insults, chronic inflammation is undoubtedly a detrimental process that is often linked to the fibrotic lesions and tissue scarring. Glomerular and interstitial inflammation, regardless of the initial causes, is believed to play a critical role in the genesis and progression of CKD.4 Clinical studies also reveal that the decline in renal function in patients with CKD often correlates closely with the extent of inflammation.1 Inflammatory cells contribute to tissue damage in many ways. They are capable of producing profibrotic cytokines such as transforming growth factor-β, which in turn induces the matrix-producing myofibroblast activation and tubular epithelial to mesenchymal transition,5,6,7 thereby promoting fibrogenic process. In addition, inflammatory cells can elicit their activities by producing radical oxygen species and by releasing proinflammatory cytokines, and therefore modulate the response of renal residential cells to injurious stimuli. Finally, they are able to produce and secrete extracellular matrix components, matrix metalloproteinases, and their endogenous inhibitors, thereby contributing to a dysregulated extracellular matrix catabolism.
Given the importance of inflammation in the pathogenesis of CKD, it is conceivable to speculate that inhibition of the inflammatory response may be beneficial attenuating fibrotic lesions after injuries. Indeed, the renal protective effect of interrupting the inflammatory process by different maneuvers has been well documented.8,9,10,11,12,13 In several studies, administration of immunosuppressive agent mycophenolate mofetil prevents lymphocyte and macrophage infiltration and reduces progressive renal damage.13,14 Likewise, treatment with chemokine receptor CCR1 antagonist reduces interstitial macrophage and lymphocyte recruitment and ameliorates renal fibrosis in obstructive nephropathy.12,15 Suppression of renal inflammation has been demonstrated to be advantageous even in nonimmune models of CKD such as angiotensin II-induced nephropathy and diabetic nephropathy.16,17 These observations establish that interfering with renal inflammation may represent a promising therapeutic strategy for progressive renal fibrotic disorders.
The expression of the genes that are important to the inflammatory responses is primarily mediated by the transcription factor nuclear factor (NF)-κB activation, which leads to the production of cellular signaling proteins such as cytokines, growth factors, or chemokines. RANTES (regulated on activation, normal T cell expressed), also known as CC-chemokine ligand 5 (CCL5), is a potent chemoattractant that is a member of the CC chemokine family.1,18 Numerous studies have described the expression and implication of RANTES in animal models of CKD.19,20 It is believed that its production and accumulation on inflamed renal tubules and endothelial cells provides directional signals for the circulating leukocytes to undergo extravasation, leading to inflammatory cell infiltration.21
Hepatocyte growth factor (HGF) is a pleiotropic factor that plays an essential role in the regulation of cell proliferation, survival, and differentiation in a variety of organs.22,23 A large body of evidence shows that HGF possesses a remarkable anti-fibrotic potential and ameliorates fibrotic lesions in a wide variety of CKD.24,25,26 This is corroborated by the observations that blocking endogenous HGF signaling with neutralizing antibody markedly exacerbates renal fibrosis and dysfunction.27,28 Interestingly, the antifibrotic action of exogenous HGF is often accompanied by an attenuation of renal inflammation in these models.29,30 However, the molecular mechanism underlying HGF inhibition of renal inflammation remains incompletely understood.
We undertook the present study to test the hypothesis that HGF suppresses renal inflammation by inhibiting proinflammatory cytokine expression. Our findings in this study demonstrate that HGF is a potent anti-inflammatory cytokine that blocks proinflammatory RANTES expression in vivo and in vitro. Our results further indicate that the anti-inflammatory action of HGF is primarily mediated by disrupting NF-κB signaling.
Materials and Methods
Antibodies and Reagents
The primary antibodies were obtained from different sources: anti-RANTES (sc-1410), anti-tumor necrosis factor (TNF)-α (sc-8301), anti-IκBβ (sc-946), anti-interleukin (IL)-6 (sc-7920), and anti-actin (sc-1616) (Santa Cruz Biotechnology, Santa Cruz, CA); anti-phospho-Akt (Ser473), anti-Akt, anti-phospho-IκBα (Ser32/36), anti-IκBα, anti-phospho-p65 NF-κB (Ser536), anti-p65 NF-κB, anti-phospho-GSK-3β (Ser9), anti-GSK-3β (Cell Signaling Technology, Beverly, MA), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Ambion, Austin, TX). Recombinant human HGF, TNF-α, and IL-1β were purchased from R&D Systems (Minneapolis, MN). Cell culture media, newborn calf serum, and supplements were obtained from Invitrogen (Carlsbad, CA). Chemical inhibitor wortmannin and cell-permeable inhibitor peptide NF-κB SN50 were purchased from Calbiochem (La Jolla, CA). All other chemicals were of analytic grade and were obtained from Sigma (St. Louis, MO) or Fisher (Pittsburgh, PA) unless otherwise indicated.
Animals
Male CD-1 mice that weighed ∼18 to 22 g were purchased from Harlan Sprague Dawley (Indianapolis, IN). Unilateral ureteral obstruction (UUO) was performed using an approved protocol by the Institutional Animal Care and Use Committee at the University of Pittsburgh, as described elsewhere.31 Briefly, under general anesthesia, complete ureteral obstruction was performed by double-ligating the left ureter using 4-0 silk after a midline abdominal incision. Sham-operated mice had their ureters exposed, manipulated but not ligated. Mice were randomly assigned into three groups: 1) sham normal control, 2) UUO control, and 3) UUO receiving HGF. Delivery of human HGF gene was achieved by intravenous injection of naked HGF plasmid (pCMV-HGF) at 1 mg/kg before (day − 1) and after (day 7) UUO, respectively, as described previously.32,33 Control UUO mice were injected with empty vector pcDNA3 plasmid at the same time points in an identical manner. At day 7 and day 14 after surgery, five mice from each group were sacrificed, respectively, and the kidneys were harvested for various analyses.
Immunohistochemical and Immunofluorescence Staining
Immunohistochemical staining of kidney sections was performed by an established protocol.34 In brief, paraffin-embedded sections were stained with anti-CD3 (sc-20047, Santa Cruz Biotechnology), anti-F4/80 (14-4801-82; eBioscience Inc., San Diego, CA), and anti-RANTES (500-P118; PeproTech Inc., Rocky Hill, NJ) antibodies using the M.O.M. immunodetection kit, according to the protocol specified by the manufacturer (Vector Laboratories, Burlingame, CA). Indirect immunofluorescence staining was performed according to the procedures described previously.35 Slides were viewed with an Eclipse E600 microscope equipped with a digital camera (Nikon, Melville, NY). Nonimmune normal rabbit IgG was used to replace the primary antibody as negative control, and no staining occurred. Nuclear staining for p65 NF-κB in HKC-8 cells after various treatments was counted and calculated. CD-3, F4/80, and RANTES staining were semiquantified by a computer-aided morphometric analysis (MetaMorph; Universal Imaging Co., Downingtown, PA). Briefly, a grid containing 117 (13 × 9) sampling points was superimposed onto images of cortical high-power field (×400). The number of grid points overlying positive area (except tubular lumen and glomeruli) was counted and expressed as a percentage of all sampling points. For each kidney, 10 randomly selected, nonoverlapping fields were analyzed in a blinded manner.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
For determination of RANTES and MCP-1 mRNA expression, a semiquantitative RT-PCR was used, as described previously.36 Total RNA was prepared from kidney homogenates of various groups of mice. After reverse transcription of the RNA, cDNA was used as a template in PCR reactions using gene-specific primer pairs. After quantifying band intensities by using densitometry, the relative steady-state level of mRNA was calculated after normalizing to β-actin. The sequences of the primer sets were as follows: RANTES, 5′-GTGCCCACGTCAAGGAGTAT-3′ (sense) and 5′-GGGAAGCGTATACAGGGTCA-3′ (antisense); MCP-1, 5′-CCCACTCACCTGCTGCTAC-3′ (sense) and 5′-TTCTTGGGGTCAGCACAGA-3′ (antisense). The sequences of the β-actin primer set were described previously.36
Cell Culture and Treatment
Human proximal tubular epithelial cell line (clone 8, HKC-8) was obtained from Dr. L. Racusen (The Johns Hopkins University, Baltimore, MD) and maintained in Dulbecco’s modified Eagle’s medium/F12 medium supplemented with 5% newborn calf serum. The HKC-8 cells were seeded onto six-well culture plates to ∼60 to 70% confluence in complete medium containing 5% newborn calf serum for 16 hours, and then changed to serum-free medium. After 24 hours of serum starvation, recombinant human IL-1β or TNF-α was added to the culture at a final concentration of 5 or 2 ng per ml, respectively. Thirty minutes before addition of TNF-α or IL-1β, recombinant human HGF was added at the concentration of 40 ng/ml unless otherwise indicated. The cells were typically incubated for 24 or 48 hours after addition of cytokines, before being subjected to Western blot or immunofluorescence staining, respectively.
Western Blot Analysis
Cells were lysed with sodium dodecyl sulfate (SDS) sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mmol/L dithiothreitol, and 0.1% bromophenol blue, supplemented with protease and phosphatase inhibitor cocktails). Kidney homogenates were prepared essentially according to an established protocol, as described previously.31 Samples were heated at 100°C for 5 to 10 minutes before being loaded and separated on 10% SDS-polyacrylamide gels. The proteins were electrotransferred to Hybond-P polyvinylidene difluoride membranes (Amersham Biosciences, Piscataway, NJ) in transfer buffer containing 48 mmol/L Tris-HCl, 39 mmol/L glycine, 0.037% SDS, and 20% methanol at 4°C for 1 hour. Nonspecific binding to the membrane was blocked for 1 hour at room temperature with 5% Carnation nonfat milk in TBST buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 0.1% Tween 20). The membranes were then incubated for 16 hours at 4°C with various primary antibodies in blocking buffer containing 5% milk at the dilutions specified by the manufacturers. After extensive washing in TBST buffer, the membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hour at room temperature in 5% nonfat milk dissolved in TBST. Membranes were washed with TBST buffer and the signals were visualized using the SuperSignal West Pico chemiluminescent substrate kit (Pierce Biotechnology, Rockford, IL).
Enzyme-Linked Immunosorbent Assay
HKC-8 cells were seeded in complete medium in 12-well plates at a concentration of 1 × 105 cells per well. Twenty-four hours later, cells were changed to serum-free medium and kept in serum-free medium for an additional 24 hours, before addition of different cytokines. The supernatants of cell cultures were collected, and the levels of RANTES protein were determined by using enzyme-linked immunosorbent assay kit (Quantikine Immunoassay, R&D Systems) according to the manufacturer’s protocol.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed to analyze in vivo interactions of NF-κB and its cognate cis-acting element in RANTES promoter. This assay was performed essentially according to the protocols specified by the manufacturer (ChIP assay kit; Upstate, Charlottesville, VA). Briefly, HKC-8 cells, after various treatments as indicated, were cross-linked with 1% formaldehyde, and then resuspended in SDS lysis buffer containing protease inhibitors. The chromatin solution was sonicated, and the supernatant was diluted 10-fold. An aliquot of total diluted lysate was used for total genomic DNA as input DNA control. The anti-p65 NF-κB antibody was added and incubated at 4°C overnight, followed by incubation with protein A-agarose for 1 hour. The precipitates were washed and chromatin complexes were eluted. After reversal of the cross-linking at 65°C for 4 hours, the DNA was purified, and 1 μl of input control or ChIP samples were used as a template for PCR using the primer sets for human RANTES promoter regions (from −208 to −10) containing NF-κB response element.37 The sequences of primers used for ChIP assay were as follows: forward, 5′-TTGGTGCTTGGTCAAAGAGG-3′; and reverse, 5′-CCCTTTATAGGGCCAGTTGA-3′.
Statistical Analysis
All data examined were expressed as mean ± SEM. Statistical analysis of the data were performed using SigmaStat software (Jandel Scientific Software, San Rafael, CA). Comparison between groups was made using one-way analysis of variance, followed by Student-Newman-Keuls test. A P value of less than 0.05 was considered significant.
Results
HGF Inhibits Renal Inflammatory Infiltration
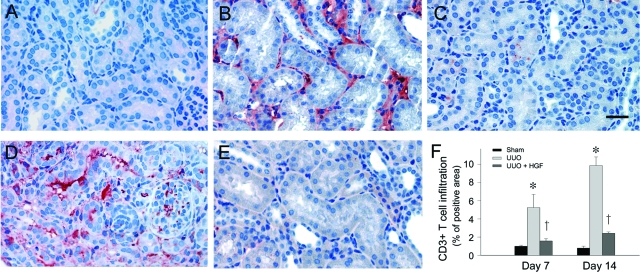
HGF has been demonstrated to be able to ameliorate renal fibrotic lesions in obstructive nephropathy,30,31 in which interstitial infiltration of inflammatory cells is a major pathological feature. To examine the potential effect of HGF on renal inflammation, we first investigated the infiltration of the CD-3-positive T cells in the obstructed kidney after UUO. As shown in Figure 1, ureteral obstruction induced substantial renal T-cell infiltration at 7 and 14 days after UUO, respectively, as illustrated by immunohistochemical staining for CD3 antigen. As reported previously,32,33,38 delivery of the HGF gene via naked plasmid vector produced a substantial amount of exogenous HGF protein in the circulation, as well as in liver and kidney. The increase in renal human HGF protein was sustained to 7 days after injection, although it peaked at 16 to 24 hours.32 We found that HGF gene therapy effectively inhibited the infiltration of T cells in the obstructed kidney at different time points (Figure 1, C and E). Quantitative analysis also demonstrated a dramatic suppression of inflammatory T-cell infiltration by HGF at 7 and 14 days after UUO, respectively, in obstructive nephropathy (Figure 1F).
Figure 1.
HGF inhibits renal infiltration of inflammatory CD3-positive T cells. A–E: Immunohistochemical staining revealed an increased infiltration of CD3-positive T cells in the obstructed kidney at 7 (B) and 14 (D) days, respectively, after UUO, compared with sham control (A). Delivery of HGF gene effectively inhibited the infiltration of T cells in the obstructed kidneys at 7 (C) and 14 (E) days after UUO, respectively, as indicated. F: Graphic presentations of quantitative data are given. Data were presented as mean ± SEM of five animals per group (n = 5). *P < 0.01 versus sham control. †P < 0.01 versus pcDNA3. Scale bar = 20 μm.
We also examined the effect of HGF on monocyte/macrophage infiltration in the obstructed kidney. As shown in Figure 2, obstructive injury caused marked infiltration and accumulation of the F4/80-positive macrophages in renal interstitium. However, delivery of the HGF gene also dramatically inhibited macrophage infiltration at 7 and 14 days after obstructive injury, respectively (Figure 2, C, E, and F). Of note, as previously reported,24,31 delivery of the HGF gene significantly ameliorated the fibrotic injury, and the total collagen content in the obstructed kidney was repressed by 48% and 44% at 7 and 14 days after UUO, respectively.
Figure 2.
HGF inhibits renal infiltration of inflammatory F4/80-positive macrophages. A–E: Immunohistochemical staining revealed an increased infiltration of F4/80-positive macrophages in the obstructed kidney at 7 (B) and 14 (D) days, respectively, after UUO, compared with sham control (A). Delivery of HGF gene effectively inhibited the infiltration of macrophages in the obstructed kidneys at 7 (C) and 14 (E) days after UUO, respectively, as indicated. F: Graphic presentation of quantitative data are given. Data were presented as mean ± SEM of five animals per group (n = 5). *P < 0.01 versus sham control. †P < 0.01 versus pcDNA3. Scale bar = 20 μm.
HGF Inhibits RANTES and MCP-1 Expression after Obstructive Injury
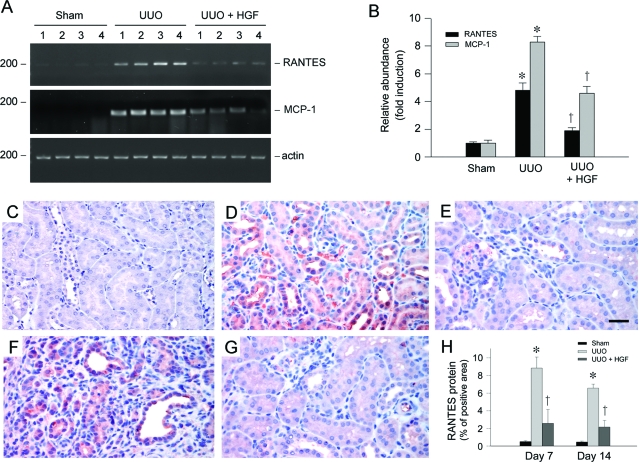
Because chemokines play an essential role in attracting and guiding the influx of inflammatory cells, we next examined the expression of RANTES in the obstructed kidney, one of the best characterized chemokines that is capable of recruiting many types of immune cells including T lymphocytes, monocytes/macrophages, and natural killer cells.18 RT-PCR analysis revealed that RANTES mRNA was markedly induced in the obstructed kidney after injury, and HGF gene therapy primarily inhibited renal RANTES mRNA induction (Figure 3A). Quantitative analysis demonstrated a more than fourfold induction of RANTES mRNA in the obstructed kidney at 7 days after UUO, compared with sham controls; and renal RANTES mRNA was inhibited by more than 60% after HGF gene administration (Figure 3B). In addition, delivery of HGF gene also significantly inhibited the mRNA expression of monocyte chemoattractant protein-1 (MCP-1), also known as CC-chemokine ligand 2 (CCL2),1,39 in the obstructed kidney (Figure 3, A and B).
Figure 3.
HGF inhibits renal RANTES and MCP-1 expression in the obstructed kidney after UUO. A and B: RT-PCR demonstrated renal RANTES and MCP-1 mRNA expression in different groups as indicated. Numbers (1 to 4) in A denote each individual animal. Relative RANTES and MCP-1 mRNA abundance (fold induction over sham controls) was calculated after normalizing with β-actin. B: Data were presented as mean ± SEM of four animals per group (n = 4). *P < 0.05 versus sham control. †P < 0.05 versus pcDNA3. C–G: Immunohistochemical staining demonstrated that RANTES protein was induced predominantly in renal tubular epithelium in the obstructed kidney at 7 (D) and 14 (F) days after UUO, compared with sham control (C). Tubular expression of RANTES was primarily suppressed by HGF at 7 (E) and 14 (G) days after UUO, respectively. H: Graphical presentation shows the quantitative determination of RANTES protein expression in different groups as indicated. Data were presented as mean ± SEM of five animals per group (n = 5). *P < 0.05 versus sham control. †P < 0.05 versus pcDNA3. Scale bar = 20 μm.
We next investigated the RANTES protein expression and its localization by immunohistochemical staining. As shown in Figure 3C, no or little RANTES protein was observed in normal, sham-operated kidney. However, RANTES expression was markedly induced in the obstructed kidney at 7 and 14 days, respectively. RANTES protein was localized predominantly in renal tubular epithelium (Figure 3, D and F). Delivery of HGF gene significantly reduced RANTES protein expression in the obstructed kidney at different time points (Figure 3, E and G). Therefore, HGF inhibition of the peritubular infiltration of inflammatory T cells is closely associated with its suppression of tubular RANTES expression in the obstructed kidney.
HGF Blocks RANTES Induction in Tubular Epithelial Cells in Vitro
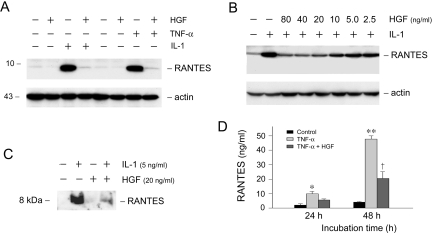
To evaluate whether HGF directly suppresses RANTES expression, we used human proximal tubular epithelial (HKC-8) cells as an in vitro model system. When HKC-8 cells were incubated with IL-1β or TNF-α, the expression of chemokine RANTES was dramatically induced (Figure 4A). Simultaneous incubation with HGF completely abolished RANTES induction by IL-1β or TNF-α in HKC-8 cells. The action of HGF appeared to be dose-dependent (Figure 4B). HGF at the concentration of 20 ng/ml effectively abrogated IL-1β-mediated RANTES expression (Figure 4B). HGF also markedly attenuated RANTES secretion into the supernatants of HKC-8 cells. Figure 4, C and D, shows the abundance of RANTES protein in the supernatants of HKC-8 cells after different treatments, as detected by Western blot analysis and enzyme-linked immunosorbent assay, respectively. These results indicate that HGF can inhibit proinflammatory RANTES expression in tubular epithelial cells in response to IL-1β or TNF-α stimulation.
Figure 4.
HGF blocks IL-1β- or TNF-α-mediated RANTES expression in tubular epithelial cells. A: HGF blocked RANTES expression induced by IL-1β and TNF-α. Human proximal tubular epithelial cells (HKC-8) were treated with IL-1β (5 ng/ml), TNF-α (2 ng/ml), HGF (40 ng/ml), or in different combinations as indicated for 48 hours. Whole cell lysates were immunoblotted with antibodies against RANTES and actin, respectively. B: HGF inhibited IL-1β-induced RANTES expression in a dose-dependent manner. HKC-8 cells were incubated with IL-1β (5 ng/ml) alone, or with IL-1β plus increasing amounts of HGF as indicated. C: HGF inhibited IL-1β-mediated RANTES secretion. The supernatant of the HKC-8 cells after various treatments as indicated was immunoblotted with anti-RANTES antibody. D: HGF inhibited TNF-α-induced RANTES secretion. The levels of RANTES secreted in the supernatant of HKC-8 cells after different treatments were measured by a specific enzyme-linked immunosorbent assay.
NF-κB Signaling Is Critical for Mediating RANTES Expression
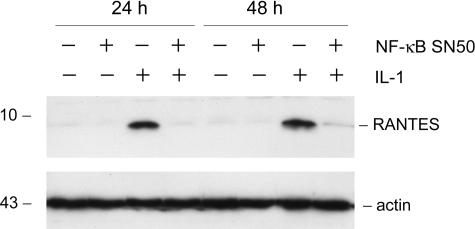
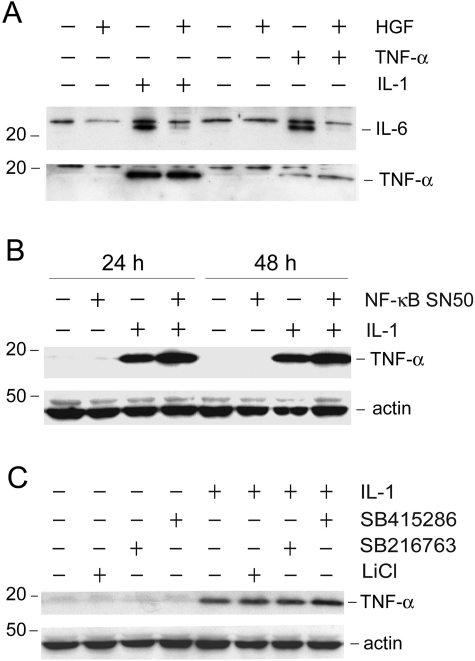
To elucidate how HGF blocks IL-1β-mediated RANTES expression, we first investigated the signal pathway leading to RANTES induction by IL-1β in tubular epithelial cells. In light of that NF-κB is the major signal mediator that plays a critical role in the regulation of proinflammatory cytokine expression, we examined the potential involvement of NF-κB signaling in the control of RANTES expression induced by IL-1β. As shown in Figure 5, pretreatment of HKC-8 cells with specific NF-κB signaling inhibitor, NF-κB SN50, completely abolished RANTES induction by IL-1β at different time points, suggesting that IL-1β-mediated RANTES induction in HKC-8 cells is dependent on an intact NF-κB signaling.
Figure 5.
Induction of RANTES expression is dependent on NF-κB signaling. HKC-8 cells were treated with IL-1β in the absence or presence of NF-κB signal inhibitor (20 μmol/L NF-κB SN50) for various periods of time as indicated. Whole cell lysates were immunoblotted with antibodies against RANTES and actin, respectively.
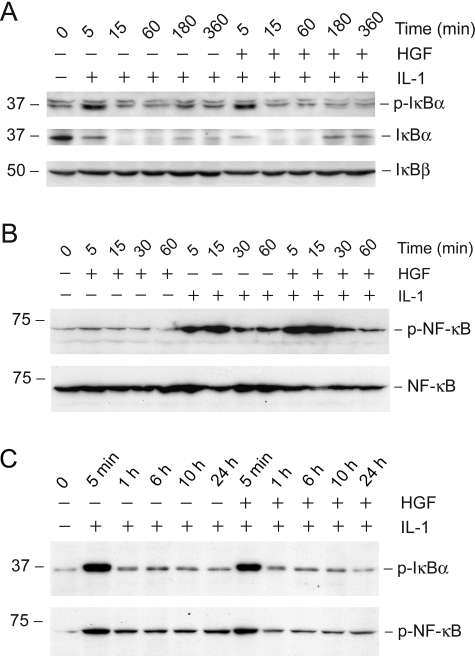
HGF Affects neither IκBα Phosphorylation nor NF-κB Activation
Having established that the NF-κB signaling is essential for mediating RANTES induction, we next examined the potential effect of HGF on IκBα and NF-κB phosphorylation in tubular epithelial cells. As presented in Figure 6A, IL-1β induced rapid phosphorylation of IκBα, which led to its ubiquitin-mediated protein degradation. The abundance of IκBα started to decrease as early as 5 minutes after IL-1β stimulation, which was consistent with its rapid phosphorylation. By 15 minutes, IκBα primarily disappeared. Pretreatment with HGF appeared to have little effect on the rate and magnitude of IκBα phosphorylation and its abundance, at least in the initial 1 hour after IL-1β treatment (Figure 6A). IκBα abundance displayed a tendency to slightly return after 3 hours. HGF treatment only marginally, if any at all, accelerated this process (Figure 6A). HGF also did not modulate IκBβ abundance in HKC-8 cells.
Figure 6.
HGF does not affect IL-1β-mediated phosphorylation of IκBα and p65 NF-κB, as well as the degradation and abundance of IκBα and IκBβ. HKC-8 cells were treated with IL-1β (5 ng/ml) alone or IL-1β plus HGF (40 ng/ml). At different time points as indicated, cells were harvested and subjected to immunoblotting with antibodies against p-IκBα, IκBα, IκBβ (Α), p-p65 NF-κB, and p65 NF-κB (B), respectively. C: Long-term incubation with HGF also did not affect the phosphorylated status of IκBα and p65 NF-κB in HKC-8 cells.
Figure 6B shows the p65 NF-κB phosphorylation after IL-1β treatment and the effects of HGF on this process in HKC-8 cells. IL-1β induced rapid phosphorylation of p65 in HKC-8 cells, which peaked at 5 to 15 minutes after stimulation. In agreement with IκBα, HGF did not significantly affect the p65 NF-κB phosphorylation and activation (Figure 6B). We also examined the effect of a prolonged incubation with HGF on IκBα and NF-κB phosphorylation in HKC-8 cells. As shown in Figure 6C, the patterns of IκBα and NF-κB phosphorylation in HKC-8 cells after incubation with IL-1β plus HGF for a long period of time ranging from 5 minutes to 24 hours were comparable with that treated with IL-1β alone.
HGF Disrupts NF-κB Signaling by Blocking p65 Binding to Its cis-Acting Element
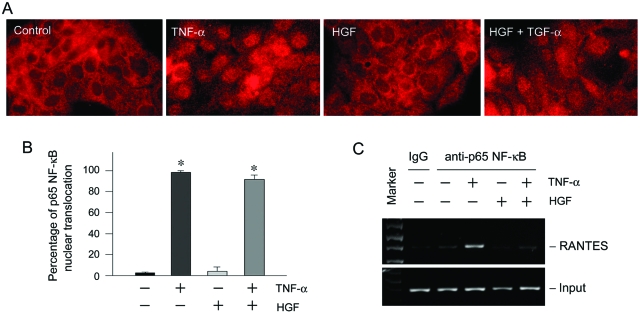
The inability of HGF to affect NF-κB activation prompted us to examine whether it blocks the p65 nuclear translocation, a critical step in NF-κB signaling. Immunofluorescence staining demonstrated that on stimulation with TNF-α, p65 NF-κB translocated into the nuclei in HKC-8 cells, and HGF did not significantly affect the nuclear translocation of p65 (Figure 7A). Quantitative analysis of the cell population with p65 nuclear staining after various treatments also revealed that HGF failed to block p65 nuclear translocation (Figure 7B), suggesting that HGF inhibition of NF-κB signaling likely takes place in the postnuclear translocation stage. We further investigated the possibility that HGF signaling might influence the in vivo binding of p65 NF-κB to RANTES promoter region. As shown in Figure 7C, TNF-α induced p65 binding to its cognate cis-acting NF-κB element in human RANTES promoter, as revealed by ChIP assay. However, treatment of HKC-8 cells with HGF completely abolished the binding of p65 to RANTES promoter induced by TNF-α. These results indicate that HGF treatment results in a disruption of the binding of p65 to its cis-element, thereby intercepting the NF-κB signaling.
Figure 7.
HGF does not block p65 NF-κB nuclear translocation but abolishes its binding to the cognate cis-acting element in RANTES promoter. A and B: HGF did not block the p65 NF-κB nuclear translocation induced by TNF-α. HKC-8 cells were treated with TNF-α or/and HGF as indicated. A: Representative micrographs showed the staining for p65 NF-κB after various treatments. B: The percentages of cell population with p65 nuclear translocation after various treatments are given. *P < 0.05 versus control. C: ChIP assay demonstrated that HGF signaling abolished the binding of p65 to its cis-acting element in the RANTES promoter. Marker, DNA size markers; IgG, precipitated with control IgG.
Suppression of RANTES Expression by HGF Is Mediated via a Phosphoinositide (PI)-3-Kinase-Dependent Pathway
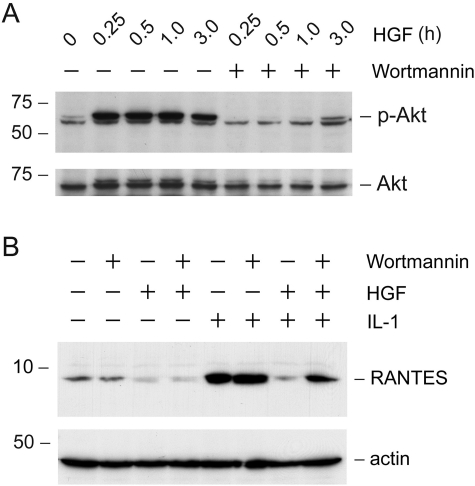
To unravel the mechanism by which HGF blocks RANTES expression, we sought to define the signaling event that is important for HGF action. We found that the suppressive effect of HGF on RANTES expression was apparently dependent on the activation of PI 3-kinase. As presented in Figure 8A, treatment of the HKC-8 cells with HGF induced activation of PI 3-kinase, which resulted in rapid phosphorylation of its downstream Akt/protein kinase B. This action of HGF was blocked by preincubation with specific PI 3-kinase inhibitor wortmannin. Interestingly, inhibition of PI 3-kinase by wortmannin abolished HGF action and restored RANTES expression in HKC-8 cells (Figure 8B).
Figure 8.
Suppression of the RANTES expression by HGF is mediated by a PI-3-kinase-dependent pathway. A: Inhibition of PI-3-kinase by wortmannin blocked Akt phosphorylation, a downstream event of PI-3-kinase activation. HKC-8 cells were pretreated with wortmannin (10 nmol/L) for 30 minutes, followed by incubation with HGF for various periods of time as indicated. Whole cell lysates were immunoblotted with antibodies against p-Akt and total Akt, respectively. B: Inhibition of PI-3-kinase abolished HGF action and restored IL-1β-mediated RANTES expression. HKC-8 cells after various treatments as indicated were subjected to immunoblotting with antibodies against RANTES and actin, respectively.
HGF-Mediated Inactivation of GSK-3β Is Crucial for Blockade of RANTES Expression
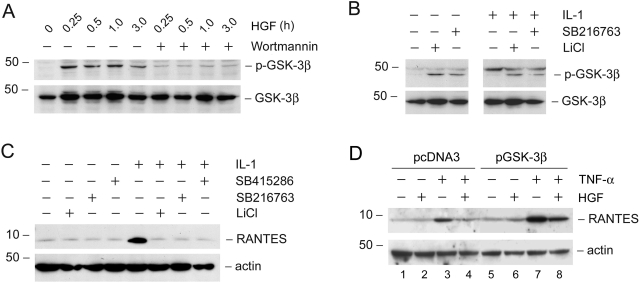
We further define the downstream pathway of PI 3-kinase that is crucial in mediating HGF suppression of the RANTES expression in tubular epithelial cells. As shown in Figure 9A, HGF induced rapid phosphorylation of GSK-3β, which would lead to its inactivation. This action of HGF was dependent on PI 3-kinase, because wortmannin abrogated GSK-3β phosphorylation (Figure 9A).
Figure 9.
HGF induces GSK-3β phosphorylation and its subsequent inactivation through PI-3-kinase-dependent pathway, and inhibition of GSK-3β suppresses RANTES induction. A: HGF induced GSK-3β phosphorylation in a PI 3-kinase-dependent manner. HKC-8 cells were pretreated with wortmannin (10 nmol/L) for 30 minutes, followed by incubation with HGF (40 ng/ml) for various periods of time as indicated. Cell lysates were immunoblotted with antibodies against p-GSK-3β and total GSK-3β. B: Inhibition of GSK-3β induces its phosphorylation. HKC-8 cells were treated with GSK-3β-specific inhibitors (5 μmol/L SB216763 and 30 mmol/L LiCl). Cell lysates were immunoblotted with antibodies against p-GSK-3β and GSK-3β, respectively. C: Inhibition of GSK-3β abolishes IL-1β-mediated RANTES expression. HKC-8 cells were treated with IL-1β for 48 hours in the absence or presence of GSK-3β inhibitors (30 μmol/L SB415286, 5 μmol/L SB216763, and 30 mmol/L LiCl). Cell lysates were immunoblotted with antibodies against RANTES and actin, respectively. D: Ectopic expression of GSK-3β enhanced TNF-α-mediated RANTES expression and primarily abolished the HGF-mediated inhibition of RANTES. HKC-8 cells were transfected with GSK-3β expression plasmid or control pcDNA3, and then treated with TNF-α or/and HGF as indicated.
To further determine whether the inactivation of GSK-3β is critical for mediating HGF suppression of RANTES expression, we examined the effects of GSK-3β inhibitors on RANTES expression. As demonstrated in Figure 9B, GSK-3β inhibitors induced its phosphorylation in either the absence or presence of IL-1β in HKC-8 cells. Furthermore, inhibition of GSK-3β activity with chemical inhibitors imitated HGF action and abolished IL-1β-mediated RANTES expression in HKC-8 cells (Figure 9C). Consistently, ectopic expression of GSK-3β expression vector sensitized HKC-8 cells to enhance RANTES induction after TNF-α stimulation (Figure 9D, lane 3 versus lane 7), when comparing to the pcDNA3 controls. GSK-3β overexpression also primarily restored RANTES expression after HGF treatment (Figure 9D, lane 4 versus lane 8). Therefore, inactivation of GSK-3β plays a fundamental role in mediating HGF suppression of RANTES expression.
HGF Specifically Blocks NF-κB-Dependent Cytokine Expression
We next examined the effect of HGF on the expression of other proinflammatory cytokines. Figure 9A shows that HGF also abolished IL-1β- and TNF-α-triggered IL-6 expression in HKC-8 cells, in addition to its inhibitory effect on RANTES (Figure 4A). However, HGF appeared to have no effect on IL-1β-induced TNF-α expression in tubular epithelial cells (Figure 10A). Similarly, HGF did not alter TNF-α-triggered autoinduction. These observations suggest that the suppressive effect of HGF on cytokine expression is gene-specific.
Figure 10.
HGF does not block IL-1β-mediated TNF-α expression which is independent on NF-κB signaling. A: HGF blocked IL-6 expression induced by IL-1β or TNF-α, but has no effect on TNF-α induction. HKC-8 cells were treated for 48 hours with IL-1β (5 ng/ml), TNF-α (2 ng/ml), HGF (40 ng/ml), or in combination as indicated. Whole cell lysates were immunoblotted with antibodies against IL-6 and TNF-α, respectively. Equal loading of the samples in each lane was confirmed by reprobing with actin (see Figure 3A). B: Induction of TNF-α expression by IL-1β in tubular epithelial cells was independent on NF-κB signaling. HKC-8 cells were pretreated with NF-κB SN50 for 30 minutes, followed by incubation with IL-1β for 24 and 48 hours, respectively. C: Blockade of GSK-3β did not affect IL-1β-mediated TNF-α expression. HKC-8 cells were treated with various GSK-3β inhibitors (30 μmol/L SB415286, 5 μmol/L SB216763, and 30 mmol/L LiCl) for 30 minutes before addition of IL-1β. Cell lysates were immunoblotted for TNF-α and actin, respectively.
To understand why HGF does not block IL-1β-mediated TNF-α expression, we first examined whether IL-1β-induced TNF-α expression is dependent on NF-κB signaling. As shown in Figure 10B, pretreatment with NF-κB inhibitor did not block IL-1β-induced TNF-α expression, suggesting that it is independent on NF-κB signaling. Likewise, treatment with GSK-3β inhibitors also did not abrogate IL-1β-mediated TNF-α expression in HKC-8 cells (Figure 10C). Therefore, HGF specifically inhibits NF-κB-mediated proinflammatory cytokine expression.
HGF Induces GSK-3β Phosphorylation in Vivo
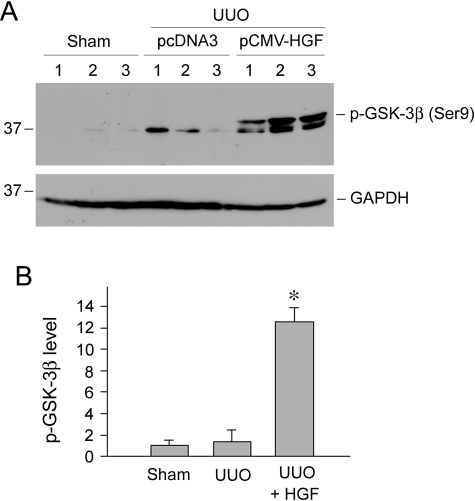
To assess the relevance of GSK-3β signaling in HGF-mediated anti-inflammation in vivo, we examined the phosphorylation status of GSK-3β in the obstructed kidney after HGF administration. As shown in Figure 11A, HGF gene therapy dramatically induced GSK-3β phosphorylation in the obstructed kidney after UUO. Quantitative analysis also revealed a marked increase in phosphorylated GSK-3β abundance (Figure 11B). Therefore, HGF inhibition of RANTES expression is closely associated with its ability to induce GSK-3β phosphorylation and subsequent inactivation in vivo.
Figure 11.
HGF induces GSK-3β phosphorylation in the obstructed kidney in vivo. A: Kidney homogenates obtained from various groups as indicated were immunoblotted with specific antibodies against phosphorylated GSK-3β and GAPDH, respectively. Numbers (1 to 3) denote each individual animal. B: Relative phosphorylated GSK-3β abundance (fold induction over sham controls) was calculated and presented as mean ± SEM of three animals per group (n = 3). *P < 0.05 versus sham or pcDNA3 controls.
Discussion
Renal inflammation is one major component of the sequential events of the tissue responses after injurious insults.2,4,40 Chronic inflammation is thought to play a pivotal role in the initiation and maintenance of the fibrotic lesions that eventually lead to end-stage kidney failure. In this study, we have demonstrated that HGF, an anti-fibrotic cytokine that has been shown to ameliorate renal fibrosis and kidney dysfunction in a variety of models,24,26,29,32 is capable of inhibiting renal infiltration of T cells and macrophages in obstructive nephropathy and suppressing proinflammatory chemokines RANTES and MCP-1 expression in vivo, consistent with numerous previous reports.30,41,42 Of note, sustained expression of the exogenous HGF gene has no adverse effect on normal kidney structure and function.32,33 We have further shown that the anti-inflammatory action of HGF is mediated by blocking NF-κB, the master signaling that is implicated in the control of many proinflammatory cytokines expression. This inhibition of NF-κB signaling by HGF is apparently operated through a mechanism that is dependent on PI 3-kinase and GSK-3β kinase. Therefore, our present study provides an intrinsic linkage between HGF and the inhibition of a major proinflammatory signaling, and offers significant insights into understanding the therapeutic efficacy of HGF for the treatment of chronic fibrotic disorders.
The influx of inflammatory cells from the peripheral circulation to the injured sites is a complex process that involves a series of interactions between soluble mediators and membrane molecules in endothelial cells and leukocytes. Chemokines, a family of chemotactic cytokines that play a vital role in immunomodulation, are believed to act as the directional signals to sort and direct effector leukocyte migration.1,40 The present study shows that proximal tubular epithelial cells are the predominant site of chemokine production after chronic kidney injury. They are susceptible to injurious stimuli in vivo and in vitro to produce a large amount of RANTES, which serves as chemotactic signals to attract the migrating leukocytes to move along its peritubular gradients to eventually reach the tubulointerstitial site of inflammation. That HGF suppresses the IL-1β- or TNF-α-induced RANTES expression in tubular epithelial cells renders it a potent inhibitor of renal inflammation. This speculation is confirmed by the observations that HGF administration attenuates renal inflammation in several models of CKD.29,41,42
The expression of proinflammatory cytokines is primarily controlled at the transcriptional level by NF-κB, a family of dimeric transcription factors that regulate many genes involved in inflammation.43 NF-κB activation has been implicated in various CKDs, in which inflammation is a major component of the pathological findings. Southwestern histochemistry has documented the presence of activated NF-κB in a variety of chronic, inflammatory renal diseases.43 In this context, it is conceivable that exogenous modulation of NF-κB activation may help to formulate new therapeutic approaches. Not surprisingly, inhibition of NF-κB signaling by numerous pharmacological maneuvers has been demonstrated to be effective in reducing inflammatory infiltration and renal injury.44,45,46,47 Our present observation that HGF specifically inhibits NF-κB signaling underscores an anti-inflammatory action of HGF that could contribute to its renal protection. The notion that HGF blocks NF-κB signaling is supported by several lines of evidence. First, HGF abolishes IL-1β- or TNF-α-mediated RANTES expression, which is dependent on NF-κB signaling (Figure 5). Second, HGF prevents NF-κB from binding to its cognate DNA element in RANTES promoter in a ChIP assay (Figure 7C). Third, HGF does not influence TNF-α expression induced by IL-1β in tubular epithelial cells, which is independent on NF-κB signaling (Figure 10).
The molecular mechanism underlying HGF suppression of NF-κB signaling remains incompletely understood. It is well known that NF-κB is present in the cytoplasm of virtually all cell types in an inactive form by association with a family of inhibitory proteins termed IκB in resting states.43 Activation of NF-κB requires the phosphorylation of IκB, which triggers its polyubiquitination and subsequent degradation by proteolytic cleavage via the proteasome system. The liberated NF-κB rapidly translocates to the nucleus, where it binds κB sites and activates the transcription of its targeted genes.43,48 Biochemical analysis reveals that HGF does not influence the rate and magnitude of IκBα phosphorylation, as well as the IκBα and IκBβ cellular abundance in tubular epithelial cells after IL-1β stimulation. Similarly, HGF also fails to affect the phosphorylation and activation of p65 NF-κB and its nuclear translocation. Hence, the early, prenuclear NF-κB signaling events and p65 nuclear import are still operative after HGF treatment. This is somewhat inconsistent with previous observations that HGF is able to inhibit IκB phosphorylation and its degradation as well as p65 NF-κB phosphorylation and its nuclear translocation.29,49 The reason behind this discrepancy is unknown, but it could be attributable to the cell-type specificity. Our studies suggest that HGF prevents the activated p65 NF-κB binding to the κB element in the RANTES promoter (Figure 7C), thereby sequestrating its ability to trans-activate the transcription of its targeted genes. These findings underscore that HGF works at the postnuclear stage of NF-κB signaling by disrupting the assembly of the transcription complexes of NF-κB.
The signal pathway leading to HGF inhibition of RANTES expression in tubular epithelial cells is evidently dependent on PI 3-kinase and its downstream GSK-3β kinase. In contrast to several reports demonstrating that activation of PI 3-kinase/Akt results in activation of NF-κB,50,51 our present study indicates that PI 3-kinase activation by HGF, through the phosphorylation and subsequent inactivation of GSK-3β, leads to the suppression of the NF-κB-mediated RANTES expression. This observation is in harmony with earlier studies in GSK-3β-deficient cells, in which the loss of GSK-3β results in a defect in NF-κB activation,48 and is also consistent with a previous observation that HGF inhibits GSK-3β via a PI-3 kinase/Akt pathway.42 Furthermore, this signaling pathway appears to be operative in vivo as well, because HGF also induces GSK-3β phosphorylation in the obstructed kidney after ureteral ligation (Figure 11). Previous studies show that the early steps leading to NF-κB activation such as IκB degradation and NF-κB nuclear translocation are unaffected in GSK-3β−/− cells, and therefore GSK-3β is thought to be required for NF-κB signaling at the level of the transcriptional complex.48 Our results also imply that GSK-3β inhibition by HGF could lead to disruption of the transcriptional complex formation of NF-κB at the postnuclear stage. Exactly how GSK-3β inhibition leads to blockade of NF-κB signaling remains elusive. One plausible explanation could be that GSK-3β directly phosphorylates p65, which may be prerequisite for p65 to interact with the cis-acting element in its targeted genes. This speculation is supported by the results of ChIP assay (Figure 7C), which indicate that HGF signaling prevents p65 NF-κB from binding to RANTES promoter. It should also be noted that the inhibitory effect of HGF on NF-κB signaling is gene- and promoter-specific because HGF apparently does not abolish NF-κB-mediated IκBα induction in response to IL-1β stimulation (Figure 6A).
HGF is a potent antifibrotic factor that ameliorates renal fibrosis and dysfunction in a wide variety of experimental animal models.22,23 Previous studies are primarily focused on dissection of the cellular and molecular pathways leading to HGF inhibition of fibrotic lesions. We have shown that HGF counteracts the profibrotic action of transforming growth factor-β1 by directly intercepting Smad signaling through distinctive mechanisms in diverse types of kidney cells,52,53,54 and that HGF is a survival factor that protects renal tubular epithelial cells from apoptosis.55 The present study indicates that the beneficial effects of HGF in CKD go beyond the inhibition of fibrosis and apoptosis. By inhibiting the NF-κB signaling and proinflammatory cytokine expression, HGF is obviously an anti-inflammatory factor that suppresses renal inflammation after injury. Of note, at this stage we cannot allocate the relative contribution of HGF as a cytoprotective, an anti-fibrotic, and an anti-inflammatory agent to the observed beneficial effects in CKD, because they could be interdependent each other. Nevertheless, by targeting multiple signal pathways and pathogenic events, HGF is well suited to be used as a therapeutic agent for progressive renal fibrotic diseases.
Footnotes
Address reprint requests to Youhua Liu, Ph.D., Department of Pathology, University of Pittsburgh, S-405 Biomedical Science Tower, 200 Lothrop St., Pittsburgh, PA 15261. E-mail: liuy@upmc.edu.
Supported by the National Institutes of Health (grants DK054922, DK061408, and DK064005) and the American Heart Association Greater Rivers Affiliate (postdoctoral fellowship to X.T.).
M.G., C.D., and X.T. contributed equally to this study.
This work was presented at the 37th Annual Meeting of the American Society of Nephrology and has been published in abstract form (J Am Soc Nephrol 2004, 15: 477A).
References
- Segerer S, Nelson PJ, Schlondorff D. Chemokines, chemokine receptors, and renal disease: from basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–176. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- Eddy AA. Molecular basis of renal fibrosis. Pediatr Nephrol. 2000;15:290–301. doi: 10.1007/s004670000461. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- Klahr S, Morrissey JJ. The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int Suppl. 2000;75:S7–S14. [PubMed] [Google Scholar]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, Horuk R, Grone HJ, Schlondorff D, Anders HJ. CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int. 2004;66:2264–2278. doi: 10.1111/j.1523-1755.2004.66038.x. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Belemezova E, Eis V, Segerer S, Vielhauer V, Perez de Lema G, Kretzler M, Cohen CD, Frink M, Horuk R, Hudkins KL, Alpers CE, Mampaso F, Schlondorff D. Late onset of treatment with a chemokine receptor CCR1 antagonist prevents progression of lupus nephritis in MRL-Fas(lpr) mice. J Am Soc Nephrol. 2004;15:1504–1513. doi: 10.1097/01.asn.0000130082.67775.60. [DOI] [PubMed] [Google Scholar]
- Tamada S, Nakatani T, Asai T, Tashiro K, Komiya T, Sumi T, Okamura M, Kim S, Iwao H, Kishimoto T, Yamanaka S, Miura K. Inhibition of nuclear factor-kappaB activation by pyrrolidine dithiocarbamate prevents chronic FK506 nephropathy. Kidney Int. 2003;63:306–314. doi: 10.1046/j.1523-1755.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- Pérez De Lema G, De Wit C, Cohen CD, Nieto E, Molina A, Banas B, Luckow B, Vicente AB, Mampaso F, Schlondorff D. Angiotensin inhibition reduces glomerular damage and renal chemokine expression in MRL/lpr mice. J Pharmacol Exp Ther. 2003;307:275–281. doi: 10.1124/jpet.103.053678. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Frink M, Linde Y, Cohen CD, Blattner SM, Kretzler M, Strutz F, Mack M, Grone HJ, Onuffer J, Horuk R, Nelson PJ, Schlondorff D. A chemokine receptor CCR-1 antagonist reduces renal fibrosis after unilateral ureter ligation. J Clin Invest. 2002;109:251–259. doi: 10.1172/JCI14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero F, Rodriguez-Iturbe B, Parra G, Gonzalez L, Herrera-Acosta J, Tapia E. Mycophenolate mofetil prevents the progressive renal failure induced by 5/6 renal ablation in rats. Kidney Int. 1999;55:945–955. doi: 10.1046/j.1523-1755.1999.055003945.x. [DOI] [PubMed] [Google Scholar]
- Van den Branden C, Ceyssens B, Pauwels M, Van Wichelen G, Heirman I, Jie N, Verbeelen D. Effect of mycophenolate mofetil on glomerulosclerosis and renal oxidative stress in rats. Nephron Exp Nephrol. 2003;95:e93–e99. doi: 10.1159/000074325. [DOI] [PubMed] [Google Scholar]
- Anders HJ, Vielhauer V, Schlondorff D. Chemokines and chemokine receptors are involved in the resolution or progression of renal disease. Kidney Int. 2003;63:401–415. doi: 10.1046/j.1523-1755.2003.00750.x. [DOI] [PubMed] [Google Scholar]
- Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int. 2003;63:209–216. doi: 10.1046/j.1523-1755.2003.00736.x. [DOI] [PubMed] [Google Scholar]
- Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky AM, Ahn YT. Mechanisms of disease: regulation of RANTES (CCL5) in renal disease. Nat Clin Pract Nephrol. 2007;3:164–170. doi: 10.1038/ncpneph0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlondorff D. Obstructive nephropathy in the mouse: progressive fibrosis correlates with tubulointerstitial chemokine expression and accumulation of CC chemokine receptor 2- and 5-positive leukocytes. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- Satriano JA, Banas B, Luckow B, Nelson P, Schlondorff DO. Regulation of RANTES and ICAM-1 expression in murine mesangial cells. J Am Soc Nephrol. 1997;8:596–603. doi: 10.1681/ASN.V84596. [DOI] [PubMed] [Google Scholar]
- Gröne HJ, Weber C, Weber KS, Grone EF, Rabelink T, Klier CM, Wells TN, Proudfood AE, Schlondorff D, Nelson PJ. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371–1383. [PubMed] [Google Scholar]
- Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. Am J Physiol. 2004;287:F7–F16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59:2023–2038. doi: 10.1046/j.1523-1755.2001.00717.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Blockage of tubular epithelial to myofibroblast transition by hepatocyte growth factor prevents renal interstitial fibrosis. J Am Soc Nephrol. 2002;13:96–107. doi: 10.1681/ASN.V13196. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Nakamura T. Hepatocyte growth factor suppresses interstitial fibrosis in a mouse model of obstructive nephropathy. Kidney Int. 2001;59:1304–1314. doi: 10.1046/j.1523-1755.2001.0590041304.x. [DOI] [PubMed] [Google Scholar]
- Azuma H, Takahara S, Matsumoto K, Ichimaru N, Wang JD, Moriyama T, Waaga AM, Kitamura M, Otsuki Y, Okuyama A, Katsuoka Y, Chandraker A, Sayegh MH, Nakamura T. Hepatocyte growth factor prevents the development of chronic allograft nephropathy in rats. J Am Soc Nephrol. 2001;12:1280–1292. doi: 10.1681/ASN.V1261280. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Kurosawa T, Mizuno-Horikawa Y, Nakamura T. Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney Int. 2000;57:937–948. doi: 10.1038/sj.ki.4491416. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rajur K, Tolbert E, Dworkin LD. Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int. 2000;58:2028–2043. doi: 10.1111/j.1523-1755.2000.00375.x. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Tolbert EM, Biswas P, Centracchio JN, Dworkin LD. Hepatocyte growth factor ameliorates renal interstitial inflammation in rat remnant kidney by modulating tubular expression of macrophage chemoattractant protein-1 and RANTES. J Am Soc Nephrol. 2004;15:2868–2881. doi: 10.1097/01.ASN.0000141962.44300.3A. [DOI] [PubMed] [Google Scholar]
- Gao X, Mae H, Ayabe N, Takai T, Oshima K, Hattori M, Ueki T, Fujimoto J, Tanizawa T. Hepatocyte growth factor gene therapy retards the progression of chronic obstructive nephropathy. Kidney Int. 2002;62:1238–1248. doi: 10.1111/j.1523-1755.2002.kid579.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. Hepatocyte growth factor gene therapy and angiotensin II blockade synergistically attenuate renal interstitial fibrosis in mice. J Am Soc Nephrol. 2002;13:2464–2477. doi: 10.1097/01.asn.0000031827.16102.c1. [DOI] [PubMed] [Google Scholar]
- Dai C, Yang J, Bastacky S, Xia J, Li Y, Liu Y. Intravenous administration of hepatocyte growth factor gene ameliorates diabetic nephropathy in mice. J Am Soc Nephrol. 2004;15:2637–2647. doi: 10.1097/01.ASN.0000139479.09658.EE. [DOI] [PubMed] [Google Scholar]
- Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice promotes liver and overall body growth. Hepatology. 2001;33:848–859. doi: 10.1053/jhep.2001.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Li Y, Liu Y. Paricalcitol attenuates renal interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol. 2006;17:3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Luo JH, Dedhar S, Liu Y. Tubular epithelial cell dedifferentiation is driven by the helix-loop-helix transcriptional inhibitor Id1. J Am Soc Nephrol. 2007;18:449–460. doi: 10.1681/ASN.2006030236. [DOI] [PubMed] [Google Scholar]
- Tan R, Zhang J, Tan X, Zhang X, Yang J, Liu Y. Downregulation of SnoN expression in obstructive nephropathy is mediated by an enhanced ubiquitin-dependent degradation. J Am Soc Nephrol. 2006;17:2781–2791. doi: 10.1681/ASN.2005101055. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Kim HT, Manning WC, Goralski TJ, Krensky AM. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. 2002;13:411–422. doi: 10.1681/ASN.V132411. [DOI] [PubMed] [Google Scholar]
- Schadde E, Kretzler M, Banas B, Luckow B, Assmann K, Schlondorff D. Expression of chemokines and their receptors in nephrotoxic serum nephritis. Nephrol Dial Transplant. 2000;15:1046–1053. doi: 10.1093/ndt/15.7.1046. [DOI] [PubMed] [Google Scholar]
- Vielhauer V, Eis V, Schlondorff D, Anders HJ. Identifying chemokines as therapeutic targets in renal disease: lessons from antagonist studies and knockout mice. Kidney Blood Press Res. 2004;27:226–238. doi: 10.1159/000079867. [DOI] [PubMed] [Google Scholar]
- Herrero-Fresneda I, Torras J, Franquesa M, Vidal A, Cruzado JM, Lloberas N, Fillat C, Grinyo JM. HGF gene therapy attenuates renal allograft scarring by preventing the profibrotic inflammatory-induced mechanisms. Kidney Int. 2006;70:265–274. doi: 10.1038/sj.ki.5001510. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Dworkin LD. Activation of PI3K-Akt-GSK3beta pathway mediates hepatocyte growth factor inhibition of RANTES expression in renal tubular epithelial cells. Biochem Biophys Res Commun. 2005;330:27–33. doi: 10.1016/j.bbrc.2005.02.122. [DOI] [PubMed] [Google Scholar]
- Guijarro C, Egido J. Transcription factor-kappa B (NF-kappa B) and renal disease. Kidney Int. 2001;59:415–424. doi: 10.1046/j.1523-1755.2001.059002415.x. [DOI] [PubMed] [Google Scholar]
- Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, Forbes JM. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–2151. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- Takase O, Hirahashi J, Takayanagi A, Chikaraishi A, Marumo T, Ozawa Y, Hayashi M, Shimizu N, Saruta T. Gene transfer of truncated IkappaBalpha prevents tubulointerstitial injury. Kidney Int. 2003;63:501–513. doi: 10.1046/j.1523-1755.2003.00781.x. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Kosaka T, Seta K, Asano T, Umezawa K, Hayakawa M. Novel nuclear factor kappa B activation inhibitor prevents inflammatory injury in unilateral ureteral obstruction. J Urol. 2003;169:1559–1563. doi: 10.1097/01.ju.0000045686.21766.c1. [DOI] [PubMed] [Google Scholar]
- Rangan GK, Wang Y, Tay YC, Harris DC. Inhibition of nuclear factor-kappaB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int. 1999;56:118–134. doi: 10.1046/j.1523-1755.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Gong R, Rifai A, Dworkin LD. Anti-inflammatory effect of hepatocyte growth factor in chronic kidney disease: targeting the inflamed vascular endothelium. J Am Soc Nephrol. 2006;17:2464–2473. doi: 10.1681/ASN.2006020185. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Béraud C, Henzel WJ, Baeuerle PA. Involvement of regulatory and catalytic subunits of phosphoinositide 3-kinase in NF-kappaB activation. Proc Natl Acad Sci USA. 1999;96:429–434. doi: 10.1073/pnas.96.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J Am Soc Nephrol. 2005;16:68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Hepatocyte growth factor promotes renal epithelial cell survival by dual mechanisms. Am J Physiol. 1999;277:F624–F633. doi: 10.1152/ajprenal.1999.277.4.F624. [DOI] [PubMed] [Google Scholar]