Abstract
The gastrointestinal tract (GIT) is a major target of infection with human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV). Chronic GIT disease and inflammation are common sequelae to HIV/SIV infection. Nonetheless, the molecular mechanisms that cause and maintain GIT dysfunction remain unclear. We investigated the contribution of CCAAT/enhancer-binding protein β (C/EBPβ) to GIT disease and viral replication in jejunum and colon collected at necropsy from 12 SIV-infected (group 1), or 10 uninfected macaques with chronic diarrhea (group 2), and 9 uninfected control macaques (group 3). All group 1 and 2 macaques had chronic diarrhea, wasting, and colitis, but group 1 animals had more severe lesions in the jejunum. C/EBPβ gene expression increased significantly in colon of groups 1 and 2 and in jejunum of only group 1 macaques compared with controls. In group 1 animals, CEBPβ expression was localized predominantly to macrophages and occasionally lymphocytes. Chromatin immunoprecipitation assays confirmed the binding of C/EBPβ and p65 to the SIV long terminal repeat region in colonic lamina propria cells, suggesting a mechanistic link between inflammation and activation of viral replication in vivo. This is the first in vivo study describing the transcriptional changes and immunophenotypic localization of C/EBPβ in the GIT of SIV-infected macaques. More importantly, these data provide a molecular mechanism for persistent inflammation and immune activation leading to increased SIV burden and GIT pathology in SIV-infected macaques and perhaps HIV-infected individuals.
Infection with human immunodeficiency virus (HIV), the causative agent of acquired immunodeficiency syndrome (AIDS) in humans, leads to progressive immune deficiency because of massive loss of CD4+ T cells. This observation has been confirmed and extended using the macaque model of AIDS in which rhesus macaques are infected with simian immunodeficiency virus (SIV), a close relative of HIV.1 Based on these studies, which have been confirmed in HIV-infected humans, it is clear that the most significant and rapid depletion of CD4+ T cells occurs in the gastrointestinal tract (GIT) during the first 14 to 21 days after infection.2,3,4 Associated with the loss of GIT CD4+ T cells are a variety of symptoms of GIT dysfunction that affect most HIV-infected patients. Interestingly, even in developed countries, ∼9% of HIV patients on highly active antiretroviral therapy with normal peripheral CD4+ T-cell counts still presented with opportunistic GIT disorders.5
Several opportunistic infections caused by protozoal, viral, bacterial, and fungal agents as well as possible direct effects of HIV infection have been implicated as contributing to the pathogenesis of intestinal dysfunction.6,7,8,9 Although the structural components of the GIT have not been reported to be targets for HIV/SIV, we and others have described signs of intestinal dysfunction during the first few weeks after SIV infection in macaques even before the appearance of opportunistic infection.10,11,12,13 Although the pathogenesis of this enteropathy remains unclear, there is ample evidence that severe intestinal immune dysfunction can lead directly to marked structural and functional abnormalities of the GIT.14,15 More recently, the acute depletion of mucosal CD4+ T cells and the immune dysfunction that is associated with HIV/SIV infection has been linked with disruption of the intestinal barrier and microbial translocation that would cause chronic immune activation facilitating viral replication and the development of AIDS.16 The specifics of the interplay between immune dysfunction caused by destruction of intestinal CD4+ T cells and immune activation (both of which occur in HIV/SIV infection) in mediating GIT dysfunction are poorly understood. Accordingly, these observations underscore the need to investigate the various immunological signaling pathways that mediate this interaction so that we can better understand the underlying pathogenic mechanisms to elucidate therapeutic strategies that can complement existing highly active antiretroviral therapy regimens.
Although the etiology of GIT dysfunction in HIV-infected patients remains complex, there is ample evidence to suggest that HIV/SIV infection results in the modulation of circulating levels of several host-derived factors. Among these, proinflammatory cytokines released by infected lymphoid cells and other inflammatory cells may be expected to play a central role because they can induce inflammation17 and at the same time influence viral replication.18,19 Increased production of interleukin (IL)-6, RANTES, IL-10, tumor necrosis factor-α, IL-8, and interferon-γ, at least at the mRNA level, in the intestinal mucosa of HIV-infected patients and SIV-infected rhesus macaques may play a role in the development of GIT dysfunction.20,21 We have previously reported up-regulation of IL-6 in the GIT of SIV-infected rhesus macaques with chronic diarrhea.22 IL-6 is a cytokine that has been reported to facilitate the transition from acute to chronic inflammation.23 Increases in IL-6 mRNA were also accompanied by constitutive activation of p-STAT3 and increases in SOCS-3 mRNA.22 Expression of p-STAT3 was localized to CD68-expressing macrophages and scattered CD3-expressing lymphocytes in the GIT of SIV-infected rhesus macaques with chronic diarrhea.22
Besides activating STAT3, IL-6 also induces the expression of CCAAT/enhancer binding protein β (C/EBPβ) either through a tethering mechanism involving STAT3 and CUG binding protein24 or through the Ras-Raf-MAPK signaling pathway.25 The C/EBP family of transcription factors include transcription activators C/EBPα, C/EBPβ, and C/EBP-related protein 1, and negative regulators such as C/EBPγ, liver-enriched transcriptional inhibitory protein (LIP), and C/EBP-homologous protein 10 (CHOP-10).26,27 C/EBPβ is an intronless gene and the presence of an internal ribosome entry site facilitates the production of several variants with activation or inhibitory functions.28 C/EBPβ also described as nuclear factor (NF)-IL6, NF-M, liver transcriptionally enriched activator protein (LAP), IL-6-D-element binding protein/EBP, and C/EBP-related protein 2, has three reported variants in the human: 46-kDa full-length, 41-kDa LAP, and 16-kDa LIP.29 LAP, similar to the full-length C/EBPβ activates transcription and shows reduced expression levels in normal tissues.30,31 However, inflammatory stimuli such as bacterial lipopolysaccharide (LPS), IL-6, IL-1β, or interferon-γ results in significant up-regulation of C/EBPβ.31 In addition, activation of C/EBPβ is also contingent on phosphorylation of serine or threonine residues catalyzed by various intracellular kinases.29 LIP lacks most of the transactivation domain in the N-terminal region and functions as a dominant-negative inhibitor of C/EBP-mediated transcription of target genes.28 Functionally active C/EBPβ proteins have been reported to regulate transcription by binding to their cognate DNA binding elements either in association with other C/EBP isoforms or other transcription factors such as p6532 and p50 subunit of NF-κB, CREB/ATF, AP-1, glucocorticoid receptor, hepatitis B virus X protein, and the retinoblastoma protein.31 Furthermore, the identification of C/EBPβ functional responsive elements in the promoter region of IL-1β,33 tumor necrosis factor-α,34 IL-6,35 IL-8,36 cyclooxygenase 2 (COX-2),29 as well as chemokine receptors such as CCR237 and CCR538 throws more light on the proinflammatory properties of this transcription factor.
Additionally, the inclusion of three and up to five C/EBPβ binding sites on the long terminal repeat (LTR) of HIV and SIV,39 respectively, provides an opportunity for HIV and SIV to subvert normal immune regulation to its own advantage. Further, C/EBPβ is essential for efficient HIV replication in macrophages but not in CD4+ T cells40,41,42 and has been reported to play an important role in regulating HIV/SIV replication in brain macrophages43,44 and alveolar macrophages.45,46,47 Although the GIT is well established as a major target for HIV/SIV replication, the role of C/EBPβ as a mediator of inflammation and viral replication in this tissue has not been examined. In our earlier study, we observed that all SIV-infected macaques with high IL-6 and constitutive STAT3 expression had high mucosal viral loads.22 Although constitutive STAT3 expression in the GIT was associated with inflammation,22 it does not have a binding site on the HIV/SIV LTR. Therefore, in the present study, using the SIV-rhesus macaque model of AIDS we attempted to identify mechanisms by which IL-6 promotes not only GIT inflammation/disease but also viral replication with a special focus on the role of C/EBPβ. The results of our study point to a significant increase in C/EBPβ mRNA and protein expression as a key event. C/EBPβ gene and protein expression was significantly increased in SIV-infected rhesus macaques with chronic diarrhea and was found to efficiently bind to the SIV LTR in infected cells isolated from the colonic lamina propria. We conclude that chronic uncontrolled intestinal inflammation, characterized by increased expression of C/EBPβ may lead to persistent localized immune activation, disruption of the intestinal architecture and function, and facilitate viral replication that ultimately leads to chronic diarrhea, wasting, and death.
Materials and Methods
Animals and Tissue Collection
Tissues were collected from a total of 31 animals including 12 animals infected with pathogenic strains of SIV (group 1) that use CCR5 in vivo and 19 animals not infected with SIV. Of the uninfected animals, 10 had chronic diarrhea (group 2) and 9 did not (group 3). The animals in group 2 with chronic nonresponsive diarrhea of no known infectious etiology have been described and used as a model of inflammatory bowel disease.48,49
It would be ideal to have a fourth group consisting of SIV-infected animals without diarrhea. Unfortunately, untreated SIV infection consistently leads to GIT dysfunction and diarrhea and hence it is not possible to include such a group. Jejunum and colon specimens were collected at necropsy from the 12 SIV-infected macaques with chronic diarrhea (group 1), 10 non-SIV-infected macaques with chronic diarrhea (group 2), and four uninfected control macaques (group 3) (Table 1). Chronic diarrhea and weight loss were major causes for euthanasia in all animals except those in group 3. In addition, pinch biopsies from jejunum and colon were collected from another five control macaques bringing the total number of control macaques (group 3) used in this study to nine. Colon specimens were collected for all 31 macaques. Jejunum specimens were available for 9 of 12 group 1, 6 of 10 group 2, and 9 of 9 group 3 control macaques. All animals in groups 1 and 2 were euthanized when they became unresponsive to treatment (eg, subcutaneous or intravenous fluids and antibiotics as appropriate based on a culture and sensitivity) or lost greater than 20% of their body weight. After euthanasia with an intravenous overdose of pentobarbital all animals received a complete necropsy and histopathological examination. All tissues were collected in RNAlater (Ambion, Austin, TX) for RNA quantification and confocal microscopy. According to the manufacturer, RNAlater protects both RNA and protein (by reversible inhibition of nucleases and proteases) in addition to preserving tissue architecture. Tissues were also collected in cryovials and snap-frozen by immersion in a 2-methylbutane/dry-ice mixture for protein extraction.
Table 1.
Animals, Inoculum, Viral Load, CD4+ T-Cell Count in Group 1 Macaques
| Animal group and no. | Duration of infection (days) | Inoculum | CD4 count cells/μl* | Plasma viral copies/ml × 106 | Viral copies/mg of total RNA × 106, colon | Viral copies/mg of total RNA × 106, jejunum |
|---|---|---|---|---|---|---|
| SIV-infected with diarrhea (group 1) | ||||||
| AJ82 | 232 | SIVmac251 | NA | NA | 1.95 | 0.078 |
| DD88 | 388 | SIVmac239 | 50 | 1.2 | 26.89 | 3.35 |
| CI65 | 377 | SIVmac239 | 450 | 1.5 | 4.59 | 2.5 |
| L441 | 170 | SIVmac251 and 239 | 632 | 1.26 | 15.5 | 33.2 |
| H405 | 232 | SIVmac239 | 523 | 71.4 | 17,200 | 15,480 |
| V205 | 973 | SIVmac239 | 296 | 0.3 | 8.0 | 3.45 |
| AT81 | 171 | SIVsmG932 | 640 | 0.06 | 0.5 | 0.057 |
| T56 | 1460 | SIVmac251 and 239 | 56 | 360 | 213,000 | 16,380 |
| DT56 | 265 | SIVmac239 | 931 | 1.2 | 5.9 | NA |
| DI28 | 81 | SIVmac251 | 954 | 0.018 | 41 | 1.27 |
| DE68 | 70 | SIVmac239 | 275 | 1 | 59 | NA |
| EB17 | 111 | SIVmac251 | 935 | 3.6 | 2.7 | NA |
The lower end of the normal range for CD4+ T cells/μl of blood in the rhesus macaques is 800 cells/μl of blood.
NA, not applicable.
Histopathology
GIT tissues were collected immediately after euthanasia and fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin (H&E) for analysis. Sections of jejunum and colon were examined in a blinded manner and inflammation was scored semiquantitatively on a scale of 0 to 3 as follows: 0, within normal limits; 1, mild; 2, moderate; 3, severe. In addition, the presence of crypt dilatation, villous blunting, diverticulosis, and amyloidosis were recorded (Table 2).
Table 2.
Intestinal Histopathology in Groups 1, 2, and 3 Macaques
| Animal group and no. | Intestinal histopathology*
|
|
|---|---|---|
| Colon | Jejunum | |
| SIV-infected with diarrhea (group 1) | ||
| AJ82 | 2 | 3 |
| DD88 | 1 and AMD | 1 and AMD |
| CI65 | 1 and AMD | 1 and AMD |
| L441 | 1 | 1 |
| H405 | 3 and CD | 2 |
| V205 | 3 | 1 and AMD, VB |
| AT81 | 3 | 3 and VB |
| AT56 | 3 and AMD, CD | 3 |
| DT56 | 3 and CD | 0 |
| DI28 | 1 | 1 |
| DE68† | 3 and CD | NA |
| EB17† | 1 | NA |
| Non-SIV-infected with diarrhea (group 2) | ||
| EI90 | 3 and CD | NA |
| EL45 | 3 and CD | NA |
| EC49 | 3 and CD | NA |
| EB12 | 3 and CD | NA |
| EM41 | 1 | 1 |
| EL71 | 3 and CD, DV | 0 |
| EB27 | 3 and CD, DV | 1 |
| DJ15 | 3 and CD | 1 |
| CT77 | 2 | 1 |
| EJ54 | 3 | 1 |
| Uninfected controls (group 3) | ||
| BV52 | 0 | 0 |
| EH70 | 0 | 0 |
| CB98 | 0 | 0 |
| CF33 | 0 | 0 |
| M302 | 0 | 0 |
| CC96 | 0 | 0 |
| R842 | 0 | 0 |
| EL66 | 0 | 0 |
| EH80 | 0 | 0 |
Sections of jejunum and colon were examined in a blinded fashion and inflammation was scored semiquantitatively on a scale of 0 to 3 as follows: 0, within normal limits; 1, mild; 2, moderate; 3 severe. In addition the presence of crypt dilatation (CD), villous blunting (VB), diverticulosis (DV), and amyloidosis (AMD) were recorded.
Macaques DE68 and EB17 were only used for the ChIP assay and their histopathology severity scores were not included for the statistical analysis.
NA, not applicable.
Quantitative Real-Time SYBR Green One-Step Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Gene expression for C/EBPβ in the jejunum and colon tissues was evaluated by the Quantitative Real-Time SYBR Green One-Step RT-PCR assay (QRT-PCR) (Qiagen Inc., Valencia, CA). Total RNA was extracted from both jejunum and colon samples using the SV Total RNA isolation kit (Promega Corp., Madison, WI) and a RNA sample representing the colon and jejunum from each macaque was assayed in triplicate wells. Each QRT-PCR reaction (25 μl) contained the following: 2× Master Mix without uracil-N-glycosylase (12.5 μl), reverse transcriptase (0.25 μl), target forward and reverse primer, and total RNA (200 ng) quantified spectrophotometrically based on A260:A280 ratios. Forward and reverse primer sequence for C/EBPβ including β-actin were as follows. An ∼111-bp region of C/EBPβ was PCR amplified using 500 nmol/L of forward (5′-AACCTGGAGACGCAGCACAA-3′) and reverse primer (5′-CTTGAACAAGTTCCGCAGGGTG-3′) on the ABI Prism 7700 sequence detection system (PE Applied Biosystems, Foster City, CA) using the following thermal cycling conditions: 50°C for 30 minutes, 95°C for 15 minutes followed by 40 repetitive cycles of 95°C for 15 seconds, 60°C for 30 seconds, 72°C for 30 seconds. As a normalization control for RNA loading, parallel reactions to amplify an ∼107-bp product representing β-actin mRNA were run in the same multiwell plate using 300 nmol/L forward (5′-AGGCTCTCTTCCAACCTTCCTT-3′) and reverse (5′-CGTACAGGTCTTTACGGATGTCCA-3′) primer.
Quantification of gene amplification after RT-PCR was made by determining the threshold cycle (CT) number for SYBR Green fluorescence within the geometric region of the semilog plot generated during PCR. Within this region of the amplification curve, each difference of one cycle is equivalent to a doubling of the amplified product of the PCR. The relative quantification of target gene expression across treatments was evaluated using the comparative CT method. The ΔCT value was determined by subtracting the β-actin CT value for each sample from the target CT value of that sample. Calculation of ΔΔCT involved using the highest sample ΔCT value (ie, sample with the lowest target expression) as an arbitrary constant to subtract from all other ΔCT sample values. Fold changes in the relative gene expression of target was determined by evaluating the expression, 2−ΔΔ CT.
Immunoprecipitation and Western Blotting
Small 2.5-cm2 pieces of colon from two group 1, and three group 2 and 3 animals were crushed using a disposable polypropylene pellet pestle and protein extraction was performed in a lysis buffer (Cell Signaling Technology, Inc., Beverly, MA) containing 20 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1 mmol/L Na2EDTA, 1 mmol/L EGTA, 1% Triton, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L β-glycerophosphate, 1 mmol/L Na3VO4, 1 μg/ml leupeptin, protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). Approximately 500 μg of total protein extract was first precleared with 5 μl of normal mouse immunoglobulin for ∼20 minutes and then immunoprecipitated with ∼10 μl of a mouse monoclonal antibody against C/EBPβ (Santa Cruz Biotechnology, Santa Cruz, CA), overnight at 4°C followed by incubation with 30 μl (50% w/v) of protein G agarose beads (Invitrogen Corp., Carlsbad, CA) at 4°C for 4 to 5 hours. The supernatant was removed and transferred to a separate 1.5-ml microcentrifuge tube and immunoprecipitated using goat polyclonal antibody (∼4 μl) (Santa Cruz Biotechnology) for β-actin at 4°C overnight on a shaker. Immunoprecipitated C/EBPβ and β-actin proteins were heat-denatured for 5 minutes at 100°C in sample loading buffer containing 62.5 mmol/L Tris-HCl, 5% 2-mercaptoethanol, 10% glycerol, 2% sodium dodecyl sulfate (SDS), and bromophenol blue, resolved on 10% SDS-polyacrylamide gel electrophoresis gels and transferred to 0.2-μm nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were probed with a rabbit polyclonal primary antibody against C/EBPβ (LAP) (Cell Signaling Technology, Inc.), and rabbit polyclonal antibody against β-actin (Santa Cruz Biotechnology) followed by the appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Membranes were treated with Supersignal West-Femto or Pico chemiluminescent substrate (Pierce Biotechnology Inc., Rockford, IL) for 5 minutes and the signal was developed by exposing the membrane to X-OMAT Kodak film (Eastman-Kodak, Rochester, NY) for 1 to 5 minutes. Immunoprecipitated C/EBPβ proteins from groups 1 and 2 animals were run on one gel and the control animals on another gel. Hence the images shown in Figure 3A (see below) were obtained from two separate radiographs, cropped, aligned, and fused into one composite image.
Figure 3.
A: Levels of C/EBPβ (LAP) protein are increased in the inflamed colonic mucosa of SIV-infected and noninfected rhesus macaques with chronic diarrhea. Representative expression of full-length (46 kDa) and the truncated (41 kDa) LAP isoforms of C/EBPβ along with β-actin protein in colon samples taken from two SIV-infected macaque (G1), three non-SIV-infected macaques with chronic diarrhea (G2), and three control animals (G3) are shown. B: ChIP assay of transcription factors present at the SIV LTR in SIV-infected LPCs isolated from the GIT of SIV-infected rhesus macaques. ChIP assays were performed using lysates prepared from LPCs. The protein-DNA complexes were recovered using antibodies specific for each transcription factor (p65 and C/EBPβ) and the binding was confirmed by PCR using SIV LTR-specific primers, as described in the Materials and Methods. All ChIP reactions were processed from the same chromatin isolated from the LPCs of each individual macaque.
Lamina Propria Cell (LPC) Isolation and Chromatin Immunoprecipitation (ChIP) Assay
Given that the objective of using the ChIP assay is to demonstrate in vivo binding of C/EBPβ to the SIV LTR in infected cells during inflammation, large number of cells carrying the SIV genome are required. However, isolating a pure population of SIV-infected cells from the GIT is also not technically feasible. Therefore we performed the assay using a heterogeneous population of LPCs (a mixture of T cells, plasma cells, stromal cells, macrophages, dendritic cells, and possibly neutrophils) assuming that interspersed in this population are SIV-infected cells. LPCs were isolated as previously described.2,50 Briefly, 1-cm intestinal segments were incubated with 1 mmol/L EDTA in Hanks’ balanced salt solution for 30 minutes to separate the epithelial cells from the lamina propria. This was followed by a 1-hour incubation in RPMI 1640 containing 20 U/ml of collagenase while rapidly shaking at 37°C to isolate the LPCs from the stroma. LPCs were separated from red blood cells by centrifugation using lymphocyte separation medium (LSM; MP Biomedicals, Aurora, OH).
LPCs (∼50 × 106) were suspended and cultured in 30 ml of RPMI 1640 supplemented with 25 mmol/L HEPES, 2 mmol/L glutamine, 5% fetal calf serum, and 100 U penicillin/streptomycin with or without recombinant human IL-6 (20 ng/ml) for 6 hours at 37°C in the presence of 5% CO2. Isolation of LPCs from the GIT takes a few hours and during this period transcription factors such as C/EBPβ may dissociate from their cognate binding sites on the DNA. To overcome this, we also treated the cells with rhIL-6 (20 ng/ml) so that the C/EBPβ proteins would be activated and translocate to the nucleus and remain bound to the DNA. After incubation, nuclear proteins were crosslinked to genomic DNA with 1% formaldehyde for 10 minutes at room temperature. Cross linking was stopped by adding glycine to a final concentration of 0.125 mol/L and incubating for 5 minutes at room temperature. The contents of the flask were transferred to a 50-ml tube and centrifuged to pellet the cells. The cells were washed twice with ice-cold RPMI 1640 after which they were resuspended in 750 μl of lysis buffer [1% SDS, 10 mmol/L EDTA, 50 mmol/L Tris-HCl, pH 8.0, protease inhibitor cocktail (Sigma Chemical Co.)]. The lysates were sonicated four to five times, at setting 4, for 10 seconds duration each with a 10-second break on ice between pulses using a Branson Sonifier 450 (Branson Ultrasonics, Danbury, CT) to produce DNA fragments of 300 to 1000 bp in length. Approximately 120 μl of the sonicated lysate was diluted 1:10 in ChIP dilution buffer (16.7 mmol/L Tris-HCl, pH 8.0, 1.1% Triton X-100, 1.2 mmol/L EDTA, 16.7 mmol/L NaCl, protease inhibitor cocktail) in three different 2.0-ml Dolphin microcentrifuge tubes (Light Laboratory Systems, Dallas, TX) and immunoprecipitations were performed overnight at 4°C with 4 μg of polyclonal antibodies specific to C/EBPβ (sc-150), p65 (sc-109) (Santa Cruz Biotechnology), and control rabbit immunoglobulin (Biomeda Corp., Foster City, CA). A fourth tube was used as the input control. The immune complexes were captured by incubation with MagnaBind Protein G beads (Pierce Biotechnology) for ∼3 hours at 4°C and separated from the supernatant by magnetic separation. The beads were then washed sequentially for 5 minutes with 1 ml of the following buffers: low-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mmol/L EDTA, 150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 8.1), high-salt wash buffer (0.1% SDS, 1% Triton X-100, 2 mmol/L EDTA, 500 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 8), and LiCl wash buffer (0.25 mmol/L LiCl, 1% Nonidet P-40, 1% sodium deoxycholate, 1 mmol/L EDTA, 10 mmol/L Tris-HCl, pH 8.0). Finally, the beads were washed twice with 1 ml of TE buffer (1 mmol/L EDTA, 10 mmol/L Tris-HCl, pH 8.0). The immune-complexes were then eluted by adding 100 μl of elution buffer (1% SDS, 100 mmol/L NaHCO3) and incubating for 15 minutes at room temperature. After sedimenting the magnetic beads, the supernatant was collected and the elution was repeated. The supernatants were combined and the cross-linking was reversed by adding NaCl to a final concentration of 200 mmol/L and incubating overnight at 65°C. The remaining proteins were digested by adding proteinase K (final concentration, 40 μg/ml) and incubation for 1 hour at 45°C. Genomic DNA fragments were recovered (MO BIO Laboratories, Carlsbad, CA), resuspended in Tris-HCl, pH 8.0 buffer (50 μl), and analyzed by PCR using the primers specific to the SIV LTR (forward: 5′-GTCAGGCCTGTCAGAGGAAG-3′ and reverse: 5′-AGAGGGCTTTAAGCAAGCAA-3′), for C/EBPβ and p65, in a 25-μl reaction containing either 5 μl (DE68) or 20 μl (EB17) of eluted DNA sample, 300 nmol/L dNTP, 300 mmol/L each primer, 1.25 U of Pfx Accuprime TaqDNA polymerase (Invitrogen Corp.). The reaction contents were heated to 95°C for 2 minutes for polymerase activation followed by 35 (DE68) and 40 (EB17) cycles at 94°C for 15 seconds, 56°C for 30 seconds. The amplified products were resolved on a 2.5% agarose gel. The amplified PCR product (∼346 bp) was gel purified and its identity was further confirmed by nested PCR using a second set of primers (forward: 5′-AGATGAGACAGCAGGGACTTTCCA-3′ and reverse: 5′-GCAGAGCGACTGACTACATAGCAA-3′) to generate a 127-bp product.
Confocal Microscopy
To determine the cell types in the GIT expressing C/EBPβ we performed double-label confocal microscopy. Tissues collected in RNAlater were first fixed in 2% paraformaldehyde for 4 hours, cryopreserved overnight in 30% sucrose solution, and embedded in Tissue Tek OCT (Sakura Finetechnical Company, Tokyo, Japan). Embedded tissues were sectioned at 10 μm and stained with the appropriate primary and secondary antibody and also H&E to microscopically assess the histological status of the tissue. Briefly, slides were blocked with 100 μl of blocking buffer (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 3% bovine serum albumin, 10% normal goat serum, and 0.1% Triton X-100) for 1 hour followed by a 1-hour incubation at 4°C with rabbit polyclonal C/EBPβ primary antibody (1:25 dilution, Santa Cruz Biotechnology). The slides were washed three times in buffer (10 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.1% Triton X-100) followed by addition of goat anti-rabbit secondary antibody conjugated to Alexa Fluor 568 (1:1000, Invitrogen Corp.). This was followed by mouse anti-CD68 (IgG1 macrophage clone KP1 at 1:20), or mouse anti-CD3 (IgG1 clone F 7.2.38 at 1:10) (DAKO, Carpinteria, CA) at room temperature for 1 hour. The slides were washed three times and incubated for 1 hour with goat anti-mouse secondary antibody conjugated with Alexa Fluorophor 488 (1:1000). Confocal microscopy was performed using a Leica TCS SP2 confocal microscope equipped with three lasers (Leica Microsystems, Exton, PA). Individual optical slices represent 0.2 μm and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. NIH Image (version 1.62) and Photoshop (version 7.0; Adobe Systems, San Jose, CA) were used to assign colors to the three channels collected: Alexa 568 (Invitrogen Corp.) is red, Alexa 488 (Invitrogen Corp.) is green, and the differential interference contrast image is in gray scale. The three channels were collected simultaneously. Co-localization of antigens is demonstrated by the addition of colors as indicated in the figure legends.
Quantitation of Mucosal Viral Loads
Total RNA samples from all group 1 animals were subjected to a quantitative real-time TaqMan two-step RT-PCR analyses to determine the viral load in SIV-infected colon and jejunum samples. Briefly, primers and probes specific to the SIV LTR sequence were designed and used in the real-time TaqMan PCR assay. Probes were conjugated with a fluorescent reporter dye (FAM) at the 5′ end and a quencher dye at the 3′ end. Fluorescence signal was detected with an ABI Prism 7700 sequence detector (PE Applied Biosystems). Data were captured and analyzed with Sequence Detector Software (PE Applied Biosystems). Viral copy number was determined by plotting CT values obtained from the colon and jejunum samples against a standard curve (y = −3.351x + 40.377) (r2 = 0.998) generated with in vitro-transcribed RNA representing known viral copy numbers. Plasma viral loads for 11 of 12 group 1 macaques were performed using the b-DNA assay for SIV viral RNA (Bayer Diagnostics, Tarrytown, NY).
Statistical Analysis
Gene expression for C/EBPβ among groups were compared using the Kruskal-Wallis one-way analysis of variance by ranks for nonparametric independent group comparisons. When the results of Kruskal-Wallis one-way analysis of variance by ranks indicated a significant difference at the P < 0.001 level between the groups examined, Dunn’s multiple comparison procedure was then used to identify the groups that differed at the P < 0.05 significance level. Histopathology severity scores for jejunum and colon in group 1, 2, and 3 animals were analyzed using Kruskal-Wallis one-way analysis of variance by ranks for nonparametric independent group comparisons. Within each group histopathology severity scores between colon and jejunum were analyzed using the Mann-Whitney test. The presence of other histopathological lesions such as villous blunting, crypt dilatation, and amyloidosis in the jejunum and colon of group 1 and 2 animals were analyzed using the two-tailed Fisher exact test.
Results
Intestinal Histopathology
Histological evaluation of H&E-stained sections of jejunum and colon (Table 2) revealed the presence of enterocolitis in most animals in groups 1 and 2 but not in group 3. When examining jejunum and colon separately, there did not appear to be much difference between groups 1 and 2 with regard to the presence of colitis (12 of 12 group 1 animals and 10 of 10 group 2 animals). However, the severity of the inflammation based on semiquantitative histopathological grading criteria and blinded analysis by a board certified pathologist (A.A.L.) suggested that differences do exist (Table 3 and see Supplemental Figure S1 at http://ajp.amjpathol.org). Specifically, the inflammation in the GIT of group 1 animals was fairly uniform in the jejunum and colon whereas in group 2 animals the colon was much more severely affected (P = 0.004) (Tables 2 and 3). These differences, based primarily on the extent and severity of the inflammatory infiltrates were mirrored by the incidence of other intestinal lesions (Table 3) such as villous blunting, crypt dilatation, and abscess and amyloidosis in the jejunum of group 1 and not in group 2 animals. However, the presence of other intestinal lesions in the jejunum of group 1 macaques lacked statistical significance. Opportunistic pathogens such as cytomegalovirus and Mycobacterium avium intracellulare complex were detected in only 3 of 10 group 1 macaques (see Supplemental Table S1 at http://ajp.amjpathol.org). Campylobacter coli, Campylobacter jejuni, or Shigella flexneri were positively isolated from the colon at necropsy in 2 of 10 group 1 and 5 of 10 group 2 macaques (see Supplemental Table S1 at http://ajp.amjpathol.org).
Table 3.
Comparison and Statistical Significance of Histopathological Lesions
| Tissue | Animal and score details | Group 1 | Group 2 | Group 3 | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| G1 versus G2 | G1 versus G3 | G2 versus G3 | Colon versus Jej − G1 | Colon versus Jej − G2 | |||||
| Colon | No. of animals with colitis/total number of animals examined | 10/10 | 10/10 | 0/7 | NSD | <0.005 | <0.0005 | NSD | =0.004 |
| Total scores | 21 | 27 | 0 | ||||||
| Average scores | 2.1 | 2.7 | 0 | ||||||
| No. of animals with other lesions/total number of animals examined* | 5/10 | 4/10 | NA | NSD | NA | NA | NSD | NSD | |
| Jejunum | No of animals with enteritis/total number of animals examined | 9/9 | 5/6 | 0/7 | NSD | <0.0008 | <0.005 | ||
| Total scores | 16 | 5 | 0 | ||||||
| Average scores | 1.78 | 0.83 | 0 | ||||||
| No. of animals with other lesions/total number of animals examined* | 4/10 | 0/6 | NA | NSD | NA | NA | |||
Lesions such as villous blunting, crypt dilation/abscesses, amyloidosis, diverticulosis, and so forth.
NSD, not significantly different; NA, not applicable.
Plasma and Mucosal Viral Loads and CD4+ T-Cell Counts
The viral loads in the plasma, jejunum, and colon of all of the SIV-infected macaques (group 1) are shown in Table 1 and were obtained at necropsy. All SIV-infected macaques (group 1) had substantial mucosal and plasma viral loads. Viral loads in the jejunum ranged from 0.057 × 106 to 16,380 × 106 copies/mg total RNA with a median of 3.35 × 106 copies/mg total RNA. The colon generally had higher viral loads than the jejunum for the same animal with a range from 0.5 × 106 to 213,000 × 106 copies/mg total RNA with a median of 11.75 × 106 copies/mg total RNA. Peripheral CD4+ T-cell counts obtained at necropsy were available for 11 of 12 group 1 macaques (Table 1). Of the 12 group 1 macaques, at least 8 had low CD4+ T-cell counts.
Mucosal Gene Expression for C/EBPβ Is Significantly Increased in the GIT of SIV-Infected and Noninfected Macaques with GIT Inflammation
To verify that the occurrence of diarrhea in SIV-infected macaques was associated with a marked expression of C/EBPβ, QRT-PCR was performed using total RNA extracted from jejunal and colonic mucosal samples from all macaques. Individual fold changes in gene expression calculated as described in the Materials and Methods for C/EBPβ in all three groups are shown in Table 4. Figure 1, A and B, shows averaged group wise fold differences for C/EBPβ in the colon and jejunum, respectively. In the colon, C/EBPβ gene expression in group 1 animals was significantly increased compared with group 2 and normal controls (P < 0.05) (Figure 1A). Further, C/EBPβ gene expression in group 2 animals was also significantly increased compared with normal controls (P < 0.05) (Figure 1A). In the colon of group 2 animals, a statistically significant correlation between histopathology severity scores (Table 3) and C/EBPβ gene expression (r2 = 0.69) (0.005 < P < 0.0005) was observed (Tables 2, 3, and 4).
Table 4.
Individual Fold Differences in C/EBPβ Gene Expression
| Group | Animal no. | C/EBPβ*
|
|
|---|---|---|---|
| Colon | Jejunum | ||
| SIV-infected with diarrhea (group 1) | AJ82 | 3 | 4 |
| DD88 | 8 | 12 | |
| CI65 | 11 | 11 | |
| L441 | 1.12 | 1.1 | |
| H405 | 5 | 3 | |
| V205 | 5 | 5 | |
| AT81 | 26 | 18 | |
| AT56 | 13 | 4 | |
| DT56 | 3 | NA | |
| DI28 | 3 | 3 | |
| Non-SIV infected with diarrhea (group 2) | EI90 | 4 | NA |
| EL45 | 4 | NA | |
| EC49 | 6 | NA | |
| EB12 | 6 | NA | |
| EM41 | 2 | 2 | |
| EL71 | 7 | 1.3 | |
| EB27 | 3 | 5 | |
| DJ15 | 5 | 2 | |
| CT77 | 2 | 5 | |
| EJ54 | 3 | 3 | |
| Control animals (group 3) | BV52 | 1 | 2 |
| CB98 | 2 | 1.3 | |
| CF33 | 2 | 3 | |
| M302 | 5 | 3 | |
| CC96 | 1.4 | 1 | |
| R842 | 3 | 2 | |
Fold changes in the relative gene expression was determined by evaluating the expression, 2ΔΔCT. Colon and jejunum specimens from EH70 (group 3) were not used for QRT-PCR analysis.
NA, not applicable.
Figure 1.
Relative abundance in gene expression of C/EBPβ detected using quantitative real-time SYBR green one-step RT-PCR. A and B represent colon and jejunum, respectively, from SIV-infected animals with diarrhea, non-SIV-infected with diarrhea, and uninfected control macaques. The fold differences in gene expression were calculated as described in the Materials and Methods. The relative fold increases for each group was summed and averaged to obtain a single figure that is shown on top of each bar graph. The error bars represent average fold difference for each group ± SEM. Animal groups that differ statistically (P < 0.05) are indicated using horizontal brackets.
In the jejunum, C/EBPβ gene expression in group 1 macaques differed significantly from group 2 and normal controls (Figure 1B) whereas there was no statistical difference between groups 2 and 3. This can be explained by the fact that the inflammation associated with SIV infection in group 1 animals affects the small and large intestine equally whereas in group 2 animals the colon was more severely affected than the jejunum (Table 2). Further, in the jejunum of group 2 animals, a statistically significant correlation was also observed between the low histopathology severity scores and the low C/EBPβ gene expression values (r2 = 0.56) (0.05 < P < 0.025) (Tables 2, 3, and 4).
Lamina Propria Mononuclear Cells Express High Levels of C/EBPβ in the Colon of SIV-Infected and Non-SIV-Infected Macaques with Diarrhea
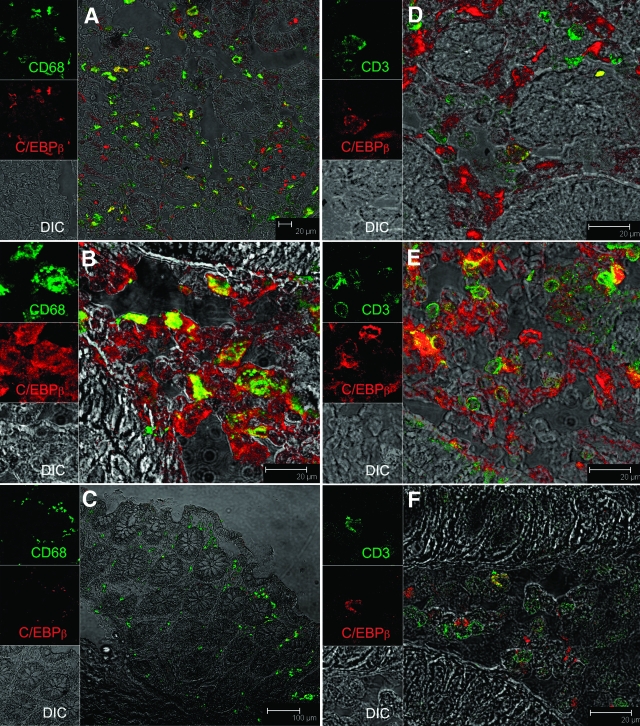
Although the RT-PCR assay demonstrated expression of C/EBPβ mRNA in tissue homogenates from jejunum and colon, it provided no information on the specific cell types expressing C/EBPβ. Accordingly, we performed confocal microscopic analysis on colon tissue from three SIV-infected macaques with diarrhea (H405, AT56, DI28), two non-SIV-infected macaques with diarrhea (DJ15, EL45), and two uninfected control macaques (EH70, BV52). The photomicrographs shown in Figure 2 are representative of the three groups. In SIV-infected (Figure 2A) and noninfected macaques (Figure 2B), numerous CD68+ macrophages expressed C/EBPβ. A small number of CD3+ T cells in the lamina propria were also found to express C/EBPβ (Figure 2D) in the SIV-infected macaque. However, in the non-SIV-infected macaque with diarrhea, CD3+ cells expressing C/EBPβ were much more frequent (Figure 2E). C/EBPβ was mainly localized to the cytoplasm of macrophages and lymphocytes with few cells exhibiting nuclear localization. In contrast to animals with chronic diarrhea, in normal controls there were very few cells expressing C/EBPβ (Figure 2, C and F). Further, unlike group 1 and 2 macaques, in the control animal, C/EBPβ signal was very weak and increased laser strength had to be used to detect any signal.
Figure 2.
Immunophenotype of C/EBPβ-positive cells in the colon of a SIV-infected macaque with diarrhea (H405, A and D), non-SIV-infected macaque with diarrhea (DJ15, B and E), and an uninfected control macaque (EH70, C and F). All panels involve double labels with C/EBPβ (red) and either CD68 for macrophages (A–C) or CD3 for T cells (D–F), which appear green. For each panel the individual channels [green for CD3 or CD68, red for C/EBPβ, and gray for differential interference contrast to reveal tissue architecture] appear on the left with a larger merged image on the right. Co-localization of labels appear yellow. Note that in SIV-infected animals with diarrhea (A and D) most of the cells positive for C/EBPβ are CD68+ macrophages whereas in the non-SIV-infected animal with diarrhea (B and E) both CD68+ macrophages and CD3+ T cells are C/EBPβ+. Interestingly, in the normal control animal (C and F) few C/EBPβ+ cells were observed and the staining is very faint.
C/EBPβ Protein Expression Is Increased in the GIT of SIV-Infected Rhesus Macaques and Can Bind to the SIV Long Terminal Repeat
To identify the different isoforms of the C/EBPβ protein expressed in the GIT during inflammation we performed immunoprecipitation/Western blotting in two group 1 and three group 2 and 3 animals. A significant increase in C/EBPβ protein expression was observed in the colon of group 1 and 2 animals. As shown in Figure 3A, two strong bands with molecular weights of ∼46 and ∼41 kDa, respectively, were observed in both group 1 and 2 animals. These bands correspond to the full-length (46 kDa) and truncated transcriptionally active variant of C/EBPβ (41 kDa) referred to as liver-enriched transcription activating protein (LAP). The C/EBPβ variant known as liver transcriptionally enriched inhibitory protein (LIP, ∼16 kDa) was not observed. In contrast, no C/EBPβ protein expression was observed in normal controls (Figure 3A).
To examine if the C/EBPβ protein was biologically active, binding of C/EBPβ and p65 to the SIV LTR in group 1 animals was additionally evaluated by ChIP assay. Sonicated chromatin was precipitated with antibodies against C/EBPβ or p65. Nonimmune rabbit IgG was used as control. The region of the SIV LTR pulled down by immunoprecipitation was identified by PCR using SIV LTR-specific primers described in Materials and Methods. In both SIV-infected macaques (DE68 and EB17), in the absence of any treatment (Unt), antibodies against C/EBPβ precipitated the C/EBPβ-containing LTR sequence (Figure 3B). Even though a very faint band was observed in the lanes representing precipitated protein-DNA complexes using the control IgG and p65, this band was very weak in intensity compared with the intense PCR product obtained after precipitation using the anti-C/EBPβ antibody. However, in the second SIV-infected animal (EB17) the LTR sequence was precipitated only by the anti-C/EBPβ antibody. The p65 and the control rabbit IgG failed to precipitate the SIV LTR sequence in this animal. Further, in the SIV-infected macaque (DE68), after 6 hours of treatment with 20 ng/ml of rhIL-6, antibodies against both p65 and C/EBPβ precipitated the C/EBPβ- and p65-containing LTR sequence, respectively (Figure 3B). Lastly, based on histopathological examination, the colonic lamina propria of macaque DE68 was characterized by severe lymphoplasmacytic and histiocytic colitis whereas macaque EB17 had mild colitis (Table 2).
Discussion
The GIT contains enormous numbers of activated CD4+ T lymphocytes of the memory phenotype that makes it a major target for infection with HIV/SIV resulting in rapid CD4+ T-cell depletion in this tissue.2,3,4,50,51 In addition to rapid and selective loss of mucosal CD4+ T cells, infection with HIV/SIV is characterized by chronic immune activation and GIT inflammation16,52,53,54,55,56,57,58 that may contribute to disruption of the intestinal epithelial barrier enabling translocation of GIT bacteria and their components (eg, LPS).59 Bacterial LPS, a known activator of several proinflammatory cytokines and their signaling transcription factors (eg, NF-κB, C/EBPβ, NFAT, AP-1), was shown to be elevated in the circulation of HIV/SIV-infected humans and macaques during all stages of infection59 and implicated as a major cause for chronic immune activation. The transcription factors activated, directly or indirectly by LPS, in addition to regulating immune responses and inflammation also have binding sites on the LTR of HIV and SIV. Consequently, although immunological activation in response to inflammatory stimuli (eg, bacterial, viral, fungal, parasitic infections) is necessary to elicit an immune response, paradoxically this event may function as a trigger to drive viral replication and disease progression in HIV/SIV-infected individuals. A clear understanding of the cellular and molecular pathways by which various inflammatory stimuli promote immune activation and viral replication in the GIT is required to facilitate the development of novel therapeutic strategies.
Earlier, using the rhesus macaque model of AIDS we demonstrated that SIV-infection and other causes of intestinal inflammation resulted in dysregulation of the IL-6-JAK-STAT3-SOCS-3 pathway and that the entire GIT was impacted in SIV infection in contrast to intestinal inflammation of other causes.22 Although IL-6 is known to mediate chronic inflammation, immune activation,23 and HIV replication in vitro,19 the potential mechanism(s) promoting viral replication in the GIT remain unexplored. In the present study, jejunum and colon from SIV- and non-SIV-infected rhesus macaques with chronic diarrhea were examined to assess the role played by C/EBPβ, a proinflammatory and proviral transcription factor activated by IL-6, IL-1β, and interferon-γ in the development of GIT inflammation/disease. Our findings suggest that C/EBPβ, known to be activated in response to IL-6 and other proinflammatory cytokine signaling in the GIT may not only be a mediator of chronic diarrhea and inflammation accompanying HIV/SIV infection but also may facilitate HIV/SIV replication.
The activity and expression level of C/EBPβ is regulated by a number of inflammatory agents including LPS and a range of cytokines such as IL-6, IL-1β, and tumor necrosis factor-α.30,31,59 Interestingly, C/EBPβ expression is detectable in the intestine even under uninduced conditions.31 The latter observation is supported by the present study because C/EBPβ mRNA, but not protein, was detected in the jejunum and colon of group 3 animals (Figures 1 and 3). However, in group 1 and 2 animals with chronic diarrhea and intestinal inflammation, C/EBPβ mRNA expression was significantly increased in the colon compared with group 3 controls. In the jejunum, elevations in C/EBPβ were only found in SIV-infected animals with diarrhea. These animals also had more severe histopathological lesions in the jejunum compared with the other groups (Tables 2 and 3). Further, group 1 macaques showed significant elevation of C/EBPβ mRNA expression relative to group 2 macaques suggesting that SIV infection has a particularly dramatic impact on expression levels of C/EBPβ. Although the factors that trigger this marked increase in C/EBPβ expression remain unclear, the high mucosal viral loads in group 1 animals could be a major driving force. Additionally, increased translocation of bacterial LPS from a leaky intestinal epithelial barrier recently reported to occur in chronic HIV-infected humans and SIV-infected macaques58 can strongly induce the expression of C/EBPβ.31,59 Increased expression of C/EBPβ has also been detected in peripheral blood mononuclear cells of HIV-infected patients38 and has been strongly associated with increased viral replication in HIV patients with pulmonary tuberculosis45 and in the development of AIDS dementia.60 Interestingly, Silver and colleagues61 had earlier reported a strong association between GIT inflammation (ulcerative colitis) and high systemic viral burden with a dramatic reversal of both high viral burden and low CD4+ T-cells counts after surgical resection of inflamed areas of the colon.61 This study clearly demonstrates an association between inflammation and viral burden and shows an increased accumulation of HIV-1-infected cells within the inflamed mucosa. The significantly increased expression of C/EBPβ observed in the GIT of SIV-infected rhesus macaques offers a molecular pathological mechanism linking GIT inflammation and immune activation with increased viral burden in SIV-infected macaques and conceivably in HIV-infected individuals.
To determine the cell types expressing C/EBPβ we then used multilabel confocal microscopy with cell-type-specific and anti-C/EBPβ antibodies. C/EBPβ expression was restricted to the mononuclear cell population present in the lamina propria. Using cell-specific surface markers such as CD3 and CD68 we found both lymphocytes (CD3) and macrophages (CD68), both of which are targets for SIV infection, to be expressing C/EBPβ. In the non-SIV-infected macaque with diarrhea the expression of C/EBPβ was equally distributed between macrophages and lymphocytes (Figure 2, B and E). However, in SIV-infected macaques, C/EBPβ expression was detectable predominantly in macrophages (Figure 2, A and D). The reason for this difference may be related to the selective destruction of CD3+CD4+ T cells by SIV that would not occur in group 2 animals. In contrast to group 1 and 2 animals, very little C/EBPβ expression was observed in control (group 3) animals. The strong expression of C/EBPβ by intestinal macrophages in SIV-infected animals is significant because, macrophage numbers increase tremendously during inflammation primarily because of the increased trafficking of monocytes that express CD4, CCR5, and CXCR462 from the blood into the inflamed GIT mucosa and submucosa.63 C/EBPβ is a strong transactivator of the CCR5 gene (approximately seven binding sites),38 the HIV/SIV LTR (approximately three to five sites)39 in addition to regulating the expression of several proinflammatory genes such as COX-2,29 iNOS., and so forth. Hence the direct consequences of persistently elevated C/EBPβ would likely be the generation of more HIV/SIV receptor-binding sites, target cells, increased viral burden, reservoir cells, immune activation, and progression to AIDS.
Given the fact that the GIT is a major site of viral replication and that most HIV/SIV-infected individuals develop GIT inflammation/disease, it is of considerable interest to identify host cellular factors that promote HIV/SIV replication in this pivotal tissue. The presence of three and up to five putative binding sites for C/EBPβ in the 5′-LTR of HIV and SIV,39 respectively, further prompted us to investigate the role of C/EBPβ proteins in regulating viral replication in the GIT. Using the ChIP assay we observed that in SIV-infected LPCs isolated from an inflamed colon, both p65 and C/EBPβ may either independently or synergistically bind to the SIV LTR (Figure 3B). These findings provide a plausible link between viral replication, inflammation, and immune activation. Similar in vivo binding of C/EBPβ in cooperation with p65 and p50 to the LTR has been demonstrated in the brain of SIV-infected rhesus macaques.44 Although these data do not indicate if this binding is stimulatory or inhibitory, the presence of high viral loads in the jejunum and colon of group 1 animals (Table 1) together with the strong expression of the 46- and 41-kDa transcription activating isoforms (LAP) (Figure 3A) suggests a stimulatory role. Aspirin and its in vivo metabolite sodium salicylate at pharmacological concentrations has been shown to selectively inhibit C/EBPβ activation by blocking C/EBPβ phosphorylation catalyzed by a number of kinases, that includes p90 ribosomal S6 kinase (RSK), which preferentially phosphorylates C/EBPβ at Thr266.64,65 Based on our findings it is logical to hypothesize that incorporation of anti-inflammatory drugs targeting C/EBPβ might improve antiretroviral treatment outcomes. Collectively, our findings suggest that increased expression of C/EBPβ helps maintain a proinflammatory environment locally in the GIT. Such an inflammatory milieu, if allowed to persist could be expected to facilitate damage to the mucosal barrier, thus setting up a positive feed forward mechanism involving viral replication, inflammation, increased GIT microbial translocation, and immune activation.
Acknowledgments
We thank Dr. Ronald S. Veazey for providing biopsy samples from uninfected healthy macaques; Ms. Robin Rodriguez for assistance with images; and Ms. Terri Rasmussen, Lisa Bowers, Tamika Bridges, and Drs. Preston Marx and Cristian Apetrei for their valuable assistance in the study.
Footnotes
Address reprint requests to Andrew A. Lackner, D.V.M., Ph.D., Tulane National Primate Research Center, 18703 Three Rivers Rd., Covington, LA 70433. E-mail: alackner@tulane.edu.
Supported by the National Institutes of Health (grants DK50550, RR00164, RR16930, and AI065325).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mónkemüller KE, Lazenby AJ, Lee DH, Loudon R, Wilcox CM. Occurrence of gastrointestinal opportunistic disorders in AIDS despite the use of highly active antiretroviral therapy. Dig Dis Sci. 2005;50:230–234. doi: 10.1007/s10620-005-1587-z. [DOI] [PubMed] [Google Scholar]
- Miao YM, Gazzard BG. Management of protozoal diarrhoea in HIV disease. HIV Med. 2000;1:194–199. doi: 10.1046/j.1468-1293.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- Wilcox CM. Gastrointestinal manifestations of AIDS. Nutr Clin Pract. 2004;19:356–364. doi: 10.1177/0115426504019004356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A, Bruzzese E, De Marco G, Buccigrossi V. Management of gastrointestinal disorders in children with HIV infection. Paediatr Drugs. 2004;6:347–362. doi: 10.2165/00148581-200406060-00003. [DOI] [PubMed] [Google Scholar]
- Sestak K. Chronic diarrhea and AIDS: insights into studies with non-human primates. Curr HIV Res. 2005;3:199–205. doi: 10.2174/1570162054368084. [DOI] [PubMed] [Google Scholar]
- Heise C, Vogel P, Miller CJ, Halsted CH, Dandekar S. Simian immunodeficiency virus infection of the gastrointestinal tract of rhesus macaques. Functional, pathological, and morphological changes. Am J Pathol. 1993;142:1759–1771. [PMC free article] [PubMed] [Google Scholar]
- Heise C, Miller CJ, Lackner A, Dandekar S. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- Kewenig S, Schneider T, Hohloch K, Lampe-Dreyer K, Ullrich R, Stolte N, Stahl-Hennig C, Kaup FJ, Stallmach A, Zeitz M. Rapid mucosal CD4(+) T-cell depletion and enteropathy in simian immunodeficiency virus-infected rhesus macaques. Gastroenterology. 1999;116:1115–1123. doi: 10.1016/s0016-5085(99)70014-4. [DOI] [PubMed] [Google Scholar]
- MacDonald TT, Spencer J. Cell-mediated immune injury in the intestine. Gastroenterol Clin North Am. 1992;21:367–386. [PubMed] [Google Scholar]
- Field M. T cell activation alters intestinal structure and function. J Clin Invest. 2006;116:2580–2582. doi: 10.1172/JCI29985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- Poli G. Laureate ESCI award for excellence in clinical science 1999. Cytokines and the human immunodeficiency virus: from bench to bedside. Eur J Clin Invest. 1999;29:723–732. doi: 10.1046/j.1365-2362.1999.00525.x. [DOI] [PubMed] [Google Scholar]
- Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease—a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–180. [PubMed] [Google Scholar]
- McGowan I, Elliott J, Fuerst M, Taing P, Boscardin J, Poles M, Anton P. Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr. 2004;37:1228–1236. doi: 10.1097/01.qai.0000131846.12453.29. [DOI] [PubMed] [Google Scholar]
- Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan M, Aye PP, Borda JT, Alvarez X, Lackner AA. Gastrointestinal disease in SIV-infected rhesus macaques is characterized by proinflammatory dysregulation of the IL-6-JAK-STAT3 pathway. Am J Pathol. 2007;171:1952–1965. doi: 10.2353/ajpath.2007.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(Suppl 2):S3:1–6. doi: 10.1186/ar1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehof M, Streetz K, Rakemann T, Bischoff SC, Manns MP, Horn F, Trautwein C. Interleukin-6-induced tethering of STAT3 to the LAP/C/EBPbeta promoter suggests a new mechanism of transcriptional regulation by STAT3. J Biol Chem. 2001;276:9016–9027. doi: 10.1074/jbc.M009284200. [DOI] [PubMed] [Google Scholar]
- Ito H. IL-6 and Crohn’s disease. Curr Drug Targets Inflamm Allergy. 2003;2:125–130. doi: 10.2174/1568010033484296. [DOI] [PubMed] [Google Scholar]
- Johnson PF, Williams SC. CCAAT/enhancer binding (C/EBP) proteins. EF Tronche, Yaniv M., editors. Austin: RG Landes Company,; Liver Gene Expression. 1994:p 231–258. [Google Scholar]
- Cooper C, Henderson A, Artandi S, Avitahl N, Calame K. Ig/EBP (C/EBP gamma) is a transdominant negative inhibitor of C/EBP family transcriptional activators. Nucleic Acids Res. 1995;23:4371–4377. doi: 10.1093/nar/23.21.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descombes P, Schibler U. A liver-enriched transcriptional activator protein. LAP, and a transcriptional inhibitory protein LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Wu KK, Liou JY, Cieslik K. Transcriptional control of COX-2 via C/EBPbeta. Arterioscler Thromb Vasc Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee R, Sawaya BE, Khalili K, Amini S. Association of p65 and C/EBPbeta with HIV-1 LTR modulates transcription of the viral promoter. J Cell Biochem. 2007;100:1210–1216. doi: 10.1002/jcb.21109. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rom WN. Regulation of the interleukin-1 beta (IL-1 beta) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993;13:3831–3837. doi: 10.1128/mcb.13.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope RM, Leutz A, Ness SA. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Invest. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Takeshima H, Hamada K, Nakao M, Kino T, Nishi T, Kochi M, Kuratsu J, Yoshimura T, Ushio Y. Cloning and functional characterization of the 5′-flanking region of the human monocyte chemoattractant protein-1 receptor (CCR2) gene. Essential role of 5′-untranslated region in tissue-specific expression. J Biol Chem. 1999;274:4646–4654. doi: 10.1074/jbc.274.8.4646. [DOI] [PubMed] [Google Scholar]
- Rosati M, Valentin A, Patenaude DJ, Pavlakis GN. CCAAT-enhancer-binding protein beta (C/EBP beta) activates CCR5 promoter: increased C/EBP beta and CCR5 in T lymphocytes from HIV-1-infected individuals. J Immunol. 2001;167:1654–1662. doi: 10.4049/jimmunol.167.3.1654. [DOI] [PubMed] [Google Scholar]
- Nonnemacher MR, Hogan TH, Quiterio S, Wigdahl B, Henderson A, Krebs FC. Identification of binding sites for members of the CCAAT/enhancer binding protein transcription factor family in the simian immunodeficiency virus long terminal repeat. Biomed Pharmacother. 2003;57:34–40. doi: 10.1016/s0753-3322(02)00334-7. [DOI] [PubMed] [Google Scholar]
- Henderson AJ, Zou X, Calame KL. C/EBP proteins activate transcription from the human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol. 1995;69:5337–5344. doi: 10.1128/jvi.69.9.5337-5344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson AJ, Connor RI, Calame KL. C/EBP activators are required for HIV-1 replication and proviral induction in monocytic cell lines. Immunity. 1996;5:91–101. doi: 10.1016/s1074-7613(00)80313-1. [DOI] [PubMed] [Google Scholar]
- Henderson AJ, Calame KL. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4(+) T cells. Proc Natl Acad Sci USA. 1997;94:8714–8719. doi: 10.1073/pnas.94.16.8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan TH, Krebs FC, Wigdahl B. Regulation of human immunodeficiency virus type 1 gene expression and pathogenesis by CCAAT/enhancer binding proteins in cells of the monocyte/macrophage lineage. J Neurovirol. 2002;8(Suppl 2):21–26. doi: 10.1080/13550280290167911. [DOI] [PubMed] [Google Scholar]
- Barber SA, Gama L, Dudaronek JM, Voelker T, Tarwater PM, Clements JE. Mechanism for the establishment of transcriptional HIV latency in the brain in a simian immunodeficiency virus-macaque model. J Infect Dis. 2006;193:963–970. doi: 10.1086/500983. [DOI] [PubMed] [Google Scholar]
- Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. Type I interferon induces inhibitory 16-kD CCAAT/enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med. 1998;188:1255–1265. doi: 10.1084/jem.188.7.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol. 2000;165:2028–2039. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse DB, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med. 2002;195:495–505. doi: 10.1084/jem.20011614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestak K, Merritt CK, Borda J, Saylor E, Schwamberger SR, Cogswell F, Didier ES, Didier PJ, Plauche G, Bohm RP, Aye PP, Alexa P, Ward RL, Lackner AA. Infectious agent and immune response characteristics of chronic enterocolitis in captive rhesus macaques. Infect Immun. 2003;71:4079–4086. doi: 10.1128/IAI.71.7.4079-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramesh G, Alvarez X, Borda JT, Aye PP, Lackner AA, Sestak K. Visualizing cytokine-secreting cells in situ in the rhesus macaque model of chronic gut inflammation. Clin Diagn Lab Immunol. 2005;12:192–197. doi: 10.1128/CDLI.12.1.192-197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, Chalifoux LV, Sehgal PK, Lackner AA. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000;74:57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veazey RS, Lackner AA. Getting to the guts of HIV pathogenesis. J Exp Med. 2004;200:697–700. doi: 10.1084/jem.20041464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189:572–582. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- Sankaran S, Guadalupe M, Reay E, George MD, Flamm J, Prindiville T, Dandekar S. Gut mucosal T cell responses and gene expression correlate with protection against disease in long-term HIV-1-infected nonprogressors. Proc Natl Acad Sci USA. 2005;102:9860–9865. doi: 10.1073/pnas.0503463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MD, Reay E, Sankaran S, Dandekar S. Early antiretroviral therapy for simian immunodeficiency virus infection leads to mucosal CD4+ T-cell restoration and enhanced gene expression regulating mucosal repair and regeneration. J Virol. 2005;79:2709–2719. doi: 10.1128/JVI.79.5.2709-2719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MD, Verhoeven D, McBride Z, Dandekar S. Gene expression profiling of gut mucosa and mesenteric lymph nodes in simian immunodeficiency virus-infected macaques with divergent disease course. J Med Primatol. 2006;35:261–269. doi: 10.1111/j.1600-0684.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- Guadalupe M, Sankaran S, George MD, Reay E, Verhoeven D, Shacklett BL, Flamm J, Wegelin J, Prindiville T, Dandekar S. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar S. Pathogenesis of HIV in the gastrointestinal tract. Curr HIV/AIDS Rep. 2007;4:10–15. doi: 10.1007/s11904-007-0002-0. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- Bradley MN, Zhou L, Smale ST. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol Cell Biol. 2003;23:4841–4858. doi: 10.1128/MCB.23.14.4841-4858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan TH, Stauff DL, Krebs FC, Gartner S, Quiterio SJ, Wigdahl B. Structural and functional evolution of human immunodeficiency virus type 1 long terminal repeat CCAAT/enhancer binding protein sites and their use as molecular markers for central nervous system disease progression. J Neurovirol. 2003;9:55–68. doi: 10.1080/13550280390173292. [DOI] [PubMed] [Google Scholar]
- Silver S, Wahl SM, Orkin BA, Orenstein JM. Changes in circulating levels of HIV, CD4, and tissue expression of HIV in a patient with recent-onset ulcerative colitis treated by surgery. Case report. J Hum Virol. 1999;2:52–57. [PubMed] [Google Scholar]
- Smith PD, Meng G, Salazar-Gonzalez JF, Shaw GM. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukoc Biol. 2003;74:642–649. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]
- Rugtveit J, Brandtzaeg P, Halstensen TS, Fausa O, Scott H. Increased macrophage subset in inflammatory bowel disease: apparent recruitment from peripheral blood monocytes. Gut. 1994;35:669–674. doi: 10.1136/gut.35.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK. Control of COX-2 and iNOS gene expressions by aspirin and salicylate. Thromb Res. 2003;110:273–276. doi: 10.1016/s0049-3848(03)00412-2. [DOI] [PubMed] [Google Scholar]
- Wu KK. Transcription-based COX-2 inhibition: a therapeutic strategy. Thromb Haemost. 2006;96:417–422. [PubMed] [Google Scholar]