Abstract
Heme oxygenase-1 (HO-1) has been viewed as a cytoprotective protein, ameliorating the effects of inflammatory cellular damage, and as beneficial in allograft protection from acute and chronic rejection, suggesting important functions in both innate and adaptive immune responses. Mice deficient in HO-1 exhibit defective immune regulation characterized by a proinflammatory phenotype. We examined if impaired regulatory T cell (Treg) function contributes to the immunoregulatory defects observed in HO-1−/− mice. HO-1−/− mice exhibited a significantly higher proportion of Foxp3-expressing cells among total CD4+ and CD4+CD25+ cells in comparison to HO-1+/+ mice, and HO-1−/− Treg cells were at least as effective as HO-1+/+ Treg cells in suppressing proliferation of effector T cells in vitro from either HO-1+/+ or HO-1−/− mice. However, the absence of HO-1 in antigen-presenting cells abolished the suppressive activity of Treg cells on effector T cells. These findings demonstrate that HO-1 activity in antigen-presenting cells is important for Treg-mediated suppression, providing an explanation for the apparent defect in immune regulation in HO-1−/− mice.
Historically, heme oxygenase-1 (HO-1) has been viewed as a cytoprotective protein, ameliorating the effects of inflammatory cellular damage. The demonstration, however, of a beneficial role for HO-1 in allograft protection from acute and chronic rejection,1,2,3 strongly suggests an important function of this enzyme in both innate and adaptive immune responses. In the original description of a mouse model of HO-1 deficiency, Poss and Tonegawa4 noted an age-related overgrowth of the CD4+ T-cell population, suggesting impaired regulation of T-cell proliferation. Our previous work assessing immune function in HO-1−/− mice supported this notion because it showed a predominance of Th1-type cytokine secretion [eg, interleukin (IL)-1, interferon-γ, tumor necrosis factor-α, IL-6] from splenocytes after polyclonal stimulation of T cells, implying that HO-1 activity is important in modulation of lymphocyte activation.5 This is of particular interest given reports that HO-1 is constitutively expressed in the CD4+CD25+ subset of Treg cells,6 and that HO-1 levels increase even further after T-cell stimulation.7 Furthermore, the report by Song and colleagues8 demonstrated that carbon monoxide (CO), a byproduct of HO activity, has a very strong inhibitory effect on CD3-activated T-cell proliferation. Previously, we proposed a hypothetical model for the immunomodulatory effects of HO-1 in the maintenance of peripheral tolerance based on then available evidence that CO produced in regulatory T (Treg) cells may be an integral component of the suppression of T-cell activation by Treg cells in the presence of effector T (Teff) cells and antigen-presenting cells (APCs).9
The purpose of the present study was to analyze the role of HO-1 in Treg-mediated suppression. As a first step, we performed a phenotypic analysis of lymphocytes obtained from the peripheral lymphoid organs of HO-1−/− mice for potential abnormalities. In the second step, we explored the functional significance of these findings in a series of T-cell proliferation/suppression assays. Finally, we examined the possibility that HO-1 expression in the APCs may modulate the suppressive capacity of Treg cells. We found that Treg cells from HO-1-deficient mice functioned normally in the presence of wild-type APCs and Teff cells, but suppression by both HO-1-deficient and HO-1+/+ Treg cells was abolished in the presence of HO-1-deficient APCs.
Materials and Methods
Animals
Male and female HO-1−/− mice (8 to 12 weeks of age, C57BL/6 × FVB) carrying a targeted deletion of a large portion of the HO-1 gene, were selected by genotyping using tail DNA as previously described from offspring of heterozygous/homozygous mating.10 Age-matched wild-type (HO-1+/+) littermates were used as controls. The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham.
Immunofluorescence Staining and Flow Cytometry
Spleens were harvested and single cell suspensions prepared according to standard protocols. The suspended cells were stained with monoclonal antibodies against B cells (CD45R/B-220), T cells (CD3, CD4, CD8), and several markers of Treg cells (CD25, Foxp3, CTLA-4, GITR, LAG-3), coupled with biotin (CD25) or various chromogens (fluorescein isothiocyanate, phycoerythrin, PerCP, APC, and Alexa 647) and immediately analyzed. Cells were pretreated with antibodies specific for CD16/32 to inhibit nonspecific binding of phycoerythrin to FCγ receptors. For the detection of intracellular antigens, the cells were first surface stained with antibodies, then fixed, permeabilized, and stained with antibodies against Foxp3 and CTLA-4. Isotype-matched antibodies were used as negative controls. Data were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed with Winlist analysis software (Verity Software House, Topsham, MA).
Suppression Assays
CD4+CD25+ (Treg) and CD4+CD25− (Teff) were isolated from total splenocytes using magnetic bead separation kits according to the manufacturer’s instructions (Miltenyi, Auburn, CA). This methodology yields a population of CD4+-depleted cells (APCs), CD4+CD25+ cells (Treg), and CD4+CD25− cells (Teff). APCs were irradiated (3300 rad) and plated onto 96-well plates (105 cells/well). Teff cells at a constant number (104 cells/well) were then added with a varying number of Treg cells to provide Teff/Treg ratios of 1:0, 1:2, 1:1, 2:1, 4:1, and 8:1. A combination of 1 μg/ml of soluble anti-CD3 and 1 μg/ml of soluble anti-CD28 (eBioscience, San Diego, CA) provided the polyclonal stimulus for proliferation. In experiments in which purified dendritic cells (DCs) were used, stimulation was provided by incubation with soluble anti-CD3 at 250 ng/ml. Cells were incubated in RPMI with 10% fetal bovine serum in a total volume of 200 μl. At 5 days of culture, 1 μCi of 3H-thymidine (Amersham Biosciences, Piscataway, NJ) was added for the final 16 hours to assess proliferation. Suppression was determined by 3H-thymidine incorporation, with the percent suppression = [1 − (mean cpm Treg + Teff)/(mean cpm Teff) × 100%]. In some experiments, incorporation was measured by BrdU incorporation using a commercial cell proliferation enzyme-linked immunosorbent assay kit (Roche Diagnostics, Indianapolis, IN).
Isolation of Bone Marrow-Derived Dendritic Cells (BMDCs)
BMDCs were isolated using a modification of the method described by Lutz and colleagues.11 Nonadherent bone marrow cells were cultured in complete Dulbecco’s modified Eagle’s medium containing 400 U/ml GM-CSF (Peprotech, Rocky Hill, NJ). The medium was changed every third day, and the nonadherent cells were removed on day 9 and used in suppression assays as noted.
HO Enzyme Activity Assay
HO activity assays were performed in spleen microsomes as previously described.12,13 Briefly, spleen microsomes from HO-1+/+ and HO-1−/− mice were incubated with rat liver cytosol (3 mg), a source of biliverdin reductase, hemin (20 μmol/L), glucose-6-phosphate (2 mmol/L), glucose-6-phosphate dehydrogenase (0.2 U), and NADPH (0.8 mmol/L) for 1 hour at 37°C in the dark. The formed bilirubin was extracted with chloroform, change in optical density from 464 to 530 nm was measured and enzyme activity expressed as nmol of bilirubin formed per 60 minutes per mg protein.
Western Blots
Samples were prepared in Laemmli buffer and separated on a 10% sodium dodecyl sulfate-polyacrylamide gel. The gels were subsequently transferred to a Hybond-P polyvinylidene difluoride membrane. The membrane was blocked in a 5% nonfat dry milk and 1% bovine serum albumin solution for 1 hour at room temperature before addition of antibodies specific for HO-1 and HO-2 (SPA-896 and SPA-897, respectively; Stressgen Biotechnologies, Victoria, Canada). The membranes were then incubated with peroxidase-conjugated goat anti-rabbit IgG antibody for 1 hour (Jackson ImmunoResearch, West Grove, PA). Peroxidase antibodies on the membranes were detected using the ECL chemiluminescent detection system (Amersham Biosciences).
Statistical Analysis
The statistical analysis was performed using the two-tailed unpaired t-test, and a P value less than 0.05 was considered significant. Data are presented as mean ± SEM.
Results
HO-1 Deficiency Is Associated with Abnormalities in Treg Phenotype
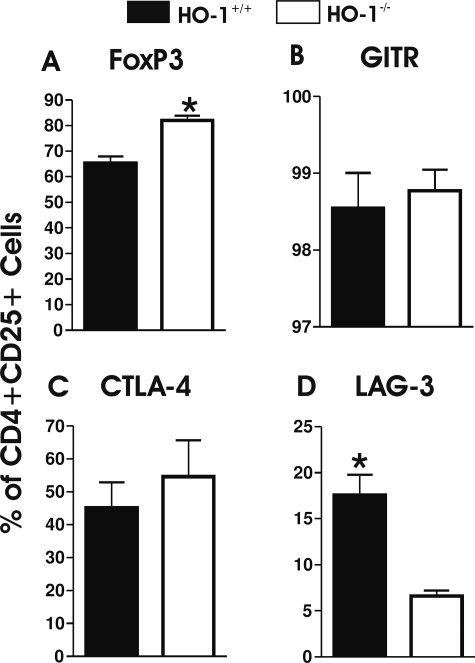
Previous work from our laboratory and others clearly demonstrated that proinflammatory tendencies are associated with HO-1 deficiency.5 Based on these observations, we characterized the phenotype of Treg cells in the spleens of HO-1-deficient mice, and examined the frequency of cells bearing a variety of putative Treg markers. We found that the overall frequency of splenic CD4+CD25+ cells was the same in both HO-1+/+ and HO-1−/− mice (2.12 ± 0.12, n = 3, and 2.27 ± 0.32%, n = 4, of gated lymphoid cells, respectively; P = 0.497, Student’s t-test). However, among these cells, the proportion of FoxP3+ cells was significantly higher in HO-1−/− animals (Figure 1A, P = 0.005). The proportion of cells expressing glucocorticoid-induced tumor necrosis factor receptor family-related gene (GITR) or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) among FoxP3+ cells was not significantly different in HO-1−/− animals in comparison to HO-1+/+ animals (Figure 1, B and C, respectively). Both GITR and CTLA-4 are involved in inhibition of the suppressive activity of Treg cells. Interestingly, however, the expression of LAG-3 (Figure 1D), a marker previously associated with Treg-suppressive function,14,15 was significantly lower in HO-1−/− mice (P = 0.010) suggesting that, in fact, HO-1−/− Treg function might be impaired.
Figure 1.
Flow cytometric analysis of gated CD4+CD25+ mouse splenocytes expressing FoxP3 (A), GITR (B), CTLA-4 (C), and LAG-3 (D) (n = 4 for HO-1−/− and n = 3 for HO-1+/+; all analyses were gated on live cells). P values were calculated using the Student’s t-test. *P < 0.05 versus HO-1+/+.
The Absence of HO-1 Expression in Treg Cells Does Not Impair Their in Vitro Suppressive Function
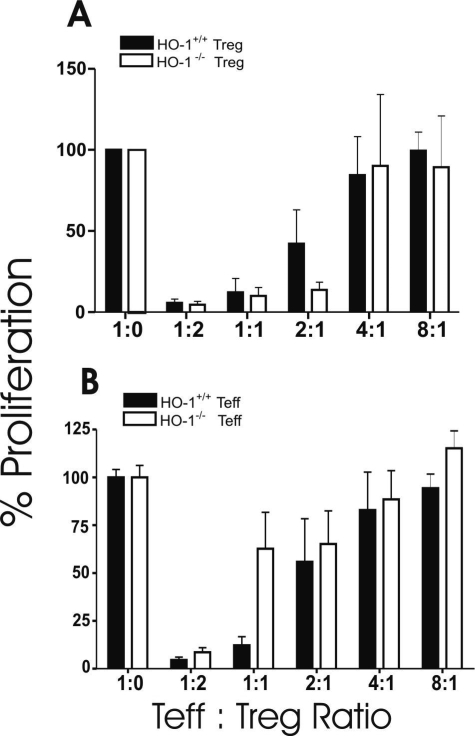
Based on the aforementioned findings, we examined the suppressive capacity of Treg cells obtained from either HO-1+/+ or HO-1−/− mice on polyclonal CD4+ T-cell proliferation in response to crosslinking of CD3 and CD28 in the presence of HO-1+/+ APCs. As depicted in Figure 2A, HO-1−/− Treg cells suppressed the proliferation of Teff cells in a dose-dependent manner, at least as efficiently as those from HO-1+/+ mice. Of note, the origin of Teff cells from either HO-1+/+ or HO-1−/− animals had no bearing on the degree of suppression (Figure 2B). These findings imply that the presence or absence of HO-1 within Treg cells does not influence their suppressive capacity.
Figure 2.
Results of in vitro suppression assays as a function of the presence or absence of HO-1 expression by Treg in the presence of HO-1+/+ Teff cells and APCs (A); the presence or absence of HO-1 expression by Teff cells in the presence of HO-1+/+ Treg cells and APCs (B). In both panels, solid bars designate wild-type cells, and open bars designate HO-1-deficient cells (Treg cells in A and Teff cells in B). At least three separate experiments were performed for each set of conditions.
The Absence of HO-1 Expression in APCs Impairs Suppressive Function of Treg Cells
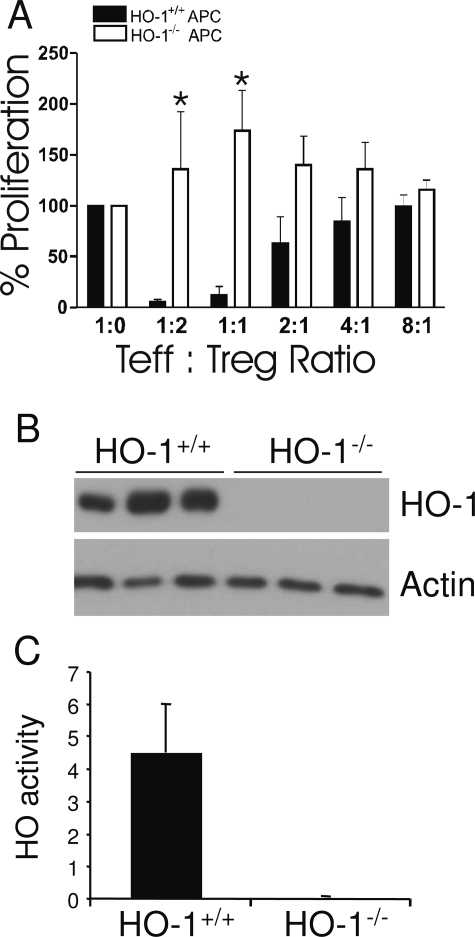
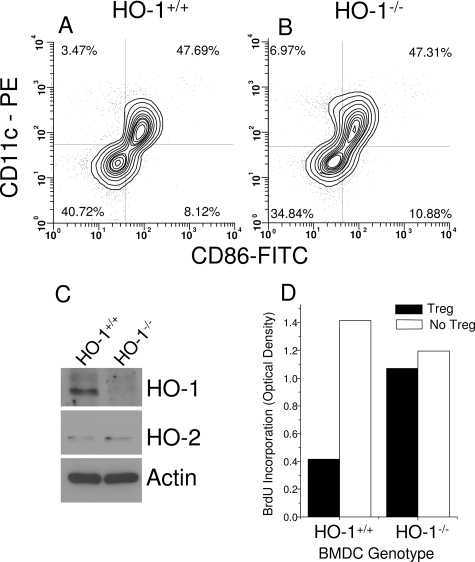
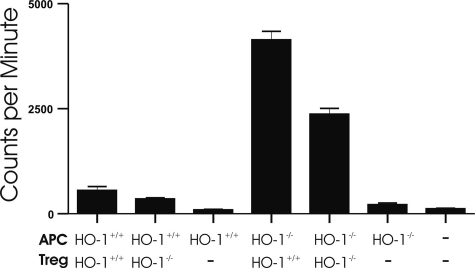
Next we evaluated the potential importance of HO-1 in APCs on the modulation of Treg function. In experiments similar to those described above, polyclonally stimulated Teff cells were co-cultured with various quantities of Treg cells in the presence of APCs obtained from either HO-1+/+ or HO-1−/− mice. As demonstrated in Figure 3A, the absence of HO-1 in APCs abolished the suppressive influence of Treg cells on Teff cell proliferation. Here also, the HO-1 genotype of Teff and Treg cells played no role (data not shown). Western blots of microsomal fractions of splenocytes confirmed that the mice were deficient in expression of HO-1 (Figure 3B). Splenic HO enzyme activity in HO-1−/− mice also showed that HO activity was undetectable (Figure 3C). In these initial experiments, the APC population was heterogeneous, consisting of monocytes, B cells, and DCs. To determine whether HO-1 expression in DCs could be important, Teff cells were co-cultured with Treg cells in the presence of BMDCs grown in culture from HO-1+/+ or HO-1−/− mice. The surface phenotype of the BMDCs isolated from HO-1−/− mouse bone marrow was the same as immature HO-1−/− DCs, with positive CD11c expression and low levels of expression of CD86 (Figure 4, A and B) and MHC class II (data not shown). Low levels of HO-1 expression were observed in DCs from HO-1+/+ mice, but not from HO-1−/− mice. HO-2 levels were low and unchanged (Figure 4C). The absence of HO-1 expression in the dendritic cells resulted in a loss of suppression by the Treg cells (Figure 4D), consistent with the results seen with APCs isolated from the spleen (Figure 3A). Treg cells proliferated readily when incubated without Teff cells in the presence of HO-1−/− APCs, whereas they did not proliferate in the presence of HO-1+/+ APCs (Figure 5). Collectively, these data suggest that the presence of HO-1 in APCs is essential for maintaining the suppressive function of Treg cells.
Figure 3.
A: Suppression of T-cell proliferation as a function of the presence or absence of HO-1 in APCs. Solid bars designate HO-1+/+ APCs, and open bars designate HO-1-deficient APCs. At least three separate experiments were performed for each set of conditions. *P < 0.05 versus HO-1+/+. B: HO-1 protein expression in splenic microsomes isolated from HO-1+/+ (n = 3) and HO-1−/− (n = 3) mice. C: HO enzyme activity in splenic microsomes from HO-1+/+ and HO-1−/− mice (n = 3 for each genotype). HO activity was measured by bilirubin generation (nmol/hour/mg protein).
Figure 4.
Surface phenotype of HO-1+/+ (A) and HO-1−/− (B) BMDCs at 5 days of culture just before addition to suppression assays. The phenotype was defined by staining with anti-CD11c-phycoerythrin and anti-CD86-fluoresecin isothiocyanate. C: Western blot of HO-1 and HO-2 expression in BMDCs on day +9 of culture. The HO-1 genotype is indicated above each lane. Actin served as a loading control. D: CD4+CD25+ Treg function is contingent on expression of HO-1 in dendritic cells. This plot depicts BrdU incorporation after 5 days of stimulation under conditions specified in the Materials and Methods. Results are derived from pooled BMDCs generated from two to three mice from each genotype.
Figure 5.
The lack of HO-1 in APCs is associated with proliferation of CD4+CD25+ Treg cells under stimulation with anti-CD3 and anti-CD28 antibodies. The plot depicts [H3]thymidine uptake results after 5 days of incubation under stated conditions. Measurements were compiled from at least three separate experiments.
Discussion
The results of this study provide insights into the nature of the immune abnormalities associated with HO-1 deficiency. We demonstrate that HO-1−/− mice exhibit significantly higher frequencies of FoxP3+ cells among CD4+CD25+ T-cell populations. The expression of lymphocyte activation gene-3 (LAG-3), a protein suggested to correlate with increased suppression by Treg cells15,16,17 was significantly lower in HO-1−/− animals. These differences suggest that Treg function could be impaired in HO-1−/− mice, which would provide an explanation for the immunoregulatory defects observed in these mice. However, our in vitro studies demonstrate that the suppressive function of Treg cells was normal in the presence of wild-type Teff cells and APCs, indicating that the immune dysregulation in HO-1−/− mice was not attributable to an intrinsic defect in Treg function. Given that Treg function is known to depend on the activity of APCs, we examined the role of HO-1 in these cells to influence Treg function as determined by in vitro suppression assays. Our results clearly demonstrate that a lack of HO-1 in APCs significantly impairs the suppressive function of Treg cells under conditions of APC excess.
Previous characterizations of HO-1−/− mice indicate that they develop chronic inflammatory changes with time, with a relative overgrowth of the CD4+ T-cell subset.4 Studies by Pae and colleagues7 have suggested that up-regulation of HO-1 in Treg cells may be related to the suppressive function of these cells. On the other hand, others have demonstrated that CD4+CD25+ cells from HO-1-deficient BALB/c mice retain their suppressive capacity both in vitro and in vivo.18 DCs actively participate in modulation of regulatory T-cell activity.19,20 As shown here and by others, immature DCs express HO-1, but apparently reduce or lose this expression as they mature.21 Furthermore, the protective effects of HO-1 induction against the development of diabetes coincides with reduced DC infiltration into islets.22,23 Therefore, it is possible that induction of HO-1 or its products can inhibit DC maturation. Our current study supports this concept in that we did not observe any defect in the in vitro suppressive activity by Treg cells from HO-1−/− mice in the presence of wild-type APCs and Teff cells. However, the absence of HO-1 in APCs resulted in a substantial loss of suppressive function by the Treg cells, indicating that the regulatory abnormality in HO-1−/− mice could be related to the induction and maintenance of Treg function by APCs rather than to an intrinsic defect of the Treg cells alone. Recent observations suggest that the up-regulation of HO-1 in APCs is essential for abolishing pathological findings in models of neuroinflammation. The protective effect of HO-1 was associated with down-regulation of MHC class II molecules, but not with changes in the infiltration of Treg cells into the lesions.24,25 HO-1 mediated alteration of the expression of either MHC molecules or co-stimulatory molecules and subsequent changes in APC/Treg interactions may be the underlying explanation for the differences in Treg suppressive activity and could explain findings reported in other experimental systems.26,27,28
It is well established that the expression of MHC and co-stimulatory molecules, as well as the cytokine milieu affect lymphocyte stimulation.29 We have previously demonstrated that HO-1 deficiency favors Th1 polarization of the profile of cytokines released by activated splenocytes.5 This effect was observed when lymphocytic populations were stimulated with anti-CD3/CD28 antibodies or when APCs were also activated by exposure to the Toll ligand, bacterial lipopolysaccharide. In addition, large amounts of IL-6 were released from stimulated HO-1−/− splenocytes in comparison to wild-type controls. IL-6 released by fully activated mature DCs is capable of reversing Treg-mediated suppression, likely by decreasing the susceptibility of CD4+CD25− cells to Treg influence.30 Moreover, such activated DCs can reverse the anergy of Treg,30,31 a phenomenon seen in our study in the presence of HO-1-deficient APCs. Thus, the functional status of APCs, particularly DCs, can affect both the proliferation of Teff cells as well as the function of Treg cells.32 It is conceivable that the crucial role of HO-1 activity is to modulate antigen presentation and other ancillary functions of APCs. Furthermore, these findings highlight a more general role for tolerogenic APCs in reinforcing the function of Treg. A prime example is the existence of DCs with a so-called tolerogenic phenotype that can be achieved by interference with the process of maturation.33,34 These tolerogenic DCs both suppress the activation of Teff cells and promote T-cell regulatory mechanisms. It would therefore be informative if the observations in this experimental system were dependent on cell to cell contact, soluble factors, or both. Experiments are currently underway to determine how HO-1 affects DC and T-cell interactions.
Interference with NF-κB activation in the presence of antigen is one of the described means by which DCs can acquire the tolerogenic phenotype.35,36 Interestingly, both HO-1 as well as the product of its activity, CO, have been shown to inhibit activation of the NF-κB pathway,37,38,39 and CO has been shown to inhibit polyclonal T-cell proliferation in response to anti-CD3 and anti-CD28.40 It is therefore possible that the induction of HO-1 during the early steps of DC activation may aid in the acquisition of a tolerogenic phenotype. Moreover, tolerogenic DCs, unlike mature DCs, secrete large amounts of Th2 cytokines, such as IL-10. Interestingly, there is a very close correlation between HO-1 activity and IL-10 secretion. HO-1 activity can result in increased IL-10 production.41 On the other hand, the anti-inflammatory properties of IL-10 require the expression of HO-1 in immune cells.42 The latter was demonstrated in our recent work showing that IL-10 prevented the immune processes associated with chronic allograft rejection, an effect dependent on systemic expression of HO-1.3
We conclude that the activity of HO-1 is an important regulatory mechanism affecting multiple levels of the immune response. The elucidation of its effects on specific immune cell populations will aid the development of therapeutic strategies for a variety of inflammatory disorders, including autoimmune diseases and transplant rejection. Conversely, inhibition of HO activity may serve an adjuvant effect to increase Teff responses in cases of cancer or infection by persistent pathogens.
Footnotes
Address reprint requests to James F. George, Ph.D., LHRB 790, University of Alabama at Birmingham, 1530 3rd Ave. South, Birmingham, AL 35294. E-mail: jgeorge@uab.edu.
Supported by the National Institutes of Health (grants K08 AI 57362 to M.H.K., and R01 DK 075532 and R01 HL068157 to A.A).
J.F.G. and A.B. contributed equally to this study.
A guest editor acted as editor-in-chief for this manuscript. No person at the University of Alabama at Birmingham was involved in the peer review process or final disposition for this article.
References
- Katori M, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation. 2002;74:905–912. doi: 10.1097/00007890-200210150-00001. [DOI] [PubMed] [Google Scholar]
- Soares MP, Bach FH. Heme oxygenase-1 in organ transplantation. Front Biosci. 2007;12:4932–4945. doi: 10.2741/2439. [DOI] [PubMed] [Google Scholar]
- Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B-M, Pae H-O, Jeong Y-R, Kim Y-M, Chung H-T. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem Biophys Res Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- Pae HO, Oh GS, Choi BM, Chae SC, Chung HT. Differential expressions of heme oxygenase-1 gene in CD25− and CD25+ subsets of human CD4+ T cells. Biochem Biophys Res Commun. 2003;306:701–705. doi: 10.1016/s0006-291x(03)01037-4. [DOI] [PubMed] [Google Scholar]
- Song Y, Shi Y, Ao LH, Harken AH, Meng XZ. TLR4 mediates LPS-induced HO-1 expression in mouse liver: role of TNF-alpha and IL-1beta. World J Gastroenterol. 2003;9:1799–1803. doi: 10.3748/wjg.v9.i8.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusko TM, Wasserfall CH, Agarwal A, Kapturczak MH, Atkinson MA. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol. 2000;278:F726–F736. doi: 10.1152/ajprenal.2000.278.5.F726. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rèossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, Vercellotti GM. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, Powell JD, Pardoll DM, Drake CG, Vignali DA. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. The phenotypic characterization of naturally occurring regulatory CD4+CD25+ T cells. Cell Mol Immunol. 2006;3:189–195. [PubMed] [Google Scholar]
- Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- Zelenay S, Chora A, Soares MP, Demengeot J. Heme oxygenase-1 is not required for mouse regulatory T cell development and function. Int Immunol. 2007;19:11–18. doi: 10.1093/intimm/dxl116. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007;46:159–167. doi: 10.1016/j.jdermsci.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hubert P, Jacobs N, Caberg J-H, Boniver J, Delvenne P. The cross-talk between dendritic and regulatory T cells: good or evil? J Leukoc Biol. 2007;82:781–794. doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- Li M, Peterson S, Husney D, Inaba M, Guo K, Terada E, Morita T, Patil K, Kappas A, Ikehara S, Abraham NG. Interdiction of the diabetic state in NOD mice by sustained induction of heme oxygenase: possible role of carbon monoxide and bilirubin. Antioxid Redox Signal. 2007;9:855–863. doi: 10.1089/ars.2007.1568. [DOI] [PubMed] [Google Scholar]
- Li M, Peterson S, Husney D, Inaba M, Guo K, Kappas A, Ikehara S, Abraham NG. Long-lasting expression of HO-1 delays progression of type I diabetes in NOD mice. Cell Cycle. 2007;6:567–571. doi: 10.4161/cc.6.5.3917. [DOI] [PubMed] [Google Scholar]
- Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, Chora A, Rodrigues CD, Gregoire IP, Cunha-Rodrigues M, Portugal S, Soares MP, Mota MM. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med. 2007;13:703–710. doi: 10.1038/nm1586. [DOI] [PubMed] [Google Scholar]
- Wu J, Ma J, Fan S-T, Schlitt HJ, Tsui T-Y. Bilirubin derived from heme degradation suppresses MHC class II expression in endothelial cells. Biochem Biophys Res Commun. 2005;338:890–896. doi: 10.1016/j.bbrc.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Martins PNA, Kessler H, Jurisch A, Reutzel-Selke A, Kramer J, Pascher A, Pratschke J, Neuhaus P, Volk HD, Tullius SG. Induction of heme oxygenase-1 in the donor reduces graft immunogenicity. Transplant Proc. 2005;37:384–386. doi: 10.1016/j.transproceed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lee SS, Gao W, Mazzola S, Thomas MN, Csizmadia E, Otterbein LE, Bach FH, Wang H. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007;21:3450–3457. doi: 10.1096/fj.07-8472com. [DOI] [PubMed] [Google Scholar]
- Beier KC, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J. 2007;29:804–812. doi: 10.1183/09031936.00094506. [DOI] [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Kubo T, Hatton RD, Oliver J, Liu X, Elson CO, Weaver CT. Regulatory T cell suppression and anergy are differentially regulated by proinflammatory cytokines produced by TLR-activated dendritic cells. J Immunol. 2004;173:7249–7258. doi: 10.4049/jimmunol.173.12.7249. [DOI] [PubMed] [Google Scholar]
- Groux H, Fournier N, Cottrez F. Role of dendritic cells in the generation of regulatory T cells. Semin Immunol. 2004;16:99–106. doi: 10.1016/j.smim.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–483. doi: 10.1046/j.1440-1711.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-Acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-{kappa}B inhibition. J Immunol. 1999;162:2569–2574. [PubMed] [Google Scholar]
- Cong Y, Konrad A, Iqbal N, Hatton RD, Weaver CT, Elson CO. Generation of antigen-specific, Foxp3-expressing CD4+ regulatory T cells by inhibition of APC proteosome function. J Immunol. 2005;174:2787–2795. doi: 10.4049/jimmunol.174.5.2787. [DOI] [PubMed] [Google Scholar]
- Megías J, Busserolles J, Alcaraz MJ. The carbon monoxide-releasing molecule CORM-2 inhibits the inflammatory response induced by cytokines in Caco-2 cells. Br J Pharmacol. 2007;150:977–986. doi: 10.1038/sj.bjp.0707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murali NS, Ackerman AW, Croatt AJ, Cheng J, Grande JP, Sutor SL, Bram RJ, Bren GD, Badley AD, Alam J, Nath KA. Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am J Physiol. 2007;292:F837–F844. doi: 10.1152/ajprenal.00254.2006. [DOI] [PubMed] [Google Scholar]
- Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol. 2007;170:1820–1830. doi: 10.2353/ajpath.2007.061093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, Chung HT. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]