Abstract
TAR DNA-binding protein (TDP-43) has been recently described as a major pathological protein in both frontotemporal dementia with ubiquitin-positive inclusions (FTLD-U) and amyotrophic lateral sclerosis. However, little is known about the relative abundance and distribution of different pathological TDP-43 species, which include hyperphosphorylated, ubiquitinated, and N-terminally cleaved TDP-43. Here, we developed novel N-terminal (N-t) and C-terminal (C-t)-specific TDP-43 antibodies and performed biochemical and immunohistochemical studies to analyze cortical, hippocampal, and spinal cord tissue from frontotemporal dementia with ubiquitin-positive inclusions and amyotrophic lateral sclerosis cases. C-t-specific TDP-43 antibodies revealed similar abundance, morphology, and distribution of dystrophic neurites and neuronal cytoplasmic inclusions in cortex and hippocampus compared with previously described pan-TDP-43 antibodies. By contrast, N-t-specific TDP-43 antibodies only detected a small subset of these lesions. Biochemical studies confirmed the presence of C-t TDP-43 fragments but not extreme N-t fragments. Surprisingly, immunohistochemical analysis of inclusions in spinal cord motor neurons in both diseases showed that they are N-t and C-t positive. TDP-43 inclusions in Alzheimer’s disease brains also were examined, and similar enrichment in C-t TDP-43 fragments was observed in cortex and hippocampus. These results show that the composition of the inclusions in brain versus spinal cord tissues differ, with an increased representation of C-t TDP-43 fragments in cortical and hippocampal regions. Therefore, regionally different pathogenic processes may underlie the development of abnormal TDP-43 proteinopathies.
Frontotemporal lobar degeneration (FTLD) is the diagnostic term for a group of clinically, genetically, and neuropathologically heterogeneous neurodegenerative disorders characterized by prominent atrophy of the frontal and anterior temporal lobes. FTLD is the second most common neurodegenerative cause of dementia after Alzheimer’s Disease (AD) under age 65.1,2 The most prevalent clinical form of FTLD is frontotemporal dementia, which primarily manifests as changes in social and personal behavior, including disinhibition, and a progressive disorder of language.3 Affected individuals can develop a movement disorder including Parkinsonism and/or motor neuron disease (MND). The neuropathology of FTLD can be broadly divided into those with tau-positive inclusions known as tauopathies, and those with ubiquitin-positive, tau-negative, and synuclein-negative inclusions (UBIs) referred to as FTLD-U, which is the most common underlying neuropathology in FTLD.3 Recent studies also classified FTLD-U cases into four major subtypes based on the distribution of UBIs detected by immunohistochemistry (IHC).4,5,6
Amyotrophic lateral sclerosis (ALS) is an adult-onset progressive neurodegenerative disease that involves selective death of the upper and lower motor neurons, leading to muscle atrophy, paralysis, and death.7 Approximately 90% of ALS cases are sporadic and have no known cause, but the remaining 10% have a genetic basis.8 Significantly, some ALS patients also develop a dementia consistent with FTLD.9 The neuropathology of ALS is characterized by UBIs formed by abnormal accumulations of ubiquitin-positive filamentous skeins or compact, round Lewy body-like inclusions in the cytoplasm of degenerating motor neurons.10 Moreover, there is evidence showing that ALS is a multisystem neurodegenerative disease, with pathological inclusions present in additional regions, including hippocampal and neocortical structures.11,12
Recently, TAR-DNA binding protein (TDP-43) was identified as the major disease protein in UBIs of sporadic and familial forms of FTLD-U and of sporadic and familial forms of ALS with the notable exception of autosomal dominantly inherited ALS caused by SOD1 mutations.13,14,15 These important findings suggest that FTLD-U and ALS represent a clinicopathological spectrum of the same neurodegenerative disorder. Furthermore, the presence of pathological TDP-43 in these diseases was rapidly confirmed by others.16,17 TDP-43 is a ubiquitously expressed nuclear protein that has been reported to facilitate RNA splicing and transcriptional repression,18,19 although much of the biology and functions of TDP-43 remain enigmatic. Pathological TDP-43 is distinct from its normal counterpart because it is hyperphosphorylated, ubiquitinated, and cleaved to generate C-terminal (C-t) fragments in affected brain and spinal cord of FTLD-U and ALS.15 Furthermore, these N-terminal (N-t) truncated TDP-43 species are only recovered in sarkosyl-insoluble fractions, suggesting that they could serve as a nidus for the aggregation and formation of UBIs. To test this hypothesis, we generated antibodies specific to the N-t and C-t of TDP-43 and used them to assess the relative abundance and distribution of full-length (FL) and N-t truncated C-t fragments of TDP-43. Our findings demonstrate that although FL-TDP-43 pathology is similarly represented in spinal cord motor neurons of FTLD-U and ALS cases, brain UBIs contain predominantly C-t fragments. Our data suggest that the pathogenic mechanisms of brain and spinal cord TDP-43 inclusion formation may be different.
Materials and Methods
Case Selection and Pathological Assessment
Frozen brain tissues and fixed, paraffin-embedded tissue blocks were obtained from the Center for Neurodegenerative Disease Research Brain Bank at the University of Pennsylvania. Consent for autopsy was obtained from legal representatives for all subjects in accordance with local institutional review board requirements. All cases were diagnosed by trained neuropathologists in accordance with published guidelines for ALS20 and different pathological variants of FTLD.2,3 Three disease groups were defined, representing FTLD-U with and without MND and ALS cases. Within the FTLD-U cases, they were further subdivided into subtypes I, II, and III4 (Supplemental Table 1, see http://ajp.amjpathol.org). Additionally, two cases of subtype IV5 with VCP mutations were also used, but because of limited availability of tissue, the data are only shown in Supplemental Figure 1 (see http://ajp.amjpathol.org). Age-matched normal cases were included as nondisease controls. Demographics of the patients, including diagnoses, age at onset, disease duration, and gender are summarized in Supplemental Table 1. In addition, cases diagnosed with a progressive dementia and showing typical Alzheimer’s disease (AD) pathology (CERAD stage C and Braak & Braak stages V–VI) by neuropathological examination were studied. These cases fulfilled the criteria for high likelihood of AD according to the National Institute on Aging criteria.21
Generation of TDP-43 Antibodies
Two polyclonal antibodies (pAb) were produced by immunizing rabbits (Covance Research Products, Inc., Denver, PA). The first (designated as N-t TDP-43) was raised against a synthetic peptide near the N-t of human TDP-43 corresponding to amino acid residues 6 to 24. The other antibody (designated as C-t TDP-43) was raised against an extreme C-terminal synthetic peptide, corresponding to amino acid residues 394 to 414 of human TDP-43. The resulting immune sera were affinity purified, and their specificities were characterized using recombinant full-length and C-t truncated T-7 tagged human TDP-43 (ProteinTech Group, Chicago, IL), QBI293 cell lysates, and sarkosyl-insoluble fractions from all three types of FTLD-U brains. A murine C-t monoclonal antibody (mAb) was generated by immunizing mice with the same synthetic peptide used for the C-t TDP-43 pAb, as previously described.22 Fusion was conducted by using spleen lymphocytes from immunized BALB/c mice and SP2 cells to produce hybridomas. Resulting hybridoma supernatants were screened by enzyme-linked immunosorbent assay using plates coated with the immunizing peptide, and their specificity was determined by immunoblot and IHC on paraffin-embedded sections of FTLD-U cortex known to contain TDP-43-positive inclusions.
Immunohistochemical Staining
The harvesting, fixation, and further processing of the tissue specimens used in this study were conducted as previously described.4,15 Briefly, fresh tissues from brain and spinal cord of FTLD-U or ALS cases were fixed with either 70% ethanol in 150 mmol/L NaCl or phosphate-buffered 3.65% formaldehyde, infiltrated with paraffin, and cut into 6-μm serial sections. Antigen retrieval was done by boiling the sections in 10 mmol/L citrate buffer (pH 6.0) in a microwave oven. IHC was performed using the avidin-biotin complex (ABC) detection system (Vector Laboratories, Burlingame, CA) and 3,3′-diaminobenzidine as described previously.4 Briefly, sections were deparaffinized and rehydrated, endogenous peroxidases were quenched with 5% H2O2 in methanol for 30 minutes, and sections were blocked in 0.1 mol/L Tris with 2% fetal bovine serum for 5 minutes. Primary antibodies were incubated for either 1 or 2 hours at room temperature or overnight at 4°C. The following primary antibodies were used: mouse anti-ubiquitin (1510; 1:40,000; Chemicon, Temecula, CA), mAb and affinity-purified rabbit polyclonal anti-TDP-43 (TDP-43 mAb and pan TDP-43 pAb; 1:50,000; Abnova Corp., Taiwan; and 1:4500; ProteinTech Group, respectively), C-t TDP-43 pAb (1:60,000), N-t TDP-43 pAb (1:60,000), and C-t TDP-43 mAb supernatant (1:30). After washing, sections were sequentially incubated with biotinylated secondary antibodies for 1 hour and avidin-biotin complex for 1 hour. Bound antibody complexes were visualized by incubating sections in a solution containing 100 mmol/L Tris, pH 7.6, 0.1% Triton X-100, 1.4 mmol/L diaminobenzidine, 10 mmol/L imidazole, and 8.8 mmol/L H2O2. Sections were then lightly counterstained with hematoxylin, dehydrated, and coverslipped.
Double-labeling immunofluorescence (IF) analyses were performed as previously described15 using Alexa Fluor 488- and 594-conjugated secondary antibodies (Molecular Probes, Eugene, OR), treated for autofluorescence with Sudan Black solution,23 and coverslipped with Vectashield-DAPI mounting medium (Vector Laboratories). Digital images were obtained using an Olympus BX 51 microscope (Tokyo, Japan) equipped with bright-field and fluorescence light sources using a ProgRes C14 digital camera (Jenoptik AG, Jena, Germany) and Adobe Photoshop, version 9.0 (Adobe Systems, San Jose, CA) or digital camera DP71 (Olympus) and DP manager (Olympus).
Quantification of Pathology
Semiquantitative analysis of pan TDP-43, C-t TDP-43, or N-t TDP-43 pAb-immunoreactive pathology was performed on sections with the most robust pathology from neocortex, hippocampus, and spinal cord following a modified protocol described previously.24 For all TDP-43 antibodies, the severity of neocortical and hippocampal pathology was rated semiquantitatively: 0, no inclusions; 1, rare; 2, mild; 3, moderate; and 4, severe. The scoring system for lower motor neuron cytoplasmic inclusions was based on total numbers in a single section of spinal cord. A total score including filamentous, round, or irregular shaped inclusions or small dense granules was calculated using the following scoring system: 0, none; 1, 1 inclusion per section; 2, 2–5 inclusions per section; 3, 6–10 inclusions per section; and 4, >10 inclusions per section. Sections were primarily scored by one investigator (L.M.I.), with validation performed by different researchers (K.U., M.N., J.Q.T.). Statistical nonparametric analysis of neuropathological scores was performed using Kruskal-Wallis test followed by Dunn’s multiple comparison post test.
Immunoblot Analysis
Postmortem brain tissue from FTLD-U and control cases was dissected, weighed, and sequentially extracted with buffers of increasing strength, as described previously.15 For Western blot analysis, protein extracts were resolved by Tris-glycine 5 to 20% gradient SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with primary and secondary antibodies (horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG; Jackson ImmunoReasearch, West Grove, PA). Blots were developed with Renaissance Enhanced Luminol Reagents (NEN Life Science Products, Inc., Boston, MA), and digital images were acquired using a Fujifilm Intelligent Darkbox II (Fuji Systems USA, Stamford, CT). Where indicated, TDP-43 was dephosphorylated by dialysis (20 mmol/L Tris and 0.2 mmol/L EDTA, pH 8.0) and treated with Escherichia coli alkaline phosphatase (Sigma-Aldrich, St. Louis, MO) for 2 hours at 56°C.
Results
Characterization of Termini-Specific Rabbit Polyclonal Antibodies
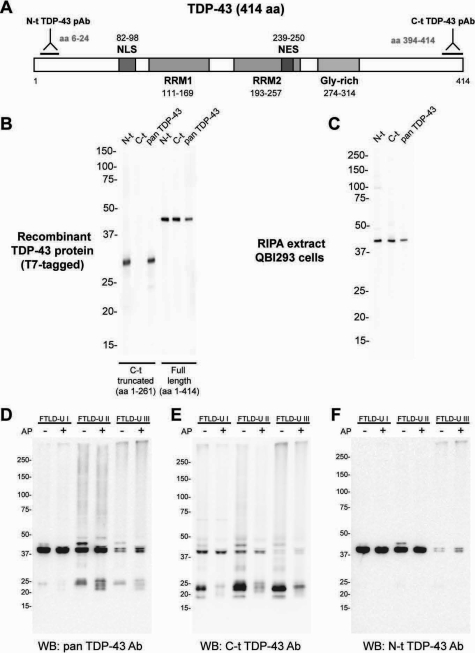
The identification of one or more extreme C-t fragments recovered from the sarkosyl-insoluble fractions of FTLD-U brains15 led us to investigate the relative abundance of FL, C-t, or N-t TDP-43 fragments in UBIs of FTLD-U and ALS. To accomplish this, we generated pAbs that recognize epitopes at the N-t and extreme C-t of the TDP-43 protein (Figure 1A). To demonstrate the specificities of these antibodies to detect the N-t and C-t of the TDP-43 protein, we tested these TDP-43 pAbs using recombinant full-length or C-t truncated TDP-43 protein in immunoblots. A commercially available TDP-43 rabbit pAb (pan TDP-43) that was raised to recombinant TDP-43 and used in previous published reports15,17 was included in all studies as a positive control. Although both N-t and C-t TDP-43 pAbs recognize FL-TDP-43, N-t and not C-t TDP-43 pAb recognizes the C-t truncated TDP-43 fragment (Figure 1B). Furthermore, both N-t and C-t TDP-43 pAbs detected endogenous TDP-43 from QBI293 cell extracts (Figure 1C), and indirect IF studies confirm that the N-t- and C-t-specific antibodies detect the endogenous nuclear TDP-43 protein in human QBI293 cells (data not shown).
Figure 1.
Biochemical characterization of novel termini-specific TDP-43 polyclonal antibodies. A: Schematic diagram of TDP-43 indicating the relative position of the peptides used to generate the pAbs against N-t and C-t regions. The diagram also shows, in scale, the most prominent features of the protein (domains and predicted localization signals). NLS, nuclear localization signal; NES, nuclear export signal; RRM, RNA-recognition motif; Gly-rich, glycine-rich domain. B and C: Representative images showing the immunoblotting pattern for TDP-43 antibodies using T7-tagged recombinant human TDP-43 (C-terminally truncated or full length) (B) or radioimmunoprecipitation assay (RIPA) lysates from QBI293 cells (C). D–F: TDP-43 immunobotting of sarkosyl-insoluble fractions from different FTLD-U subtype cases before (−) and after (+) alkaline phosphatase (AP) treatment. Proteins from frontal cortex of FTLD-U cases were sequentially extracted with buffers of increasing strength and subjected to SDS-polyacrylamide gel electrophoresis. Dephosphorylation of FTLD-U urea extracts with AP followed by immunoblotting with pan TDP-43 (D) and C-t TDP-43 pAb (E) collapsed the 45-kDa band into the 43-kDa band and separated TDP-43 fragments into four immunoreactive ∼23- to ∼27-kDa bands. Immunoblotting with N-t TDP-43 pAb (F) shows the collapse of the 45-kDa band, but no evidence for low molecular weight species. This indicates that pathological, sarkosyl-insoluble TDP-43 is abnormally phosphorylated and that low molecular weight pathological bands contain extreme C-t TDP-43 fragments.
Previous studies have identified a disease-specific biochemical signature of pathologically altered TDP-43 in sarkosyl-insoluble but urea-soluble extracts of gray matter from sporadic and familial FTLD-U cases of different subtypes.15 To evaluate the specificity and affinity of our antibodies in detecting this pathological signature, we conducted immunoblot analyses on urea fraction extracted from frontal cortices of FTLD-U type I-III cases (Figure 1, D–F). Although both C-t- and N-t-specific antibodies detected FL-TDP-43, C-t but not N-t TDP-43 pAb detected C-t fragments from all three subtypes. Interestingly, the C-t pAb showed less affinity for FL-TDP-43 compared with the N-t and pan TDP-43 pAbs, but it detected the C-t fragments better than the pan TDP-43 pAb. Both C-t and N-t pAbs detected phosphorylated FL TDP-43 at 45 kDa, which upon dephosphorylation collapsed into the 43-kDa protein band (Figure 1, D–F). Unexpectedly, although the C-t TDP-43 pAb was raised to a nonphosphorylated synthetic peptide, dephosphorylation of the C-t fragments reduced the affinity of the antibody for these fragments. Finally, the C-t detected much more high Mr ubiquitinated smear than the N-t TDP-43 pAb from all three FTLD-U subtypes (Figure 1, D–F). Thus, these termini-specific pAbs exhibit the desirable specificities for IHC studies described below.
TDP-43 Neuropathology Is Comprised Mostly of C-Terminal Fragments in Dentate Gyrus and Frontal and Temporal Cortices of FTLD-U Cases
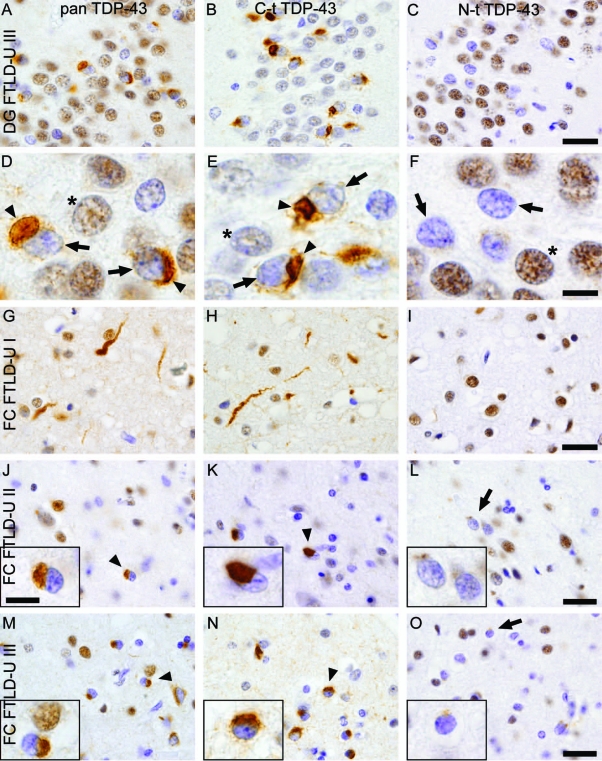
To determine whether TDP-43 inclusions in different brain regions of FTLD-U cases comprise mostly FL or C-t fragment of TDP-43, we conducted IHC studies using serial sections from FTLD-U cases (Supplemental Table 1) and probed with C-t, N-t, or pan TDP-43 pAbs. Analysis of hippocampal tissue sections from FTLD-U cases showed robust staining of UBIs in granule cells of the dentate gyrus with the C-t and the pan TDP-43 pAb but not the N-t TDP-43 pAb (Figure 2, A–F). Consistent with previous observations,15 nuclear clearance of endogenous TDP-43 was observed when cells contained TDP-43 inclusions (arrowheads), but most albeit not all nuclei of normal cells were immunostained (compare with normal TDP-43 staining in nonaffected neurons).
Figure 2.
Anti-TDP-43 C-t antibodies detect more neuropathology than N-t antibodies in dentate gyrus and frontal cortex of FTLD-U subtypes. A-F: Adjacent sections of hippocampal tissue from FTLD-U cases show robust labeling of neuronal cytoplasmic inclusions (arrowheads) in the dentate gyrus (DG) using both pan TDP-43 antibody (A and D) and C-t TDP-43 pAb (B and E), in contrast to marginal staining with N-t TDP-43 pAb (C and F). D–F: High-power views of the fields depicted in A–C. Note clearing of nuclear TDP-43 (arrows) in inclusion-bearing neurons compared with that of nonaffected neurons (*). G–O: Adjacent sections of frontal cortex (FC) in FTLD-U cases show the characteristic features of type I pathology (G–I), with long and tortuous dystrophic neurites with relatively few neuronal cytoplasmic inclusions and no neuronal intranuclear inclusions. Type II cases (J–L) present numerous cytoplasmic inclusions and infrequent neuritic profiles. Type III cases (M–O) have numerous neuronal cytoplasmic inclusions and dystrophic neurites and occasional intranuclear inclusions in lamina II. Note the similar immunoreactivity pattern of characteristic TDP-43 pathology, ie, cytoplasmic inclusions (arrowheads) and dystrophic neurites, for pan TDP-43 pAb (G, J, and M) and C-t TDP-43 pAb (H, K, and N). Use of N-t TDP-43 pAb (I, L, and O) revealed remarkably reduced staining for both types of pathology (arrows) but robust nuclear staining of normal cells. Insets in J–O show a high-power detail of representative staining. Scale bars: 25 μm (A–C and G–O); 7 μm (D–F and insets in J–O).
Next, we examined whether cortical TDP-43 pathology as seen in the different FTLD-U subtypes also showed differential immunostaining. We analyzed cases representing types I, II, and III as previously described using ubiquitin IHC.4,15 Tissue sections of frontal or temporal cortices from type I, II, and III FTLD-U cases were analyzed, and we found that cortical pathology presented a similar differential staining as those in the hippocampus with robust staining of inclusions using C-t but not N-t TDP-43 pAb. Pan TDP-43 pAb detected similar neuropathology as C-t TDP-43 pAb detecting dystrophic neurites (Figure 2, G and H) and neuronal cytoplasmic inclusions (Figure 2, J, K, M, and N). Nonetheless, N-t TDP-43 pAb did detect some albeit reduced levels of TDP-43 lesions in all three FTLD-U types (Figure 2, I, L, and O). Analysis of temporal cortex samples representing the different FTLD-U types showed remarkably similar results (data not shown). Interestingly, N-t TDP-43 pAb presented a higher immunoreactivity for endogenous nuclear TDP-43 than C-t pAb, possibly due to a higher availability of the epitope; this was consistent with the results from antibody characterization by immunoblot (Figure 1, D–F). In addition, glial inclusions were mostly positive for both C-t and N-t TDP-43 antibodies (data not shown). IHC performed using both a harsher antigen retrieval method (formic acid pretreatment), and frozen sections showed a similar differential pattern of immunostaining (data not shown).
In addition to sporadic or familial FTLD-U cases with unknown genetic cause, IHC was also conducted in familial FTLD-U cases, including those with progranulin (GRN) and valosin-containing protein (VCP) mutations.5,25,26 C-t- but not N-t TDP-43 pAb detected cytoplasmic and neuritic inclusions in cases with GRN and VCP mutations (Supplemental Figure 1, A–C see http//ajp. amjpathol.org), however, neuronal intranuclear inclusions were consistently recognized by both C-t and N-t TDP-43 pAbs (Supplemental Figure 1, D–F, see http//ajp. amjpathol.org). Although the number of familial GRN and VCP cases available for IHC was small, this observation suggests differences in the composition of TDP-43 protein fragments in intranuclear versus that of cytoplasmic and neuritic inclusions.
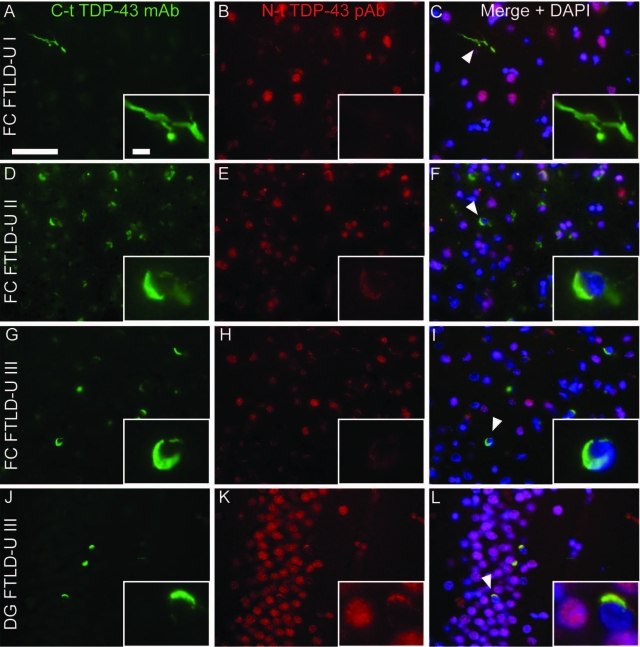
To further demonstrate that brain TDP-43 UBIs are detected more readily by C-t and not N-t antibodies, we conducted double-labeling IF studies using a combination of the three TDP-43 pAbs and a newly developed mAb to the same C-t synthetic peptide used to generate the C-t TDP-43 pAb. Immunoblot and IHC analysis demonstrate that C-t TDP-43 mAb has similarly specificities as those of the C-t TDP-43 pAb (Supplemental Figure 2; see http://ajp.amjpathol.org). Significantly, N-t TDP-43 pAb only colocalized with a small subset of cytoplasmic and neuritic inclusions detected by the C-t TDP-43 mAb in the various subtypes of FTLD-U brains [Figure 3, A–I (frontal cortex) and J–L (dentate gyrus)]. On the other hand, both N-t pAb and C-t mAb detected round and lentiform nuclear inclusions in VCP cases (Supplemental Figure 1, G–R, see http//ajp.amjpathol.org).
Figure 3.
Colocalization studies of C-t and N-t TDP-43 antibodies in FTLD-U. Double-label immunofluorescence of FTLD-U cases with a C-t TDP-43 mAb (A, D, G, and J) and N-t TDP-43 pAb (B, E, H, and K) shows almost complete lack of N-t pAb staining for the C-t-positive inclusions in the presence of normal nuclear TDP-43 staining (Merge + DAPI panels: C, F, I, and L). This was observed in frontal cortices from all FTLD-U types analyzed (A–I) and, to a lesser extent, in hippocampal granule cells (J–L). Scale bars: 50 μm (A–L); 5 μm (insets).
Additional studies were also conducted to document that the TDP-43 inclusions detected by the N-t and C-t TDP-43 pAbs are also ubiquitinated. To accomplish this, we performed double-labeling IF using our C-t TDP-43 pAb and an anti-ubiquitin mAb and demonstrated almost perfect colocalization (arrowheads) of UBIs in neurons of the dentate gyrus (Supplemental Figure 3; see http://ajp.amjpathol.org). By contrast, N-t TDP-43 pAb only recognized weakly a subset of UBIs detected by the ubiquitin mAb. Interestingly, a number of dystrophic neurites were also detected by the C-t TDP-43 pAb but not the ubiquitin mAb (data not shown). Taken together, our data suggest that the TDP-43 neuropathology in FTLD-U brains are enriched in TDP-43 C-t fragments and that only low levels of FL-TDP-43 might be present in these inclusions.
TDP-43 Neuropathology in Spinal Cord Motor Neurons of ALS Cases Comprise Mostly Full-Length TDP-43
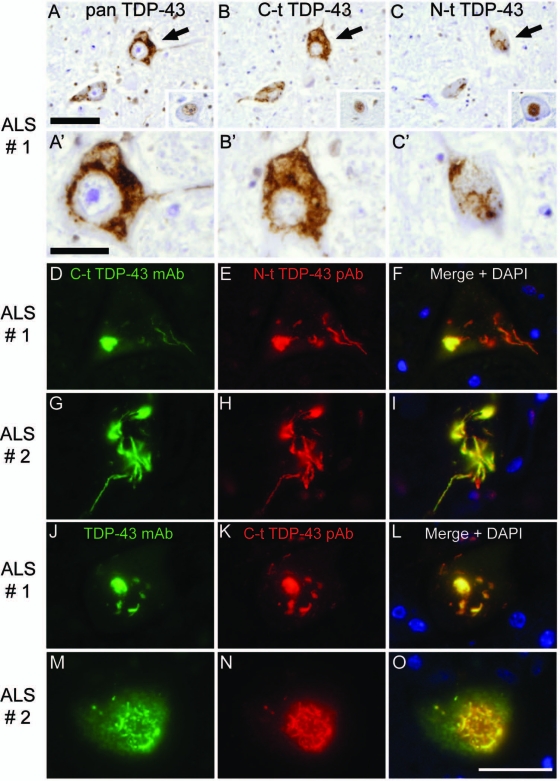
To examine whether the TDP-43-positive filamentous skeins and Lewy-body-like inclusions in ALS motor neurons are also enriched in TDP-43 C-t fragments, we conducted IHC using the same pAbs as described above to immunostain spinal cord sections from ALS cases. All three antibodies showed variable IR for normal nuclear TDP-43 in anterior horn cells [Figure 4, A–C (insets)]. Surprisingly, both C-t and N-t TDP-43 pAb immunostained almost equivalent numbers of inclusion-bearing neurons in serial sections of ALS brains (Figure 4, A–C). Moreover, double IF staining using a combination of C-t mAb and N-t pAb for TDP-43 as well as C-t pAb with a previously described commercial TDP-43 mAb confirmed and extended these findings (Figure 4, D–O). Thus, these results suggest that the lesions in ALS motor neurons comprise primarily the FL-TDP-43 protein, although it cannot be excluded that equal amounts of C-t and N-t fragments are present in the motor neuron inclusions to account for these findings.
Figure 4.
Single-color immunohistochemistry and double-label immunofluorescence of neuronal cytoplasmic inclusions in ALS spinal cord. A–C: Adjacent sections of spinal cord in sporadic ALS cases show that the same motor neurons display inclusions positive for all three antibodies tested, pan TDP-43 (A), C-t TDP-43 (B), and N-t TDP-43 pAb (C). Note positive nuclear staining for all three antibodies (insets). A high-power view of one motor neuron (arrow) from A–C is shown (A′–C′). D–I: Double-label immunofluorescence shows that both filamentous and compact, round cytoplasmic inclusions in sporadic ALS cases display complete colocalization (F and I) of C-t TDP-43 mAb (D and G) and N-t TDP-43 pAb (E and H) staining. For comparison, the same cases were stained with a combination of commercial TDP-43 mAb (J and M) and C-t TDP-43 pAb (K and N), which also show complete colocalization (L and O). Scale bars: 50 μm (A–C); 20 μm (A′–C′ and D–O).
Differential Enrichment of C-Terminal Fragments versus Full-Length TDP-43 in Brain and Spinal Cord of FTLD-U and ALS Cases
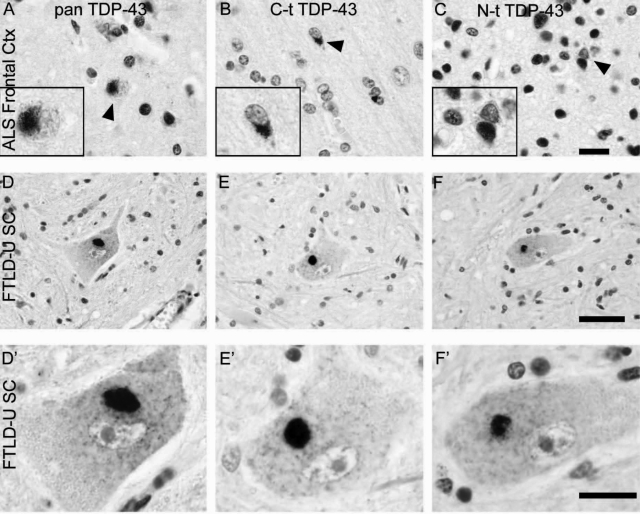
Because many FTLD-U patients develop MND and because some ALS patients show signs of frontotemporal dementia, we next asked whether the differential immunostaining patterns of TDP-43 neuropathology seen with C-t and N-t TDP-43 pAbs correlated with brain versus spinal cord of FTLD-U versus ALS cases. To do this, we immunostained frontal cortices and hippocampus of ALS cases (with and without dementia) and spinal cord of FTLD-U cases (with and without MND). Our results showed that TDP-43 inclusions in dentate gyrus and frontal cortex of ALS cases were detected primarily by C-t but not N-t TDP-43 pAbs whereas motor neurons in spinal cord of FTLD-U are recognized by both C-t and N-t TDP-43 pAbs (Figure 5), suggesting that brain TDP-43 inclusions are enriched in C-t fragments, whereas spinal cord inclusions comprise mostly FL-TDP-43.
Figure 5.
TDP-43 pathology in FTLD-U with MND spinal cord and ALS frontal cortex. A set of three adjacent sections of ALS frontal cortex (A–C) or FTLD-U with MND spinal cord tissue (D–F) were stained with pan TDP-43 (A and D), C-t TDP-43 (B and E), or N-t TDP-43 pAb (C and F). Whereas the pathology staining pattern for FTLD-U with MND spinal cord was similar for all three antibodies (D–F), the cortical pathology in ALS cases with dementia showed reduced N-t TDP-43 immunoreactivity compared with the other two antibodies (arrowheads; A–C). High-power images are shown in D′–F′ and insets on A–C. Scale bars: 20 μm (A–C and D′–F′); 50 μm (D–F); 6 μm (insets in A–C).
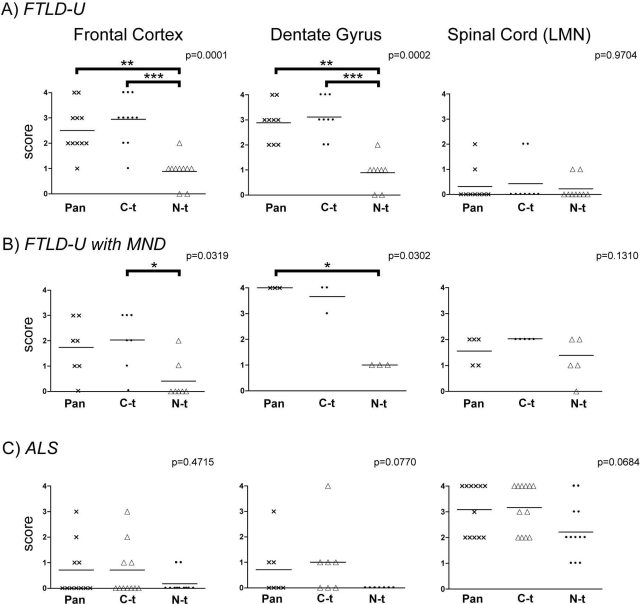
Finally, we conducted semiquantitative IHC analysis in 19 FTLD cases with and without MND and 12 ALS cases with and without dementia using the C-t and N-t TDP-43 pAbs. We observed statistical differences between TDP-43 neuropathology detected with the C-t versus the N-t TDP-43 pAbs in FTLD-U with and without MND in both frontal cortex and dentate gyrus (Figure 6). However, although a similar trend of differential immunostaining of TDP-43 inclusions can be seen in the same brain regions of ALS cases, it did not reach statistical significance because of the lower abundance and the variability of the pathology. Similar variability in the amount of TDP-43 motor neuron inclusions also was noted when we examined the spinal cord of FTLD-U cases with and without MND and ALS cases. Nevertheless, the semiquantitative scores between the TDP-43 inclusions detected by the C-t and N-t TDP-43 pAbs are less disparate. Analysis of normal controls displayed no pathological pan, C-t, or N-t immunoreactivity. Taken together, these observations are consistent with the notion that C-t fragments are concentrated in TDP-43 inclusions in hippocampus and frontal cortex, whereas motor neuron inclusions comprise primarily FL-TDP-43.
Figure 6.
Semiquantitative analysis of TDP-43-positive pathology in FTLD-U and ALS cases reveal differential enrichment of C-t fragments in brain regions. Scatter plots illustrating individual values and means of the neuropathological immunoreactivity scores for pan-TDP-43 (crosses), C-t TDP-43 (dots), and N-t TDP-43 (triangles) polyclonal antibodies. A: TDP-43 pathology score values from FTLD-U cases in frontal cortex gray matter (left), hippocampal dentate gyrus (middle), and lower motor neurons (LMN) in spinal cord (right). B: Comparable data sets compiled from FTLD-U with MND cases. C: Data from ALS cases. *P < 0.05; **P < 0.01; ***P < 0.001 (Dunn’s multiple comparison post test).
C-t Fragments Are Enriched in TDP-43 Inclusions in AD Hippocampus and Frontal Cortex
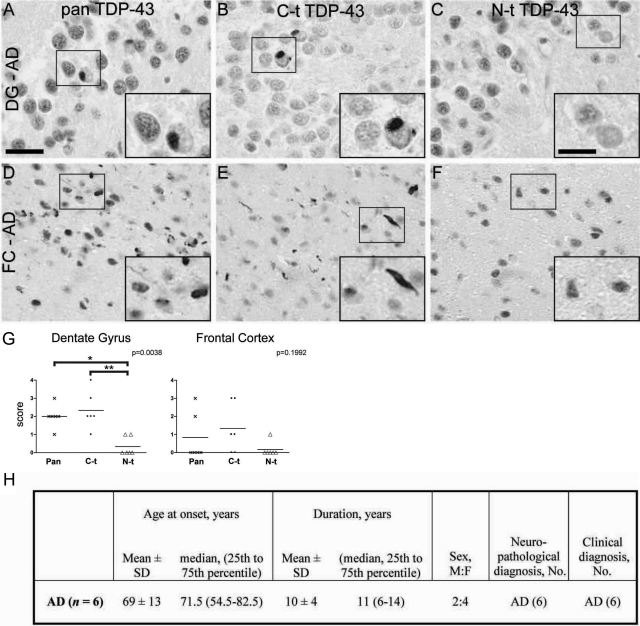
Because previous studies have shown the presence of TDP-43 neuropathology in other neurodegenerative diseases including AD,27 we asked whether TDP-43 C-t fragments are also enriched in AD. To this end, we examined the hippocampus and frontal cortex of six AD cases with TDP-43 lesions and showed that C-t-specific TDP-43 antibodies detected many more inclusions than N-t antibodies (Figure 7), whereas spinal cord TDP-43 pathology was virtually absent in the AD spinal cord samples. Thus, these data provide additional support for our view that the disparate distribution of C-t versus N-t TDP-43 immunoreactivities is specific to brain regions and not the neurodegenerative disorder.
Figure 7.
Differential C-t and N-t anti-TDP-43 immunoreactivities in AD brain. A–F: Adjacent sections of hippocampus (A–C) or frontal cortex (FC; D–F) from AD brains show robust labeling of neuronal cytoplasmic inclusions in the dentate gyrus (DG) and dystrophic neurites in FC using both pan TDP-43 antibody (A and D) and C-t TDP-43 pAb (B and E); in contrast, only marginal pathological staining is revealed with N-t TDP-43 pAb (C and F). High-power images corresponding to the boxed regions are shown in insets in A–F. Scale bars: 25 μm (A–F); 12 μm (all insets). G: Scatter plots showing individual values and means of the neuropathological immunoreactivity scores for pan-TDP-43 (crosses), C-t TDP-43 (dots), and N-t TDP-43 (triangles) pAbs. The diagrams display TDP-43 pathology score values from AD cases in hippocampal dentate gyrus (left) and frontal cortex gray matter (right). *P < 0.05; **P < 0.01 (Dunn’s multiple comparison post test). H: Summary of demographic characteristics of AD patients used in this study. F, female: M, male; No, number.
Discussion
TDP-43 has recently emerged as a novel pathological protein that defines a new family of neurodegenerative disorders, ie, TDP-43 proteinopathies,28 and abnormal TDP-43 is present in the signature lesions in a spectrum of diseases that includes FTLD-U, FTLD with MND, and ALS.5,14,15,17,24 However, little is known about the consequences of pathologically altered TDP-43 or about the differential distribution of abnormal TDP-43 in affected regions of brain and spinal cord in FTLD-U, FTLD with MND, and ALS. The purpose of this study was to determine the regional incidence of FL and C-t versus N-t forms of TDP-43 across the neuropathological spectrum represented by FTLD-U and ALS, using a panel of novel C-t- and N-t-specific antibodies. Our results show that TDP-43 pathology found in cortical and limbic brain regions of FTLD-U and ALS cases is enriched in TDP-43 C-t fragments, and we found no biochemical evidence of N-t TDP-43 fragments in these regions. On the other hand, analysis of spinal cord tissue from affected individuals showed no C-t TDP-43 fragment enrichment relative to FL, thereby suggesting that the pathogenic mechanisms underlying inclusion formation in spinal cord and brain might be different. Remarkably, this finding correlates with which structure (ie, brain versus spinal cord) is analyzed and not with one specific disorder because brain lesions from FTLD-U, ALS, and AD are enriched in C-t TDP-43 fragments.
Semiquantitative analysis of TDP-43-positive pathology in FTLD-U and ALS cases suggested that boundaries between neurodegenerative disorders can be difficult to define at the clinico-neuropathological level, and our data here show that the abundance of N-t and C-t TDP-43-positive inclusions is comparable in the spinal cord but not in brain regions (Figure 6). Clinico-pathological definitions of FTLD-U with MND and ALS with dementia are seemingly dependent on which symptoms present first, although the underlying pathological processes may be similar.29 In that respect, it is interesting to note that, although limited in number, the ALS cases that showed brain pan- and C-t-positive TDP-43 pathology presented almost no cortical or limbic N-t TDP-43 lesions (Figure 6C). These cases included most of those clinically diagnosed as ALS with dementia and displayed a remarkably similar TDP-43 immunoreactivity profile compared with FTLD-U with MND cases. Some of these cases (ie, showing TDP-43-positive pathology in spinal cord from FTLD-U and brain inclusions in ALS) may represent examples of subtle changes developed during a prodromal stage and support the notion that these disorders probably reflect different facets of a disease spectrum. Future studies with larger series of cases from both familial and sporadic forms of these diseases will be required to elucidate the significance of these observations.
Our immunobloting data complemented and supported the immunohistochemical studies. Although biochemical analysis of urea fractions from spinal cord of ALS cases demonstrated in previous studies13,15 a disease-specific TDP-43 signature similar to that described for FTLD-U,15 the presence of C-t fragments in this region is quite low and highly variable, when compared with urea extracts from FTLD-U brains. An interesting trend can be also detected within FTLD-U cases, where we observed that subtype I generally presents a higher immunoreactivity for N-t TDP-43 pAb and a weaker C-t signature at the biochemical level (data not shown). Lastly, immunohistochemical data from this study (Supplemental Figure 1, see http//ajp.amjpathol.org) suggest that the sparse load of C-t TDP-43 species biochemically recovered from FTLD-U cases with VCP mutations5 might be explained by a predominance of nuclear inclusions mainly composed of FL TDP-43. Altogether, these observations are consistent with the notion that heterogeneity of TDP-43 inclusion composition can be assessed with these novel antibodies.
The molecular basis for the different profiles of TDP-43 pathologies in spinal cord and brain of FTLD-U and ALS is not clear. Although there are no data at present regarding the pathophysiological regulation of TDP-43 subcellular localization, it would be of interest to define potential differences in nucleocytoplasmic transport mechanisms between motor neurons and other neuronal populations. The morphology of cytoplasmic inclusions in spinal cord motor neurons is consistent across the three TDP-43 polyclonal antibodies used in this study, arguing against a different composition of skeins and Lewy-body like structures. In the brain, inclusions labeled by pan- and C-t TDP-43 antibodies have a similar distribution and morphology, further supporting the notion that both antibodies detect the same population of cortical and hippocampal UBIs enriched in C-t TDP-43 fragments.
Given that TDP-43 has been described as a predominantly nuclear protein under physiological conditions,30,31 how and why TDP-43 deposits in cytoplasmic aggregates remains enigmatic. Although there are several examples of regulation of subcellular localization by posttranslational modification (ie, phosphorylation,32 ubiquitination, and sumoylation33), it is possible that mechanisms underlying the formation of cytoplasmic inclusions involve the proteolytical processing of TDP-43 in the nucleus, which gives rise to C-t species that shuttle to the cytoplasm, due to passive diffusion or loss of a predicted nuclear localization signal in the N-t region of TDP-43. These fragments might have altered solubility properties and could be subjected to further posttranslational modifications. Conversely, translocation of full-length TDP-43 to the cytoplasm by an unknown mechanism could lead to its proteolytic processing. Interestingly, a recent report showed that TDP-43 can be proteolytically processed by caspase-3 both in vitro and in cell culture.34 An important point to address is the pathological sequence of events that leads to abnormal modifications of TDP-43 and how these modifications play a role in the aberrant subcellular distribution of TDP-43. It is also intriguing that the majority of inclusion-bearing cells display nuclei devoid of TDP-43, thereby suggesting that some of the deleterious effects of abnormal TDP-43 processing or metabolism reflect a loss of TDP-43 nuclear function in affected cells. The results presented in this study, revealing the presence of C-t but not N-t TDP-43 fragments in affected brain areas, suggest that these insoluble C-t species might have a role in inclusion formation, whereas the other protein domains, such as the N-t fragments, are degraded more quickly by proteasomal, autophagy-dependent, or other pathways.
Although TDP-43 pathology was first described and characterized in FTLD-U and ALS cases, it is now becoming increasingly evident that the full range of disorders with TDP-43 inclusions is yet to be discovered. Evidence from a number of recent studies indicate that there is concurrent TDP-43 pathology in subsets of AD patients,27 nearly all Guam-Parkinson dementia complex patients,35,36 and some Lewy body disease37,38 cases, but the list of diseases in which TDP-43 pathology co-occurs may grow with increasing research on this newly recognized disease protein.39 Further studies using these novel antibodies will certainly be informative to define similarities and differences in the pathological processes underlying accumulation of TDP-43 in diverse neurodegenerative conditions.
In summary, our data show that while brain inclusions in FTLD-U and ALS are enriched in C-t TDP-43 fragments, those found in motor neurons of spinal cord contain predominantly FL TDP-43. These findings will have implications for understanding the pathogenic mechanisms underlying TDP-43 proteinopathies, in particular those conditions that involve cognitive impairment and motor symptoms. Elucidating the different region-specific pathways that lead to abnormal TDP-43 processing, mislocalization, and inclusion formation will further our understanding of these neurodegenerative disorders and help in the identification and development of potential therapies.
Acknowledgments
We thank the families of patients whose generosity made this research possible. We thank our colleagues at the Center for Neurodegenerative Disease Research for their technical support and advice, particularly Dr. F. Geser, Dr. M. Winton, T. Schuck, C. Groomes, and J. Robinson.
Footnotes
Address reprint requests to Virginia M.-Y. Lee, Center for Neurodegenerative Disease Research, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3600 Spruce St., 3rd floor, Maloney Bldg., Philadelphia, PA 19104. E-mail: vmylee@mail.med.upenn.edu.
Supported by grants from the NIH (AG10124 and AG17586) and from the German Federal Ministry of Education and Research (01GI0704). V.M.-Y.L. is the John H. Ware III Chair of Alzheimer’s Research, and J.Q.T. is the William Maul Measey-Truman G. Schnabel, Jr., MD Professor of Geriatric Medicine and Gerontology.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Snowden JS, Neary D, Mann DM. Frontotemporal dementia. Br J Psychiatry. 2002;180:140–143. doi: 10.1192/bjp.180.2.140. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VMY, Hatanpaa KJ, White CL, III, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, Bruce J, Grossman M, Trojanowski JQ, Lee VMY. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–1352. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Mackenzie IR, Cairns NJ, Boyer PJ, Markesbery WR, Smith CD, Taylor JP, Kretzschmar HA, Kimonis VE, Forman MS. TDP-43 in the ubiquitin pathology of frontotemporal dementia with VCP gene mutations. J Neuropathol Exp Neurol. 2007;66:152–157. doi: 10.1097/nen.0b013e31803020b9. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DM. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Gros-Louis F, Gaspar C, Rouleau GA. Genetics of familial and sporadic amyotrophic lateral sclerosis. Biochim Biophys Acta. 2006;1762:956–972. doi: 10.1016/j.bbadis.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Talbot K, Ansorge O. Recent advances in the genetics of amyotrophic lateral sclerosis and frontotemporal dementia: common pathways in neurodegenerative disease. Hum Mol Genet. 2006;15:R182–R187. doi: 10.1093/hmg/ddl202. [DOI] [PubMed] [Google Scholar]
- Leigh PN, Whitwell H, Garofalo O, Buller J, Swash M, Martin JE, Gallo JM, Weller RO, Anderton BH. Ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis: morphology, distribution, and specificity. Brain. 1991;114:775–788. doi: 10.1093/brain/114.2.775. [DOI] [PubMed] [Google Scholar]
- Wightman G, Anderson VE, Martin J, Swash M, Anderton BH, Neary D, Mann D, Luthert P, Leigh PN. Hippocampal and neocortical ubiquitin-immunoreactive inclusions in amyotrophic lateral sclerosis with dementia. Neurosci Lett. 1992;139:269–274. doi: 10.1016/0304-3940(92)90569-s. [DOI] [PubMed] [Google Scholar]
- Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman LB, McCluskey L, Xie SX, Lee VMY, Trojanowski JQ. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008;65:636–641. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- Mackenzie IR, Bigio EH, Ince PG, Geser F, Neumann M, Cairns NJ, Kwong LK, Forman MS, Ravits J, Stewart H, Eisen A, McClusky L, Kretzschmar HA, Monoranu CM, Highley JR, Kirby J, Siddique T, Shaw PJ, Lee VMY, Trojanowski JQ. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Ann Neurol. 2007;61:427–434. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, Nishizawa M, Kakita A, Takahashi H. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–542. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, Mackenzie IR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VMY. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Davidson Y, Kelley T, Mackenzie IR, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DM. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain the TAR DNA-binding protein TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–878. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–1097. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VMY. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47:719–730. doi: 10.1177/002215549904700601. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, III, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VMY, Mackenzie IR. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Mackenzie IR, Cairns NJ, Swanson E, Boyer PJ, Drachman DA, Jhaveri BS, Karlawish JH, Pestronk A, Smith TW, Tu PH, Watts GD, Markesbery WR, Smith CD, Kimonis VE. Novel ubiquitin neuropathology in frontotemporal dementia with valosin-containing protein gene mutations. J Neuropathol Exp Neurol. 2006;65:571–581. doi: 10.1097/00005072-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VMY. TDP-43: a novel neurodegenerative proteinopathy. Curr Opin Neurobiol. 2007;17:548–555. doi: 10.1016/j.conb.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Feldman HH. Ubiquitin immunohistochemistry suggests classic motor neuron disease, motor neuron disease with dementia, and frontotemporal dementia of the motor neuron disease type represent a clinicopathologic spectrum. J Neuropathol Exp Neurol. 2005;64:730–739. doi: 10.1097/01.jnen.0000174335.27708.0a. [DOI] [PubMed] [Google Scholar]
- Shankaran SS, Capell A, Hruscha AT, Fellerer K, Neumann M, Schmid B, Haass C. FTLD-U linked missense mutations in the progranulin gene reduce progranulin production and secretion. J Biol Chem. 2008;283:1744–1753. doi: 10.1074/jbc.M705115200. [DOI] [PubMed] [Google Scholar]
- Wang IF, Reddy NM, Shen CK. Higher order arrangement of the eukaryotic nuclear bodies. Proc Natl Acad Sci USA. 2002;99:13583–13588. doi: 10.1073/pnas.212483099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans DA, Hubner S. Regulation of protein transport to the nucleus: central role of phosphorylation. Physiol Rev. 1996;76:651–685. doi: 10.1152/physrev.1996.76.3.651. [DOI] [PubMed] [Google Scholar]
- Pichler A, Melchior F. Ubiquitin-related modifier SUMO1 and nucleocytoplasmic transport. Traffic. 2002;3:381–387. doi: 10.1034/j.1600-0854.2002.30601.x. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Dickey CA, Buratti E, Baralle F, Bailey R, Pickering-Brown S, Dickson D, Petrucelli L. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J Neurosci. 2007;27:10530–10534. doi: 10.1523/JNEUROSCI.3421-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geser F, Winton MJ, Kwong LK, Xu Y, Xie SX, Igaz LM, Garruto RM, Perl DP, Galasko D, Lee VMY, Trojanowski JQ. Pathological TDP-43 in parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam. Acta Neuropathol. 2007;115:133–145. doi: 10.1007/s00401-007-0257-y. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Arai T, Akiyama H, Nonaka T, Mori H, Hashimoto T, Yamazaki M, Oyanagi K. TDP-43 is deposited in the Guam parkinsonism-dementia complex brains. Brain. 2007;130:1386–1394. doi: 10.1093/brain/awm065. [DOI] [PubMed] [Google Scholar]
- Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VMY, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Kosaka K, Arai H. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- Dickson DW. TDP-43 immunoreactivity in neurodegenerative disorders: disease versus mechanism specificity. Acta Neuropathol. 2008;115:147–149. doi: 10.1007/s00401-007-0323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]