Abstract
Vascular endothelial growth factor C (VEGF-C) recently has been described to be a relatively specific growth factor for the lymphatic vascular system. Here we report that ectopic application of recombinant VEGF-C also has potent angiogenic effects in vivo. VEGF-C is sufficiently potent to stimulate neovascularization from limbal vessels in the mouse cornea. Similar to VEGF, the angiogenic response of corneas induced by VEGF-C is intensive, with a high density of new capillaries. However, the outgrowth of microvessels stimulated by VEGF-C was significantly longer than that induced by VEGF. In the developing embryo, VEGF-C was able to induce branch sprouts from the established blood vessels. VEGF-C also induced an elongated, spindle-like cell shape change and actin reorganization in both VEGF receptor (VEGFR)-2 and VEGFR-3-overexpressing endothelial cells, but not in VEGFR-1-expressing cells. Further, both VEGFR-2 and VEGFR-3 could mediate proliferative and chemotactic responses in endothelial cells on VEGF-C stimulation. Thus, VEGF-C may regulate physiological angiogenesis and participate in the development and progression of angiogenic diseases in addition to lymphangiogenesis.
Angiogenesis, the outgrowth of new capillaries from pre-existing vessels, is essential for embryonic development, organ formation, tissue regeneration, and remodeling (1). It also contributes to the development and progression of a variety of pathological conditions, including tumor growth and metastasis, cardiovascular diseases, diabetic retinopathy, rheumatoid arthritis, and psoriasis (2). Angiogenesis and vasculogenesis are complex multistep processes that include proliferation, migration and differentiation of endothelial cells, degradation of the extracellular matrix, tube formation, and sprouting of new capillary branches (3, 11). The complexity of the angiogenic processes suggests the existence of multiple controls of the system, which can be transiently switched on and off.
A switch of the angiogenic phenotype in tissues is thought to depend on a local change of the balance between angiogenic stimulators and inhibitors (4). Among angiogenic factors, vascular endothelial growth factor (VEGF)/vascular permeability factor is the best-characterized positive regulator with its distinct specificity for vascular endothelial cells (5–7). The biological actions of VEGF include stimulation of endothelial cell proliferation, migration, differentiation, tube formation, increase of vascular permeability, and maintenance of vascular integrity (8–11, 35). The angiogenic responses induced by VEGF are mediated by two structurally related tyrosine kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR/Flk-1), both of which are expressed primarily on vascular cells of the endothelial lineage (8, 12, 13). The biological responses via these two receptors to VEGF appear to be different (14). For example, activation of VEGFR-2 leads to proliferation and migration of endothelial cells, whereas VEGFR-1 is unable to transduce such signals when stimulated with VEGF (14).
VEGF is the prototype of an enlarging family of growth peptides that includes four other structurally related members. These recently identified VEGF-related molecules are placenta growth factor (PlGF) (15), VEGF-B/VEGF-related factor (16, 17), VEGF-C (18, 19), and c-fos-induced growth factor (FIGF/VEGF-D) (20, 39, 40). They show a striking similarity in their primary sequences, especially in the platelet-derived growth factor-like domain containing the conserved eight cysteine residues. PlGF is predominantly expressed in the placenta and binds to VEGFR-1, but not to VEGFR-2 (21, 22). VEGF-B has been identified as a weak mitogen for endothelial cells and a robust expression is particularly detected in skeletal and cardiac muscle tissues (16). Both PlGF and VEGF-B modulate VEGF activity via formation of heterodimers (16, 23). VEGF-C is a ligand for two receptors, VEGFR-2 and VEGFR-3 (Flt-4) (18, 24). The latter differs from VEGFR-1 and VEGFR-2 by being predominantly expressed in lymphatic endothelial cells in adult tissues, but at low levels in most other vascular endothelial cells (25, 44). VEGF-C recently has been characterized to be a fairly selective growth factor for lymphatic vessels (26, 27). In addition, proteolytic processing is involved in the regulation of VEGF-C activity (24). FIGF/VEGF-D, which is drastically induced by c-fos activation (20), also binds to VEGFR-2 and VEGFR-3 (40).
Although these factors are believed to stimulate endothelial cell growth in vitro, their in vivo angiogenic effects have not yet been fully characterized. In the present study, we demonstrate that recombinant VEGF-C is an angiogenic factor in corneas and chicken embryo chorioallantoic membrane (CAM). These results raise the possibility that VEGF-C also may be involved in the regulation of physiological and pathological blood vessel growth.
MATERIALS AND METHODS
Reagents, Cells, and Animals.
Recombinant mature form of human VEGF-C was expressed in pichia pastoris yeast cells and purified as described (24). Recombinant human VEGF165 was prepared as described (23). Recombinant human fibroblast growth factor (FGF)-2 was obtained from Scios Nova (Mountainview, CA). Stable porcine aortic endothelial (PAE) cell lines expressing VEGFR-1, VEGFR-2, and VEGFR-3 were established as reported (14, 24) and maintained in Ham′s F12 medium supplemented with penicillin/streptomycin and 10% fetal calf serum (FCS). Male 5- to 6-wk-old C57BL6/J mice were acclimated and caged in groups of four or fewer. Animals were anesthetized in a methoxyflurane chamber before all procedures and killed with a lethal dose of methoxyflurane. All animal studies were reviewed and approved by the animal care and use committee of the Stockholm Animal Board.
Mouse Corneal Micropocket Assay.
The mouse corneal assay was performed according to procedures previously described (28–30). Corneal micropockets were created with a modified von Graefe cataract knife in both eyes of each male 5- to 6-wk-old C57BL6/J mouse. A micropellet (0.35 × 0.35 mm) of sucrose aluminum sulfate (Bukh Meditec, Copenhagen, Denmark) coated with hydron polymer type NCC (IFN Sciences, New Brunswick, NJ) containing 160 ng of VEGF-C or VEGF, or 80 ng of FGF-2 was implanted into each pocket. The pellet was positioned 0.6–0.8 mm from the corneal limbus. After implantation, erythromycin/ophthalmic ointment was applied to each eye. Eyes were examined by a slit-lamp biomicroscope on day 5 after pellet implantation. Vessel length and clock hours of circumferential neovascularization were measured.
Cell Shape Assay and Actin Staining.
PAE/VEGFR-1, PAE/VEGFR-2, and PAE/VEGFR-3 cells were grown on coverslips in 6-well plates to about 60–70% confluency in Ham′s F12 medium supplemented with 10% FCS. The medium was removed and replaced with fresh Ham′s F12 medium containing 10% FCS with or without 100 ng/ml of VEGF and VEGF-C. After 12 h incubation, cells were fixed with 3% paraformaldehyde in phosphate buffer (pH 7.5) for 30 min at room temperature. After rinsing three times with PBS, the cells were permeabilized with 0.5% Triton X-100 for 30 min. The cells then were washed three times with PBS and stained for 30 min at room temperature with 1 μg/ml of rhodamine-phalloidin (Sigma) in PBS. After washing with PBS five times, the coverslips were mounted in a mixture of glycerol and PBS (90:10), and the cells were examined under light and fluorescence microscopes.
Chicken Embryo CAM Assay.
Three-day-old fertilized white Leghorn eggs (OVA Production, Sörgården, Sweden) were cracked, and chicken embryos with intact yolks were carefully placed in 20 × 100 mm plastic Petri dishes. After 6 days of incubation in 3% CO2 at 37°C, a disk of methylcellulose containing 2.5 μg of VEGF-C or VEGF dried on a nylon mesh (3 × 3 mm) was implanted on the CAM of individual embryos. The nylon mesh disks were made by desiccation of 10 μl of 0.45% methylcellulose (in H2O). After 4–5 days of incubation, embryos and CAMs were examined for the formation of new blood vessels in the field of the implanted disks by a stereoscope. Disks of methylcellulose containing PBS were used as negative controls.
Endothelial Cell Proliferation Assay.
PAE/VEGFR-1, PAE/VEGFR-2, and PAE/VEGFR-3 cells were grown in 25-cm2 flasks in Ham′s F12 medium supplemented with 10% FCS. Cells were trypsinized and resuspended in Ham′s F12 medium containing 1% FCS. Approximately 104 cells were seeded in each well of 24-well plates. VEGF-C and VEGF at various concentrations were added in a triplicate of each sample to the cells. After incubation for 72 h at 37°C with 5% CO2, cells were trypsinized and resuspended in Isoton II solution (Coulter) and counted with a Coulter counter.
Chemotaxis.
The motility response of cells to VEGF-C and VEGF was assayed by a modified Boyden chamber technique (36) as described (14, 38) by using micropore nitrocellulose filters (8 mm thick, 8-μm pores) coated with type-1 collagen solution at 100 μg/ml (Vitrogen 100, Collagen Corp.). Cells were trypsinized and resuspended at a concentration of 1 × 106 cells/ml in serum-free medium containing 0.2% BSA. The cells (50,000 cells per well) were placed in the upper chamber in serum-free medium containing 0.2% BSA with or without VEGF or VEGF-C in the lower chamber. The number of cells migrating through the filter was counted and plotted as number of migrating cells per optic field (×32). After 4 h at 37°C, the medium was removed and cells attached to the filter were fixed in 99% ethanol and stained with Giemsa solution. All experiments were performed in triplicate.
RESULTS
Characterization of Recombinant VEGF-C.
Recombinant human VEGF-C was expressed in pichia pastoris yeast cells as described (24) and purified to homogeneity. Under nonreducing conditions, recombinant VEGF-C appeared as dimers with the expected molecular mass of about 40 kDa (Fig. 1, lane 2). The dimeric forms of VEGF-C were converted to monomers of 21 kDa under reducing conditions in the presence of DTT (Fig. 1, lane 3). Thus, recombinant VEGF-C expressed in yeast cells was appropriately folded to form covalently linked dimers.
Figure 1.
SDS/PAGE analysis of VEGF-C preparation. Recombinant mature form of human VEGF-C was expressed in pichia pastoris yeast cells. Three micrograms of the purified VEGF-C was analyzed on a 10–20% gradient SDS-polyacrylamide gel followed by staining with Coomassie blue. Dimeric (lane 2) and monomeric (lane 3) forms of VEGF-C were detected under nonreducing (lane 2) and reducing/alkylating conditions (lane 3). Molecular mass markers are indicated on the left (lane 1).
Induction of Neovascularization in Mouse Corneas.
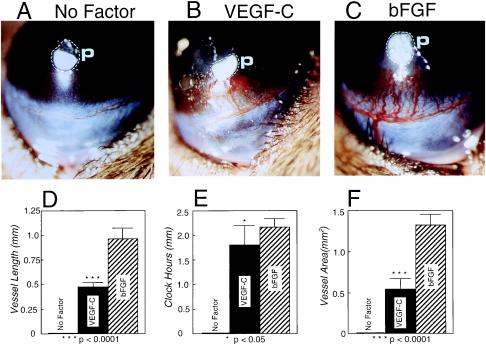
Micropellets of aluminum sulfate coated with the slow release polymer-hydron containing VEGF-C, VEGF, and FGF-2 were surgically implanted into the corneas of C57BL6/J mice. Stimulation of new blood vessel growth was examined on day 5 after implantation. The angiogenic response of corneas stimulated by 160 ng of VEGF-C was intensive with a high density of capillary sprouts (Figs. 2A and 3B). The limbal vessels were markedly dilated in the VEGF-C-implanted corneas. The capillary vessel length of about 0.5 mm in corneas implanted with VEGF-C was significantly greater than that in the VEGF-implanted corneas (≈0.35 mm) (Fig. 2C). A statistically significant difference in circumferential clock hours was observed between corneas implanted with VEGF-C or VEGF (Fig. 2D). No significant difference in neovascularization area was found between VEGF-C and VEGF (Fig. 2E).
Figure 2.
Corneal neovascularization stimulated by VEGF-C and VEGF. Pellets of sucrose aluminum sulfate and hydron polymer containing 160 ng of VEGF-C (A) and VEGF (B) were implanted into corneal micropockets of C57BL6/J mice as described in Materials and Methods. Corneas were photographed with a slit-lamp stereomicroscope on day 5 after growth factor implantation. Dashed lines encircle the area of the implanted pellets. Photographs represent ×20 amplification of the mouse eye. Quantitation of corneal neovascularization. (C) Maximal vessel length. (D) Clock hours of circumferential neovascularization. (E) Area of neovascularization. Graphs represent mean values (±SEM) of 10 eyes (five mice) in each group. The P values were calculated by two-tailed Student t test analysis using the statistical instat 1.1 and Microsoft excel 5 programs.
Figure 3.
Angiogenic effects of VEGF-C and FGF-2 in the cornea. Pellets containing 160 ng of VEGF-C (B), 80 ng of FGF-2 (C), and PBS alone without growth factors (A) were implanted into corneal micropockets of C57BL6/J mice. Photographs represent ×20 amplification of the mouse eye. p, pellets. Corneal neovascularization was quantitated on day 5 after pellet implantation. (D) Maximal vessel length. (E) Clock hours of circumferential neovascularization. (F) Area of neovascularization. Graphs represent mean values (±SEM) of 10 eyes of five mice in each group.
In contrast, the length (Fig. 3 C and D) and area (Fig. 3F) of new vessels stimulated with FGF-2 were significantly greater over the VEGF-C-induced corneal angiogenesis. In FGF-2-implanted corneas, new microvessels crossed the cornea from the limbus toward the pellet, occasionally penetrating into the pellet. In addition, the FGF-2-induced microvessels were heavily dilated (Fig. 3C) as compared with VEGF-C- (Figs. 2A and 3B) and VEGF- (Fig. 2B) stimulated vessels. Thus, angiogenic patterns in corneas stimulated by VEGF-C, VEGF, and FGF-2 were different from each other. We should emphasize the heparin binding properties of FGF-2 and VEGF165 may influence their angiogenic patterns in our assay because they can be sequestered by heparan sulfate proteoglycans in the corneal tissue and prevented from diffusion. The mature and fully processed VEGF-C did not bind to heparin (data not shown). Pellets containing sucrose aluminum sulfate alone without growth factors did not induce corneal neovascularization (Fig. 3A).
Stimulation of Neovascularization in the Chicken Embryo.
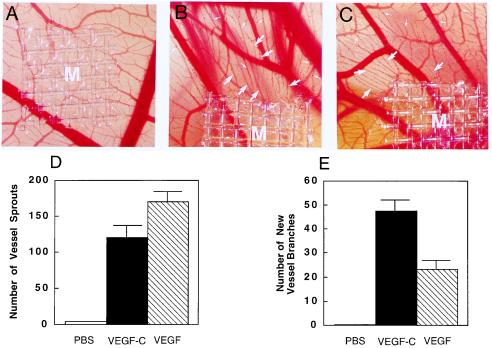
To further study the angiogenic activity in vivo, VEGF-C was tested on the chicken embryo CAMs. The CAM assay is one of the most widely used in vivo angiogenic assays, which detects angiogenic activity of growth factors during embryonic development (31). VEGF-C and VEGF at the concentration of 2.5 μg/disk were analyzed in each chicken embryo. A dramatic increase of neovascularization with a high vessel density was observed in the surrounding areas of VEGF-C and VEGF implants (Fig. 4 B and C). VEGF-C and VEGF in some regions also induced the formation of new branches from the existing vessels that grew toward the implanted pellets (Fig. 4 A and B, large arrows). In addition, both VEGF-C and VEGF were able to induce vessel sprouts (small arrows in Fig. 4 B and C). These vessel sprouts appeared as “red dots” budding from blood vessels adjacent to the implanted factors. To quantify the angiogenic response induced by VEGF-C and VEGF, the area was defined within 85 mm2 surrounding the implants. The new vessel branches and sprouts within this area were counted. Of interest, the number of new vessel branches induced by VEGF-C was significantly greater than that induced by VEGF. Thus, VEGF-C stimulated new vessel formation in this in vivo system. Likewise, VEGF also induced a similar angiogenic response in CAMs (Fig. 4C). In contrast, pellets without growth factors did not seem to stimulate neovascularization in chicken embryos (Fig. 4A). The angiogenic patterns of VEGF-C and VEGF in our CAM assay were highly reproducible because similar results have been obtained in three independent experiments with more than 20 embryos per sample.
Figure 4.
Angiogenic effect of VEGF-C and VEGF on CAMs. Nylon meshes (9.3 mm2) coated with methylcellulose containing 2.5 μg of VEGF-C and VEGF were implanted on CAMs of 7-day-old chicken embryos. After 4-day implantation, the formation of new blood vessels was examined under a stereoscope. (A) A control CAM with a methylcellulose mesh containing PBS without growth factors. An example of VEGF-C- (B) and VEGF-implanted CAM (C). New blood vessel branches are marked by large arrows. Small arrows indicate the new microvessel sprouts. At the concentration of 2.5 μg/disk, the number of vessel sprouts (D) and the number of new vessel branches (E) stimulated by VEGF-C, VEGF, and PBS were quantitated within a defined area of 85 mm2 surrounding the implanted mesh. Seven embryos in each group were used for quantitative studies. Data represent mean values (±SEM).
Cell Shape Changes and Actin Reorganization.
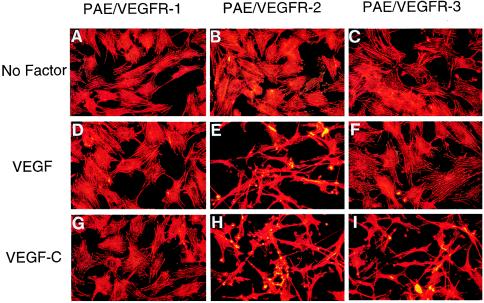
Because the mature form of VEGF-C was reported to bind to the angiogenic receptor VEGFR-2 (24), which has been known to mediate VEGF-stimulated biological responses including membrane ruffling, cell migration, and proliferation, we determined the biological effect of VEGF-C on PAE/VEGFR-2 cells. The addition of 100 ng/ml of VEGF-C to VEGFR-2 overexpressing cells for 12 h resulted in an elongated cell shape change with distinct spindle-like processes as determined by fixation and staining with rhodamine-conjugated phalloidin (Fig. 5H). A similar cellular shape alteration also was observed in the VEGF-stimulated PAE/VEGFR-2 cells (Fig. 5E). Under identical conditions, VEGF-C and VEGF did not induce cell shape changes in PAE/VEGFR-1 cells (Fig. 5 G and D). These data are consistent with our in vivo findings that VEGF-C is an angiogenic molecule.
Figure 5.
VEGF-C- and VEGF-induced endothelial cell shape changes and actin reorganization. VEGF-C and VEGF were added to approximately 70% confluent VEGF receptor-expressing PAE cells at a final concentration of 100 ng/ml for 12 h, fixed with paraformaldehyde, permeabilized with Triton X-100, and stained with rhodamine-phalloidin. In response to VEGF-C (G–I) and VEGF (D–F), PAE/VEGFR-2 cells (H and E) undergo dramatic spindle-like shape changes, leading lamellae, lamellipodium extensions and actin reorganization. PAE/VEGFR-3 cells also show this striking cell shape change in response to VEGF-C stimulation (I). PAE/VEGFR-1 cells lack such a cell shape change in the presence of VEGF-C (G) and VEGF (D). All VEGFR-expressing cells also lack cell morphological changes in the absence of growth factors (A–C).
To study whether VEGF-C also could induce a similar cell shape change via the VEGFR-3, VEGF-C was incubated with PAE/VEGFR-3 cells for 12 h. Interestingly, VEGF-C-stimulated PAE/VEGFR-3 cells underwent striking cell morphological changes with long spindle processes (Fig. 5I), which are virtually indistinguishable from VEGF-C- (Fig. 5H) and VEGF- (Fig. 5E) challenged PAE/VEGFR-2 cells. The cell shape change appeared to be independent of the addition of bovine serum, because a similar change was observed in the presence and absence of serum (data not shown). These data suggested that other serum factors were not required for the morphological effect. In contrast, VEGF did not induce such a morphological change in PAE/VEGFR-3 cells (Fig. 5F). Similarly, all three VEGFR-expressing PAE cell lines did not change their shapes in the absence of growth factors (Fig. 5 A–C). These data demonstrated that although VEGF-C and VEGF shared an overlapped biological activity via VEGFR-2, VEGF-C displays a unique activity through VEGFR-3.
VEGF-C-stimulated PAE/VEGFR-2 (Fig. 5H) and VEGFR-3 (Fig. 5I) cells as well as VEGF-stimulated PAE/VEGFR-2 cells (Fig. 5E) showed actin reorganization, membrane ruffles, and lamellipodium extensions, suggesting that these receptors mediate chemotactic migration (see below), and perhaps, the sprouts of endothelial cells in vivo. Thus, these data are in agreement with the finding that VEGFR-2 and VEGFR-3 mediate VEGF-C factor-stimulated angiogenesis and lymphangiogenesis in vivo.
Stimulation of Endothelial Cell Proliferation.
Previous studies showed that VEGF-C was able to stimulate blood vessel endothelial cell proliferation (18, 24). To investigate which receptor mediated the proliferative effect of VEGF-C on endothelial cells, we incubated VEGF-C and VEGF with PAE/VEGFR-1, R-2, and R-3 cells. Both VEGF-C and VEGF were able to stimulate the proliferation of PAE/VEGFR-2 cells (Fig. 6B) in a dose-dependent manner. This data supported the previous finding that VEGFR-2 transduced the mitogenic signals of VEGF. Interestingly, VEGF-C, but not VEGF, elicited a dose-dependent stimulation of the growth of PAE/VEGFR-3 cells (Fig. 6C). Thus, VEGF-C could elicit a dual effect on proliferation of both blood and lymphatic endothelial cells, expressing VEGFR-2 and VEGFR-3, respectively. Neither VEGF-C nor VEGF was able to induce proliferation of PAE/VEGFR-1 cells (Fig. 6A). These findings indicate that both VEGFR-2 and VEGFR-3 when stimulated with adequate ligands are able to transduce the cell shape change and proliferation signals.
Figure 6.
Cell proliferation. The proliferative effects of VEGF-C (■) and VEGF (○) were assayed on PAE/VEGFR-1 (A), PAE/VEGFR-2 (B), and PAE/VEGFR-3 (C) cells. VEGF-C and VEGF at various concentrations in a triplicate of each sample were incubated with cells in the presence of 1% FCS. After 72 h incubation, cell numbers were counted by a Coulter counter, and values represent the mean (±SEM) of a triplicate of each sample. PBS was used as the negative control.
Chemotactic Activity.
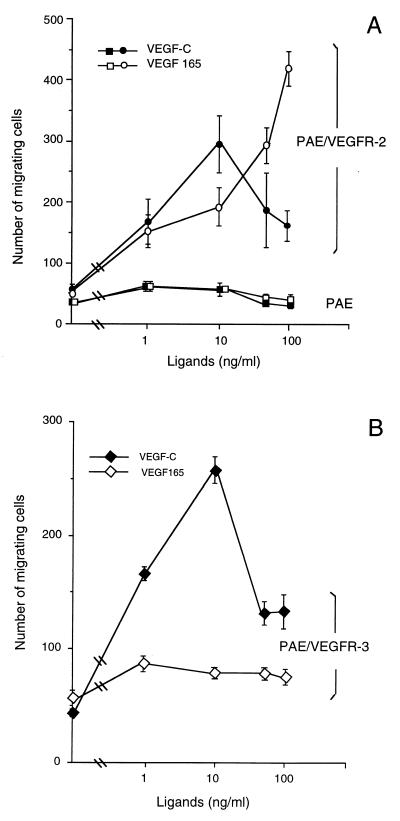
In addition to stimulation of cell proliferation and the above-mentioned effects on endothelial cell shape, we studied the chemotactic effect of VEGF-C on PAE cells in a modified Boyden chamber assay (14, 38). The migration of the receptor-expressing and nontransfected PAE cells through collagen-coated micropore filters toward chemoattractants was scored in the absence of serum. As shown in Fig. 7, both VEGF-C and VEGF stimulated the migration of PAE/VEGFR-2 cells in a dose-dependent manner (Fig. 7A). VEGF-C and VEGF exhibited a comparable chemotactic activity on PAE/VEGFR-2 cells. Of interest, VEGF-C reached a maximal chemotactic effect at the concentration of 10 ng/ml and higher concentrations resulted in reduced motility. However, VEGF displayed a dose-dependent stimulation even at high concentrations (10–100 ng/ml). These data demonstrate that VEGF-C and VEGF exhibited different motility effects on PAE/VEGFR-2 cells although both are sufficiently potent to induce cell migration. Both factors were unable to stimulate the migration of nontransfected PAE cells. To further investigate whether VEGF-C also induced cell motility through other receptors, we determined its chemotactic effect on PAE/VEGFR-3 cells. Similar to PAE/VEGFR-2 cells, PAE/VEGFR-3 cells migrated efficiently in response to VEGF-C stimulation (Fig. 7B). The maximal activity was observed at a concentration of 10 ng/ml and higher concentrations led to decreased motility. In contrast, VEGF had no effect on PAE/VEGFR-3 cells.
Figure 7.
Chemotactic activity. (A) Chemotactic response of VEGFR-2/PAE cells (circles) and nontransfected PAE cells (squares) to VEGF (open symbols) and VEGF-C and (filled symbols). (B) Chemotactic response of VEGFR-3 cells to VEGF-C (○) and VEGF (■). Cells were incubated in serum-free Ham′s F-12 medium containing 0.2% BSA with or without growth factors for 4 h at 37°C in a modified Boyden chamber. Migrating cells were counted after staining with Giemsa and plotted in absolute number per optic field. Data represent means (±SEM) of a triplicate of each sample.
DISCUSSION
Results presented in this work show that recombinant VEGF-C is a potent angiogenic factor. In mouse corneas, VEGF-C induced an angiogenic pattern that appeared to be different from VEGF and FGF-2. VEGF-C also stimulated the outgrowth of embryonic microvessels in CAMs with new branches sprouting from the existing blood vessels. Similar to VEGF, VEGF-C induced a dramatic spindle-like cell shape change, actin reorganization, membrane ruffles, and lamellipodium extension in PAE/VEGFR-2 cells, but not in VEGFR-1 cells. VEGFR-3-expressing cells also showed similar striking changes in cell morphology and actin reorganization on VEGF-C stimulation, whereas they lacked such responses at stimulation by VEGF. In addition, VEGF-C also exhibited potent stimulatory effects on proliferation and chemotaxis of both PAE/VEGFR-2 and PAE/VEGFR-3 cells. These findings demonstrate that the ectopically applied mature form of VEGF-C can be as potent as its structurally related VEGF prototype in the stimulation of angiogenesis.
Two structurally related tyrosine kinase receptors, VEGFR-2 and VEGFR-3, have been reported to bind to and to become activated by VEGF-C (24). In adults, VEGFR-3 is predominantly expressed on lymphatic endothelial cells, whereas VEGFR-2 also is expressed in the blood vascular endothelial cell lineage. However, directed overexpression of VEGF-C in the skin tissue of transgenic mice induces only lymphangiogenesis rather than blood vessel angiogenesis (26). Similarly, a robust lymphangiogenic response and only weak angiogenic response was obtained in response to VEGF-C application to the mature CAM (27).
The apparent discrepancy between the previous result and our present data could be explained as follows, although further work should be carried out to test these hypotheses. (i) The angiogenic activity of VEGF-C might be modulated by other angiogenic factors including its structural relative, VEGF. Because of the similarity of their primary sequences, especially the eight conserved cysteine residues in the platelet-derived growth factor-like domain, VEGF-C is likely to form heterodimers with some members of the VEGF family. Such heterodimers exist between VEGF and PlGF (23, 29) and between VEGF and VEGF-B (16). The angiogenic activity of VEGF also can be modulated by heterodimers. For example, PlGF down-regulates the angiogenic activity of VEGF (23, 29). It is possible that VEGF-C interacts intracellularly with VEGF-D or other members of the VEGF family by the formation of heterodimers when two or more factors are coexpressed in the same cell. Thus, transgenic expression of VEGF-C may result in a different angiogenic response than the topical administration of the protein. (ii) The angiogenic response to VEGF-C in various tissues and during different stages of development may be different depending on the switch of expression patterns of different receptors. For example, when both VEGFR-2 and -3 are present in the same tissue, VEGF-C preferentially binds to VEGFR-3 with a higher affinity (24). Low concentrations of radiolabeled VEGF-C decorated predominantly lymphatic vessels in human skin, whereas radioactive VEGF bound mainly to blood vessels (42). Thus, when both types of receptors are present, the angiogenic effect of VEGF-C on lymphatic vessels may become more prominent than its effect on blood vessels. Indeed, recombinant VEGF-C displays a strong lymphangiogenic activity during late stages of embryonic development (27). In addition, the receptor levels may be regulated in the target tissue by physiological and pathological stimuli, such as tissue hypoxia, which is known to increase VEGFR-2 levels (43). (iii) Native VEGF-C has to be processed to release the most active form of the protein (24). Thus, proteolytic processing may function as a switch of the angiogenic and lymphangiogenic activity of VEGF-C. (iv) Overexpression of VEGF-C in a tissue may not necessarily induce angiogenesis even though VEGF-C is an angiogenic factor. The in vivo angiogenic activity of a growth factor depends on the net balance between angiogenic factors and inhibitors in a tissue. For example, high levels of expression of VEGF have been detected in several normal tissues including pancreas, adrenal gland, and brain (9). However, these highly VEGF-expressing tissues do not undergo angiogenesis under physiological conditions. It is believed that endogenous angiogenesis inhibitors also are expressed in these tissues. Thus, the net outcome is no angiogenesis in these tissues. It is possible that VEGF-C is unable to induce angiogenesis in the skin tissue where endogenous angiogenesis inhibitors are also overexpressed in the same tissue. We should emphasize that mechanisms underlying the relative lack of angiogenesis in the transgenic model might be more complex and involved in several of the above-mentioned possibilities.
To date, there are only a few growth factors that are sufficiently potent to induce angiogenic responses in mouse corneas. These include FGF-2, FGF-1, and VEGF (28–30). The outgrowth of limbal vessels stimulated with VEGF-C was significantly longer than that induced with VEGF, suggesting that VEGF-C can be a potent chemotactic factor. In agreement with this finding, we show that VEGF-C is a potent chemotactic factor for endothelial cells. The enhanced migration of capillary sprouts is also consistent with the finding that recombinant VEGF-C stimulates the migration of bovine capillary endothelial cells in collagen gels (18). However, the clock hours of VEGF-C-induced neovascularization were significantly less than that of VEGF. These data imply that VEGF-C and VEGF may serve different, although overlapping, functions in the remodeling of vessel development.
A prominent expression pattern of VEGF-C mRNA in embryos has been detected in the developing mesentery, lung, heart, and kidney (25). The CAM assay allowed us to study the angiogenic effect of VEGF-C in the embryo. VEGF-C induces new sprouts from established vessels suggests that it may act as a branching factor during development of the vascular tree of the embryo. The angiogenic activity and the pattern of expression of VEGF-C in relation to VEGFR-3 and VEGFR-2 expression then would suggest the existence of a paracrine stimulation of both lymphangiogenesis and blood angiogenesis during embryonic development (25, 32).
The high affinity interaction of VEGF-C with VEGFR-2 is consistent with our present finding that VEGF-C can act as an angiogenic factor in vivo. In addition to stimulation of cell proliferation and cell shape changes, VEGF-C is a potent chemotactic factor for VEGFR-2-expressing endothelial cells (18). The chemotactic and proliferative effects of VEGF-C appear to be at least as potent as VEGF. If cell motility is correlated with vessel length in vivo, the length of VEGF-C-induced blood vessels is at least comparable with that of VEGF-stimulated vessels. In addition, VEGF-C, but not VEGF, is capable of inducing the migration of VEGFR-3-overexpressing endothelial cells, suggesting that it probably serves as a natural chemoattractant for lymphangiogenesis in vivo.
The activation of VEGFR-2 by both VEGF and VEGF-C suggests redundancy in the VEGF family in the regulation of angiogenesis. However, genetically deficient mice with mutations that inactivate a single allele of the VEGF gene die during embryonic development between days 11 and 12 of embryogenesis (33, 34). This finding indicates that other members of the VEGF family cannot replace the functions of VEGF. One of the key differences between VEGF and VEGF-C is that the former also binds to VEGFR-1. VEGFR-1 has an important role in vessel organization during development (37).
Acknowledgments
Y.C. is supported by Swedish Medical Research Council Grant K97-12P-11819-02B. This work was supported by Swedish Medical Research Council Grant K97-12X-12185-01A (to Y.C.), Swedish Cancer Foundation Grant 3811-B96-01XAB (961607) (to Y.C.), the Fredrik and Ingrid Thurings Foundation, the Karolinska Institutet Foundation, the Magnus Bergvalls Foundation, the Harald Jeanssons Foundation, and Swedish Cancer Society Grant 3820-B96–01XAB (to L.C.-W.). K.A.’s laboratory was supported by the Finnish Cancer Organizations, the Finnish Academy, the Sigfrid Juselius Foundation, the University of Helsinki Hospital (TYH 8105), the State Technology Development Center, and European Union Biomed programs BMH4-CT96-0669 and 98-3380.
ABBREVIATIONS
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- PlGF
placenta growth factor
- FGF
fibroblast growth factor
- PAE cells
porcine aortic endothelial cells
- FCS
fetal calf serum
- CAM
chorioallantoic membrane
References
- 1.Folkman J, Shing Y. J Biol Chem. 1992;267:10931–10934. [PubMed] [Google Scholar]
- 2.Folkman J. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 5.Senger D R, Galli S J, Dvorak A M, Perruzzi C A, Harvey V S, Dvorak H F. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N, Henzel W J. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 7.Gospodarowicz D, Abraham J A, Schilling J. Proc Natl Acad Sci USA. 1989;86:7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mustonen T, Alitalo K. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N, Davis-Smyth T. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 10.Thomas K. J Biol Chem. 1996;271:603–606. doi: 10.1074/jbc.271.2.603. [DOI] [PubMed] [Google Scholar]
- 11.Risau W. Nature (London) 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 12.De Vries C, Escobedo J A, Ueno H, Huck K, Ferrara N, Williams L T. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 13.Terman B I, Dougher-Vermazen M, Carrion M E, Dimitrov D, Armellino D C, Gospodorawicz D, Bohlen P. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 14.Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Oncogene. 1998;16:359–367. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 15.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico M G. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson R F, Alitalo K, Eriksson U. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimmond S, Lagencrantz J, Drinkwater C, Silins G, Townson S, Pollock P, Gotley D, Carson E, Rakar S, Nordenskjiöd M, et al. Genome Res. 1996;6:124–131. doi: 10.1101/gr.6.2.124. [DOI] [PubMed] [Google Scholar]
- 18.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Gray A, Yuan J, Luoh S-M, Avraham H, Wood W I. Proc Natl Acad Sci USA. 1996;93:1988–1992. doi: 10.1073/pnas.93.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlandini M, Marconcini L, Ferruzzi R, Oliviero S. Proc Natl Acad Sci USA. 1996;93:11675–11680. doi: 10.1073/pnas.93.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maglione D, Guerriero V, Viglietto G, Ferraro M G, Aprelikova O, Alitalo K, Vecchio S D, Lei K-J, Chou J Y, Persico M G. Oncogene. 1993;8:925–931. [PubMed] [Google Scholar]
- 22.Park J E, Chen H E, Winer J, Ferrara N. J Biol Chem. 1994;269:25646–25654. [PubMed] [Google Scholar]
- 23.Cao Y, Chen H, Zhou L, Chiang M-K, Anand-Apte B, Weatherbee J A, Wang Y, Fang F, Flanagan J G, Tsang M L-S. J Biol Chem. 1996;271:3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 24.Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kukk E, Lymbousaki A, Taira S, Kaipainen A, Jeltsch M, Joukov V, Alitalo K. Development (Cambridge, UK) 1996;122:3829–3837. doi: 10.1242/dev.122.12.3829. [DOI] [PubMed] [Google Scholar]
- 26.Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain R K, Alitalo K. Science. 1997;276:1423–1425. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- 27.Oh S J, Jeltsch M M, Birkenhager R, Mccarthy J E G, Weich H A, Christ B, Alitalo K, Wilting J. Dev Biol. 1997;188:96–109. doi: 10.1006/dbio.1997.8639. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Chen C, Weatherbee J A, Tsang M, Folkman J. J Exp Med. 1995;182:2069–2077. doi: 10.1084/jem.182.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Y, Linden P, Shima D, Browne F, Folkman J. J Clin Invest. 1996;98:2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenyon B M, Voest E E, Chen C, Flynn E, Folkman J, D’Amato R J. Invest Ophthal Visual Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 31.Nguyen M, Shing Y, Folkman J. Microvasc Res. 1994;47:31–40. doi: 10.1006/mvre.1994.1003. [DOI] [PubMed] [Google Scholar]
- 32.Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico M G, Terman B I, Alitalo K. J Exp Med. 1993;178:2077–2088. doi: 10.1084/jem.178.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kiechens L, Gertsenstein M, Fahrig M, Vandenhoek A, Harpal K, Eberhardt C, et al. Nature (London) 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 34.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea K S, Powell-Braxton L, Hillan K J, Moore M W. Nature (London) 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 35.Breier G, Risau W. Trends Cell Biol. 1997;6:454–456. doi: 10.1016/0962-8924(96)84935-x. [DOI] [PubMed] [Google Scholar]
- 36.Boyden S. J Exp Med. 1962;115:453–466. doi: 10.1084/jem.115.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fong G-H, Rossant J, Gertsenstein M, Breitman M L. Nature (London) 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 38.Auerbach R, Auerbach W, Polakowski I. Pharmacol Ther. 1991;51:1–11. doi: 10.1016/0163-7258(91)90038-n. [DOI] [PubMed] [Google Scholar]
- 39.Yamada Y, Nesu J O, Shimane M, Hirata Y. Genomics. 1997;42:483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 40.Achen M G, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks A F, Alitalo K, Stacker S A. Proc Natl Acad Sci USA. 1998;95:548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jain R K, Schlenger K, Hockel M, Yuan F. Nat Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- 42.Lymboussaki A, Partanen T, Olofsson B, Thomas-Crusells J, Fletcher C D M, de Waal R M W, Kaipainen A, Alitalo K. Am J Pathol. 1998;153:395–403. doi: 10.1016/S0002-9440(10)65583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kremer C, Breier G, Risau W, Plate K H. Cancer Res. 1997;57:3852–3859. [PubMed] [Google Scholar]
- 44.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh V W M, Fang G-H, Dumont D, Breitman M, Alitalo K. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]