Abstract
Intravascular hemolysis results in the release of massive amounts of hemoglobin and heme into plasma, where they are rapidly bound by haptoglobin and hemopexin, respectively. Data from haptoglobin and hemopexin knockout mice have shown that both proteins protect from renal damage after phenylhydrazine-induced hemolysis, whereas double-mutant mice were especially prone to liver damage. However, the specific role of hemopexin remains elusive because of the difficulty in discriminating between hemoglobin and heme recovery. To study the specific role of hemopexin in intravascular hemolysis, we established a mouse model of heme overload. Under these conditions, both endothelial activation and vascular permeability were significantly higher in hemopexin-null mice compared with wild-type controls. Vascular permeability was particularly altered in the liver, where congestion in the centrolobular area was believed to be associated with oxidative stress and inflammation. Liver damage in hemopexin- null mice may be prevented by induction of heme oxygenase-1 before heme overload. Furthermore, heme-treated hemopexin-null mice exhibited hyperbilirubinemia, prolonged heme oxygenase-1 expression, excessive heme metabolism, and lack of H-ferritin induction in the liver compared with heme-treated wild-type controls. Moreover, these mutant mice metabolize an excess of heme in the kidney. These studies highlight the importance of hemopexin in heme detoxification, thus suggesting that drugs mimicking hemopexin activity might be useful to prevent endothelial damage in patients suffering from hemolytic disorders.
Heme (ferrous protoporphyrin-IX) is the most important iron complex in the body because it is responsible for oxygen and electron transfer. However, free heme is highly toxic because it catalyzes free radical reaction, thus promoting oxidative damage.1,2 Excess of heme occurs in many pathological conditions associated to intravascular hemolysis such as hemoglobinopathies, paroxysmal nocturnal hemoglobinuria, trauma, and bacterial infections. Because of enhanced rates of red blood cell hemolysis, the endothelium of these patients is exposed to higher levels of reactive oxygen species catalyzed by plasma hemoglobin, heme, and free iron. Oxidative stress induces the expression of adhesion molecules on endothelial cells, which results in the binding of leukocytes.3 Moreover, hemoglobin released from damaged cells reduces nitric oxide bioavailability, thus promoting vasoconstriction and impairing downstream homeostatic vascular functions of nitric oxide, such as inhibition of platelet activation and aggregation and transcriptional repression of the cell adhesion molecules.4 Oxidative stress, endothelial cell activation, and inflammation are the main factors responsible for vaso-occlusions that frequently occur in hemolytic disorders.5,6
The organism defends itself against reactive heme released during hemolysis by inducing haptoglobin and hemopexin (Hx), the plasma scavengers of hemoglobin and heme, respectively.7,8 Moreover, the vasculature and organs up-regulate the expression of two cytoprotective genes, heme oxygenase (HO)-1 and ferritins. HO-1 is the rate-limiting enzyme in the catabolism of heme. It breaks down the porphyrin ring to yield equal molar amounts of biliverdin, free iron, and carbon monoxide. The induction of HO-1 is accompanied by the induction of apoferritin that inhibits iron-mediated oxidative damage by binding nonreactive Fe3+.1,9
Induction of HO-1 has been shown to protect tissues and cells against ischemia/reperfusion injury, oxidative stress, inflammation, transplant rejection, apoptosis, and cell proliferation.10,11,12 Conversely, humans and mice deficient in HO-1 are especially prone to oxidant-mediated injury.13,14,15 Recently, Belcher and co-workers16 demonstrated that HO-1 induction in a mouse model of sickle cell disease prevented vascular stasis.
On the other hand, little is known on the protective roles of plasma scavengers of heme and hemoproteins. After massive hemolysis both haptoglobin and Hx synthesis are induced, but the proteins rapidly disappear from the bloodstream because of the accelerated uptake of the hemoglobin-haptoglobin and heme-Hx complexes, respectively. Previous works have shown that, after phenylhydrazine-induced hemolysis, haptoglobin-null mice as well as Hx-null mice suffered from severe renal damage, whereas double-mutant mice were especially prone to develop liver damage.17,18,19 However, the phenylhydrazine model did not allow to study in detail the role of Hx because it resulted in a massive release of hemoglobin, thus making it difficult to discriminate between hemoglobin and heme recovery. Here, we established a model of heme overload in mice that reproduces what occurs in human hemolytic disorders when free hemoglobin overcomes the binding capacity of haptoglobin and, consequently, is degraded into the bloodstream, thus increasing free heme. Our results show that lack of Hx promotes endothelial activation and enhances vascular permeability. The liver is the most susceptible organ to heme overload when Hx is lacking because it develops congestion in the centrolobular area associated with inflammation and oxidative stress. We also show that Hx is necessary to mediate heme-iron recovery into hepatocytes, whereas its lack results in heme-iron recovery in Kupffer cells and proximal tubular cells of the kidney.
Materials and Methods
Mice and Treatments
Hx-null mice, on a 129Sv genetic background, were previously generated in our laboratory.19 The mice used in these studies were 8 to 12 weeks old. All of the mice were maintained on a standard chow diet. Hemin (Sigma-Aldrich, Milano, Italy) was dissolved in 0.1 N of NaOH and diluted in phosphate-buffered saline (PBS) at a final concentration of 20 mmol/L. pH was adjusted at 7.4 with 0.1 N of HCl. Freshly prepared hemin was injected into the tail vein of mice at a dose of 70 μmol/kg (high dose) or of 35 μmol/kg (low dose). Control mice were injected with PBS. Mice were sacrificed at different times after hemin injection. Hemin preconditioning was performed by injecting mice intraperitoneally with hemin at a dose of 40 μmol/kg/day for 3 days. One day after preconditioning, the high dose of hemin was injected into the tail vein and mice sacrificed 6 hours later. All experiments were approved by the animal studies committee of the University of Torino (Torino, Italy).
Western Blotting
Tissue extracts were prepared in 1% Triton X-100 in Tris-buffered saline plus protease inhibitors (Roche Diagnostics Corp., Milano, Italy). Protein concentration was determined using the Bio-Rad protein assay system (Bio-Rad, München, Germany). Plasma was collected from the tail vein. One μl of plasma or 50 μg of total protein extracts were separated on 10 to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting using antibodies against Hx (mAb 3D6/E12 produced in our laboratory), low-density lipoprotein receptor-related protein (LRP),20 HO-1 (Stressgen, Victoria, Canada), intercellular adhesion molecule (ICAM)-1 (Stemcell Technologies Inc., Vancouver, Canada), vascular cell adhesion molecule (VCAM)-1 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), L- and H-Ferritin (Ft),21 actin (Santa Cruz Biotechnology Inc.), vinculin (Sigma-Aldrich).
In Vivo Permeability Assay
A 0.5% solution of Evans blue dye (Sigma-Aldrich) was injected into the tail vein of mice at a dose of 20 mg/kg. One hour after dye injection, mice were anesthetized with Avertin (2,2,2-tribromoethanol; Sigma-Aldrich), transcardially perfused with PBS, and organs quickly harvested. Evans blue dye was then extracted from tissues by formamide (4 ml/g tissue) at 37°C for 24 hours. After measurement of optical density at 620 to 740 nm in a spectrophotometer, the concentration of Evans blue dye (ng/mg) was calculated based on a standard curve of known amounts of Evans blue dye.
Histology and Immunohistochemistry
Animals were anesthetized with Avertin (Sigma-Aldrich) at a dose of 2 mg/kg body weight and transcardially perfused with PBS. Tissues were collected, fixed in 10% formalin in PBS overnight at room temperature, and embedded in paraffin. Microtome sections, 7 to 10 μm thick, were stained with hematoxylin and eosin (H&E). For immunohistochemistry, tissue sections were analyzed with the following antibodies: rabbit antibody to HO-1 (Stressgen), rat monoclonal antibody to CD18 antigen (BMA Biomedicals AG, Augst, Switzerland), rat monoclonal antibody to F4/80 antigen (Serotec, Oxford, UK). The following secondary antibodies were used: biotinylated swine anti-rabbit IgG and biotinylated rabbit anti-rat IgG (DakoCytomation, Milano, Italy). Immunoreactivity was detected with the StreptABComplex/HRP system (DakoCytomation) and developed with diaminobenzidine (Roche Diagnostics Corp.). Liver cells counts were made on a microscope at ×20 or ×40 magnification using an image analyzer (Image Pro Plus 4.0; Media Cybernetics, Bethesda, MD). Positive cells were counted on four randomly chosen fields within an area of 7 × 10−2 mm2 per section. At least four animals for each experimental point were counted.
Organ Heme Content
The heme content of livers, kidneys, and lungs was measured in organ homogenates by the pyridine-hemochrome method as previously described.22 Data were expressed as μg heme/μg DNA.
Serum Bilirubin Measurement
Blood was collected from anesthetized mice by retro-orbital bleeding. Total serum bilirubin was measured colorimetrically using the QuantiChrom bilirubin assay kit DIBR-180 from BioAssay Systems (Hayward, CA), according to the manufacturer’s instructions. Serum bilirubin concentrations were expressed as mg/dl.
Lipid Peroxidation Assay
Lipid peroxidation from tissue extracts was measured using the colorimetric assay kit Bioxytech LPO-586 from Oxis International (Portland, OR) according to the manufacturer’s instructions. Briefly, tissue samples were homogenized (20% w/v) in ice-cold 20 mmol/L phosphate buffer (pH 7.4) containing 5 mmol/L butylated hydroxytoluene and protein content was determined using the Bio-Rad protein assay system. A 200-μl quantity of sample was assayed for malonaldehyde (MDA) content in hydrochloride using the chromogenic reagent N-methyl-2-phenylindole. Absorbance was measured at 586 nm and the results were expressed as pmol MDA per mg of protein.
Statistical Analysis
Results were expressed as mean ± SD or mean ± SEM. Statistical analyses were performed using one-way analysis of variance followed by the Bonferroni correction for multiple group comparisons. An unpaired Student’s t-test was used when only two groups were compared (see data shown in Figure 4B, below). A P value of less than 0.05 was considered significant.
Figure 4.
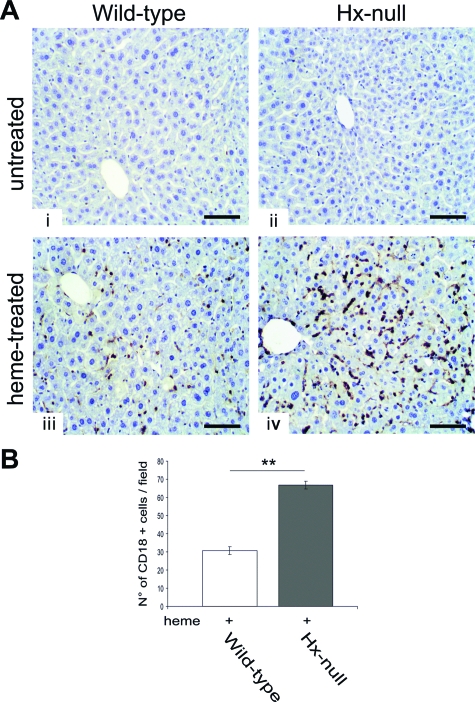
Increased liver inflammation in Hx-null mice after heme overload. A: Liver sections of wild-type and Hx-null mice untreated (i, ii) or treated with the high dose of hemin for 6 hours (iii, iv), stained with an antibody to CD18 antigen. Note the higher number of CD18-positive cells in Hx-null liver after hemin treatment. B: Quantification of CD18-positive cells on liver sections of wild-type and Hx-null mice. Cells were counted as reported in the Materials and Methods. Data represent mean ± SD; n = 4 for each genotype; **P < 0.01. Scale bars = 100 μm.
Results
Establishment of an in Vivo Model of Heme Overload
To study the protective role of Hx under pathological conditions associated with the massive release of heme into the bloodstream, we generated an in vivo model of heme overload in mice by injecting hemin into the tail vein at a dose of 70 μmol/kg (high dose) or of 35 μmol/kg (low dose). Both doses caused a strong reduction of plasma Hx level in wild-type mice at 6 hours after injection (not shown), thus indicating that Hx is involved in heme detoxification. The residual amount of Hx still detectable 6 hours after treatment in the plasma of wild-type mice indicated that the hemin doses used in our experiments did not fully saturate Hx. The analysis of LRP, known as the Hx receptor,20 in liver extracts did not show alterations in LRP expression levels after heme overload in wild-type and in Hx-null mice (not shown). Hx-null and wild-type mice were injected with the high dose of hemin and analyzed 6 hours after the treatment. Control mice were injected with PBS. In some experiments the low dose of hemin was used and mice sacrificed 6 or 24 hours after the treatment.
Increased Endothelial Activation and Vascular Permeability in Hx-Null Mice after Heme Overload
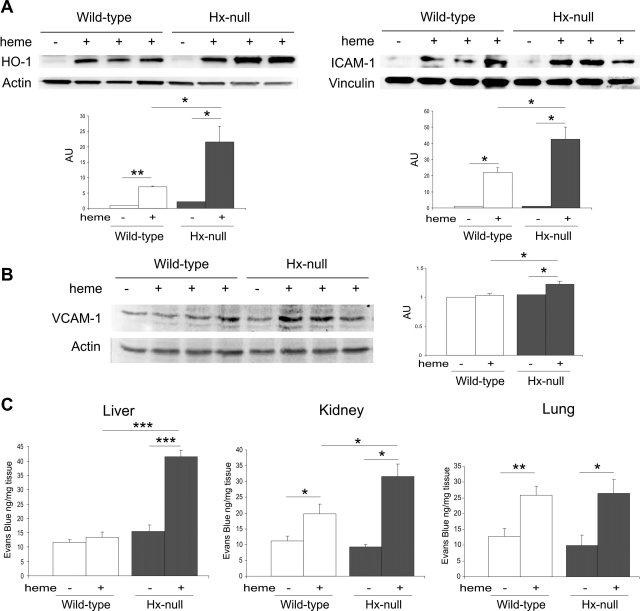
To assess vascular activation after heme treatment, we analyzed the expression of HO-1 and ICAM-1 in extracts of aorta from wild-type and Hx-null mice 6 hours after hemin injection. HO-1 and ICAM-1 were undetectable under basal conditions and strongly induced by hemin in both Hx-null and control mice. Both HO-1 and ICAM-1 induction were significantly higher in Hx-null aorta than in wild-type counterpart (Figure 1A). To further investigate the state of endothelial activation in the vasculature, we analyzed the expression of VCAM-1 in liver extracts. As shown in Figure 1B, VCAM-1 was slightly, but significantly induced 6 hours after hemin injection in the liver of Hx-null mice, but not in that of wild-type animals.
Figure 1.
Endothelial activation and vascular permeability after heme overload. A: Western blotting analysis of HO-1 (left) and ICAM-1 (right) expression on extracts of aortas from wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin for 6 hours (+). B: Western blotting analysis of VCAM-1 expression on liver extracts from wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin for 6 hours (+). In A and B a representative experiment for each protein is shown. Band intensities were measured by densitometry and normalized to actin or vinculin expression. Densitometry data represent mean ± SEM; n = 3 for each genotype. *P < 0.05; **P < 0.01. Results shown are representative of three independent experiments; in each experiment at least three mice per genotype were analyzed. C: Evans blue dye content of liver, kidney, and lung of wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin (+) for 6 hours. Data represent mean ± SEM; n = 6 for each group. *P < 0.05; **P < 0.01; ***P < 0.0001.
Vascular permeability was analyzed by Evans blue dye diffusion assay. To determine whether heme treatment altered vascular permeability, we injected mice with hemin and, 5 hours later, with Evans blue dye into the tail vein. Animals were sacrificed 1 hour after Evans blue treatment and dye content in liver, kidney, and lung was determined. Compared with PBS mock treatment, injection with hemin significantly increased the accumulation of dye in the liver of Hx-null mice by approximately threefold, but not in that of wild-type controls. Vascular permeability was weakly, but significantly increased in the kidney of heme-treated, wild-type mice and strongly augmented in the corresponding organ of Hx-null animals. In the lung, hemin injection caused a comparable increase in vascular permeability in both wild-type and Hx-null mice (Figure 1C).
Increased Oxidative Stress in Hx-Null Mice after Heme Overload
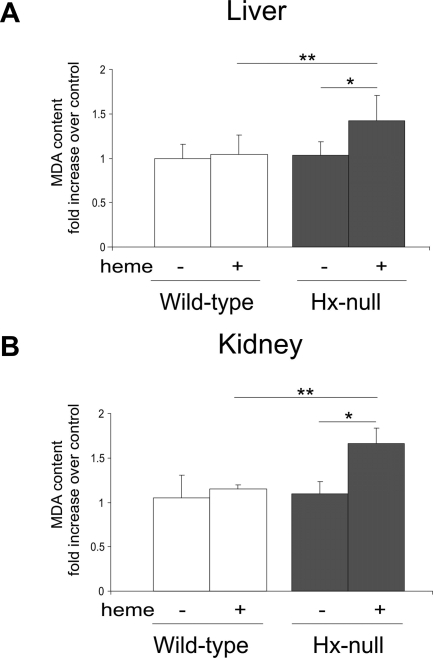
Because heme is a strong pro-oxidant agent, we measured MDA tissue level as an index of lipid peroxidation consequent to an oxidative damage. This analysis was performed on liver and kidney that had shown an altered permeability after heme overload in Hx-null mice compared with wild-type animals. Liver and kidney MDA levels were comparable in Hx-null and wild-type mice under basal conditions. Hemin injection caused a significant increase in MDA levels in Hx-null livers and kidneys (Figure 2, A and B), but not in wild-type organs.
Figure 2.
Increased lipid peroxidation in Hx-null mice after heme overload. Lipid peroxidation, estimated as MDA levels, of liver (A) and kidney (B) homogenates of wild-type and Hx-null mice untreated or treated with the high dose of hemin for 6 hours. Data are expressed as the fold increase over a control sample (an untreated wild-type mouse). Data represent mean ± SD; n = 7 for each group; *P < 0.05; **P < 0.01.
Liver Congestion in Hx-Null Mice after Heme Overload
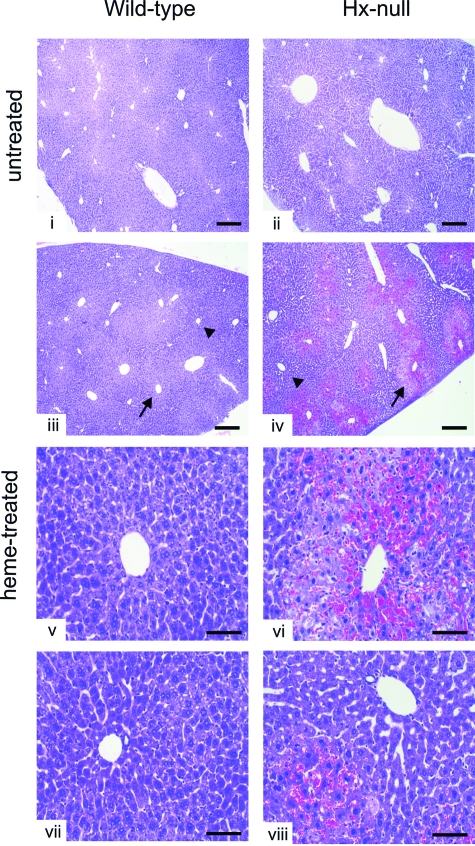
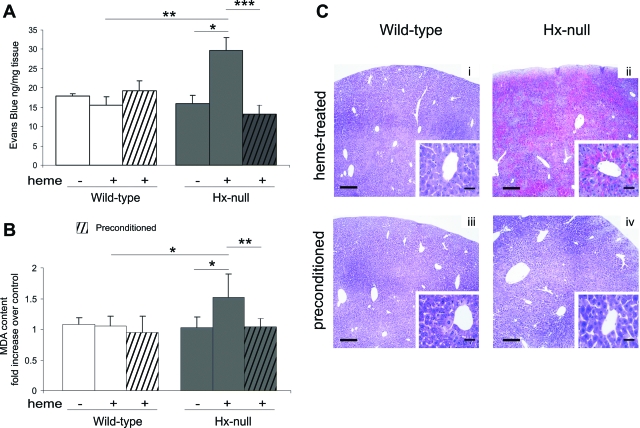
Data reported in the previous paragraphs showed alterations in vascular permeability and oxidative injury after heme overload in liver and kidney of Hx-null mice. To evaluate if these alterations resulted in tissue abnormalities, we analyzed organ histopathology. The kidney of hemin-treated Hx-null animals showed no obvious abnormalities (not shown). In the liver of Hx-null mice we observed red blood cell congestion in sinusoids around the centrolobular vein (Figure 3). The red blood cell congestion was estimated by measurement of organ heme content by the pyridine-hemochrome method. The livers of hemin-treated Hx-null mice had approximately sevenfold more heme than those of normal mice. On the other hand, heme content in other organs, such as kidney, lung, and skeletal muscle, did not significantly change after hemin injection both in Hx-null and wild-type mice (Table 1). Congested livers were characterized by areas of cell necrosis and inflammation. The latter was estimated by counting CD18-positive infiltrating cells in the liver of Hx-deficient and wild-type mice. We observed a twofold increase in the number of CD18-positive cells in the liver of hemin-treated Hx-null mice compared with the liver of hemin-treated wild-type animals (Figure 4, A and B).
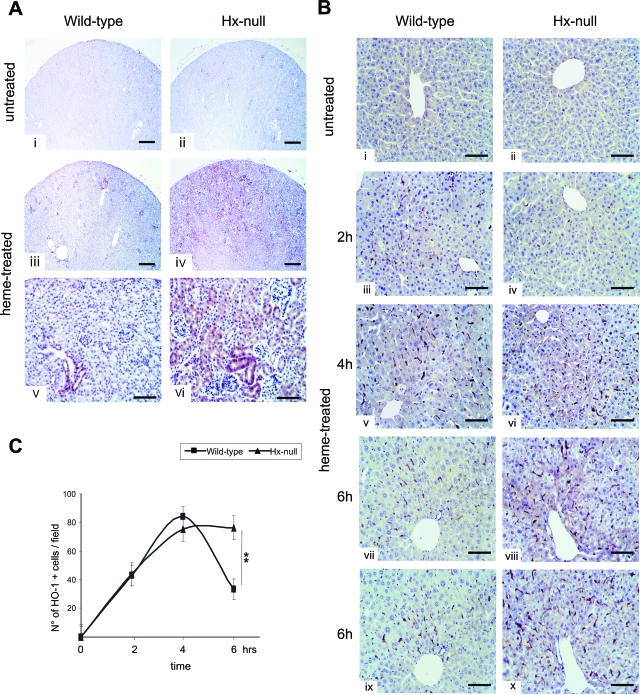
Figure 3.
Liver congestion in Hx-null mice after heme overload. Liver sections of wild-type and Hx-null mice untreated (i, ii) or treated with the high dose of hemin for 6 hours (iii–viii), stained with H&E. Note congestion around the centrolobular vein (arrows), but not in the periportal area (arrowheads), only in Hx-null mouse. Scale bars: 300 μm (i–iv); 100 μm (v–viii).
Table 1.
Organ Heme Content
| Mice | Heme content (μg heme/μg DNA)
|
|||
|---|---|---|---|---|
| Liver | Kidney | Lung | Muscle | |
| Untreated wild-type | 1.52 ± 0.34 | 2.29 ± 0.54 | 3.69 ± 0.4 | 2.68 ± 1.13 |
| Heme-treated wild-type | 4.63 ± 1.3 | 2.25 ± 0.59 | 6.08 ± 0.71 | 2.15 ± 1.04 |
| Untreated Hx-null | 1.59 ± 0.23 | 2.13 ± 0.51 | 3.65 ± 1.4 | 2.14 ± 0.5 |
| Heme-treated Hx-null | 34.19 ± 13.45* | 2.28 ± 0.31 | 5.96 ± 1.48 | 1.26 ± 0.46 |
The organs of untreated or heme-treated wild-type and Hx-null mice were homogenized, and the heme content was measured by the pyridine-hemochrome method, as described in the Materials and Methods. Data represent mean ± SEM; n = 5 for each group.
P < 0.05, heme-treated versus untreated Hx-null mice, heme-treated Hx-null mice versus heme-treated wild-type animals.
Altered HO-1 Induction in Hx-Null Mice after Heme Overload
Because heme is a strong inducer of HO-1, we measured serum bilirubin level as an index of HO activity. Hemin injection caused an increase in serum bilirubin both in Hx-null and wild-type mice. However, the increase resulted much greater in Hx-null mice than in wild-type controls (Table 2). Then, we analyzed HO-1 expression by immunohistochemistry. In kidney, hemin treatment induced a strong expression of HO-1 in Hx-null mice, but only a weak expression in wild-type animals. HO-1 was mainly induced in proximal tubular cells (Figure 5A). HO-1 was also strongly induced in the liver of both Hx-null and wild-type mice. HO-1-positive cells present irregular shape and dendritic extensions. Comparison of consecutive liver sections stained with an antibody to the macrophage marker F4/80 and an antibody to HO-1, respectively, demonstrated that HO-1 was induced in Kupffer cells in both Hx-null and wild-type mice (Figure 5B, vii to x). To assess if Hx-null and wild-type mice differed in HO-1 expression, we quantified HO-1 induction by counting immunoreactive cells on liver sections. The number of HO-1-positive cells increased in the liver of Hx-null and wild-type mice by 2 hours after hemin injection and peaked at 4 hours with no significant differences between mice of the two genotypes. By 6 hours after heme overload, the number of HO-1-positive cells decreased in wild-type mice but remained high in Hx-null mice (Figure 5, B and C). The same results were obtained with the low dose of hemin. Again, the number of HO-1-positive cells was higher in Hx-null liver than in wild-type controls at both 6 and 24 hours after hemin injection (not shown).
Table 2.
Serum Bilirubin
| Mice | Serum bilirubin (mg/dl) |
|---|---|
| Untreated wild-type | 0.96 ± 0.14 |
| Heme-treated wild-type | 3.03 ± 0.53† |
| Untreated Hx-null | 0.83 ± 0.23 |
| Heme-treated Hx-null | 9.18 ± 1.66*† |
Serum bilirubin was measured as reported in the Materials and Methods. Data represent mean ± SEM; n = 4 for each group.
P < 0.05: heme-treated Hx-null mice versus heme-treated wild-type animals.
P < 0.001: heme-treated versus untreated wild-type and Hx-null mice.
Figure 5.
HO-1 induction after heme overload. A: Kidney sections of wild-type and Hx-null mice untreated (i, ii) or treated with the high dose of hemin for 6 hours (iii–vi), stained with an antibody to HO-1. Note the induction of HO-1 in proximal tubular cells of Hx-null kidney after hemin injection. B: Liver sections of wild-type and Hx-null mice untreated (i, ii) or treated with the high dose of hemin for 2 hours (iii, iv), 4 hours (v, vi), or 6 hours (vii–x), stained with an antibody to HO-1 (i–viii) or an antibody to F4/80 (ix–x). Note the increased number of HO-1-positive cells in Hx-null liver 6 hours after hemin injection. The last four panels show consecutive sections of wild-type (vii–ix) and Hx-null (viii–x) livers: the anti-HO-1 and anti-F4/80 antibodies stain the same cell type, ie, Kupffer cell. C: Quantification of HO-1-positive cells on liver sections of wild-type and Hx-null mice. Cells were counted as described in the Materials and Methods. Data represent mean ± SD; n = 3 for each experimental point; **P < 0.01. Scale bars: 300 μm (A, i–iv); 100 μm [A, (v, vi) B].
Differential Regulation of Ferritins in Hx-Null Mice after Heme Overload
Because heme catabolism results in the release of iron ions, we analyzed ferritin expression on tissue extracts. Ferritins are ubiquitous and highly conserved iron-binding proteins, consisting of two subunits, termed L-Ferritin (L-Ft) and H-Ferritin (H-Ft). H-Ft has a ferroxidase activity needed for sequestering iron. Twenty-four Ft subunits assemble to form the apo-Ft shell that can sequester up to ∼4500 iron atoms. The H:L ratio is not fixed, but is rather quite plastic: it depends on the iron status of the cell and it is modified in many inflammatory and infectious conditions.23
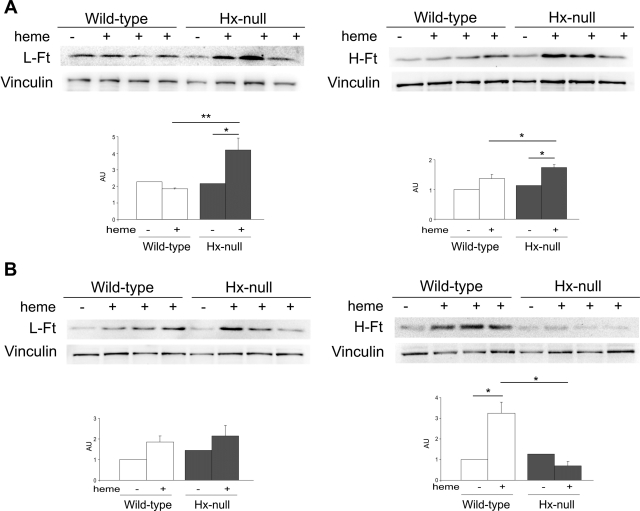
Ft expression was analyzed by Western blotting. In liver and kidney L- and H-Ft were expressed at similar levels in Hx-null and wild-type mice under basal conditions. In the kidney hemin injection caused a slight, but significant induction of L- and H-Ft only in Hx-null mice (Figure 6A). In the liver L-Ft was weakly, but not significantly, induced by hemin treatment in both Hx-null and wild-type animals. On the other hand, H-Ft was strongly induced in wild-type liver, but not significantly modulated in Hx-null organ (Figure 6B).
Figure 6.
L- and H-Ft expression in kidney and liver after heme overload. Western blotting analysis of L-Ft (left) and H-Ft (right) expression on kidney (A) and liver (B) extracts from wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin for 6 hours (+). A representative experiment for each protein is shown. Band intensities were measured by densitometry and normalized to vinculin expression. Densitometry data represent mean ± SEM; n = 3 for each genotype. *P < 0.05; **P < 0.01. Results shown are representative of three independent experiments; in each experiment at least three mice per genotype were analyzed.
Hemin Preconditioning Prevents Liver Damage in Heme-Overloaded Hx-Null Mice
Data reported in the previous sections demonstrated that the liver is the most compromised organ after heme overload in Hx-null mice. We also showed that HO-1 is induced by heme overload and that induction is maintained for a longer time in Hx-null mice than in wild-type animals. Because HO-1 and its products have documented anti-inflammatory properties, a key question is whether additional up-regulation of HO-1 expression can prevent liver damage. To test this possibility, Hx-null and wild-type mice were injected intraperitoneally with hemin at 40 μmol/kg/day for 3 days before intravenous injection of the high dose of hemin. Hemin preconditioning for 3 days caused a strong induction of HO-1 in the liver of both Hx-null and wild-type mice (not shown). We evaluated vascular permeability, oxidative damage, and liver histology in preconditioned and hemin-treated mice compared with hemin-treated ones. As shown in Figure 7, preconditioning prevented Evans blue dye accumulation in the liver of hemin-treated Hx-null mice (Figure 7A) as well as MDA level increase (Figure 7B) and liver congestion (Figure 7C). These data demonstrated that preconditioning may induce cytoprotection, thus overcoming the negative effects because of the lack of Hx.
Figure 7.
Prevention of liver damage by preconditioning. A: Evans blue dye content of the liver of wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin (+) for 6 hours without (open and solid bars) or with preconditioning (open and solid hatched bars). Data represent mean ± SEM; n = 8 for each group. *P < 0.05; **P < 0.01; ***P < 0.0001. B: Lipid peroxidation, estimated as MDA levels, of liver homogenates of wild-type and Hx-null mice untreated (−) or treated with the high dose of hemin (+) for 6 hours without (open and solid bars) or with preconditioning (open and solid hatched bars). Data are expressed as the fold increase over a control sample (an untreated wild-type mouse). Data represent mean ± SD; n = 8 for each group; *P < 0.05; **P < 0.01. C: Liver sections of wild-type and Hx-null mice treated with the high dose of hemin for 6 hours without (i, ii) or with (iii, iv) preconditioning, stained with H&E. Note congestion around the centrolobular vein in heme-treated Hx-null mouse, but not in preconditioned animal. Scale bars: 300 μm (C); 50 μm (C, insets).
Discussion
These data highlight the critical importance of Hx in preventing vascular inflammation and vaso-occlusion in a murine model of heme overload. Physiologically, the vascular endothelium is nonadhesive for leukocytes. However, once activated by inflammatory stimuli, endothelial cells increase the expression of specific adhesion molecules that mediate the recruitment of leukocytes.24,25,26,27 Heme has been shown to induce the expression of ICAM-1, VCAM-1, and E-selectin on endothelial cells, which results in the binding of blood cells to endothelial cells.3 Moreover, infusion of heme in mice has already been shown to increase vascular permeability and leukocyte recruitment.28 These toxic effects of heme are exacerbated in Hx-null mice, indicating that Hx has an important protective role in plasma. Moreover, none of the plasma proteins able to bind heme, ie, albumin, high- and low-density lipoproteins, α1-microglobulin,7 may substitute for Hx after heme overload.
Oxidative stress has already been shown to induce vascular HO-1 expression in rats, mice, and humans.29,30,31 Consistently, we found that hemin treatment induces HO-1 in extracts of aorta from wild-type and Hx-null mice. Even if HO-1 induction is significantly higher in the aorta of Hx-null mice than in that of wild-type animals, it cannot prevent endothelial damage. On the other hand, the induction of HO-1 before heme overload preserve endothelial integrity in Hx-null mice, thus indicating that the lack of Hx may be tolerated if the cells are equipped to metabolize an excess of heme. These data demonstrate that Hx and HO-1 work in sequence to counteract the toxic effect of heme, Hx being the first line of defense.
Despite systemic endothelial damage, we observed a dramatic increase in vascular permeability, accompanied by congestion and inflammation only in the liver of Hx-null mice. This may be explained by the particular architecture of the fenestrated sinusoidal endothelium that probably renders it more susceptible to heme overload. Consistently, haptoglobin and Hx double-mutant mice exhibited liver necrosis and fibrosis after phenylhydrazine-induced hemolysis.18
The histological features of Hx-null liver after hemin injection resemble those observed in patients suffering from veno-occlusive disease (VOD). VOD is attributable to hepatic venous outflow obstruction and is characterized by hepatomegaly, marked hyperbilirubinemia, and at histological level, narrowing of central veins and sinusoids, sinusoidal congestion, and hepatocellular necrosis.32
VOD is attributable to a nonthrombotic sinusoidal obstruction, which occurs as a result of sinusoidal endothelial cell injury. This disease is most frequently caused by hematopoietic stem cell transplantation and is also seen after solid organ transplantation. In these cases VOD is attributed to toxicity of cytoreductive agents in the conditioning therapy, that cause oxidative damage to endothelium.33 Interestingly, patients with thalassemia major undergoing bone marrow transplantation are particularly prone to developing VOD. In thalassemia intravascular hemolysis is increased and vascular hemoglobin and heme scavengers levels are reduced, thus suggesting that heme may potentiate cytotoxic effect of cytoreductive agents.34 In addition, Srivastava and co-authors35 have shown that a polymorphism in the glutathione S-transferase gene, affecting the anti-oxidant capacity of the enzyme, increases the incidence of VOD in thalassemia major patients, again suggesting that reactive oxygen species-mediated endothelial damage is the principal pathogenic cause of VOD.
Histologically, VOD is very similar to Budd-Chiari syndrome (BCS). BCS is generally associated with myeloproliferative disorders, polycythemia vera, and paroxysmal nocturnal hemoglobinuria. Interestingly, BCS is seen in up to 12% of patients with paroxysmal nocturnal hemoglobinuria and is the main cause of mortality in this disorder.36 Intravascular thrombosis of hepatic veins and/or inferior vena cava is the major cause of BCS and most patients who suffers from BCS have an underlying condition that predisposes to blood clotting.37 Our data, together with the high incidence of BCS in paroxysmal nocturnal hemoglobinuria patients, suggest that heme is a predisposing factor. We demonstrated that Hx may efficiently counteract heme toxic effects. Thus, agents that enhance the heme detoxifying potentialities might be helpful in the prevention of BCS or related diseases.
Painful vaso-occlusive crises are also frequently associated with hemoglobinopathies. In particular, in sickle cell disease, vascular occlusions are the major causes of the pain, morbidity, and mortality.38,39,40 Even in this case, the enhanced rate of intravascular hemolysis results in heme overload, that in turn causes endothelial damage and vaso-occlusions.5,6,41
In summary, the phenotype of Hx-null mice after heme overload indicates that heme is mainly responsible for vaso-occlusion, at least at the hepatic level, and that Hx is crucial to counteract its toxic effects. Excess of heme would cause oxidative damage and promote endothelial injury, adhesion of leukocytes to endothelium, and/or thrombosis. We thus speculate that drugs mimicking Hx activity might be useful in prevention and treatment of vaso-occlusive crisis in patients suffering from hemolytic disorders. In this respect Hx-null mice represent an useful model to test new therapeutic approaches.
In the liver hepatocytes and Kupffer cells are able to metabolize heme. Both cell types express the heme-Hx complex receptor, LRP.20,42 However, hepatocytes have a much greater iron storage capacity than Kupffer cells. We showed that H-Ft is strongly induced in the liver of wild-type mice, but not in that of Hx-null animals. Because hepatocytes represent more than 90% of liver cells, our Western blots report protein expression in this population. Up-regulation of H-Ft in wild-type hepatocytes indicates a strong iron detoxifying capacity, because of its ferroxidase activity, and an active iron storage.43 Conversely, lack of H-Ft induction in Hx-null liver demonstrates that this detoxifying activity is deficient in Hx-null mice. We conclude that Hx is crucial to mediate heme-iron recovery in hepatocytes.
Kupffer cells are able to acquire non-Hx-bound heme, and, indeed, they remained activated longer in Hx-null mice than in wild-type animals. It is likely that an up-regulation of ferritins occurred also in this cell population, but it is undetectable in whole liver extracts. Prolonged HO-1 induction in Kupffer cells would be responsible for hyperbilirubinemia. Heme that overwhelmed Kupffer cells’ recovery capacity would be responsible for endothelial damage.
Furthermore, we observed a stronger induction of HO-1 and ferritins in the kidney of Hx-null mice compared with wild-type counterpart. This indicates that excess of heme-iron is recovered by the kidney. This result is in agreement with renal iron loading observed in Hx-null kidney after phenylhydrazine-induced hemolysis.19 Proximal tubular cells express the megalin/cubilin receptor complex able to take up hemoglobin from glomerular ultrafiltrate, and divalent metal transporter-1 that recovers iron and other ions.44,45 The recently identified heme transporters heme carrier protein-1 and feline leukemia virus subgroup C cellular receptor are expressed in the kidney.46 Therefore, it is possible to speculate that the kidney may take up excess of heme from circulation and store iron. This would represent a mechanism to prevent iron loss as occurs in haptoglobin-null mice that recover free hemoglobin and store iron in proximal tubular cells.47,48
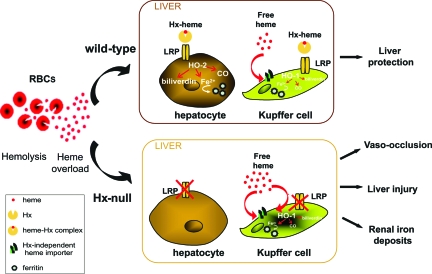
In conclusion, our results demonstrate that Hx is essential to mediate heme recovery by hepatocytes, thus preventing vaso-occlusion, liver damage, and renal iron loading as summarized in the model depicted in Figure 8. We suggest that agents that increase and/or mimic Hx activity may provide new therapies to prevent or treat endothelial damage in patients suffering from hemolytic disorders.
Figure 8.
A model of Hx-mediated heme recovery. In wild-type mice (top) the heme-Hx complex is taken up by hepatocytes and Kupffer cells through the LRP receptor. The latter are also able to recover free heme in an Hx-LRP-independent way. Once into cells, heme is degraded by HO-2 (hepatocytes) and HO-1 (Kupffer cells) and iron stored in ferritins. Because hepatocytes represent more than 90% of liver cells, they account for the recovery of most heme, thus preventing heme toxicity. In Hx-null mice (bottom), lack of Hx prevents heme recovery in hepatocytes. Heme is taken up by Kupffer cells that remain activated longer than in wild-type counterpart. Heme that overwhelms the recovery capacity of Kupffer cells promotes vaso-occlusion and liver damage. Part of heme is recovered by proximal tubular cells of the kidney.
Acknowledgments
We thank Sonia Levi for the gift of anti-ferritin antibodies, Soren Moestrup for the anti-LRP antibody, Clara Camaschella for the helpful discussion, and Sharmila Fagoonee and Donatella Barisani for the critical reading of the manuscript.
Footnotes
Address reprint requests to Emanuela Tolosano, Ph.D., Molecular Biotechnology Center, Via Nizza 52, 10126 Torino, Italy. E-mail: emanuela.tolosano@unito.it.
Supported by the Italian Ministry of University and Research (to E.T. and F.A.), the Regione Piemonte, and the Telethon grant GGP04181.
References
- Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Wagener FA, Volk HD, Willis D, Abraham NG, Soares MP, Adema GJ, Figdor CG. Different faces of the heme-heme oxygenase system in inflammation. Pharmacol Rev. 2003;55:551–571. doi: 10.1124/pr.55.3.5. [DOI] [PubMed] [Google Scholar]
- Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood. 2000;95:3442–3450. [PubMed] [Google Scholar]
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood. 2002;100:879–887. doi: 10.1182/blood.v100.3.879. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Bryant CJ, Nguyen J, Bowlin PR, Kielbik MC, Bischof JC, Hebbel RP, Vercellotti GM. Transgenic sickle mice have vascular inflammation. Blood. 2003;101:3953–3959. doi: 10.1182/blood-2002-10-3313. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Vilback AE, Sonbol KM, Kalambur VS, Bowlin PR, Bischof JC, Hebbel RP, Vercellotti GM. Critical role of endothelial cell activation in hypoxia-induced vasoocclusion in transgenic sickle mice. Am J Physiol. 2005;288:H2715–H2725. doi: 10.1152/ajpheart.00986.2004. [DOI] [PubMed] [Google Scholar]
- Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, Beringhelli T, Fasano M. Hemoglobin and heme scavenging. IUBMB Life. 2005;57:749–759. doi: 10.1080/15216540500380871. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Altruda F. Hemopexin: structure, function, and regulation. DNA Cell Biol. 2002;21:297–306. doi: 10.1089/104454902753759717. [DOI] [PubMed] [Google Scholar]
- Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 9:2119–2137. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Surh YJ. Heme oxygenase-1 as a potential therapeutic target for hepatoprotection. J Biochem Mol Biol. 2006;39:479–491. doi: 10.5483/bmbrep.2006.39.5.479. [DOI] [PubMed] [Google Scholar]
- Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SK, Kim H, Lim SK, bin Ali A, Lim YK, Wang Y, Chong SM, Costantini F, Baumman H. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–1877. [PubMed] [Google Scholar]
- Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, Altruda F. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood. 2002;100:4201–4208. doi: 10.1182/blood-2002-04-1270. [DOI] [PubMed] [Google Scholar]
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood. 1999;94:3906–3914. [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Santambrogio P, Cozzi A, Levi S, Rovida E, Magni F, Albertini A, Arosio P. Functional and immunological analysis of recombinant mouse H- and L-ferritins from Escherichia coli. Protein Expr Purif. 2000;19:212–218. doi: 10.1006/prep.2000.1212. [DOI] [PubMed] [Google Scholar]
- Berry EA, Trumpower BL. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal Biochem. 1987;161:1–15. doi: 10.1016/0003-2697(87)90643-9. [DOI] [PubMed] [Google Scholar]
- Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- Mukherjee TK, Mishra AK, Mukhopadhyay S, Hoidal JR. High concentration of antioxidants N-acetylcysteine and mitoquinone-Q induces intercellular adhesion molecule 1 and oxidative stress by increasing intracellular glutathione. J Immunol. 2007;178:1835–1844. doi: 10.4049/jimmunol.178.3.1835. [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Batkai S, Hasko G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, Pacher P. CB2-receptor stimulation attenuates TNF-alpha-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol. 2007;293:H2210–H2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting HJ, Stice JP, Schaff UY, Hui DY, Rutledge JC, Knowlton AA, Passerini AG, Simon SI. Triglyceride-rich lipoproteins prime aortic endothelium for an enhanced inflammatory response to tumor necrosis factor-alpha. Circ Res. 2007;100:381–390. doi: 10.1161/01.RES.0000258023.76515.a3. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, Figdor CG. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- Hu C, Dandapat A, Chen J, Liu Y, Hermonat PL, Carey RM, Mehta JL: Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis (in press) [DOI] [PubMed] [Google Scholar]
- Lee YM, Cheng PY, Hong SF, Chen SY, Lam KK, Sheu JR, Yen MH. Oxidative stress induces vascular heme oxygenase-1 expression in ovariectomized rats. Free Radic Biol Med. 2005;39:108–117. doi: 10.1016/j.freeradbiomed.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: three similar syndromes. World J Gastroenterol. 2007;13:1912–1927. doi: 10.3748/wjg.v13.i13.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzolo M, Germani G, Cholongitas E, Burra P, Burroughs AK. Veno occlusive disease: update on clinical management. World J Gastroenterol. 2007;13:3918–3924. doi: 10.3748/wjg.v13.i29.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonkuzhali B, Srivastava A, Quernin MH, Dennison D, Aigrain EJ, Kanagasabapathy AS, Krishnamoorthy R, Chandy M. Pharmacokinetics of oral busulphan in children with beta thalassaemia major undergoing allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;24:5–11. doi: 10.1038/sj.bmt.1701814. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, Krishnamoorthy R. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574–1577. doi: 10.1182/blood-2003-11-3778. [DOI] [PubMed] [Google Scholar]
- Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253–1258. doi: 10.1056/NEJM199511093331904. [DOI] [PubMed] [Google Scholar]
- Aydinli M, Bayraktar Y. Budd-Chiari syndrome: etiology, pathogenesis and diagnosis. World J Gastroenterol. 2007;13:2693–2696. doi: 10.3748/wjg.v13.i19.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn H, Li CS, Wang W. Sickle cell hepatopathy: clinical presentation, treatment, and outcome in pediatric and adult patients. Pediatr Blood Cancer. 2005;45:184–190. doi: 10.1002/pbc.20317. [DOI] [PubMed] [Google Scholar]
- Dampier C, Setty BN, Eggleston B, Brodecki D, O'Neal P, Stuart M. Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates. J Pediatr Hematol Oncol. 2004;26:785–790. [PubMed] [Google Scholar]
- Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Marker PH, Weber JP, Hebbel RP, Vercellotti GM. Activated monocytes in sickle cell disease: potential role in the activation of vascular endothelium and vaso-occlusion. Blood. 2000;96:2451–2459. [PubMed] [Google Scholar]
- Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–1893. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts; molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- Gburek J, Verroust PJ, Willnow TE, Fyfe JC, Nowacki W, Jacobsen C, Moestrup SK, Christensen EI. Megalin and cubilin are endocytic receptors involved in renal clearance of hemoglobin. J Am Soc Nephrol. 2002;13:423–430. doi: 10.1681/ASN.V132423. [DOI] [PubMed] [Google Scholar]
- Abouhamed M, Gburek J, Liu W, Torchalski B, Wilhelm A, Wolff NA, Christensen EI, Thevenod F, Smith CP. Divalent metal transporter 1 in the kidney proximal tubule is expressed in late endosomes/lysosomal membranes: implications for renal handling of protein-metal complexes. Am J Physiol. 2006;290:F1525–F1533. doi: 10.1152/ajprenal.00359.2005. [DOI] [PubMed] [Google Scholar]
- Latunde-Dada GO, Simpson RJ, McKie AT. Recent advances in mammalian haem transport. Trends Biochem Sci. 2006;31:182–188. doi: 10.1016/j.tibs.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Fagoonee S, Gburek J, Hirsch E, Marro S, Moestrup SK, Laurberg JM, Christensen EI, Silengo L, Altruda F, Tolosano E. Plasma protein haptoglobin modulates renal iron loading. Am J Pathol. 2005;166:973–983. doi: 10.1016/S0002-9440(10)62319-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marro S, Barisani D, Chiabrando D, Fagoonee S, Muckenthaler MU, Stolte J, Meneveri R, Haile D, Silengo L, Altruda F, Tolosano E. Lack of haptoglobin affects iron transport across duodenum by modulating ferroportin expression. Gastroenterology. 2007;133:1261–1271. doi: 10.1053/j.gastro.2007.07.004. [DOI] [PubMed] [Google Scholar]