Abstract
Visual awareness has been proposed to depend on recurrent processing in early visual cortex areas including the primary visual cortex (V1). Here, we address this hypothesis with high spatiotemporal resolution magnetoencephalographic recordings in subjects performing a substitution masking paradigm. Neural activity reflecting awareness is assessed by directly comparing the neuromagnetic response elicited by effectively and ineffectively masked targets after the proportion of trials leading to masking was individually adjusted to match the proportion of trials without masking. This revealed a modulation of recurrent activity in the primary visual cortex rapidly after the onset of the feedforward sweep of processing in striate and extrastriate areas but significantly before the onset of attention-dependent recurrent modulations in V1. Our data provide direct support for the notion that (i) recurrent processing in V1 correlates with visual awareness and (ii) that attention and awareness involve distinct recurrent processing operations.
Keywords: magnetoencephalography, visual attention, visual awareness
There is considerable evidence suggesting that attention influences activity in early visual cortex areas, including the primary visual cortex via feedback-driven modulations (1–4). Data from single cell recordings in the monkey have revealed that such feedback may operate via selective rate enhancements in V1 that label the segmentation of relevant input for priority for further processing (5, 6). Conversely, there is evidence suggesting that attentional feedback operates by suppressing irrelevant input, thereby increasing the relative prominence of relevant input representations in V1 (7).
Beyond its undoubted role for attentional selection, feedback processing from extrastriate to primary visual cortex has also been implicated as an important factor for mediating visual awareness (6, 8–11). Empirical evidence for this claim has been provided by a number of experimental approaches. While recording from V1 neurons in the macaque, Lamme et al. (12) observed that backward masking by texture patterns did not influence the initial feedforward activity elicited by the figure but caused a suppression of the delayed figure-ground modulation of neural firing, the latter being assumed to be mediated by feedback connections. Furthermore, Super et al. (13) could show that the delayed modulation reflecting figure-ground segregation was reduced in V1 when the monkey failed to identify the figure-ground stimulus.
Ress and colleagues (14, 15) report related observations with fMRI in human observers. They observed that better discrimination performance at visual detection thresholds correlated with a blood oxygenation level dependent (BOLD) response enhancement in primary visual cortex (14). This was the case even when subjectively perceived stimuli were physically absent (15). Although Ress and Heeger (15) discuss an explanation in terms of bottom-up fluctuations of sensory processing in V1, they also entertain the possibility that top-down modulatory influences may play a critical role.
The strongest available evidence in humans to date for a link between feedback projections to V1 and visual awareness has been provided by transcranial magnetic stimulation (TMS) studies in humans (10, 11, 16). Pascual-Leone et al. (10) report that TMS applied to V5 typically produces the perception of a moving phosphene. This perception was, however, disrupted when a TMS pulse over V5 was followed by a second pulse over V1 5–45 ms later. In addition, Silvanto et al. (11) observed that moving phosphene perception appears when a suprathreshold TMS pulse over V1 was applied 10–40 ms after a subthreshold TMS pulse over V5, with the latter not producing movement perception when applied alone. These observations were taken to suggest that back projections from V5 to V1 are critical for motion awareness.
Here, we use magnetoencephalographic (MEG) recordings, a method that permits to assess neural activity with practically unlimited temporal resolution, to provide evidence that stimulus awareness correlates with rapid modulations of recurrent activity that significantly predate attention-dependent recurrent modulations in the primary visual cortex (V1). Recordings were taken while subjects performed a visual search task in which the target was masked by object substitution, a type of masking that is believed to arise as a consequence of feedback processing in early visual cortex areas (17, 18). Masking by object substitution (in short, “substitution masking”) refers to the observation of a substantial decline in the ability to identify a search item when it is surrounded by small dots (the mask) that persist beyond the offset of the search items. When search items and the mask offset simultaneously, no (or a much smaller) performance decrease is seen. Substitution masking has been shown to represent a masking phenomenon different from backward pattern masking or metacontrast masking (17, 18). It has been hypothesized to appear because feedback from higher level visual cortex enters early visual cortex areas after the target disappeared from screen, forcing a readout of information from the trailing mask that conflicts with the initial feedforward representation in visual working memory (18).
Results
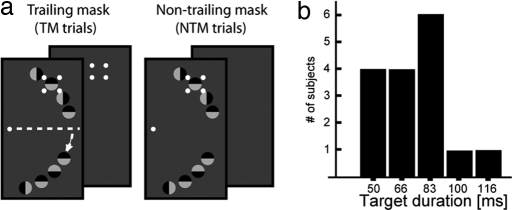
While fixating on the center of the screen, subjects were required to discriminate the orientation of one among eight similar items placed at iso-eccentric locations along an imaginary half-circle in the right visual field (VF; see Fig. 1a). The location of the target was indicated by a four-dot mask that appeared around the target simultaneously with the onset of the search items. In 67% of trials, the mask remained on the screen (fixed duration of 250 ms) after the search items disappeared ≈83 ms after search frame onset (trailing mask trials, TM trials). In the remaining 33% of the trials, the search items and the mask had common offset [non-trailing mask (NTM) trials].
Fig. 1.
Stimulus setup. (a) Four small dots are placed around one search item; the dots designate the target and serve to mask it. In 67% of the trials the dots remain on screen after the search items disappear from screen (TM trials). In the remaining trials, search items and the dots disappear simultaneously (NTM trials). The small arrow indicates that search items in the lower quadrant are displaced away from the horizontal meridian to avoid potentially ambiguous magnetic field configurations (see Experimental Procedures). (b) Summary of the target duration individually adjusted for each subject (staircase procedure).
To identify neural activity that reflects successful target identification, we compared the event-related magnetic field (ERMF) response to TM trials when subjects were or were not able to discriminate the target. For this comparison to be reasonable, it is crucial to obtain a sufficiently high proportion of hits. We, therefore, adjusted the presentation time of the search items for each subject individually based on a staircase procedure (see Experimental Procedures). This adjustment yielded on average 57% hits in TM trials and 66% hits in NTM trials, with a resulting median target duration of 83 ms. The histogram in Fig. 1b summarizes the target duration across subjects. After adjusting the number of correct TM trials, performance was still better in NTM trials, indicating that there was a significant substitution masking effect (chance performance would be 25% in this four alternative choice task). To confirm this statistically, a repeated-measures ANOVA (rANOVA) with the factor trial type (TM, NTM) was computed on the proportion of correct responses. This analysis revealed a significant effect of trial-type (F[1,15] = 63.4, P < 0.001), indicating that subjects performed significantly better on NTM than TM trials.
General Waveforms and Distributions.
Before turning to the direct comparison of successful and unsuccessful target identifications, we will shortly review the general time course and distribution of the ERMF response elicited by the search frames to provide a reference frame for the subsequent evaluation of the time course of awareness related activity.
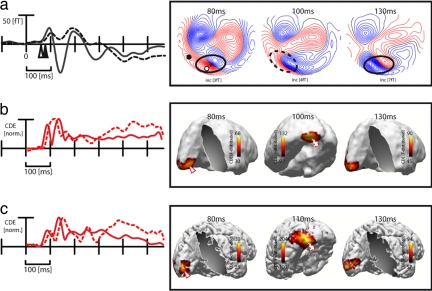
Fig. 2a shows average ERMF waveforms elicited by TM trials, collapsed across all conditions, at representative sensor sites over the occipital (solid) and the left inferior-temporal cortex (dashed) together with ERMF distribution maps at three representative time points after stimulus onset. Fig. 2b provides corresponding current source localization results [standardized low-resolution electromagnetic tomography (sLORETA) estimates; see Experimental Procedures for details], with the red waveforms representing the (normalized) time course of source activity at current density maxima (arrowheads) in the calcarine fissure (solid) and the extrastriate cortex (dashed). Fig. 2c shows analogous results in a single observer, where source density estimates were constrained by individual anatomical data (see Experimental Procedures). When inspecting the red waveforms from the occipital site in Fig. 2a, the first obvious effect is a positive deflection that starts roughly 70 ms after stimulus onset and reaches its peak shortly before 100 ms. t tests against baseline activity computed for subsequent time samples between 50 and 100 ms revealed that the earliest significant difference (P < 0.05) arises at 71 ms after search frame onset. From the distribution map at 80 ms, it is visible that this first deflection reflects the efflux component of an efflux-influx field configuration over the central-occipital cortex (solid ellipse). This distribution, whose current source is expected to originate from the cortex directly underneath the efflux-influx field transition (asterisk), reflects the initial activity elicited by the feedforward response in early visual cortex areas. sLORETA estimates of the total average (Fig. 2b) and the single observer (Fig. 2c) confirm this impression. The initial current maximum appears in the left calcarine fissure, a structure that coincides with the primary visual cortex (V1).
Fig. 2.
General waveforms/distributions and source localization results. (a) ERMF waveforms (total average across subjects) averaged across correct and incorrect TM trials at a central occipital (solid) and lateral occipitotemporal sensor site (dashed). The approximate location of the sensor sites are indicated by white and black circles in the distribution map. The arrowheads mark the onset of significant activity differences against baseline. Distribution maps at 80, 100, and 130 ms after search frame onset are shown on the right side. (b) Time course and distribution of sLORETA estimates of the total average response across subjects and conditions (correct TM trials). The red traces represent the time course of source activity at the source maximum in V1 (solid trace) and the left extrastriate cortex (dashed trace). (c) Time course and distribution of sLORETA estimates in a single subject.
When inspecting the distribution map at 100 ms in Fig. 2a, an efflux-influx field transition is visible over left lateral occipitotemporal sensor sites (dashed ellipse). The ERMF waveform taken from the efflux component (dashed trace), as well as the corresponding source waveform (dashed trace in Fig. 2b) show that it builds up with a delay relative to the onset of activity at the occipital sensor (arrowheads). Step-wise t tests between 50 and 100 ms against baseline activity yielded the first significant ERMF difference (P < 0.05) at 87 ms after stimulus onset. This 16-ms delay relative to the onset of the occipital response at 71 ms is consistent with the feedforward propagation of stimulus-elicited activity from early visual cortex toward higher-level extrastriate areas. sLORETA estimates at 100 ms yield a current source maximum in the lateral inferior-temporal cortex, which confirms that activity has propagated toward extrastriate regions after ≈100 ms. Finally, ERMF distributions and sLORETA estimates at 130 ms reveal that the source density maximum in the calcarine fissure region seen between 70 and 100 ms reappears with the same distribution in the subsequent time range between 110 and 170 ms, indicating that this activity represents recurrent activity in primary visual cortex.
Correlates of Successful Target Identification (TM Trials).
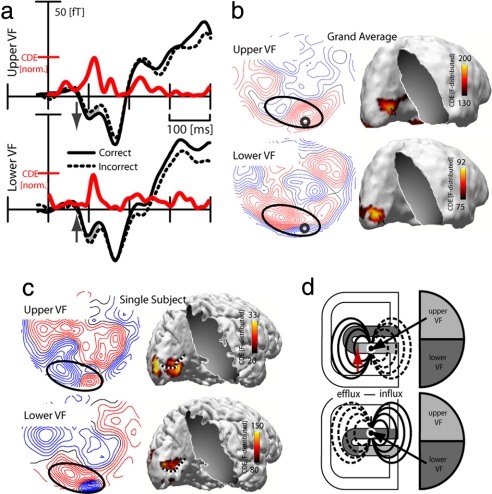
To assess activity that correlates with stimulus awareness, we performed a direct comparison of the magnetic response elicited by correct and incorrect TM trials. Fig. 3a shows waveforms of correct (solid) and incorrect (dashed) TM trials separately for targets in the upper and the lower VF at a representative sensor site. Figure 3b shows field distributions of corresponding correct-minus-incorrect ERMF differences at 110 ms. The red traces in Figure 3a represent the time course of source activity at the sLORETA maxima in the upper and lower bank of the calcarine fissure shown in Fig. 3b (arrows). An inspection of the ERMF waveforms revealed that the correct/incorrect distinction leads to an ERMF modulation between ≈100 and 120 ms (gray areas) with a reversal of relative polarity for upper versus lower VF targets (see arrows). In the source waveforms (red), this modulation was visible as a peak response at ≈110 ms. Importantly, the initial feedforward response in the earlier time range between 70 and 100 ms does not show such modulation. To confirm this statistically, rANOVAs with the factor performance (correct and incorrect) were computed for the 70–100 and 100–120 ms time ranges, which revealed a significant effect of performance between 100 and 120 ms in the upper (F[1,15] = 6.8, P < 0.05), and in the lower VF (F[1,15] = 6.3, P < 0.05) but no effect between 70 and 100 ms (upper VF: F[1,15] = 0.31; lower VF: F[1,15] = 1.26).
Fig. 3.
Awareness related effects. (a) Average ERMF waveforms (black traces) for correct and incorrect TM trials of targets in the upper and lower VF. The gray areas highlight the time range of significant differences. The red traces illustrate the time course of source activity (estimates based on the correct-minus-incorrect difference of TM trials) at the calcarine fissure maxima for upper and lower VF targets (red arrows in b). (b and c) ERMF maps and sLORETA estimates of the correct-minus-incorrect difference for TM trials in the upper and lower VF at 110 ms. The total average across subjects and the single subject are shown in b and c, respectively. The small circle in b marks the sensor position from which the waveforms in a were recorded. The dashed line in c marks the localization of the calcarine fissure. (d) Illustration of the cruciform model. The horizontal meridian is slightly displaced toward the lower bank of the calcarine fissure (red arrow; see Experimental Procedures for details).
A comparison of the distribution maps (correct-minus-incorrect difference) for upper and lower VF targets of the total average (Fig. 3b) and the single subject (Fig. 3c) showed that the modulation between 100 and 120 ms corresponded with an efflux-influx configuration over occipital sites (ellipses). Importantly, this configuration reversed polarity for upper versus lower VF targets, with lower VF targets producing a left-side efflux (red field lines) plus a right-side influx field (blue field lines) and upper VF targets producing a right-side efflux plus left-side influx field. What is important about this polarity reversal is that it provides strong evidence for a V1 origin of the generating current source. Fig. 3d illustrates the anatomical background of this reasoning based on the so called “cruciform model” (see Experimental Procedures for more details). The way visual quadrants are mapped onto parts of the calcarine fissure entails that a stimulus in the lower VF (dark gray area) elicited activity in the upper bank of the calcarine fissure. Conversely, a stimulus in the upper VF (bright gray area) produces activity changes in the lower bank. Because the pyramidal cells in the calcarine fissure reverse direction between the upper and the lower bank, a corresponding current dipole would reverse orientation accordingly. Consequently, the resulting ERMF distribution would flip efflux-influx components, as observed in the present data. Moreover, as visible from the sLORETA estimates of the single subject (Fig. 3c), the source density maxima at 110 ms appeared in the left calcarine fissure region (dashed line), with a maximum in the lower bank for upper VF targets and a maximum in the upper bank for lower VF targets, a distribution that is perfectly in line with the prediction of the cruciform model (Fig. 3d). To determine the onset of this modulation in V1 more precisely, we computed t tests of the correct-minus-incorrect difference against baseline activity for subsequent time samples between 70 and 120 ms, which revealed the first significant difference at 98 ms (P = 0.045) after stimulus onset.
In sum, the source localization results, together with the observation of a field polarity reversal for upper versus lower VF targets, suggest that the first activity modulation reflecting target awareness arose in the primary visual cortex. With a time range of 100–120 ms, this modulation arose soon (i.e., 27 ms) after the onset of the initial feedforward-driven activity in V1 and very rapidly (11 ms) after the onset of activity in extrastriate areas. Importantly, it appeared much earlier than attention-related recurrent activity modulations in V1, which have been found to arise not until after 150–250 ms after stimulus onset (2–5, 19). The considerable difference in the time course of awareness and attention-related modulations suggests that both represent distinct recurrent processing operations in V1.
Attention-Related Activity Modulations in V1.
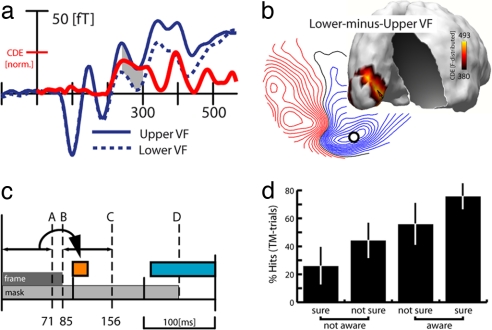
To assess attention-related activity in the present experiment, we compared ERMF activity elicited by upper versus lower VF targets. This would, of course, reflect differences in the spatial position of the mask, but it would also reveal differences in the spatial distribution of attention. Fig. 4a shows waveforms for upper and lower VF targets (correct TM trials) at an occipital sensor. The red trace shows the time course of sLORETA estimates of the lower-minus-upper field difference at the left calcarine fissure maximum (red arrow in Fig. 4b). Obviously, this comparison reveals a delayed activity difference, arising after ≈220 ms, whereas there was no visible difference between 100 and 120 ms or earlier. The distribution map of the lower-minus-upper field difference at 280 ms (Fig. 4b) shows that the delayed effect appears as efflux-influx field configuration over the occipital cortex, and corresponding sLORETA estimates show that the underlying current source originates in the vicinity of the left calcarine fissure, consistent with V1. These observations were confirmed statistically by rANOVAs with the factor VF (upper, lower) computed for the average ERMF response between 100 and 120 and 250 and 300 ms, which revealed a significant effect in the delayed time range (F[1,15] = 11.33;P < 0.005) but no effect between 100 and 120 ms (F[1,15] = 1.11).
Fig. 4.
Attention-related effects. (a) Average ERMF waveforms (blue traces) elicited by correct TM trials of targets in the upper and the lower VF. The red trace represents the time course of source activity (estimates of the lower-minus-upper VF difference) at the calcarine maximum (red arrow in b). (b) ERMF distribution of the lower-minus-upper VF difference and corresponding sLORETA estimates at 280 ms. The small circle marks the location of the sensor site where the waveforms in a were recorded. (c) Illustration of the timing of frame B and mask D relative to the onset of the feedforward-driven activity in V1 A, the time range of significant awareness-related (orange) and attention-related (blue) activity. C marks the time point at which the frame-offset transient would arrive in V1 (frame duration of 85 ms plus 71 ms for the visual input to arrive in V1). (d) Control experiment. Percentage of correct target discriminations as a function of the subsequent awareness judgement of the observers.
Taken together, the results of the upper/lower comparison are in line with observations of previous studies showing that attention modulates activity in V1 with a substantial delay of more than 200 ms after stimulus onset. Importantly, this effect appears roughly 100 ms after the response in V1 that correlates with awareness, suggesting that recurrent activity reflecting attention is distinct from awareness-related activity under the present experimental conditions. Of course, the upper/lower VF comparison did not provide a completely clean estimate of the attention-related response, because it also reflects activity changes attributable to the mask appearing in the upper versus lower VF. However, the fact that there was no upper/lower effect between 100 and 120 ms strongly suggests that attention did not influence recurrent processing in V1 in this early time range.
Control Experiment.
It should be noted that our conclusions regarding the neural correlates of awareness critically depend on the notion that successful target discrimination expresses visual awareness. That is not necessarily the case (20), and blindsight phenomena have revealed that there may be a considerable degree of discrimination processing without awareness (21). It is, thus, conceivable that the discrimination performance of subjects reflects the effectivity of processing at some subconscious level not directly linked to awareness. To address this possibility, we ran an extended behavioral version of the experiment (no MEG recorded) based on 13 of the 16 subjects who took part in the main MEG experiment. Subjects performed as in the main experiment but with the additional requirement to explicitly indicate whether and with what confidence they had been aware of the target. Specifically, 1 s after search frame offset (≈600 ms after the response of the subject to the target), subjects were prompted to rate with a four-alternative button press whether they were (i) aware of the target with confidence, (ii) aware but not sure, (iii) not aware but not sure, and (iv) confidently unaware and, therefore, guessing. With a proportion of 59% hits in TM trials versus 71% hits in NTM trials, the effect of substitution masking was significant (F[1,12] = 36.3;P < 0.0001) and almost identical to the main MEG experiment. Fig. 4d shows the proportion of correct TM trials separately for each of the four rating categories. The condition in which subjects were confidently aware of the target contained 75% hits, whereas the condition in which subjects were sure to be unaware of the target contained 26% hits, the latter being almost exactly chance level in the present experiment. Thus, the judgments of subjects about being aware of the target were highly correlated with the proportion of hits during target discrimination, indicating that stimulus awareness is in fact reliably reflected by our discrimination performance.
Discussion
The present data show that a retinotopically specific modulation of recurrent activity in the primary visual cortex correlates with whether subjects become aware of the target in the present substitution masking experiment. This modulation appeared between 100 and 120 ms after search frame onset, that is, 27 ms after the onset of the initial feedforward sweep of processing in V1 at 71 ms. With a delay of only 11 ms relative to the onset of activity in extrastriate areas, this recurrent modulation arose rather fast and much earlier than the typical onset of attention driven recurrent effects in V1. Previous event-related potential (ERP) experiments addressing the time course of attention with location precuing have observed modulatory effects in such early time range (22). These attention effects, however, have been found to arise from extrastriate areas and not from V1. In fact, recurrent modulations attributable to attention in the primary visual cortex have not been seen before 150 ms after stimulus onset, with the majority of effects seen beyond 200 ms (2–5, 19). Thus, the present findings suggest that recurrent activity that correlates with target awareness in V1 is distinct from attention-related recurrent activity.
One important question is whether this early recurrent activity modulation in V1 reflects feedback influences from higher-level extrastriate areas. Recent ERP observations suggest that the propagation of activity from striate to extrastriate areas is rather fast and condensed (23, 24), and feedback projections from extrastriate cortex may take less than 10 ms to reach back to the striate cortex (10, 25). Thus, the 11-ms delay relative to the onset of feedforward activity in extrastriate cortex is clearly compatible with a feedback-driven origin of this recurrent modulation. On the other hand, it is possible that the recurrent activity between 100 and 120 ms reflects a fluctuation within the (feedforward) laminar sequence of activations in V1 proper (e.g., the propagation of activity from the granular to the extragranular layers) (26, 27). Although the ultimate processing nature of this effect may not be clarifiable in that respect here, our data clearly show that rapid recurrent processing in the primary visual cortex correlates with successful target discrimination and appears significantly before, and thus independent of, recurrent activity attributable to attention in V1.
Such early onset would be compatible with a facilitating role of this modulation, in that it boosts the neural representation of the target plus mask in V1, which then increases the chance to discriminate the target. Alternatively, it may serve to attenuate neural activity representing the trailing mask after target offset in V1. It is possible that it facilitates target identification by attenuating the neural signature of the trailing mask in V1 before reentrant processing reads out “conflicting” information from the mask. The fact that attention-related activity arose roughly 100 ms later in V1 is consistent with this interpretation. In fact, as illustrated in Fig. 4c, our modulation appeared to arise in V1 even before information about the trailing mask (the frame offset) would have arrived from the retina. If we took the onset latency of 71 ms of the initial response in V1 (A) as a rough measure of the time it takes to transfer information from the eye to V1, and added 85 ms until frame offset (B), the earliest possible neural indication of the mask remaining on screen would not appear in V1 until after 156 ms after stimulus onset (A and B, see arrows). Thus, it is possible that our recurrent modulation between 100 and 120 ms served to attenuate the trailing mask by temporarily weakening the neural excitability of V1 just before the target-offset transient reaches V1 and rendered the trailing mask apparent. The neural trace left by the trailing mask would then be less likely to interfere with the subsequent attention-driven recurrent processing in V1.
This notion is in line with the object substitution model (18, 28). This model posits that attention driven reentrant activity in early visual cortex serves to (iteratively) test the “perceptual prediction” built up during the initial feedforward sweep of processing in higher-level extrastriate regions. In visual search, the first feedforward sweep of processing would generate a scene representation that necessarily encompasses more than the target, including the mask and presumably most of the other nontarget items. By the time attention settles onto the target [typically with a delay of roughly 200 ms according to recent observations (29)], only the mask remains, and replaces the current representation. The attention-related reentrant signal in V1 would boost the representation of the mask, and the target would be more likely to lose the competition for signal strength in working memory (18). What our findings may be taken to suggest along this framework is that the recurrent activity modulation between 100 and 120 ms counters this loss of relative signal strength of the target by attenuating the trailing mask representation in the primary visual cortex significantly before recurrent processing refreshes visual working memory.
It should be mentioned that we do not believe that stimulus-contingent recurrent activity in V1 represents the only factor relevant for visual awareness. Ongoing trial-by-trial fluctuations of “sensory readiness” (i.e., the internal state of the observer) may be another important determinant of successful stimulus detection, as has been recently demonstrated in the monkey (30).
To conclude, our observations add to a growing body of data suggesting that recurrent processing plays an important role for mediating visual awareness (8, 9, 31–33). Although the present data may not ultimately clarify whether the observed recurrent activity modulations in V1 reflect feedback influences from higher-level extrastriate cortex or modulations within the laminar sequence of activations in V1 proper, they clearly demonstrate a close link between those rather early modulations and visual awareness. In recurrent processing, the early visual cortex, and particularly V1, has been proposed to serve as an “active blackboard” (8, 33) that integrates computations in higher level areas. The present observations from substitution masking in visual search suggest that recurrent processing may not represent a single operation but involve a sequence of multiple processes that serve different processing goals while cooperating to optimize performance. Rapid recurrent modulations in this framework may serve to “tailor” the neural representation of the input (e.g., by attenuating distracting information or enhancing relevant input) before subsequent attention-related feedback operations gain access to this information. Importantly, our data suggest a dissociation of neural processing reflecting awareness and attention, a notion that has recently been emphasized by others (9, 29, 31, 34–36). Woodman and Luck (29), for example, have observed typical ERP signs of attentional focusing onto the target in visual search between 200 and 400 ms even when target discrimination was impaired by substitution masking. Kanai et al. (35) have demonstrated that feature-based attention influences the magnitude of orientation-adaptation even when masked by continuous flash suppression.
Our findings fit into recent concepts of the relation between attention and awareness (9, 34), which propose that attention does not directly operate on the primary input representations but on already highly processed descriptions of the input (34). Although attention may be seminal for making such descriptions accessible to conscious vision via delayed feedback processing, it may not be involved in shaping these descriptions primarily (9, 31). Rapid recurrent processing in V1, which ultimately correlates with awareness, shapes early visual descriptions, which are then accessed by attention via further recurrent processing to generate an accessible description in visual working memory.
Experimental Procedures
Subjects.
Sixteen observers (mean age, 24.6 years; two males) took part in this experiment. All observers were neurologically normal, gave informed consent, and were paid for participation. The experiment was approved by the ethics committee of the Otto-von-Guericke University Magdeburg.
Stimuli and Task.
Each search frame contained an array of eight black–white circles placed at iso-eccentric locations (7.5° of visual angle) along an imaginary half-circle in the right VF (see Fig. 1a). The diameter of each circle was 0.9°. Four circles appeared in the upper visual quadrant at positions 5°, 20°, 35°, and 50° (directional angle) away from the horizontal meridian. Four circles were presented at 30°, 45°, 60°, and 75° positions from the horizontal meridian in the lower visual quadrant. From trial to trial, the orientation of the white half of each circle varied randomly along the vertical or horizontal direction. Simultaneously with the onset of the search items, four white dots (diameter of one dot 0.2°) appeared at the corners of an imaginary square around one of the search items. The dots subtended a distance of 0.9° from the center of the circle and designated this item as the target. Subjects had to discriminate the orientation of the target with a four alternative forced button press with the right hand (index, left; middle, right; ring, up; small, down) immediately after search frame onset. In 67% of trials (1,024 in total), the four dots remained on the screen for 250 ms (trailing mask trials, TM trials). In the remaining 33% of the trials (512 in total), the search items and the mask disappeared simultaneously at ≈83 ms after search frame onset (nontrailing mask trials, NTM trials).
For a direct comparison of neural activity during successful versus unsuccessful target discrimination, it was important to gain a roughly balanced number of trials in both situations. To this end, the presentation duration of the search items for each subject was adjusted individually based on a short pilot experiment (200 TM trials) run immediately before the MEG experiment. The individual frame duration was determined by an interleaved double staircase procedure starting with 100 and 200 ms. Depending on the identification performance of the subject, the frame duration was shortened or prolonged by units of the refresh rate of the projector (16.6 ms) in a simple one-up, one-down manner. In the end, the frame-duration resulting from both staircases was averaged (Fig. 1b) and taken as the value to be used in the subsequent MEG experiment.
To verify V1 as the origin of neural activity, we resort to the particular morphology of the calcarine fissure (a structure that coincides with V1). The form of the calcarine fissure entails a polarity inversion of the magnetic field response when elicited by a current source in its upper versus the lower bank. Fig. 3d illustrates this based on the so called cruciform model (37). Activity in the upper bank (bright gray) produces an ERMF response whose polarity is opposite (efflux left, influx right) to an ERMF response arising from the lower bank (efflux right, influx left). Visual quadrants are orderly mapped onto calcarine fissure with the lower quadrant represented in the upper bank and the upper quadrant represented in the lower bank. Observing a polarity reversal for stimuli presented in opposite quadrants is thus strong evidence for an underlying current origin in V1. Please note that previous studies (38) have established that the transition from the upper to the lower VF is somewhat displaced toward the lower bank of the calcarine fissure (red arrow in Fig. 3d). Stimuli at lower VF locations close to the horizontal meridian may already elicit an upper-VF polarity pattern. To avoid such ambiguity, we placed circles in the lower visual quadrant 25° farther from the horizontal meridian than in the upper VF.
Data Acquisition and Analysis.
The MEG signal was recorded by using a BTi Magnes 2500 whole-head MEG system (Biomagnetic Technologies) with 148 magnetometers. Data were recorded at a sampling rate of 509 Hz. Data processing including offline noise reduction, artifact rejection, and low-pass filtering (DC, 100 Hz) followed a protocol that has been described previously in detail (39).
Average ERMF waveforms were computed for each subject, time-locked to search frame onset, relative to a 100-ms prestimulus baseline interval. Separate averages were computed for correct and incorrect TM trials, and activity reflecting the success of target identification was derived by subtracting the ERMF response to incorrect trials from the response to correct trials. The temporal onset of significant ERMF waveform effects was determined by successive sample-by-sample t tests against baseline activity (sliding t tests) with the criterion that the first sample in a row of at least four significant time samples marks the onset latency of the ERMF effect (40).
For source localization, sLORETA (41) was used as available with the neuroimaging software Curry 5.08 (Compumedics Neuroscan). sLORETA represents an extension of the minimum norm least squares method, in which current density estimates at each source location are weighted by their measurement error [standardized current densities with zero localization error (41)]. sLORETAs were computed based on total average ERMF data, as well as on data from a single representative subject. To maximize the precision of source localization, realistic anatomical constraints were derived from 3D surface segmentations of the standard MNI brain (Montreal Neurological Institute) for the total average across subjects. For the single subject, source localization was constrained by a segmentation of an individual high-resolution MR scan (T1-weighted 3D spoiled gradient echo sequence; 256 × 256 matrix; field of view, 25 × 25 cm; 124 slices; slice thickness, 1.5 mm; echo time, 6 ms; repetition time, 20 ms; flip angle, 30°). The 3D segmentations [based on the boundary element method as included in the Curry package, (42)] provided (i) the gray matter compartment that served to constrain possible source localizations and (ii) a segmentation of the cerebrospinal fluid space that served to build the volume conductor model.
Acknowledgments.
This research was supported by Deutsche Forschungsgemeinschaft Grant HE 1531/9-1 and Bundesministerium für Bildung und Forschung Grant 01GO00202, CAI.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Martinez A, et al. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- 2.Noesselt T, et al. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- 3.Di Russo F, Martinez A, Hillyard SA. Source analysis of event-related cortical activity during visuo-spatial attention. Cereb Cortex. 2003;13:486–499. doi: 10.1093/cercor/13.5.486. [DOI] [PubMed] [Google Scholar]
- 4.Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: Distribution and timing of effects across visual areas. Cereb Cortex. 2000;10:343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- 5.Roelfsema PR, Lamme VAF, Spekreijse H. Object-based attention in the primary visual cortex of macaque monkey. Nature. 1998;395:376–381. doi: 10.1038/26475. [DOI] [PubMed] [Google Scholar]
- 6.Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 7.Hopf JM, et al. Direct neurophysiological evidence for spatial suppression surrounding the focus of attention in vision. Proc Natl Acad Sci USA. 2006;103:1053–1058. doi: 10.1073/pnas.0507746103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bullier J. Feedback connections and conscious vision. Trends Cognit Sci. 2001;5:369–370. doi: 10.1016/s1364-6613(00)01730-7. [DOI] [PubMed] [Google Scholar]
- 9.Lamme VA. Why visual attention and awareness are different. Trends Cognit Sci. 2003;7:12–18. doi: 10.1016/s1364-6613(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 11.Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. Nat Neurosci. 2005;8:143–144. doi: 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- 12.Lamme VA, Zipser K, Spekreijse H. Masking interrupts figure-ground signals in V1. J Cognit Neurosci. 2002;14:1044–1053. doi: 10.1162/089892902320474490. [DOI] [PubMed] [Google Scholar]
- 13.Super H, Spekreijse H, Lamme VAF. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- 14.Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3:940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- 15.Ress D, Heeger DJ. Neuronal correlates of perception in early visual cortex. Nat Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juan CH, Walsh V. Feedback to V1: A reverse hierarchy in vision. Exp Brain Res. 2003;150:259–263. doi: 10.1007/s00221-003-1478-5. [DOI] [PubMed] [Google Scholar]
- 17.Enns FA, Di Lollo V. Object substitution: A new form of masking in unattended visual locations. Psychol Sci. 1997;8:135–139. [Google Scholar]
- 18.Di Lollo V, Enns JT, Rensink RA. Competition for consciousness among visual events: The psychophysics of reentrant visual processes. J Exp Psychol Gen. 2000;129:481–507. doi: 10.1037//0096-3445.129.4.481. [DOI] [PubMed] [Google Scholar]
- 19.Martinez A, et al. Putting spatial attention on the map: Timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vision Res. 2001;41:1437–1457. doi: 10.1016/s0042-6989(00)00267-4. [DOI] [PubMed] [Google Scholar]
- 20.Lau HC, Passingham RE. Relative blindsight in normal observers and the neural correlate of visual consciousness. Proc Natl Acad Sci USA. 2006;103:18763–18768. doi: 10.1073/pnas.0607716103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiskrantz L, Barbur JL, Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1) Proc Natl Acad Sci USA. 1995;92:6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangun GR. Neural mechanisms of visual selective attention. Psychophysiology. 1995;32:4–18. doi: 10.1111/j.1469-8986.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- 23.Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans. A framework for defining “early” visual processing. Exp Brain Res. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- 24.Tzelepi A, Ioannides AA, Poghosyan V. Early (N70m) neuromagnetic signal topography and striate and extrastriate generators following pattern onset quadrant stimulation. NeuroImage. 2001;13:702–718. doi: 10.1006/nimg.2000.0735. [DOI] [PubMed] [Google Scholar]
- 25.Hupe JM, et al. Feedback connections act on the early part of the responses in monkey visual cortex. J Neurophysiol. 2001;85:134–145. doi: 10.1152/jn.2001.85.1.134. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- 27.Maunsell JHR, Gibson JR. Visual response latencies in striate cortex of the macaque monkey. J Neurophysiol. 1992;68:1332–1344. doi: 10.1152/jn.1992.68.4.1332. [DOI] [PubMed] [Google Scholar]
- 28.Enns JT, Di Lollo V. What's new in visual masking? Trends Cognit Sci. 2000;4:345–352. doi: 10.1016/s1364-6613(00)01520-5. [DOI] [PubMed] [Google Scholar]
- 29.Woodman GF, Luck SJ. Dissociations among attention, perception, and awareness during object-substitution masking. Psychol Sci. 2003;14:605–611. doi: 10.1046/j.0956-7976.2003.psci_1472.x. [DOI] [PubMed] [Google Scholar]
- 30.Super H, van der Togt C, Spekreijse H, Lamme VA. Internal state of monkey primary visual cortex (V1) predicts figure- ground perception. J Neurosci. 2003;23:3407–3414. doi: 10.1523/JNEUROSCI.23-08-03407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamme VA. Separate neural definitions of visual consciousness and visual attention; a case for phenomenal awareness. Neural Netw. 2004;17:861–872. doi: 10.1016/j.neunet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8:679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullier J. Integrated model of visual processing. Brain Res Brain Res Rev. 2001;36:96–107. doi: 10.1016/s0165-0173(01)00085-6. [DOI] [PubMed] [Google Scholar]
- 34.Cavanagh P. Attention routines and the architecture of selection. In: Posner MI, editor. Cognitive Neuroscience of Attention. New York: Guilford; 2004. pp. 13–28. [Google Scholar]
- 35.Kanai R, Tsuchiya N, Verstraten FA. The scope and limits of top-down attention in unconscious visual processing. Curr Biol. 2006;16:2332–2336. doi: 10.1016/j.cub.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Koch C, Tsuchiya N. Attention and consciousness: Two distinct brain processes. Trends Cognit Sci. 2007;11:16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Jeffreys DA, Axford JD. Source locations of pattern-specific components of human visual evoked potentials. I. Component of striate cortical origin. Exp Brain Res. 1972;16:1–21. doi: 10.1007/BF00233371. [DOI] [PubMed] [Google Scholar]
- 38.Clark VP, Fan S, Hillyard SA. Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp. 1995;2:170–187. [Google Scholar]
- 39.Hopf JM, Boelmans K, Schoenfeld A, Luck SJ, Heinze H-J. Attention to features precedes attention to locations in visual search: Evidence from electromagnetic brain responses in humans. J Neurosci. 2004;24:1822–1832. doi: 10.1523/JNEUROSCI.3564-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- 41.Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):5–12. [PubMed] [Google Scholar]
- 42.Fuchs M, Drenckhahn R, Wischmann HA, Wagner M. An improved boundary element method for realistic volume-conductor modeling. IEEE Trans Biomed Eng. 1998;45:980–997. doi: 10.1109/10.704867. [DOI] [PubMed] [Google Scholar]