Abstract
Plants exhibit an exceptional adaptability to different environmental conditions. To a large extent, this adaptability depends on their ability to initiate and form new organs throughout their entire postembryonic life. Plant shoot and root systems unceasingly branch and form axillary shoots or lateral roots, respectively. The first event in the formation of a new organ is specification of founder cells. Several plant hormones, prominent among them auxin, have been implicated in the acquisition of founder cell identity by differentiated cells, but the mechanisms underlying this process are largely elusive. Here, we show that auxin and its local accumulation in root pericycle cells is a necessary and sufficient signal to respecify these cells into lateral root founder cells. Analysis of the alf4–1 mutant suggests that specification of founder cells and the subsequent activation of cell division leading to primordium formation represent two genetically separable events. Time-lapse experiments show that the activation of an auxin response is the earliest detectable event in founder cell specification. Accordingly, local activation of auxin response correlates absolutely with the acquisition of founder cell identity and precedes the actual formation of a lateral root primordium through patterned cell division. Local production and subsequent accumulation of auxin in single pericycle cells induced by Cre-Lox-based activation of auxin synthesis converts them into founder cells. Thus, auxin is the local instructive signal that is sufficient for acquisition of founder cell identity and can be considered a morphogenetic trigger in postembryonic plant organogenesis.
Keywords: cell identity, branching, development, pericycle, plant hormones
Plants, unlike animals, exhibit the remarkable ability to continue organogenesis throughout their entire life cycle. During embryogenesis only shoot and root apical meristems are formed; however, lateral organs such as axillary shoots, lateral roots, leaves, and flowers initiate during the subsequent growth and development of the adult plant (1). The first event in the formation of a new plant organ involves specification of founder cells, which upon activation start to divide and form a primordium (2). Although specification of founder cells is a key event in postembryonic organ formation, there is very little knowledge about the mechanisms regulating this process. It has been known for decades that plant organogenesis is under the control of long-range signaling by plant hormones, prominent among them auxin (3). Auxin promotes organ formation (1, 4, 5), and locally increased levels of auxin response have been reported to mark positions of organ initiation and distal tips of developing organ primordia (6, 7). In shoot apical meristem, specification of founder cells for leaf formation involves down-regulation of a highly conserved class of homeobox genes related to KNOTTED1 (KNOX) (8). It has been shown that the ASYMMETRIC LEAVES1 (AS1) gene, which encodes a Myb protein, restricts KNOTTED expression to cells that are destined to form leaf primordia and is a key factor in leaf founder cell recruitment (9). Thus, this interaction between AS1 and KNOX functions to distinguish between stem cells and founder cells within the shoot apical meristem. Similar functions have been revealed for AS1 gene orthologs NARROW SHEAT1 in maize (10) or PHANTASTICA in Anthirinium (11). In roots, knowledge concerning founder cell specification is less consistent. Lateral root founder cells are recruited from the pericycle cells adjacent to the xylem pole. In contrast to leaf initiation, which in Arabidopsis involves recruitment of ≈30 founder cells from the periphery of the shoot apical meristem (10), lateral roots are formed from a minimum of three or six founder cells depending on whether the initiation is of a longitudinal unicellular or bicellular type (12). Xylem pole pericycle cells, from which founder cells are recruited, carry cytological features such as dense cytoplasm, large nuclei, and small vacuoles typical of meristematic cells (13). In addition, it has been shown that these cells are capable of fast entry into the cell cycle because of sustained expression of cell cycle genes such as CDKA;1 or CycA2;1 (14, 15). However, only very few cells of the pericycle tissue layer are recruited to become founder cells, and until now no founder cell-specific marker or mutants affected specifically in founder cell specification have been found (14).
Here, we demonstrate that the plant hormone auxin is the local instructive signal for specification of founder cells that give rise to lateral roots. Our analysis of the alf4–1 mutant suggests that acquisition of founder cell identity and activation of patterned cell division can be genetically separated. Time-lapse experiments show that the auxin-responsive promoter DR5 is the earliest marker for founder cells and its activation absolutely correlates with subsequent primordium formation. Furthermore, a Cre-Lox-based mosaic expression of an enzyme for auxin synthesis in β-glucuronidase (GUS)-labeled sectors demonstrates that local auxin accumulation in a single pericycle cell converts it into a founder cell. Thus, auxin is sufficient to trigger acquisition of founder cell identity in postembryonic organogenesis in plants.
Results and Discussion
Local Auxin Response Correlates with Founder Cell Specification.
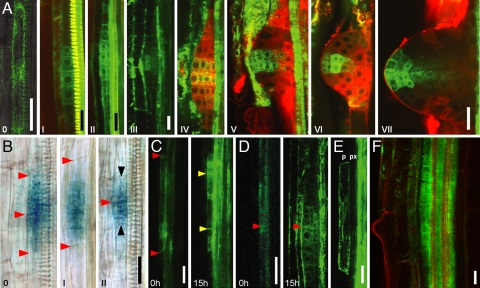
To gain insights into the mechanism of cell reprogramming and founder cell specification we analyzed available molecular markers and screened enhancer trap libraries for reporter expression associated with early stages of lateral root primordium (LRP) initiation. The earliest activity identified was that of the synthetic promoter DR5, which is an established marker for auxin response and indirectly for auxin accumulation (16, 17). DR5 is active at all stages of LRP development in both Arabidopsis (ref. 6 and Fig. 1A) and tomato (Fig. 1B). The earliest detectable DR5 (DR5rev::GFP; ref. 18) expression was either in single or two longitudinally abutted xylem-adjacent pericycle cells (Fig. 1 C and D). These DR5-expressing cells exhibited all attributes of LRP founder cells: they were found only in the xylem-adjacent pericycle where all LRPs initiate and were distal to the youngest LRPs, which is consistent with the acropetal pattern of LRP initiation (12, 14, 19). To test whether these DR5-expressing pericycle cells were in fact founder cells, we followed the fate of GFP-positive cells by performing a time-lapse experiment in live roots. In intervals of ≈15 h, we scanned roots of DR5rev::GFP seedlings and closely followed fate of all DR5-positive cells and the origin of all initiating LRP. In 13 tested roots, all pericycle cells that showed DR5rev::GFP expression developed into LRPs (Fig. 1 C and D); conversely, not a single LRP was initiated from a GFP-negative cell. These data show that DR5 activation completely correlates with the acquisition of founder cell identity and that the local activation of auxin response precedes the initiation of LRP formation.
Fig. 1.
Auxin response-marked specification of lateral root founder cells precedes cell cycle activation in the pericycle. (A and B) DR5 activity throughout the lateral root formation starting from a presumptive founder cell (0); roman numbers are developmental stages in accordance with ref. 30. (A) Arabidopsis roots of the DR5rev::GFP line (n = 58). (B) Tomato roots of the DR5::GUS line (n = 46). Red arrowheads denote end walls of pericycle founder cells; black arrowheads denote periclinal cell walls. (A) CLSM images. (B) Nomarski optics. 0, merged Nomarski and CLSM; 0 and III, live unstained roots; I and II, live roots stained with neutral red; IV–VII, fixed roots. (C and D) Time-lapse analysis of live roots showing that pericycle founder cells are always accompanied by increased DR5 auxin response. Longitudinal unicellular (C) and bicellular (D) types of lateral root initiation. (Left) Pericycle cells at the beginning of the experiment. (Right) Images taken at the same focal plane 15 h later, showing primordia formed. Red arrowheads indicate end walls of founder cells. Yellow arrowheads indicate new cell walls formed. At the beginning of the experiments plants were 6 days old (C) and 7 days old (D). (E) DR5 activation in presumptive founder cell in 10-day homozygous alf4–1 mutant plant (n = 10); p, pericycle; px, protoxylem. (F) In Arabidopsis, DR5 can be activated in all pericycle cells by auxin treatment (10 μM NAA, 6 h); live roots were stained with neutral red. (Scale bars: A, B, and F, 20 μm; C–E, 25 μm.)
Acquisition of Founder Cell Identity Is Genetically Separable from Activation of Cell Division.
To further analyze whether specification of founder cells precedes cell division in primordium morphogenesis, we analyzed DR5 activity in the alf4–1 Arabidopsis mutant, which is blocked in pericycle cell division that leads to LRP formation (20, 21). In alf4–1 roots, we consistently observed the presence of DR5-active pericycle cells (Fig. 1E) that were distributed along the root in a pattern comparable to LRP distribution in WT roots; however, these cells did not develop into LRPs (data not shown). In 10-day-old homozygous alf4–1 plants, the number of pericycle DR5 activation events (21.0 ± 4.0, n = 3, mean ± SD) was similar to the number of lateral roots and LRPs in 10-day-old WT plants (19.9 ± 3.7, n = 11, mean ± SD, Student's t test P = 0.412). This observation suggests that acquisition of founder cell identity marked by DR5 activation precedes activation of patterned cell division for LRP development.
Auxin Production in a Single Pericycle Cell Triggers Its Conversion into a Founder Cell.
As established, DR5 is expressed in response to activated auxin signaling in a given cell and thus, indirectly, DR5 expression positively correlates with cellular auxin levels (6, 16, 17). Indeed, increased DR5 activity in embryos and roots has been previously correlated with local auxin accumulation as visualized by anti-indole-3-acetic acid (IAA) antibody (6, 18). In line with this, we observed uniform activation of DR5rev::GFP expression in all xylem-adjacent pericycle cells after treatment with different natural and synthetic auxins such as IAA, 1-naphthaleneacetic acid (NAA), and 2,4-dichlorophenoxyacetic acid (2,4-D), demonstrating comparable auxin response in these cells [Fig. 1F and supporting information (SI) Fig. S1]. This observation suggests that increased DR5rev::GFP activity in single pericycle cells at the positions of lateral root initiation (as shown in Fig. 1 A–C and E) does not reflect higher sensitivity of auxin signaling but rather increased cellular auxin levels in these cells. In addition, these DR5-positive cells after auxin treatment become proliferatively active, and eventually, formed lateral root primordia (6). Based on these results, we propose a scenario where local accumulation of auxin in single pericycle cells is the signal that induces specification of LRP founder cells.
To test this model, we created transgenic plants that allowed us to stimulate auxin production in random single cells and identify these same cells and their progeny by GUS activity. In brief, these plants carry a heat shock-inducible Cre recombinase gene that, when induced, creates clonal sectors that simultaneously express indoleacetic acid tryptophan monooxygenase (iaaM) and the GUS reporter. iaaM catalyzes a critical step in the conversion of tryptophan (Trp) into auxin (22). Where Trp levels are limiting such as in roots, exogenous Trp application to iaaM-expressing lines has been shown to increase auxin production (23). Thus in these Arabidopsis Cre/Lox≫iaaM lines after heat shock treatment, seedlings will randomly form sectors that both express iaaM and can be visualized with the GUS reporter (Fig. 2A). These same sectors will have increased auxin production in the presence of Trp. Such a system would, in theory, allow inducible activation of auxin production in marked random sectors.
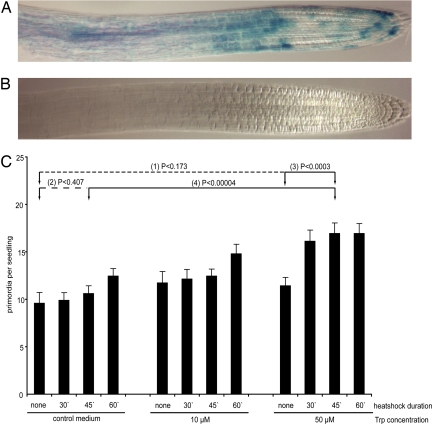
Fig. 2.
Heat shock-induced Cre/Lox based random iaaM expression. (A) Sectors of GUS-marked iaaM-expressing cells in root meristem induced by heat shock. (B) No sectors of GUS-marked iaaM expression were observed without the heat shock treatment. (C) LRP initiation was not affected by 30- or 45-min heat shock durations (Student's t test 2, P = 0.407), nor by 10- or 50-μM Trp treatments (Student's t test 1, P = 0.173). Simultaneous iaaM activation by 45-min heat shock and 50-μM Trp treatment led to a significant increase of LRP initiation over the heat shock alone (Student's t test 4, P < 0.00004) or Trp alone (Student's t test 3, P < 0.0003). The frequency of LRP initiation was scored 62 h after the heat shock in 10–15 seedlings per treatment (mean ± SE).
First, we tested in detail different aspects of this system. The generated expression sectors were, as expected, originally of single cell size. No sectors of GUS-marked iaaM expression were observed in untreated seedlings (Fig. 2B). The iaaM expression under more general RPS5 promoter (Fig. S2) or when large sectors were induced (data not shown) indeed lead to increased auxin production as manifested by typical auxin overproduction phenotypes, including long hypocotyls. These phenotypes were shown to correlate with higher levels of free auxin in iaaM-activated lines (22, 23). To optimize the conditions for in vivo auxin biosynthesis in the random sectors, the effects of different heat shock and Trp treatments on LRP initiation were tested. LRP initiation was not significantly affected by 30- or 45-min heat shock durations, nor by 10- or 50-μM Trp treatments alone (Fig. 2C). Simultaneous iaaM activation by 45-min heat shock and 50-μM Trp treatment led to a significant increase of LRP initiation over heat shock alone or Trp alone. Based on these analyses, 45-min heat shock and 50-μM Trp treatment were used for all further studies (Fig. 2C).
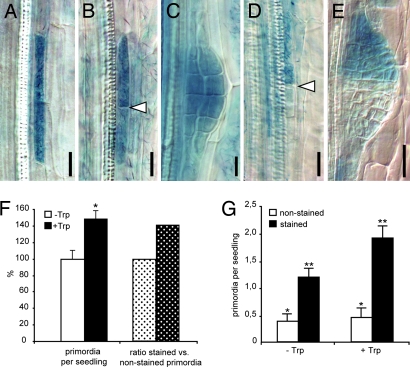
To examine the consequences of locally stimulated auxin production on lateral root formation, we scored the frequency of LRP initiation 48 h after the heat shock in the absence or presence of Trp. In addition, GUS staining revealed which of the LRPs originated from iaaM-expressing cells. Indeed, among the LRPs that arose after Trp treatment, GUS-positive LRPs were found as expected for primordia arising from one activated founder cell (Fig. 3 A–C). In some cases, half-stained primordia were found corresponding to only one of two adjacent founder cells being activated through iaaM expression (Fig. 3 D and E), confirming the clonal character of the sectors and showing that auxin production in a single cell leads to the recruitment also of the neighboring cells into founder cells. In total, Trp-treated roots formed 48% more LRP than untreated roots (Figs. 2C and 3F). This increase in primordia initiation proportionally correlated with an increase of GUS-labeled LRPs (Fig. 3G). Thus, the additional LRPs that were initiated after Trp treatment originated mostly from the auxin-producing pericycle sectors, showing that auxin production in pericycle cells triggers LRP initiation.
Fig. 3.
Local auxin production in pericycle cells triggers primordium formation. (A–E) Examples of sectors of GUS-marked iaaM-expressing cells induced by heat shock. (A) Sector of a single xylem-adjacent pericycle cell is shown. (B) After cell division two longitudinally abutted cells are formed; arrowhead indicates the new cell wall formed. (C) Such activation leads to formation of a fully stained primordium. (D and E) Activation of iaaM expression in only one of two longitudinally abutted cells can recruit the neighboring cell as the second founder cell and leads to staining of the progeny of only one founder cell (D) and development of a half-stained primordium (E). Arrowhead in D shows the position of end walls of a heat shock-activated and an independently specified pericycle founder cells. (F) Seedlings with random sectors of iaaM expression initiate 48% more primordia when auxin synthesis is induced by Trp treatment (black bar) as compared with untreated controls (white bar) (Student's t test; *, P < 0.0008). Accordingly, the proportion of GUS-positive primordia (indicating auxin-producing sectors) is 40% higher in Trp-treated seedlings (dotted black bar) as compared with untreated controls (dotted white bar). (G) Scoring of stained (iaaM expressing) and unstained primordia reveals that Trp treatment does not affect the iaaM-independent initiation of primordia, as the number of unstained primordia is not changed compared with the untreated controls (white bars) (Student's t test; *, P = 0.6,). The significant increase in the number of stained primordia (black bars) upon Trp treatment (Student's t test; **, P < 0.005) suggests that the local increase in the auxin level as a consequence of iaaM activity in pericycle sectors stimulates primordia initiation. The number of primordia was scored in 35–40 seedlings from two independent experiments (mean ± SE). (Scale bars: A–C, 25 μm; D and E, 40 μm.)
Next, we addressed the positions, where these additional LRPs were initiated. It has been shown that LRP initiation follows a regular left and right alternating pattern (24). In Trp-treated seedlings with random activation of iaaM expression, we observed increased frequency of deviations from this natural positioning pattern. These included initiation of two LRPs in close proximity at the same xylem pole (Fig. 4) or directly opposite each other (data not shown). Activation of iaaM in Trp-treated seedlings increased frequency to 0.63 positioning defects per seedling (LRP, n = 145) as compared with 0.30 positioning defects per seedling (LRP, n = 94) in untreated seedlings. Thus, as expected for random activation of iaaM expression, the regular patterning of LRP initiation was disrupted and some primordia initiated irregularly. Notably, similar defects, albeit with lower frequency, can be induced by a general increase of auxin levels either by auxin or Trp treatment, which result in increased LRP initiation (0.39 defects in Trp-treated seedlings, LRP, n = 125). Altogether, these results suggest that an increased auxin level in individual pericycle cells is a sufficient signal for the pericycle cell fate change into founder cell identity.
Fig. 4.
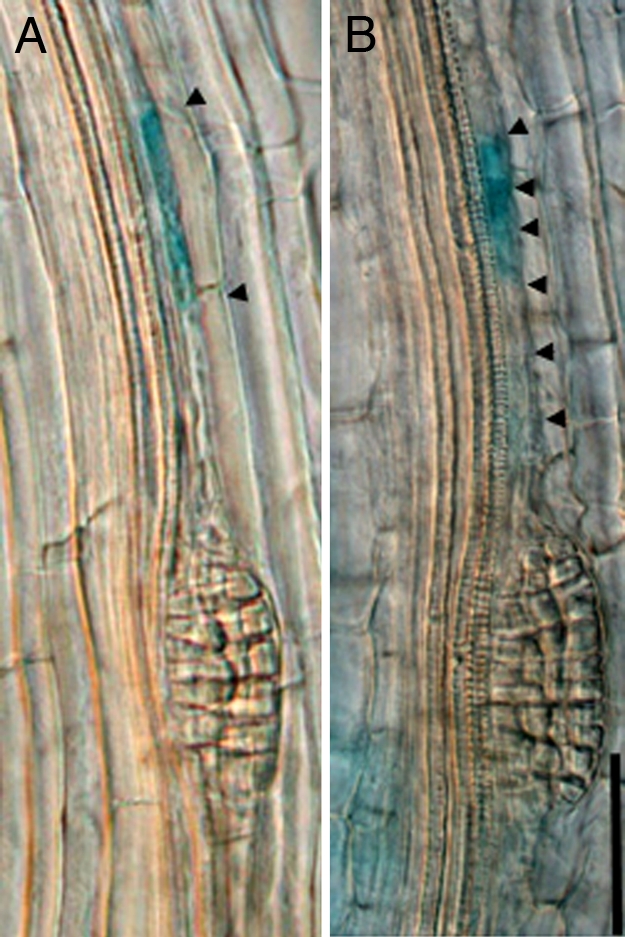
Random activation of IaaM leads to increased frequency of defects in LRP positioning. (A) In non-Trp-treated control, IaaM activated pericycle cell in close proximity of other LRP at the same xylem pole without LRP iniation. (B) In Trp-treated seedlings coincidental activation of IaaM in pericycle cell in close proximity of other LRP at the same xylem pole results in primordia initiation. Arrowheads indicate pericycle end walls. A total of 30–35 seedlings were analyzed 48 h after heat shock incubation. (Scale bar: 55 μm.)
Conclusion
In summary, our results show that an increase in auxin levels and signaling in individual pericycle cells always accompanies lateral root organogenesis, and that such increases are sufficient for the acquisition of lateral root founder cell identity. This process is not directly coupled to subsequent division of the founder cells, as the specification event can be genetically separated from the patterned division during primordium morphogenesis. The local accumulation of auxin in individual xylem pericycle cells could result from either directed transport or local synthesis and serves as a local instructive signal for cell fate reprogramming and the onset of organogenesis. This mechanism of local auxin maxima can thus “select” given pericycle cells and convert them into founder cells, thereby determining a spatial pattern of lateral root formation.
The fact that lateral root founder cell specification and patterned cell division in the pericycle can be separated both temporally and genetically indicates that the primary event during LRP initiation is not an auxin-induced activation of the cell cycle as has been proposed previously (14). Instead, we propose a model whereby auxin serves as a morphogenetic trigger. Although the criteria defining a morphogen in animal systems (25, 26) do not completely fit to plant systems (3, 18), we define a morphogenetic trigger as the factor or signal that induces, through unequal distribution of its activity, acquisition of a new developmental fate in a cell or a group of cells. The current study shows that auxin IAA is such morphogenetic trigger for lateral root initiation.
Previous studies showed that local auxin application on the shoot apical meristem of tomato was sufficient to induce leaf formation from the adjacent peripheral zone of the meristem (5). Taken together with the detection of auxin response maxima correlating with shoot- and root-derived organ initiation in different plant species (5, 6, 7), the results presented here suggest a general, evolutionary conserved auxin-based mechanism for acquisition of founder cell identity in plant organogenesis.
Materials and Methods
Transgenic Lines and Growth Conditions.
DR5rev::GFP and DR5::GUS Arabidopsis lines have been described (6, 18). The male-sterile alf4–1 mutant was crossed with the DR5rev::GFP line, and individual homozygous alf4–1 plants expressing DR5rev::GFP were selected from the F2 generation. Plants were genotyped (Fig. S3) by PCR. DNA was isolated from rosette leaves with a PUREGENE kit (Gentra Systems); the 137-bp product for the alf 4–1 allele and the 149-bp product for the WT allele were amplified by using 5′-GTAATTTGTTTTCTGGGTGG-3′ forward and 5′-CAAAGTCTTGAAATCCTCCG-3′ reverse primers that span a 12-bp deletion in the alf4–1 mutant allele. The PCR products were resolved in 10% polyacrylamide gel (PAAG). Plants were grown on vertical Petri dishes on solid medium under conditions described (27). The DR5::GUS construct (17) was introduced into Agrobacterium tumefaciens strain EHA 105, and stable transformation of tomato (Solanum lycopersicum) was performed as described (28). The lines with the strongest GUS expression were used. Lateral root development and founder cell specification were analyzed within the primary root of Arabidopsis plants and within first-order lateral roots of tomato plants. GUS staining was performed as in ref. 19. Auxin treatments were performed with 10 μM NAA or 20 μM IAA for 6 h and 2.5 μM 2,4-D for 6.5 h.
Cre-Lox-Based Stimulation of Auxin Biosynthesis.
Transgenic plants harboring a CRE recombinase under control of a heat shock promoter and an empty pCB1 vector [a 35S promoter separated from GAL4::VP16 coding sequence by a spacer flanked with lox P sites (29)] were crossed with pEF iaaM plants that contain both the iaaM and GUS genes under control of an UAS promoter (pSDM7010) (23) to obtain lines with a heat shock-inducible, CRE recombinase-mediated mosaic of GUS-labeled cells expressing iaaM. Seedlings homozygous for the above constructs were germinated and grown for 4 days on MS medium without or with 5 μM N-(1-naphthyl)phthalamic acid NPA to prevent LRP initiation before iaaM activation. The seedlings were then heat shock-treated for 45 min (unless otherwise noted) and subsequently incubated in liquid MS medium or MS medium supplemented with 50 μM tryptophan (Trp) for 48 or 60 h. Roots were stained for GUS and cleared as described (30). LRP initiation was scored with a Zeiss Axiophot microscope using Nomarski optics. To examine positioning defects in lateral root patterning, frequencies of nonstandard initiation events were scored like two LRPs in close proximity at the same xylem pole or directly opposite each other. Seedlings 48 and 60 h after heat shock treatment were analyzed. The number of analyzed plants is given in the figure legends.
Microscopy and Time-Lapse Experiments.
Live or fixed (4–6 h in 4% formaldehyde either in PBS or in phosphate buffer, pH 6.5, supplemented with 1 μg·ml−1 propidium iodide) roots expressing DR5rev::GFP were analyzed with a Zeiss LSM 510 Meta confocal laser scanning microscope (CLSM) equipped with an argon laser. Zeiss ×40 (NA 0.75, Plan Neofluar) dry and ×63 (NA 1.2, C-Apochromat) water immersion objectives were used. Some live roots were stained with Neutral red at pH 5.6 (27). For time-lapse experiments, 6- or 7-day-old plants were mounted over a thin layer of 0.4% agar plant growth medium in a custom-made chamber with a coverslip at the bottom. The roots of the plants were covered with solidified 0.4% agar medium, and liquid medium was added to the bottom of the chamber. To minimize evaporation, the chamber's borders were sealed with Vaseline and covered with a piece of glass. Founder cells were detected in the young root differentiation zone by GFP fluorescence. A total of 32–40 serial optical sections were taken both above and below the middle image. In the same focal planes, Nomarski CLSM images were acquired. The chamber was left overnight on the microscope stage, and 14–16 h later images were taken within the same focal planes and the same confocal settings. To confirm that a GFP-expressing cell became a part of an LRP, merged Nomarski and CLSM images were analyzed, and root hairs or cell walls were used as landmarks to verify that the focal plane was not changed over the experimental period (Fig. S4). For illustration purposes, the signal of the green channel was increased (Fig. 1 C and D) by using Adobe Photoshop. Images with original signal intensity are shown in Fig. S3. The number of plants analyzed is indicated in the figure legends.
Supplementary Material
Acknowledgments.
We thank G. Hagen for the DR5::GUS construct. This research was supported by the Odysseus program of the Fonds Wetenschappelijk Onderzoek (J.F. and M.S.), the European Molecular Biology Organization Young Investigator Program (J.F. and E.B.), U.S. Department of Agriculture National Research Initiative Competitive Grants Program Grant 2006-03434 (to M.G.I.), the Dirección General de Asuntos del Personal Académico–Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (J.G.D.), de la Universidad Nacional Autónoma de México Grants IN 210202 and IN225906 (to J.G.D.), Consejo Nacional de Ciencia y Technología Grant 49267 (to J.G.D.), and Deutscher Akademischer Austauschdienst (J.G.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712307105/DCSupplemental.
References
- 1.Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell Mol Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beveridge CA, Mathesius U, Rose RJ, Gresshoff PM. Common regulatory themes in meristem development and whole plant homeostasis. Curr Opin Plant Biol. 2007;10:44–51. doi: 10.1016/j.pbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Friml J. Auxin transport: Shaping the plant. Curr Opin Plant Biol. 2003;6:7–12. doi: 10.1016/s1369526602000031. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinhardt D, et al. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:256–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- 6.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 7.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Jackson D, Veit B, Hake S. Expression of maize KNOTTED1-related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- 9.Byrne ME, et al. Asymmetric leaves 1 mediates axis leaf patterning and stem cell fate in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- 10.Scanlon MJ. NARROW SHEATH1 functions from two meristematic foci during founder-cell recruitment in maize leaf development. Development. 2000;127:4573–4585. doi: 10.1242/dev.127.21.4573. [DOI] [PubMed] [Google Scholar]
- 11.Waites R, Selvadurai HR, Oliver IR, Hudson A. The PHANTASTICA gene encodes a MYB transcription factor involved in growth and dorsoventrality of lateral organs in Antirrhinum. Cell. 1998;93:779–789. doi: 10.1016/s0092-8674(00)81439-7. [DOI] [PubMed] [Google Scholar]
- 12.Dubrovsky JG, Rost TL, Colon-Carmona A, Doerner P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta. 2001;214:30–36. doi: 10.1007/s004250100598. [DOI] [PubMed] [Google Scholar]
- 13.Parizot B, et al. Diarch symmetry of the vascular bundle in Arabidopsis root encompasses the pericycle and is reflected in distich lateral root initiation. Plant Physiol. 2007 doi: 10.1104/107.107870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Smet I, Vanneste S, Inze D, Beeckman T. Lateral root initiation or the birth of a new meristem. Plant Mol Biol. 2006;60:871–887. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- 15.Roudier F, et al. The Medicago species A2-type cyclin is auxin regulated and involved in meristem formation but dispensable for endoreduplication-associated developmental programs. Plant Physiol. 2003;131:1091–1103. doi: 10.1104/pp.102.011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 17.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friml J, et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- 19.Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiol. 2000;124:1648–1657. doi: 10.1104/pp.124.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- 21.DiDonato RJ, et al. Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J. 2004;37:340–353. doi: 10.1046/j.1365-313x.2003.01964.x. [DOI] [PubMed] [Google Scholar]
- 22.Romano CP, Robson PR, Smith H, Estelle M, Klee H. Transgene-mediated auxin overproduction in Arabidopsis: Hypocotyl elongation phenotype and interactions with the hy6–1 hypocotyl elongation and axr1 auxin-resistant mutant. Plant Mol Biol. 1995;27:1071–1083. doi: 10.1007/BF00020881. [DOI] [PubMed] [Google Scholar]
- 23.Weijers D, et al. Maintenance of embryonic auxin distribution for apical-basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell. 2005;17:2517–2526. doi: 10.1105/tpc.105.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Smet I, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- 25.Gurdon JB, Bourillot PY. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 26.Tabata T, Takei Y. Morphogens: Their identification and regulation. Development. 2004;131:703–712. doi: 10.1242/dev.01043. [DOI] [PubMed] [Google Scholar]
- 27.Dubrovsky JG, et al. Neutral red as a probe for confocal laser scanning microscopy studies of plant roots. Ann Bot. 2006;97:1127–1138. doi: 10.1093/aob/mcl045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park SH, Morris JL, Park JE, Hirschi KD, Smith RH. Efficient and genotype-independent Agrobacterium-mediated tomato transformation. J Plant Physiol. 2003;160:1253–1257. doi: 10.1078/0176-1617-01103. [DOI] [PubMed] [Google Scholar]
- 29.Heidstra R, Welch D, Scheres B. Mosaic analyses using marked activation and deletion clones dissect Arabidopsis SCARECROW action in asymmetric cell division. Genes Dev. 2004;18:1964–1969. doi: 10.1101/gad.305504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.