Abstract
Nuclear PI3K and its downstream effectors play essential roles in a variety of cellular activities including cell proliferation, survival, differentiation, and pre-mRNA splicing. Aly is a nuclear speckle protein implicated in mRNA export. Here we show that Aly is a physiological target of nuclear PI3K signaling, which regulates its subnuclear residency, cell proliferation, and mRNA export activities through nuclear Akt phosphorylation and phosphoinositide association. Nuclear Akt phosphorylates Aly on threonine-219, which is required for its interaction with Akt. Aly binds phosphoinositides, and this action is regulated by Akt-mediated phosphorylation. Phosphoinositide binding but not Akt phosphorylation dictates Aly's nuclear speckle residency. Depletion of Aly results in cell growth suppression and mRNA export reduction. Inhibition of Aly phosphorylation substantially decreases cell proliferation and mRNA export. Furthermore, disruption of phosphoinositide association with Aly also significantly reduces these activities. Thus, nuclear PI3K signaling mediates both cell proliferation and mRNA export functions of Aly.
Keywords: nuclear PI 3-kinase, nuclear speckle, nuclear Akt, T219 phosphorylation
PI3K and Akt predominantly locate in the cytoplasm, but they also occur in the nucleus or translocate there upon stimulation. Most of phosphoinositol lipid metabolic enzymes and PI3K signaling machinery occur in the nucleus. For example, PI-PLC (1, 2), phosphatidylinositol phosphate kinases (3, 4), PDK1 (5), PTEN (6–8), and Akt (9, 10). Nuclear phosphoinositides play critical roles in cell growth and differentiation (11). In addition to nuclear membrane, phosphoinositides also associate with the nuclear speckles, a peculiar nuclear subcompartment enriched in small ribonucleoprotein (RNP) particles and various splicing factors (12), where many elements of nuclear phosphoinositide metabolism, including PI(4,5)P2, are concentrated (4, 13, 14). Nuclear phosphoinositides may be implicated in pre-mRNA splicing and chromatin structure (11). It has been shown before that nuclear PI(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing (14). Elements of the transcriptional and pre-mRNA processing machinery interact with this pool of nuclear PI(4,5)P2, and PI(4,5)P2 immunoprecipitates contain intermediates and products of the splicing reaction. Alternative splicing of pre-mRNAs can be regulated by extracellular signals such as growth factors, cytokines, hormones, and stress stimuli (15). For instance, insulin-activated PI3K signaling has been shown to implicate in mammary epithelial–mesenchymal interaction, which regulates fibronectin alternative splicing (16).
Splicing of pre-mRNA and export of mRNA are normally coupled (17–19). Pre-mRNA splicing has been proposed to stimulate mRNA export (17, 20). Based on genetic and biochemical evidence, Reed et al. (21) proposed a model for mRNA export: nascent pre-mRNA is first packaged into heterogeneous nuclear RNP (hnRNP) particles. During spliceosome assembly, exons are packaged by non-hnRNP spliceosome components such as SR proteins. The spliced messenger RNP is targeted for export by factors recruited during the splicing pathway, in particular the mRNA export factor Aly. Other non-hnRNP factors present in the spliced messenger RNP, such as SR proteins, may also be involved in linking splicing and export or may simply serve a packaging function to prevent binding of nuclear retention factors such as hnRNP proteins. The non-hnRNP factors form a splicing-dependent messenger RNP complex that specifically targets mature mRNA for export, whereas hnRNP proteins retain introns in the nucleus (21). In yeast, the RNA-binding proteins Yra1 and Mex67, also known as Aly and TAP in mammalian cells, are required for mRNA export (22, 23). Recently, it has been shown that Yra1 is required for S phase entry and affects Dia2 binding to replication origins. Thus, it has a role in DNA replication distinct from its role in mRNA export (24). Although Aly directly binds UAP56 and couples it to spliced mRNA, REF/Aly is dispensable for mRNA export in Drosophila (25) and Caenorhabditis elegans (26).
In this report we show that Aly is a downstream target of nuclear PI3K signaling cascade. Nuclear Akt directly binds Aly and phosphorylates it on the T219 residue. Interestingly, Aly directly interacts with nuclear PI(4,5)P2 and PI(3,4,5)P3, which is essential for its nuclear speckle residency. Depletion of Aly markedly blocks cell cycle progression and reduces cell growth and mRNA export, and these processes are regulated by Akt phosphorylation and PI(3,4,5)P3 binding.
Results
Akt Phosphorylates Aly on T219 Residue.
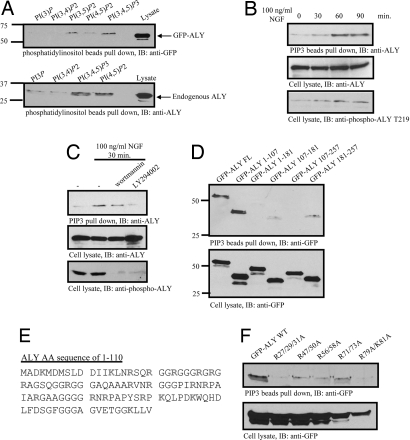
Aly contains an RNA recognition domain (RRM) and GR (glycine-arginine)-rich domain in both the N and C termini. In exploring the sequence of Aly, we noticed that amino acids 29–34, RGRAGS, and amino acids 214–219, GTRRGT, correspond to a motif that is identified as a consensus Akt phosphorylation element present in numerous Akt substrates (Fig. 1A). In vitro Akt kinase revealed that both N-terminal (amino acids 1–107 and amino acids 1–181) and C-terminal (amino acids 107–257 and amino acids 181–257) fragments were robustly phosphorylated by active Akt. By contrast, the middle RRM domain (amino acids 107–181) was not phosphorylated (Fig. 1B Upper). Mutation with S34A or T219A in Aly abolished the phosphorylation of fragments 1–107 and 181–257, suggesting that both residues can be phosphorylated by Akt in vitro (Fig. 1C Upper). To explore whether Aly can be phosphorylated by Akt in intact cells, we performed a metabolic labeling assay with HEK293 cells transfected with GST-Aly. The transfected cells were pretreated with PI3K inhibitors, wortmannin and LY294002, respectively, and followed by EGF stimulation. Compared with control, EGF elicited potent Aly phosphorylation, which was substantially blocked by PI3K inhibitors (Fig. 1D, first panel). Immunoblotting with phospho-T219 antibody verified this observation (Fig. 1D, last panel). Furthermore, metabolic labeling revealed that NLS-Akt provoked strong phosphorylation in wild-type Aly. The phosphorylation was slightly decreased in S34A and almost completely abolished in T219A mutant (Fig. 1E, first panel), indicating that T219 residue in Aly is a major Akt phosphorylation site. Interestingly, T219A but not S34A mutation abolished Akt association by Aly (Fig. 1E, second panel), fitting with the observation that T219 but not S34 is the predominant phosphorylation site. The interaction between Akt and Aly was further explored in supporting information (SI) Fig. S1 and SI Text. Aly phosphorylation was markedly decreased when Akt1 was depleted by its siRNA compared with control siRNA. Overexpression of active plasma membrane myristoylated Akt substantially enhanced Aly phosphorylation, underscoring that Akt is the physiological upstream kinase for Aly (Fig. 1F Left). Aly T219 phosphorylation was selectively diminished in Akt1 but not Akt2 knockout MEF cells (Fig. 1F Right), indicating that Akt1 specifically mediates Aly phosphorylation. Collectively, these data support that Aly is a physiological substrate of Akt.
Fig. 1.
Aly is an Akt substrate in vitro and in vivo. (A) Schematic diagram of Aly. Aly is a 257-aa protein containing an RNA recognition motif (RRM). Aly has several Akt phosphorylation motifs. (B) Aly N terminus and C terminus are phosphorylated by Akt. Except GST-ALY 107–181 (RRM domain), both C and N termini of Aly were phosphorylated by Akt (Upper). Coomassie brilliant blue staining of used GST recombinant proteins is shown in Lower. (C) S34 and T219 residues in Aly are Akt phosphorylation sites. S34A and T219A mutation disrupted Aly phosphorylation by Akt in vitro. (D) ALY phosphorylation by EGF in vivo. The transfected HEK293 cells were serum-starved and fed with 32P-orthophosphate for 4 h. The cells were treated with 100 ng/ml EGF for 20 min after wortmannin (100 nM) or LY294002 (10 μM) treatment. GST-Aly was pulled down and separated on SDS/PAGE and analyzed by autoradiography or Western blotting. (E) T219 is the major phosphorylation site by Akt in vivo. GST-Aly wild type, S34A, and T219A construct transfected HEK293 cells were treated with NLS-Akt adenovirus. After 36 h of infection, cells were metabolically labeled. GST-Aly were pulled down and analyzed by autoradiography and immunoblotting. S34A mutation weakly decreased Aly phosphorylation, and T219A mutation substantially abolished Aly phosphorylation (first panel). The T219A mutant did not bind to Akt (second panel). (F) Ablation of Akt abolishes Aly phosphorylation. Knocking down of Akt eliminated Aly T219 phosphorylation, whereas overexpression of active myristoylated Akt enhanced Aly phosphorylation (top panel in Left). Aly T219 phosphorylation was selectively diminished in Akt1 but not Akt2 knockout MEF cells (top panel in Right).
Nuclear Phosphoinositides Bind Aly.
Nuclear phosphoinositides associate with pre-mRNA splicing machinery (19). To investigate whether Aly binds to phosphoinositol lipids, we conducted an in vitro binding assay with extracts from HEK293 cells transfected with GFP-Aly. Aly bound avidly to PI(3,4,5)P3, weakly to PI(3,5)P2 and PI(4,5)P2, and not at all to other phosphoinositol lipids (Fig. 2A Upper). Endogenous Aly robustly interacted with both PI(3,4,5)P3 and PI(4,5)P2, but it failed to bind either PI(3)P or PI(3,4)P2 (Fig. 2A Lower).
Fig. 2.
Aly binds to phosphatidylinositol-(3,4,5)-Tris-phosphate. (A) Aly binds to phosphatidylinositol lipids. GFP-Aly transfected HEK293 cells were harvested, mixed with phosphatidylinositol lipid-conjugated beads, and incubated for 3 h at 4°C. Lipid-bound proteins were analyzed by immunoblotting. Exogenous and endogenous Aly associated with phospholipids, especially PI(4,5)P2 and PI(3,4,5)P3. (B) NGF mediates the interaction between PI(3,4,5)P3 and Aly. PC12 cells were treated with NGF for various time points. The cell lysates were incubated with PI(3,4,5)P3-conjugated beads. After extensive washing, the bead-bound proteins were analyzed by immunoblotting. (C) T219 phosphorylation is required for Aly/PI(3,4,5)P3 association. (D) The N terminus of Aly is the PI(3,4,5)P3 binding site. (E) Amino acid sequence of N-terminal Aly. Positive amino acid residues were indicated in gray. (F) R27/29/31 and R79/K81 are important for Aly's PI(3,4,5)P3 binding activity. R27/29/31A and R79A/K81A mutants lost its PI(3,4,5)P3 interaction activity.
To explore whether Aly/PI(3,4,5)P3 association is regulated by growth factors, we incubated PI(3,4,5)P3-conjugated beads with the nuclear extracts from PC12 cells, pretreated with NGF for various time points. Immunoblotting analysis revealed that binding occurred at 30 min, which peaked at 60 min and partially decayed at 90 min (Fig. 2B Top), suggesting that NGF mediates the interaction between Aly/PI(3,4,5)P3. Aly phosphorylation correlated with its binding activity to PI(3,4,5)P3 beads (Fig. 2B Bottom). Preincubation with PI3K inhibitors substantially decreased the association between PI(3,4,5)P3 and Aly. Consequently, Aly phosphorylation by Akt was markedly blocked by PI3K inhibitors (Fig. 2C), underscoring that Aly phosphorylation by Akt is necessary for the binding. To map which portion is required for its association with PI(3,4,5)P3, we prepared various Aly truncates. PI(3,4,5)P3 selectively interacted with full-length Aly and the N-terminal fragment (amino acids 1–107) but not other fragments (Fig. 2D Upper), indicating that the N terminus of Aly is required for the binding activity. Aly N terminus contains a GR-rich domain. All of the positive residues are highlighted in red (Fig. 2E). To determine which R or K residues are essential for Aly binding to PI(3,4,5)P3, we prepared numerous point mutants with K or R changed into A. R27/29/31A and K79A/K81A mutation disrupted the association between Aly and PI(3,4,5)P3, whereas other mutants exhibited slightly decreased affinity (Fig. 2F). Taken together, these data demonstrate that Aly strongly binds nuclear PI(3,4,5)P3 and that Akt phosphorylation mediates its affinity to PI(3,4,5)P3.
Depletion of Aly Suppresses Cell Proliferation and Reduces mRNA Export.
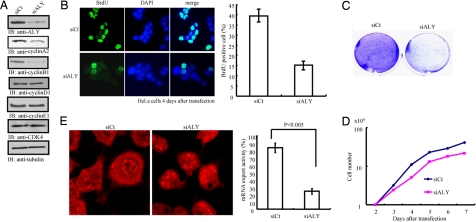
Yra1, a yeast homolog of Aly, is required for S phase entry and plays a role in DNA replication in yeast (24). To explore whether Aly mediates cell proliferation, we knocked it down with its specific siRNA. Ablation of Aly selectively decreased the expression levels of both cyclin A (also called cyclin A2) and cyclin B1 but not other G1 phase cyclins including cyclin D1 or E1. Cdk4 expression level also remained constant (Fig. 3A). Cyclin A is expressed during S and G2 phases, and cyclin B1 is mainly expressed during G2/M phases. These results suggest that Aly selectively regulates S and G2/M phases but not G1 phase cyclin expression. To further evaluate its role in cell growth, we conducted a BrdU incorporation assay. Depletion of Aly substantially attenuated DNA replication (Fig. 3B Left), confirming its cell growth activity in yeast. Knockdown of Aly reduced BrdU incorporation from ≈40% to <15% (Fig. 3B Right). Colony formation assay demonstrated that inactivation of Aly markedly inhibited cell proliferation (Fig. 3C). To further examine the effect of Aly on cell proliferation, we monitored the cell growth curve. Indeed, ablation of Aly decreased HeLa cell proliferation (Fig. 3D). Thus, Aly is necessary for cell proliferation. To explore whether Aly is required for mRNA export in HeLa cells, we performed an in vitro mRNA export assay with biotinylated oligo-dT(50) followed by staining with rhodamine-streptavidin. Compared with control siRNA, depletion of Aly significantly decreased mRNA export (Fig. 3E). Thus, these results demonstrate that Aly is required for cell proliferation and mRNA export.
Fig. 3.
Aly regulates cell cycle progression and cell proliferation. (A) Aly knockdown alters the expression of cell cycle regulators. HeLa cells were transfected with a control scrambled RNA or Aly siRNA and incubated for 48 h. The cell lysate was separated by SDS/PAGE and analyzed by immunoblotting. Cyclin A2 and cyclin B1 protein expression levels were selectively decreased. (B). siAly inhibits S phase progression. HeLa cells were transfected with control or siAly and incubated for 4 days. Cells were treated with BrdU (20 μM) for 1 h at 37°C and then fixed by 3% formaldehyde. The fixed cells were treated with 2 M HCl and neutralized by boric acid (pH 8.4); cells were analyzed by immunostaining analysis against BrdU antibody. Depletion of Aly decreases BrdU incorporation. Results are expressed as mean ± SEM from three independent experiments. (C) Aly knockdown prevents cell proliferation. Twenty-four hours after transfection, HeLa cells were split into six-well dishes (5 × 104) and incubated for 5 days. After cells were fixed by 3% formaldehyde, cells were stained with crystal violet solution. (D) Ablation of Aly decreases cell growth. (E) Knockdown of Aly decreases mRNA export. Results are expressed as mean ± SEM from three independent experiments (P < 0.005, Student's t test).
Akt Phosphorylation of Aly Mediates Its mRNA Export and Cell Proliferation Activities.
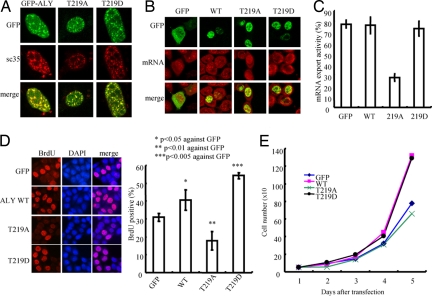
To investigate whether Akt phosphorylation mediates its subnuclear localization, we transfected GFP-Aly wild type and various mutants into MEF cells. Immunofluorescent staining showed that all transfected Aly proteins tightly colocalized with SC-35, a marker for nuclear speckle, demonstrating that Akt phosphorylation does not influence its subnuclear residency (Fig. 4A). To assess the effect of Akt phosphorylation on Aly's mRNA export activity, we transfected HeLa cells with various GFP-Aly constructs. GFP-Aly T219D displayed comparable activity to GFP control and wild-type Aly, indicating that Aly phosphorylation by Akt is sufficient to enhance mRNA export. Noticeably, the unphosphorylated T219A mutant markedly decreased mRNA export (Fig. 4 B and C), suggesting that Akt phosphorylation is required for Aly's mRNA export activity.
Fig. 4.
Akt phosphorylation mediates Aly's effect in cell proliferation. (A) Aly phosphorylation does not alter Aly nuclear speckle residency. (B and C) Akt phosphorylation on Aly T219 is required for mRNA export activity (B). Quantitative analysis of mRNA export is shown in C. (D) Akt phosphorylation regulates Aly's cell proliferation activity. T219 phosphorylation mimetic mutants enhanced BrdU incorporation, whereas unphosphorylated mutant T219A displayed decreased cell proliferation activity. Results are expressed as mean ± SEM from three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.005 (Student's t test). (E) Cell proliferation assay. HeLa cells, transfected with various Aly phosphorylation mutants, grew in six-well plates. The cell numbers were counted at the indicated time points.
To explore whether Aly phosphorylation by Akt contributes to its cell growth effect, we transfected GFP-Aly wild-type and phosphorylation mutants into HeLa cells. A BrdU incorporation assay showed that overexpression of wild-type Aly increased cell proliferation compared with GFP control. The strongest cell growth stimulatory effect occurred to the Aly T219D mutant. By contrast, the unphosphorylated Aly T219A mutant robustly blocked BrdU incorporation (Fig. 4D Left). Quantitative analysis revealed >40% BrdU-positive cells in wild-type Aly transfected cells, which was increased to ≈55% in T219D transfected cells. In contrast, BrdU incorporation was decreased to ≈15% in T219A transfected cells (Fig. 4D Right). The cell growth curve further supported that Akt phosphorylation of Aly enhanced its mitogenic activity, whereas inhibition of Akt phosphorylation suppressed cell growth (Fig. 4E). Hence, Akt phosphorylation of Aly is required for its mRNA export and cell proliferation activities.
Phosphoinositides Binding by Aly Regulates Its Subnuclear Residency, mRNA Export, and Cell Proliferation Activities.
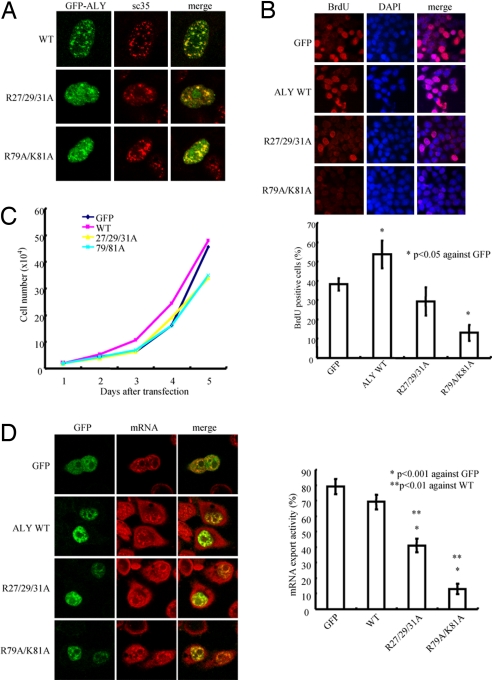
To determine whether phosphoinositide interaction regulates Aly's subnuclear localization, we conducted immunofluorescent staining. Whereas wild-type Aly resided exclusively in the nuclear speckles, Aly mutants R27/29/31A and R79A/K81A, which failed to associate with PI(3,4,5)P3, partially colocalized with SC-35. Interestingly, these mutants aggregated in the nucleoplasm outside the nuclear speckles (Fig. 5A), indicating that PI(3,4,5)P3 binding somehow regulates its nuclear speckle distribution. The BrdU incorporation assay showed that both Aly mutants R27/29/31A and R79A/K81A evidently suppressed cell proliferation compared with wild-type Aly and GFP control (Fig. 5B). The cell growth curve further verified this observation (Fig. 5C), underscoring that nuclear phosphoinositide association dictates Aly's cell growth stimulatory effect.
Fig. 5.
Phosphoinositide binding of Aly regulates its subnuclear residency, mRNA export, and cell proliferation. (A) PI(3,4,5)P3 binding mutants localize outside of nuclear speckles. Wild-type Aly distributed in the nuclear speckles, colocalizing with SC35. R27/29/31A and R79A/K81A partially localized in the nuclear speckles and aggregated in nucleoplasm. (B) BrdU incorporation assay. Aly mutants lacking PI(3,4,5)P3 binding affinity suppressed BrdU incorporation. Results are expressed as mean ± SEM from three independent experiments. *, P < 0.05 (Student's t test). (C) Aly/PI(3,4,5)P3 interaction is implicate in mediating cell proliferation. (D) Aly/PI(3,4,5)P3 interaction is essential for its mRNA export activity. GFP, GFP-Aly wild type, and mutants deficient of PI(3,4,5)P3 binding were transfected into HeLa cells, respectively, and then incubated for 48 h, followed by mRNA export assay. R79/K81A mutant displayed crippled mRNA export activity compared with wild type and R27/29/31A mutant. Results are expressed as mean ± SEM from three independent experiments. *, P < 0.001; **, P < 0.01 (Student's t test).
Nuclear phosphoinositides influence pre-mRNA splicing and chromatin structure (11). To investigate whether PI(3,4,5)P3 association is required for Aly's mRNA export activity, we transfected Aly R27/29/31A and R79A/K81A mutants lacking PI(3,4,5)P3 binding affinity into HeLa cells and performed an mRNA export assay. Aly mutants displayed a decreased activity in mRNA export compared with wild-type Aly and GFP control (Fig. 5D), suggesting that these basic residues are essential for this effect. Taken together, our data suggest that the N terminus of Aly is critical for nuclear PI(3,4,5)P3 binding and that disruption of this interaction impairs Aly's cell proliferation and mRNA export activities.
Discussion
In the present study we demonstrate that Aly is a physiological substrate of nuclear Akt. Active Akt tightly associates with Aly, and T219 phosphorylation in Aly by Akt is critical for the complex formation. Noticeably, Aly potently interacts with phosphoinositides, for which its N terminus is necessary. In addition to mediating mRNA export, we surprisingly uncovered that Aly played a critical role in cell proliferation. Blocking T219 phosphorylation or crippling PI(3,4,5)P3 binding strongly suppresses cell proliferation. Moreover, T219 phosphorylation is indispensable for its mRNA export activity. On the other hand, Aly R79A/K81A and R27/29/31/A mutants, which fail to bind PI(3,4,5)P3, display a marked decrease in mRNA export activity. Thus, nuclear PI3K signaling regulates Aly's cell proliferation and mRNA export activities.
PI3K is important for both transition from G1 to S phase and S-phase progression (27, 28). Activation of PI3K-C2β and a parallel increase of PI(3)P during the G2/M phase of the cell cycle occur in the nuclei, whereas no change is observed in the cytosol (29), fitting with the current paradigm that the nuclear and cytoplasmic phosphoinositide cycles function independent of each other. Depletion of Aly selectively eliminates cyclin A and cyclin B1 but not other G1 cyclin expression. In addition, BrdU incorporation is significantly decreased in HeLa cells when Aly is inactivated. These results support that Aly is required for cell cycle progression. This finding corroborates with the recent report that Yra1, a yeast homolog of Aly, is required for S phase entry and affects Dia2 binding to replication origins (24). Thus, Aly mediates cell cycle and has a role in DNA replication. To explore whether nuclear Akt phosphorylation of Aly plays any role in mediating Aly's cell cycle activity, we found that T219 phosphorylation strongly enhanced cell proliferation, and blockade of T219 phosphorylation profoundly blocked BrdU incorporation and cell proliferation (Fig. 4). In addition, the cell growth activity of Aly is also regulated by phosphoinositides. Overexpression of Aly mutants deficient of binding to nuclear PI(3,4,5)P3 substantially suppresses cell proliferation. Concomitantly, these mutants aggregated in the nucleoplasm outside the nuclear speckles (Fig. 5). Conceivably, Aly nuclear speckle residency is necessary for its cell growth activity. Accumulative evidence supports that cell cycle progression and nuclear phosphoinositide metabolism are linked. Although the levels of total cellular phosphoinositides remain constant throughout the cell cycle, nuclear phosphoinositides fluctuate significantly in a cell-cycle-dependent manner (30, 31). Our finding that Aly is a downstream target of nuclear PI3K, namely phosphorylation by nuclear Akt and association with nuclear phosphoinositol lipids, provides a molecular mechanism for how nuclear PI3K mediates cell cycle progression and cell proliferation.
In our previous effort to search for nuclear receptors for PI(3,4,5)P3 we identified nucleophasmin/B23 as one of the binding targets (32). Aly was also one of the binding proteins identified. In current study we provided further evidence demonstrating that Aly directly binds phosphoinositides. Mapping experiments suggest that the N terminus of Aly is involved in this activity (Fig. 2). Protein sequence analysis reveals that this region is composed of numerous basic residues. Mutation of R27/29/31 or R79/K81 into alanine abolishes Aly's binding affinity to PI(3,4,5)P3, suggesting that these residues play an essential role in binding to the heavily negatively charged phosphoinositide head groups. Presumably, the clusters of positively charged arginine residues in the N terminus and arginine-79/lysine-81 residues might form a pocket that harbors the phosphorylated inositol head group from phosphoinositol lipids. Alteration of one cluster of positively charged residues results in deformation of the delicate three-dimensional conformation, leading to loss of binding affinity by Aly to phosphoinositides. Interestingly, T219 phosphorylation is indispensable for Aly's phosphoinositol lipid binding activity, indicating that nuclear Akt mediates Aly's affinity to PI(3,4,5)P3.
Nuclear speckles are highly dynamic, and their morphology is tightly linked to the state of mRNA transcription. Inhibition of mRNA transcription induces these structures to become larger and fewer in number; the phosphatidylinositol phosphate kinases and PI(4,5)P2 reorganize identically (33). The presence of PI(4,5)P2 in the speckle domains of the nucleus has been related to its involvement in pre-mRNA splicing (14). In fact, when PI(4,5)P2 was immunoprecipitated from HeLa cell nuclear extracts, some proteins were pulled down with it, resulting in a specific inhibition of pre-mRNA splicing in the extracts (14). Here we show that PI(4,5)P2 and PI(3,4,5)P3 robustly bind to Aly in the speckles and thus the involvement by nuclear phosphoinositides in pre-mRNA splicing or mRNA export might be a direct effect. Nevertheless, the possibility that PI(4,5)P2 or PI(3,4,5)P3 binds nuclear matrix proteins and serves as a structural interface between the enzymatic core of the spliceosome and the matrix itself cannot be excluded either. Identification of Aly, a nuclear speckle protein implicated in mRNA export, as one of nuclear phosphoinositol lipid binding targets is certainly of great help in understanding the exact and multifaceted functions of these nuclear lipids. Collectively, our study establishes that Aly is a physiological downstream target of nuclear PI3K signaling and that nuclear PI(3,4,5)P3 and Akt coordinately mediate the nuclear effector through the concerted interaction and phosphorylation. This finding provides a molecular mechanism of how the cell cycle progression, cell proliferation, and mRNA export activities of Aly are regulated by nuclear PI3K.
Materials and Methods
Cells and Reagents.
HEK293, HeLa, MEF, Akt1, Akt2, and Akt1/2 null cells were maintained in DMEM including 10% FBS and 100 units of penicillin-streptomycin. PC12 cells were maintained in DMEM including 10% horse serum, 5% FBS, and 100 units of penicillin-streptomycin. All cells were maintained at 37°C with 5% CO2 atmosphere in a humidified incubator. EGF, NGF, avidin-rhodamine, and biotin-16–2′-deoxy-uridine-5′-triphosphate were from Roche. Anti-cdk4 and BrdU antibodies were from Calbiochem. Wortmannin, LY294002, rottlerin, staurosporin, etoposide, BrdU, anti-tubulin, SC-35, GST-HRP, and HA-HRP antibodies were from Sigma. Terminal transferase was from New England Biolabs. Anti-phospho-Akt S473 antibody was from Cell Signaling Technology. Anti-ALY (NB100-670) was from NOVUS. Anti-cyclin A2, cyclin B1, cyclin D1, Akt, HA, and GFP antibodies were from Santa Cruz Biotechnology. Purified active Akt was from Upstate Biotechnology. Phosphatidylinositol lipid-conjugated beads were from Echeron Research Laboratory. siRNA of ALY (1757609) was from Qiagen. The target sequence is TGGGAAACTGCTGGTGTCCAA.
In Vitro Akt Kinase Assay.
Purified Aly fragments or its mutants (0.5 μg) were incubated with active Akt in kinase reaction buffer (20 mM Tris, pH 7.5/10 mM MgCl2) containing 25 μM ATP and 2.5 μCi of [γ-32P]ATP for 30 min at 30°C. Reactions were terminated by addition of Laemmli's sample buffer and boiling for 10 min. A portion of the sample (20 μl) was separated by SDS/PAGE and analyzed by autoradiography.
In Vivo ALY Phosphorylation Assay and Metabolic Labeling.
Transfected HEK293 cells were serum-starved for 24 h, and in some experiments cells were infected by adenovirus and incubated for 24 h. Cells were washed with phosphate-free medium and incubated for 90 min in phosphate-free medium. Cells were treated with 250 μCi/ml 32P-orthophosphate for 4 h. The labeled cells were treated with or without inhibitors for 30 min and then treated with EGF for 20 min. Cells were washed with ice-cold PBS three times and lysed and then mixed with GSH beads for overnight at 4°C. After washing, protein complexes were separated by SDS/PAGE and analyzed by autoradiography.
Coimmunoprecipitation and in Vitro Binding Assay.
A 10-cm plate of transfected HEK293 cells was washed once in PBS, lysed in 1 ml of lysis buffer (50 mM Tris, pH 7.4/40 mM NaCl/1 mM EDTA/0.5% Triton X-100/1.5 mM Na3VO4/50 mM NaF/10 mM sodium pyrophosphate/10 mM sodium β-glycerophosphate/protease inhibitor mixture), and centrifuged for 10 min at 16,000 × g at 4°C. The supernatant was transferred to a fresh tube and mixed with a variety of antibody or GSH beads or phosphatidylinositol lipid-conjugated beads. After SDS/PAGE the samples were transferred to a nitrocellulose membrane. Western blotting analysis was performed with a variety of antibodies.
Cell Cycle Analysis.
For cell counting analysis, transfected HeLa cells were incubated for 24 h and 1 × 105 cells were split into six-well plate; on the indicated days trypsinized cells were counted by hemacytometer. For crystal violet analysis, 5 × 104 transfected cells were split into six-well plates and incubated for 5 days. Cells were fixed by 3% formaldehyde in PBS and stained by crystal violet solution. In the BrdU incorporation assay, transfected cells were grown in 20 μM BrdU including medium for 2 h and fixed by 3% formaldehyde in PBS. After washing with PBS, cells were treated with prewarmed 2 M HCl for 15 min at 37°C and neutralized by 0.1 M boric acid (pH 8.4) for 15 min at 37°C twice. Then cells were blocked by 2% FBS/PBS and stained by anti-BrdU antibody. Finally, cells were counterstained with DAPI to visualize the nuclei. For cell cycle regulator protein expression check, cells were transfected and incubated for 24 h and split into six-well plates, then incubated for 48 h. The cells were lysed in lysis buffer, and the protein amount was determined by Bradford solution. After the protein amount was adjusted, proteins were loaded onto SDS/PAGE and analyzed by immunoblotting.
Fluorescent in Situ Hybridization for mRNA Export.
HeLa cells were transfected with various GFP-tagged Aly constructs or siRNA and incubated for 24 h. Cells were fixed by 3% formaldehyde in PBS/diethyl pyrocarbonate (DEPC) for 10 min at room temperature. After washing with ice-cold PBS/DEPC, cells were permeabilized by 0.5% Triton X-100 in PBS/DEPC for 5 min on ice. After cells were washed with ice-cold PBS/DEPC three times, coverslips were transferred into 2× SSC and incubated at room temperature for 10 min. Cells were blocked by blocking mixture (2× SSC, 1 mg/ml tRNA, 10% dextran sulfate, and 25% formamide) at room temperature for 30 min and then hybridized in hybridization mixture [50 mg/ml biotinylated oligo-dT(50), 2× SSC, 1 mg/ml tRNA, 10% dextran sulfate, and 25% formamide] at 37°C overnight. Cells were washed with 2× SSC for 10 min three times and then washed with 0.5× SSC for 10 min once. After blocking with 2% FBS in PBS for 30 min at room temperature, cells were stained by rhodamine-streptavidin for 20 min at room temperature. After cells were washed with 0.2% Triton X-100/PBS for 10 min three times, cells were washed with PBS twice. Fluorescent images were taken by using a confocal fluorescence microscope.
Supplementary Material
Acknowledgments.
We are thankful to Dr. Rozanne M. Sandri-Goldin (University of California, Irvine, CA) for EGFP-ALY and Flag-ALY constructs and Dr. Michael Green from (University of Massachusetts, Worcester, MA) for the pRSETC-BEF/ALY construct. This work was supported by National Institutes of Health Grant RO1 NS045627 (to K.Y.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802533105/DCSupplemental.
References
- 1.Cocco L, Martelli AM, Gilmour RS, Rhee SG, Manzoli FA. Nuclear phospholipase C and signaling. Biochim Biophys Acta. 2001;1530:1–14. doi: 10.1016/s1388-1981(00)00169-4. [DOI] [PubMed] [Google Scholar]
- 2.Ye K, et al. Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature. 2002;415:541–544. doi: 10.1038/415541a. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, et al. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphate-containing phosphatidylinositol signaling molecules. J Biol Chem. 1997;272:17756–17761. doi: 10.1074/jbc.272.28.17756. [DOI] [PubMed] [Google Scholar]
- 4.Boronenkov IV, Loijens JC, Umeda M, Anderson RA. Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell. 1998;9:3547–3560. doi: 10.1091/mbc.9.12.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim MA, Kikani CK, Wick MJ, Dong LQ. Nuclear translocation of 3′-phosphoinositide-dependent protein kinase 1 (PDK-1): A potential regulatory mechanism for PDK-1 function. Proc Natl Acad Sci USA. 2003;100:14006–14011. doi: 10.1073/pnas.2335486100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimm O, et al. Differential nuclear and cytoplasmic expression of PTEN in normal thyroid tissue, and benign and malignant epithelial thyroid tumors. Am J Pathol. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachyankar MB, et al. A role for nuclear PTEN in neuronal differentiation. J Neurosci. 2000;20:1404–1413. doi: 10.1523/JNEUROSCI.20-04-01404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torres J, et al. Heterogeneous lack of expression of the tumour suppressor PTEN protein in human neoplastic tissues. Eur J Cancer. 2001;37:114–121. doi: 10.1016/s0959-8049(00)00366-x. [DOI] [PubMed] [Google Scholar]
- 9.Meier R, Alessi DR, Cron P, Andjelkovic M, Hemmings BA. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bbeta. J Biol Chem. 1997;272:30491–30497. doi: 10.1074/jbc.272.48.30491. [DOI] [PubMed] [Google Scholar]
- 10.Ahn JY, Rong R, Liu X, Ye K. PIKE/nuclear PI 3-kinase signaling mediates the antiapoptotic actions of NGF in the nucleus. EMBO J. 2004;23:3995–4006. doi: 10.1038/sj.emboj.7600392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martelli AM, Manzoli L, Cocco L. Nuclear inositides: Facts and perspectives. Pharmacol Ther. 2004;101:47–64. doi: 10.1016/j.pharmthera.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Dundr M, Misteli T. Functional architecture in the cell nucleus. Biochem J. 2001;356:297–310. doi: 10.1042/0264-6021:3560297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Didichenko SA, Thelen M. Phosphatidylinositol 3-kinase c2alpha contains a nuclear localization sequence and associates with nuclear speckles. J Biol Chem. 2001;276:48135–48142. doi: 10.1074/jbc.M104610200. [DOI] [PubMed] [Google Scholar]
- 14.Osborne SL, Thomas CL, Gschmeissner S, Schiavo G. Nuclear PtdIns(4,5)P2 assembles in a mitotically regulated particle involved in pre-mRNA splicing. J Cell Sci. 2001;114:2501–2511. doi: 10.1242/jcs.114.13.2501. [DOI] [PubMed] [Google Scholar]
- 15.Stamm S. Signals and their transduction pathways regulating alternative splicing: A new dimension of the human genome. Hum Mol Genet. 2002;11:2409–2416. doi: 10.1093/hmg/11.20.2409. [DOI] [PubMed] [Google Scholar]
- 16.Blaustein M, et al. Mammary epithelial-mesenchymal interaction regulates fibronectin alternative splicing via phosphatidylinositol 3-kinase. J Biol Chem. 2004;279:21029–21037. doi: 10.1074/jbc.M314260200. [DOI] [PubMed] [Google Scholar]
- 17.Luo MJ, Reed R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci USA. 1999;96:14937–14942. doi: 10.1073/pnas.96.26.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, et al. The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans. Nature. 2000;407:401–405. doi: 10.1038/35030160. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Steitz JA. Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell. 2001;7:899–905. doi: 10.1016/s1097-2765(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 20.Cullen BR. Connections between the processing and nuclear export of mRNA: Evidence for an export license? Proc Natl Acad Sci USA. 2000;97:4–6. doi: 10.1073/pnas.97.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed R, Magni K. A new view of mRNA export: Separating the wheat from the chaff. Nat Cell Biol. 2001;3:E201–E204. doi: 10.1038/ncb0901-e201. [DOI] [PubMed] [Google Scholar]
- 22.Strasser K, Hurt E. Yra1p, a conserved nuclear RNA-binding protein, interacts directly with Mex67p and is required for mRNA export. EMBO J. 2000;19:410–420. doi: 10.1093/emboj/19.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zenklusen D, Vinciguerra P, Strahm Y, Stutz F. The yeast hnRNP-Like proteins Yra1p and Yra2p participate in mRNA export through interaction with Mex67p. Mol Cell Biol. 2001;21:4219–4232. doi: 10.1128/MCB.21.13.4219-4232.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swaminathan S, Kile AC, MacDonald EM, Koepp DM. Yra1 is required for S phase entry and affects Dia2 binding to replication origins. Mol Cell Biol. 2007;27:4674–4684. doi: 10.1128/MCB.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gatfield D, Izaurralde E. REF1/Aly and the additional exon junction complex proteins are dispensable for nuclear mRNA export. J Cell Biol. 2002;159:579–588. doi: 10.1083/jcb.200207128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longman D, Johnstone IL, Caceres JF. The Ref/Aly proteins are dispensable for mRNA export and development in Caenorhabditis elegans. RNA. 2003;9:881–891. doi: 10.1261/rna.5420503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SM, Klinghoffer R, Prestwich GD, Toker A, Kazlauskas A. PDGF induces an early and a late wave of PI 3-kinase activity, and only the late wave is required for progression through G1. Curr Biol. 1999;9:512–521. doi: 10.1016/s0960-9822(99)80235-8. [DOI] [PubMed] [Google Scholar]
- 28.Dangi S, Cha H, Shapiro P. Requirement for phosphatidylinositol-3 kinase activity during progression through S-phase and entry into mitosis. Cell Signalling. 2003;15:667–675. doi: 10.1016/s0898-6568(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 29.Visnjic D, et al. Biochim Biophys Acta. 2003;1631:61–71. doi: 10.1016/s1388-1981(02)00356-6. [DOI] [PubMed] [Google Scholar]
- 30.York JD, Majerus PW. Nuclear phosphatidylinositols decrease during S-phase of the cell cycle in HeLa cells. J Biol Chem. 1994;269:7847–7850. [PubMed] [Google Scholar]
- 31.Clarke JH, et al. Inositol lipids are regulated during cell cycle progression in the nuclei of murine erythroleukaemia cells. Biochem J. 2001;357:905–910. doi: 10.1042/0264-6021:3570905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahn JY, et al. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol Cell. 2005;18:435–445. doi: 10.1016/j.molcel.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. J Biol Chem. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.