Abstract
A model of chemical thymectomy by inducible Rag ablation was used to study peripheral T cell homeostasis. Induction of Rag ablation was efficient and complete, leading to cessation of thymic T cell production within 3–4 weeks. The decay of peripheral T cells became apparent with a delay of an additional 2–3 weeks and was entirely accounted for by loss of naïve T cells, whereas numbers of memory phenotype and regulatory T cells were not decreased. Naïve CD4 T cells decayed with an average half-life of 50 days, whereas naïve CD8 T cells exhibited a considerably longer half-life. The rapid decay of naïve CD4 T cells was not caused by intrinsic survival differences compared with naïve CD8 T cells, but was caused by changes in the lymphopenic environment resulting in higher microbial load and consequential activation. This finding suggests that in lymphopenic conditions involving compromised thymic function replenishment and survival of a naïve CD4 T cell repertoire may be severely curtailed because of chronic activation. Such a scenario might play a role in the aging immune system and chronic viral infection, such as HIV infection, and contribute to loss of CD4 T cells and impaired immune function. As our data show, continued replenishment with cells from the thymus seems to be required to maintain efficient gut mucosal defense.
Keywords: homeostasis, thymus, lymphopenia, CD4 depletion, T cell life span
T cell homeostasis ensures the maintenance of constant T cell numbers in the periphery and safeguards independent coexistence of naïve and memory T cell pools in the periphery (1, 2). One important parameter in maintaining homeostasis in the face of lymphopenic incidents is the capacity of the thymus to continuously export new naïve T cells, because naïve T cells cannot be regenerated by peripheral mechanisms (3–5). However, there are physiological situations in which thymic export is compromised such as in old age (6–8) or in chronic infections, e.g., HIV infection (9–11). Because it appears that in euthymic mice export of new T cells, rather than proliferation of peripheral T cells, corrects homeostasis even after depletion of as much as 90% of the peripheral T cell pool (12), we chose to address the consequences of thymus ablation on T cell homeostasis in the periphery. Here, we have used an approach of “genetic thymectomy” using conditional gene ablation of the recombination activating gene [Rag2 (13)] to study the decay of peripheral T cells without exposing mice to surgical trauma. Mice with a floxed Rag2 allele (Rag2 fl/fl) were crossed with mice expressing Cre under control of the Mx promoter that is inducible by type I IFN (14). Injection with polyinosinic-polycytidylic acid [poly(I:C)] induces Cre expression and consequential Rag2 deletion, blocking T cell development in the thymus.
We found that ablation of the thymus resulted in a decay of peripheral T cell numbers that was entirely caused by the loss of naïve T cells, whereas memory T cells and even regulatory T cells were maintained in constant numbers. Interestingly, naïve CD4 T cells declined more rapidly than naïve CD8 T cells. However, this event was not caused by intrinsic differences in survival capacity between CD4 and CD8 T cells, but instead by increased turnover of CD4 T cells in response to endogenous antigens. Thus, immune homeostasis in the periphery is compromised when thymic export is abrogated not only by loss of T cell production but also by systemic activation of CD4 T cells, an event that could crucially exacerbate immune pathology in chronic infections, such as HIV infection, and contribute to deficient immune responses in old age.
Results
Conditional Inactivation of the Rag2 Gene and Effect on the Thymus.
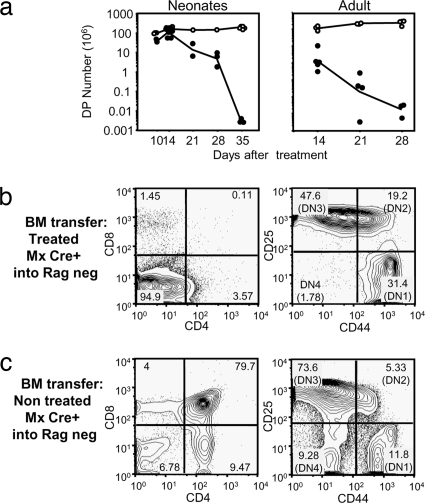
To terminate generation of T cells and their export from the thymus we used the inducible ablation of the Rag2 gene in F1 mice carrying a floxed Rag2 allele on a Rag2-deficient background and a Cre recombinase transgene under control of the IFN-inducible Mx promoter (MxCre+ Rag2 fl/−). The efficiency of Rag deletion in this system was previously ascertained on the level of B cells (13). We first determined the effect of poly(I:C) treatment on thymic cellularity. As shown in Fig. 1a Left the numbers of double positive (DP) thymocytes decreased from week 2 after treatment of neonatal mice with type I IFN and were reduced to background levels 5 weeks after treatment. In adult mice (aged 8–10 weeks) the deletion proceeded faster and was complete 4 weeks after treatment with poly(I:C) (Fig. 1a Right). Younger adults (aged 4–6 weeks) showed intermediate speed of deletion [supporting information (SI) Fig. S1], which was complete by 5 weeks similar to neonatal mice. All subsequent experiments were done using poly(I:C) treatment of 4–6 week old adult mice.
Fig. 1.
Efficiency of the deletion. (a) Absolute cell numbers of DP thymocytes after type I IFN treatment of 3-day-old MxCre- Rag2 fl/− (open symbols) or MxCre+ Rag2 fl/− (filled symbols) (Left) or after poly(I:C) treatment of 8- to 10-week-old mice (Right). (b and c) Dot plots of gated CD45.2 cells from thymus of bone marrow chimeras MxCre+ Rag2fl/−-treated with poly(I:C) 4 weeks before bone marrow isolation (b) or MxCre+ Rag2fl/− untreated bone marrow (c) injected into 5 Gy irradiated Rag2-deficient B6 CD45.1 hosts. (Left) Dot plots show CD8 vs. CD4 profiles. (Right) Dot plots show CD25 vs. CD44 profiles of the DN population with stages DN1 to DN4 marked.
To assess whether Rag deletion upon poly(I:C) treatment was complete, we reconstituted Rag-deficient mice with allotype-marked bone marrow from poly(I:C)-treated or untreated MxCre+ mice. As shown in Fig. 1b, there was no reconstitution of DP thymocytes in recipients of poly(I:C)-treated MxCre+ bone marrow, although precursors could still be seen in the DN fraction up to the DN4 stage, where the blockade of development occurs in Rag-deficient mice (15). Bone marrow from untreated MxCre+ control mice, on the other hand, fully reconstituted the thymus of Rag-deficient hosts.
Poly(I:C) treatment did not induce any deleterious effects that impacted directly on T cell homeostasis. The total number of thymocytes and DP thymocytes was not altered within 2 weeks after poly(I:C) injection, and no differences in thymocyte numbers between poly(I:C)-treated MxCre− Rag2fl/− mice and untreated Mx1Cre+ Rag2 fl/− mice was seen, confirming that poly(I:C) injection per se does not induce any loss in thymic cellularity (Fig. S2a). To address whether Cre expression induced after poly(I:C) treatment could exhibit any toxicity (16), mice expressing Cre, but not a floxed Rag locus (Mx1Cre+ Rag wt/wt) were treated with poly(I:C). We observed no alterations in total thymocytes or thymocytes subsets between MxCre+ and MxCre− Rag wt/wt mice, confirming that Cre did not exert any nonspecific toxicity (Fig. S2b).
Furthermore, the peripheral T cell numbers of MxCre+ Ragfl/− or MxCre− Ragfl/− mice showed no significant differences 2 weeks after the beginning of poly(I:C) treatment, and numbers of CD4 and CD8 T cells were comparable, whether naïve, memory, or regulatory T cell subsets were concerned (Fig. S3a). Subsequent experiments used either untreated MxCre+ Rag2fl/− mice as controls or poly(I:C)-treated MxCre− Rag2fl/− littermates as they were indistinguishable. Finally, poly(I:C) treatment did not influence expression of MHC class II, CD80, and CD86 costimulatory molecules as indicators for the activation status of dendritic cells (DC) (Fig. S3b). Altogether, our results suggest that the induction of IFN-α production caused by poly(I:C) injections on its own does not induce any significant disturbance of the peripheral T cell compartment.
Effect of Thymus Ablation on Peripheral T Cells.
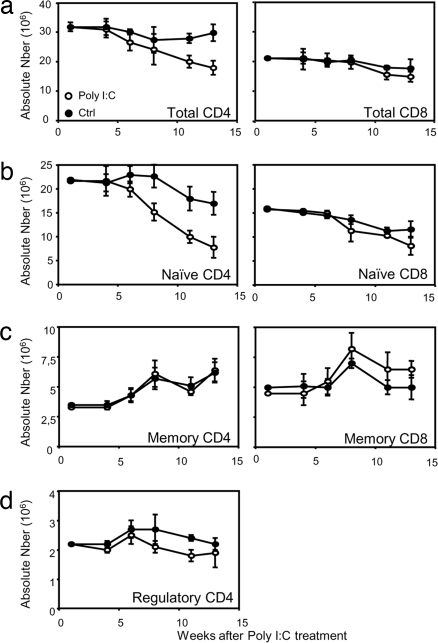
The effect of Rag deletion and thymus ablation on the peripheral T cell pool was delayed, but from ≈6 weeks after poly(I:C) treatment a gradual decline in peripheral T cell numbers was observed. Whereas for CD4 T cells an obvious decline in numbers was observed from ≈8 weeks after induction of Cre recombinase activity by poly(I:C) injection (Fig. 2a Left), CD8 T cell numbers remained constant until about weeks 11–13 before a slight decay could be detected (Fig. 2a Right). We next analyzed the effect of thymus ablation on the different T cell subsets within the CD4 and CD8 T cell subsets, defined on the basis of CD25 and/or CD44 expression. FACS profiles obtained 10 weeks after poly(I:C) treatment showed a shift in the proportion of naïve/memory (CD44hi CD25−) and regulatory T cells (CD44 intermediate, CD25+) at the expense of the naïve (CD44low CD25−) CD4 T cell subset (data not shown). In terms of T cell numbers, the cellular reduction within the CD4 T cell pool was restricted to the naïve pool (Fig. 2b Left). In contrast, the absolute number of memory (Fig. 2c Left) or regulatory (Fig. 2d) CD4 T cells did not differ between poly(I:C)-treated and untreated mice. Thus, thymic ablation induces selective loss of naïve T cells without affecting the pool size of memory and regulatory T cells. This observation applies also to the CD8 T cell compartment (Fig. 2b Right), although the reduction of naïve CD8 T cells was less marked compared with that of naïve CD4 T cells.
Fig. 2.
Peripheral T cell survival in the absence of thymic export. Peripheral T cell numbers were determined in poly(I:C)-treated (○) or untreated (●) MxCre+ Rag2fl/− mice. Numbers of CD4 (Left) and CD8 (Right) peripheral T cells (a), naïve T cells (b), memory T cells (c), and regulatory CD4 T cells (d) are shown. Mean values and SDs for three mice per time point are shown. These data are representative of three experiments using three mice per group for each experiment.
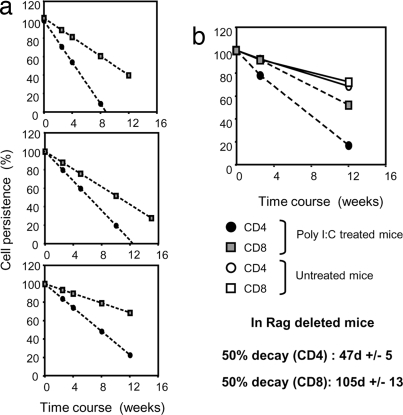
To facilitate comparisons of decay rates of each of these subsets, linear curves from numbers of naïve CD4 and CD8 T cells were plotted on a single graph representing the percentage of residual T cells on the y axis to have a common starting point. T cell numbers 5 weeks after the start of poly(I:C) treatment were set to 100%. The decay rate of naïve CD4 T cells from poly(I:C)-treated Mx Cre+ Rag2fl/− mice was markedly higher than that of naïve CD8 T cells as shown in three independent experiments (Fig. 3a).
Fig. 3.
Decay of naïve CD4 and CD8 T cells after thymus ablation. CD4 and CD8 T cell decay was extrapolated from the numbers of naïve CD4 and CD8 T cells recovered over a course of 15 weeks after poly(I:C) treatment and plotted against values obtained from untreated Mx Cre+ Rag2fl/− littermates, as shown in Fig. 2b. Cell numbers obtained 5 weeks after treatment were set as 100% value on the y axis and 0 on the x axis. (a) Trend lines for CD4 (circles) and CD8 (squares) T cell decay in poly(I:C)-treated Mx Cre+ Rag2fl/− mice in three independent experiments. (b) Average trend lines for CD4 (circles) and CD8 (squares) T cell decay in poly(I:C)-treated (filled symbols) or untreated (open symbols) Mx Cre+ Rag2fl/− mice. Note that naïve CD4 and CD8 T cells from untreated mice exhibit identical decay and therefore show overlapping trend lines on the graph. Error bars were omitted from the summary graph in the interest of visual clarity.
Fig. 3b gives an average of the three experiments together with decay rates of T cells from untreated Mx Cre+ Rag2fl/− mice. CD4 and CD8 T cells from untreated mice only showed a slight decay caused by physiological thymic involution over the time frame of 15 weeks and their decay curves were superimposable, whereas the decay rates for CD4 and CD8 T cells from poly(I:C)-treated Mx Cre+ Rag2fl/− mice were accelerated because of abrogation of production of new T cells from the thymus. Based on these data the half life of naïve CD4 and CD8 T cells after thymic ablation was 47± 5 days for CD4 T cells and 105 ± 13 days for CD8 T cells.
Higher Decay Rate of Naïve CD4 T Cells Is Determined by the Environment.
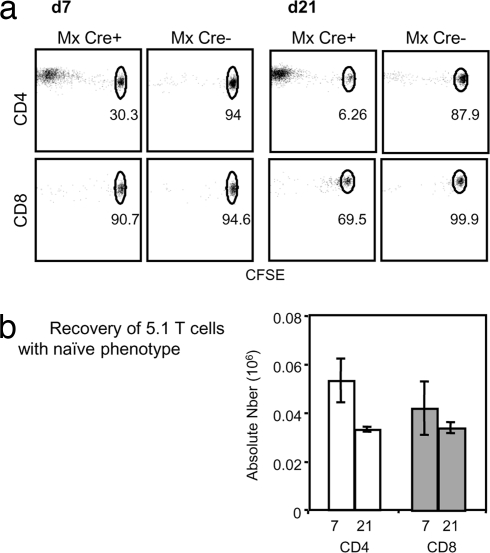
The faster decay rate of naïve CD4 compared with naïve CD8 T cells after thymus ablation suggests that naïve CD4 and CD8 T cells either have different intrinsic half-lives or are differentially affected by changes in their environment. No difference in the rate of decay of naïve CD4 and CD8 T cells from poly(I:C)-treated hosts or WT hosts were seen upon transfer into untreated WT B6 CD45.1 hosts (data not shown), allowing us to conclude that naïve CD4 and CD8 T cells do not have an intrinsic difference in their survival capacity. To investigate the possibility that CD4 T cells react differently to their environment, (5,6-carboxyfluorescein diacetate succinimidyl ester) (CFSE)-labeled naïve CD4 or CD8 T cells from WT mice were transferred into poly(I:C)-treated Mx Cre+ or Cre− hosts 20 weeks after poly(I:C) treatment. As expected, transfer of CFSE-labeled naïve T cells into nonlymphopenic Mx Cre− Rag2fl/− mice did not induce their proliferation as analyzed 7 or 21 days after transfer (Fig. 4a). Surprisingly, when transferred into poly(I:C)-treated Mx Cre+ Rag2fl/− mice, sorted naïve CD4 T cells, but not CD8 T cells, proliferated rapidly so that 70% of CD4 T cells had already lost CFSE 7 days after transfer and 94% were CFSE-negative after 21 days. Furthermore, proliferating CD4 T cells acquired activation markers, and consequently the number of recovered naïve CD4 T cells in poly(I:C)-treated Mx Cre+ Rag2fl/− hosts decreased, whereas CD8 T cells did not change their naïve phenotype as they did not undergo cell division (Fig. 4b). These results clearly indicate that CD4 T cells react to a changed environment in poly(I:C)-treated mice, whereas naïve CD8 T cells do not seem to perceive any environmental changes in poly(I:C)-treated hosts and consequently are not activated.
Fig. 4.
CD4 T cells, but not CD8 T cells, proliferate in poly(I:C)-treated MxCre+ hosts. (a) Proliferation and recovery of sorted B6 CD45.1 naïve CD4 T cells (Upper) or naïve CD8 T cells (Lower) after transfer into poly(I:C)-treated Mx Cre+ Rag2fl/− or Mx Cre− Rag2fl/− control mice. CFSE profiles were obtained 7 and 21 days after transfer. (b) Absolute numbers of recovered donor CD4 (open bars) or CD8 T cells (filled bars) with a naïve phenotype 7 and 21 days after transfer into poly(I:C)-treated Mx Cre+ Rag2fl mice. This experiment is representative of three experiments using three mice per group and per time point.
Loss of Naïve CD4 T Cells Correlates with Higher Microbial Load in the Periphery.
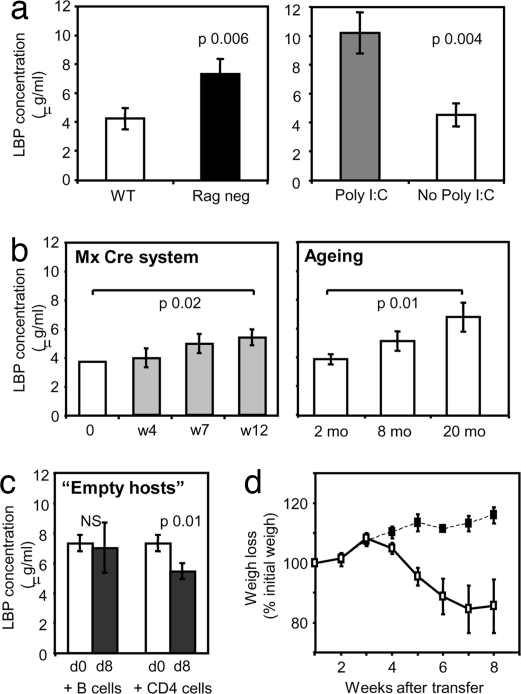
The expansion and activation of adoptively transferred polyclonal T cells in T cell-deficient hosts was shown to be caused primarily by stimulation by the gut-derived microbial flora (17). The involvement of gut-derived antigens in the activation of naïve CD4 T cells was suggested by the detection of higher concentrations of LPS binding protein (LBP) than found in normal mice (18, 19). To assess whether lymphopenic poly(I:C)-treated MxCre+ Ragfl/− mice show a corresponding change in the overall antigenic load, we measured the level of LBP in the sera of poly(I:C)-treated or untreated MxCre+ Rag2fl/− mice. Sera were collected 20 weeks after poly(I:C) treatment (i.e., the same time point that showed expansion of transferred CD4 T cells). LBP levels were compared between poly(I:C)-treated mice, WT mice, and genetically T cell-deficient Rag knockout mice.
As shown in Fig. 5a, sera from Rag-deficient mice contain higher levels of LBP than those of WT mice. Although poly(I:C)-treated mice are only partially lymphopenic, because their memory and regulatory T cell pool are not significantly decreased, the LBP concentrations in the sera of these mice were similar to those obtained in completely T cell-deficient Rag knockout mice. The increase in LBP occurred gradually over a time frame of 12 weeks (Fig. 5b) and reached statistical significance 12 weeks after poly(I:C) induction of thymus ablation. Elevated levels of LBP were not only observed in this particular experimental system of thymus ablation, but also in mice in which the thymus atrophied naturally during aging. Mice aged 20 months had significantly higher levels of LBP in their serum compared with 2-month-old controls. Adoptive transfer of naive CD4 T cells, but not B cells, into Rag-deficient mice resulted in a reduction of LBP levels 8 days after transfer (Fig. 5c), suggesting that naïve CD4 T cells play a role in controlling the levels of endogenous gut-derived antigens.
Fig. 5.
LBP levels in serum. (a) LBP concentration in the sera of 8-week-old B6 mice (open bar) and Rag2-deficient mice (black bar) (Left) and LBP concentration in serum of poly(I:C)-treated mice (gray bar) or untreated Mx Cre+ Rag2fl/− mice (open bar) 15–20 weeks after treatment (Right). This experiment is representative of two experiments using three to five mice per group. (b) LBP concentration in sera of poly(I:C)-treated MxCre+ Rag2fl/− mice at different time points after poly(I:C) treatment (Left) and LBP concentration in sera of B6 mice at 2, 8, and 20 months of age (Right). (c) LPB concentration in sera of Rag-deficient mice injected with purified B cells or naïve CD4 T cells (5 × 106 per mouse) before cell transfer at day 0 (open bars) or 8 days after cell transfer (filled bars). (d) Transfer of 1.5 × 106 naïve CD4 T cells isolated from poly(I:C)-treated (■) or untreated (□) Mx Cre+ Rag2fl/− mice into Rag-deficient hosts. The graph shows percentage of weigh loss in the adoptive hosts. These data are representative of two experiments using at least four mice per group.
Because poly(I:C)-treated MxCre+ mice gradually lose their naïve CD4 T cell pool and simultaneously exhibit an increase of LBP in their circulation, we reasoned that the residual naïve CD4 population might be depleted of T cell specificities against environmental antigens so that as a consequence the microbial load is gradually increasing. To test this hypothesis, we performed adoptive transfer experiments injecting naïve CD4 T cells from either poly(I:C)-treated (12 weeks after treatment) or untreated mice into Rag-deficient hosts. Transfer of naïve CD4 T cells devoid of regulatory T cells normally induces immune pathology, resulting in severe weight loss caused by the activation and expansion of transferred cells specific for environmental antigens. Indeed naïve CD4 T cells from nontreated hosts induced severe weight loss in the adoptive hosts (Fig. 5d). However, naïve CD4 T cells from poly(I:C)-treated mice did not induce any weigh loss or other signs of pathology (Fig. 5), suggesting that indeed residual surviving naïve T cells in these mice were purged of a repertoire that responds to environmental antigens.
Discussion
The immune system, like many other biological systems, has devised ways of controlling overall numbers of cells to safeguard both the maintenance of a diverse repertoire for control of new pathogens and an efficient memory response capable of rapid elimination of previously encountered pathogens. Whereas memory T cell numbers are homeostatically controlled independent of thymic output and will adjust to a given threshold even after severe lymphopenic incidents (20), naïve T cell numbers depend strictly on replenishment by the thymus. As thymus function wanes with old age, the periphery becomes partially lymphopenic because of the loss of naïve T cells that cannot be regenerated by homeostatic mechanisms (4, 21–23). To define what consequences the complete loss of thymic repopulation has on peripheral T cells, we used a model of induced Rag2 ablation. This model has the advantage of allowing ablation of T cell production from the thymus without surgical intervention and the concurrent trauma and furthermore allowed us to measure the decay of peripheral T cells starting out with a fully competent normal peripheral T cell compartment. The deletion efficiency of the Rag-2fl allele was previously shown to be virtually 100% in the bone marrow and ≈90% in the spleen and cells in the peritoneal cavity as determined by Southern blot analysis (13). In the thymus, deletion efficiency varies between the subpopulations, but does generally not exceed 60% 2 weeks after the start of poly(I:C) treatment. Although a reduction in thymocytes was observed as early as 1 week after Cre induction, it took 3–4 weeks before T cell production in the thymus was fully ablated as indicated by complete loss of DP thymocytes. The small residual activity of T cell differentiation is a direct consequence of the survival of nonrecombined thymocytes in the DN1 stage in which thymocytes spend on average ≈10 days before further differentiation (24). Because there is a delay of ≈14 days between the generation of mature positively selected thymocytes and their exportation (25), the effect of thymus ablation on the periphery was not noticeable until 5–6 weeks after Cre induction. Taken together, this strongly argues for a scenario in which full cessation of thymic export can be only achieved after the full replacement of intrathymic T cell progenitors with Rag2-deficient bone marrow-derived progenitors.
The rapid decay of naïve CD4 T cells does not reflect an intrinsically different survival capacity compared with CD8 T cells, but rather a selective reactivity to changes in the environment that cause their activation. The presence of increased levels of LBP suggests an increased exposure to microbial antigen, which may be responsible for this activation step. It appears that specifically the loss of newly produced naïve T cells from the thymus is associated with an increase in microbial translocation, as the mice had unaltered numbers of CD4 T cells with an activated phenotype. Given that CD8 T cells were not affected by this activation, we assume that it is extracellular antigens and predominant access to the class II presentation pathway that favored recognition by CD4 T cells.
It has previously been demonstrated that the rapid expansion and activation of polyclonal T cells adoptively transferred into T cell-deficient Rag KO or nude mice is mainly cased by a response to the endogenous gut flora (17). Interestingly, this kind of response was found upon adoptive transfer into genetically (chronically) immunodeficient hosts, but not sublethally irradiated hosts, in which T cell depletion is acutely induced over a short time frame so that presumably microbial translocation had not built up to noticeable levels within the time frames studied (17). Although one might have predicted that increased microbial load and consequential activation of CD4 T cells could lead to pathology at mucosal sites, we did not observe any gut pathology in our mice. It is conceivable that the presence of CD103-positive Tregs, which have access to mucosal sites might be sufficient to prevent pathology at least under steady conditions in a pathogen-free animal house. Finally, we cannot exclude that thymic export in some way minimizes and corrects adverse effects of peripheral activation of naïve CD4 T cells by environmental/gut-derived stimuli. The fact that CD4 T cells activated in response to environmental antigens do not seem to control the antigenic load suggests that chronic exposure to environmental antigen eventually cripples the response of activated CD4 T cells so that only a new supply of naïve CD4 T cells from the thymus can guarantee effective control of gut-derived antigens.
With advanced age there is a trend for reduction of the CD4/CD8 T cell ratio in mice and in humans (26, 27). In part this reduction was attributed to oligoclonal expansion of CD8 T cells (28) and in humans it often applies to CMV-specific T cell populations (29). In mice the oligoclonal expansion of CD8 T cells in old age has been reported, but seems to be less frequent and dependent on the barrier conditions for animal housing (28). In our experience <5% of >12-month-old mice showed oligoclonal CD8 expansions. However, the data presented here provide an alternative explanation for age-related changes in CD4/CD8 ratios that might be caused by gradual erosion of naïve CD4 T cells because of environmental antigen-driven activation once thymic output is compromised. In support of this assumption we also found increased LBP levels in mice that had naturally aged in a pathogen-free environment. Because of the complexity of the T cell repertoire it is not possible to directly prove the antigen specificity of the cells involved nor is it feasible to accurately determine the remaining repertoire after thymic ablation. Nevertheless, our data implicate gut flora in the decay of naïve T cells and thereby raise important issues for understanding disease parameters in certain infections that compromise mucosal immunity and thymic regeneration, such as HIV infection, where the association between thymic abrogation and immune activation is of particular interest. HIV infection in humans is characterized by severe CD4 T cell depletion, leading to immunodeficiency and opportunistic infections. The loss of peripheral CD4 T cells is progressive and associated with a chronic state of peripheral immune activation. However, it is clear that the loss of peripheral T cells is unlikely to be caused solely by direct cytopathic effects of the virus and the elimination of uninfected cells. It has been suggested recently that loss of CD4 T cells occurs first at the mucosal level with a massive depletion of memory-phenotype CD4 T cells in the gut and higher microbial translocation caused by disruption of gut mucosal defenses (30–32). Chronic immune activation has been so far attributed to direct effects of HIV on mucosal T cell subsets. However, chronic HIV infection also causes impairment of thymic functions (33–36). As our data show, continued replenishment with cells from the thymus seems to be required to maintain efficient gut mucosal defense.
The targeting strategy of the HIV virus, which ensures depletion of cycling CD4 T cells both at the mucosal and thymic level, may be doubly effective to reinforce its pathogenicity and exacerbate CD4 T cell loss at the mucosal level, because compromised thymic function may participate in the chronic immune activation observed in HIV. Moreover, this chronic activation will increase the percentage of target cells susceptible to viral integration. This is compatible with mathematical models that concluded that HIV infection per se is not sufficient to fully explain the rate of T cell depletion during chronic HIV infections (37), nor the tropism for CD4 T cells during depletion (38).
In conclusion, our study demonstrated that production of new naïve T cells by a functional thymus not only ensures the maintenance of a diverse peripheral repertoire, but also provides essential protection to chronic immune activation by constant replenishment of naïve cells. The mechanisms underlying protection of mucosal integrity and nature of the cells, which are involved in this process, need to be further investigated. The increased sensitivity of naïve CD4 T cells to activation caused by increased microbial translocation provides a rational explanation for alteration of CD4/CD8 ratios in aged mice and humans and may furthermore provide clues for events that are instrumental in initiating the development of gut pathology in HIV infection or gut disorders such as inflammatory bowel disease or Crohn's disease.
Materials and Methods
Mice.
Briefly, mice expressing two floxed Rag2 allele (Rag2fl/fl) (13, 39). were bred with Rag2-deficient mice expressing the Mx Cre transgene, which is inducible by type I IFN or any IFN inducers, such as poly(I:C). Mx Cre± Rag 2−/− (H-2b) mice and Rag2 fl/fl (H-2b) mice were crossed to obtain either Mx Cre± Rag2 fl/− mice or Mx Cre−/− Rag2 fl/− control littermates. C57BL/6 mice expressing CD45.1 or CD45.2 and B6 Rag2-deficient mice were used as donors and hosts, respectively. All mice were kept under specific pathogen-free conditions following institutional guidelines and Home Office regulations.
Induced Deletion of Rag2.
To induce Cre expression and subsequent Rag2 deletion, 6- to 8-week-old mice were injected i.p. three times on days 0, 3, and 6 with 400 μg of poly(I:C) (Sigma-Aldrich) or with three doses of 1 × 106 units of type I IFN given at 2-day intervals from day 3 after birth (13).
Bone Marrow Transfer.
For thymus reconstitution assays, single cell suspensions were prepared from bone marrow of MxCre+ mice treated 4 weeks before with poly(I:C) and from control untreated MxCre+ donors, followed by B and T cell depletion by magnetic beads (Miltenyi Biotech). Five million bone marrow cells were injected i.v. into 5 Gy irradiated Rag2-deficient CD45.1 hosts. Thymus repopulation was assessed 6 weeks after reconstitution.
Cell Suspension, Flow Cytometry, and Cellularity Determinations.
Lymph node and spleen cell suspensions were prepared in Iscove's modified Dulbecco medium (IMDM; Sigma). Cells were stained following usual procedures using allophycocyanin (APC) and phycoerythrin (PE)-CD4 (GK1.5); APC and FITC-TCR (H57–597); PE-CD44; biotinylated and PE Mel-14 and CD69; APC, PE and FITC-CD8 (53.6.7); biotinylated and PE-Thy1.2; PE- Thy1.1, APC-IL7Ra and APC-IgG2a isotype control. Streptavidin-peridinin chlorophyll protein (PerCP) was used to develop biotinylated antibodies. Antibodies were purchased from Pharmingen or eBioscience. Flow cytometry was performed with a FACScalibur cytometer (Becton Dickinson) and data files were analyzed with FlowJo software (Tree Star).
Cell sorting was performed on a MoFLO cell sorter (Cytomation) after negative selection by using biotinylated antibodies against CD11c, CD19, Ter119, and GR1 (eBioscience) and streptavidin dynabeads (Dynal). Naïve T cells cell were sorted as CD44low and CD25− CD4 or CD8 cells. The total number of T cells recovered from the peripheral pools was considered equal to that from spleen and pooled lymph nodes.
CFSE Labeling.
Cell division on transferred T cells was assessed by CFSE labeling (Molecular Probes) using standard methods. Cells were resuspended in PBS in a concentration of 107/ml and incubated with CFSE at final concentration of 2.5 μM for 10 min at 37°c, followed by two washes in IMDM containing 10% FCS. Labeled cells were i.v.-injected into syngeneic recipients.
LBP Assay.
LBP levels in serum were determined by ELISA (HyCult Biotechnology).
Statistical Analysis.
P values were obtained by using Mann–Whitney's two-tailed T test.
Supplementary Material
Acknowledgments.
We thank Aaron Rae and Graham Preece for cell sorting and Hannah Boyes and Trisha Norton for expert animal care. This work was supported by the Medical Research Council U.K. and grants from the Deutsche Forschungsgemeinschaft and National Institutes of Health (to K.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803732105/DCSupplemental.
References
- 1.Tanchot C, Rocha B. The peripheral T cell repertoire: Independent homeostatic regulation of virgin and activated CD8+ T cell pools. Eur J Immunol. 1995;25:2127–2136. doi: 10.1002/eji.1830250802. [DOI] [PubMed] [Google Scholar]
- 2.Jameson SC. Maintaining the norm: T cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 3.Mackall CL, Gress RE. Thymic aging and T cell regeneration. Immunol Rev. 1997;160:91–102. doi: 10.1111/j.1600-065x.1997.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 4.Ge Q, Hu H, Eisen HN, Chen J. Different contributions of thymopoiesis and homeostasis-driven proliferation to the reconstitution of naive and memory T cell compartments. Proc Natl Acad Sci USA. 2002;99:2989–2994. doi: 10.1073/pnas.052714099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berzins SP, Boyd RL, Miller JF. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: Substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. Eur J Immunol. 1998;28:1886–1893. doi: 10.1002/(SICI)1521-4141(199806)28:06<1886::AID-IMMU1886>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Miller R. A. The aging immune system: Primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF, Markert ML, Sempowski GD, Patel DD, Hale LP. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu Rev Immunol. 2000;18:529–560. doi: 10.1146/annurev.immunol.18.1.529. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 10.Hazra R, Mackall C. Thymic function in HIV infection. Curr HIV/AIDS Rep. 2005;2:24–28. doi: 10.1007/s11904-996-0005-2. [DOI] [PubMed] [Google Scholar]
- 11.Sempowski GD, et al. Naive T cells are maintained in the periphery during the first 3 months of acute HIV-1 infection: Implications for analysis of thymus function. J Clin Immunol. 2005;25:462–472. doi: 10.1007/s10875-005-5635-4. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeois C, Stockinger B. CD25+CD4+ regulatory T cells and memory T cells prevent lymphopenia-induced proliferation of naive T cells in transient states of lymphopenia. J Immunol. 2006;177:4558–4566. doi: 10.4049/jimmunol.177.7.4558. [DOI] [PubMed] [Google Scholar]
- 13.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 15.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 17.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 18.Schumann RR, et al. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 19.Zweigner J, Schumann RR, Weber JR. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes Infection. 2006;8:946–952. doi: 10.1016/j.micinf.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: Functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 21.Tanchot C, Rocha B. Peripheral selection of T cell repertoires: The role of continuous thymus output. J Exp Med. 1997;186:1099–1106. doi: 10.1084/jem.186.7.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanchot C, et al. Conversion of naive T cells to a memory-like phenotype in lymphopenic hosts is not related to a homeostatic mechanism that fills the peripheral naive T cell pool. J Immunol. 2002;168:5042–5046. doi: 10.4049/jimmunol.168.10.5042. [DOI] [PubMed] [Google Scholar]
- 23.Mackall CL, et al. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 24.Porritt HE, Gordon K, Petrie HT. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J Exp Med. 2003;198:957–962. doi: 10.1084/jem.20030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabor MJ, Godfrey DI, Scollay R. Recent thymic emigrants are distinct from most medullary thymocytes. Eur J Immunol. 1997;27:2010–2015. doi: 10.1002/eji.1830270827. [DOI] [PubMed] [Google Scholar]
- 26.Chakravarti B, Abraham GN. Aging and T cell-mediated immunity. Mech Ageing Dev. 1999;108:183–206. doi: 10.1016/s0047-6374(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 27.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 28.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 29.Khan N, et al. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. J Immunol. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 30.Brenchley JM, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 31.Haynes BF. Gut microbes out of control in HIV infection. Nat Med. 2006;12:1351–1352. doi: 10.1038/nm1206-1351. [DOI] [PubMed] [Google Scholar]
- 32.Veazey RS, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 33.Su L, et al. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 34.Schnittman SM, et al. Evidence for susceptibility of intrathymic T cell precursors and their progeny carrying T cell antigen receptor phenotypes TCRαβ+ and TCRγΔ+ to human immunodeficiency virus infection: A mechanism for CD4+ (T4) lymphocyte depletion. Proc Natl Acad Sci USA. 1990;87:7727–7731. doi: 10.1073/pnas.87.19.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JC, et al. Thymic volume, T cell populations, and parameters of thymopoiesis in adolescent and adult survivors of HIV infection acquired in infancy. AIDS. 2006;20:667–674. doi: 10.1097/01.aids.0000216366.46195.81. [DOI] [PubMed] [Google Scholar]
- 36.Bonyhadi ML, et al. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 37.Yates A, Stark J, Klein N, Antia R, Callard R. Understanding the slow depletion of memory CD4 T cells in HIV infection. PLoS Med. 2007;4:948–955. doi: 10.1371/journal.pmed.0040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro RM, Mohri H, Ho DD, Perelson AS. In vivo dynamics of T cell activation, proliferation, and death in HIV-1 infection: Why are CD4+ but not CD8+ T cells depleted? Proc Natl Acad Sci USA. 2002;99:15572–15577. doi: 10.1073/pnas.242358099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajewsky K, et al. Conditional gene targeting. J Clin Invest. 1996;98:600–603. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.