Abstract
A Phytophthora mating hormone with an array of 1,5-stereogenic centers has been synthesized by using our recently developed methodology of catalytic enantioselective conjugate addition of Grignard reagents. We applied this methodology in a diastereo- and enantioselective iterative route and obtained two of the 16 possible stereoisomers of Phytophthora hormone α1. These synthetic stereoisomers induced the formation of sexual spores (oospores) in A2 mating type strains of three heterothallic Phytophthora species, P. infestans, P. capsici, and P. nicotianae but not in A1 mating type strains. The response was concentration-dependent, and the oospores were viable. These results demonstrate that the biological activity of the synthetic hormone resembles that of the natural hormone α1. Mating hormones are essential components in the sexual life cycle of a variety of organisms. For plant pathogens like Phytophthora, sexual reproduction is important as a source of genetic variation. Moreover, the thick-walled oospores are the most durable propagules that can survive harsh environmental conditions. Sexual reproduction can thus greatly affect disease epidemics. The availability of synthetic compounds mimicking the activity of Phytophthora mating hormone will be instrumental for further unravelling sexual reproduction in this important group of plant pathogens.
Keywords: conjugate addition, oomycyte, oospore, plant pathogen, Grignard reagents
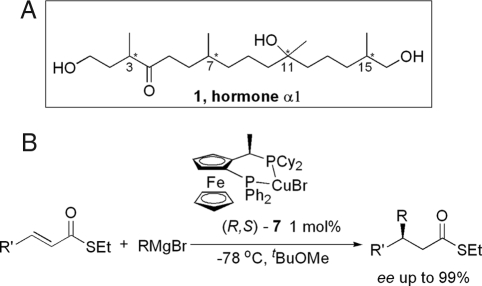
Phytophthora species are devastating plant pathogens that cause substantial yield losses in agricultural crops worldwide and destroy many indigenous plant species in natural vegetation (1). One of the most notorious species is Phytophthora infestans, the causal agent of late blight disease on potato and tomato and responsible for the Irish potato famine in the mid-19th century (2, 3). In the life cycle of Phytophthora, sexual reproduction plays an important role (2, 4). In 2005, Qi et al. (5) reported the purification of a Phytophthora mating hormone and revealed that the structure consists of an array of 1,5-stereogenic centers. Such structures can in principle be accessed by our recently developed powerful methodology of catalytic enantioselective conjugate addition of Grignard reagents (6–14) (Fig. 1). The challenging chemical structure of the mating hormone and its biological importance has prompted us to undertake the total synthesis of the target molecule and to test its biological activity.
Fig. 1.
Structure of mating hormone α1 (1) (A) and enantioselective CA of Grignard reagents to α, β-unsaturated thioesters (B).
Despite its fungal-like growth morphology (mycelium), Phytophthora is not a fungus. It belongs to the oomycetes, a diverse group in the stramenopile lineage that evolved entirely independently from fungi and includes both saprophytes and pathogens of plants, insects, fish, vertebrates, and microbes. Among the plant pathogenic oomycetes are nearly 80 Phytophthora species, a hundred or more Pythium species, and a variety of obligate biotrophs, including downy mildews and white rusts (1, 15). The closest relatives of oomycetes are brown algae and diatoms (16, 17). As most fungi, oomycetes have a vegetative and generative life cycle and propagate via spores. The thick-walled sexual spores, called oospores, are not only important as source of genetic variation; they are also crucial for surviving harsh environmental conditions. Sexual reproduction can either occur in single culture (homothallism) or requires mating of two strains that posses different sexual compatibility types (heterothallism). In heterothallic Phytophthora species, two mating types are known, A1 and A2 (18). Analysis of mating type inheritance in two species, P. infestans and Phytophthora parasitica, showed that A1 is governed by a heterozygous locus carrying one dominant and one recessive allele, whereas A2 is homozygous recessive (19, 20). In P. infestans, positioning of the mating type locus on a genetic linkage map (20, 21) and on a contig of genomic clones (22) showed that the locus is hemizygous and, in several strains, is linked to genetic abnormalities such as balanced lethality and translocations (19, 23, 24). Although the precise determinants of mating type in Phytophthora are still unknown, the mating type locus is thought to regulate either the synthesis of a mating hormone or the response to this hormone.
Qi et al. (5) purified a compound from culture filtrate from Phytophthora nicotianae that can induce oospore formation in a P. nicotianae A2 mating type strain. They determined the structure of the purified compound and designated it mating hormone α1 (MH-α1). The structure of MH-α1 is shown in Fig. 1A (1). Purification of 1.2 mg of MH-α1 required >1,800 liters of culture filtrate suggesting that P. nicotianae produces only minute amounts. The activity spectrum of MH-α1 was not limited to P. nicotianae; in other heterothallic Phytophthora species, MH-α1 also induced oospore formation. This is consistent with the phenomenon that an A1 strain of one Phytophthora species can induce oospores in an A2 strain of another species, which points to conservation of mating hormones throughout the genus.
Although Qi et al. (5, 25, 26) reported the structural characterization of MH-α1, the relative and absolute configurations were not assigned, and the optical rotation was not determined. The four stereocenters in MH-α1 can lead to 16 possible stereoisomers. In the absence of stereochemical information for 1, chemical synthesis requires a route that allows independent control of the configuration of each individual stereogenic center. Recently, we developed a highly enantioselective conjugate addition (CA) of Grignard reagents, in particular MeMgBr, to α,β-unsaturated thioesters catalyzed by a copper bromide complex of Josiphos ligand (Fig. 1B) and applied this methodology in a diastereo- and enantioselective iterative route to deoxypropionate chains (6–14).
The aim of the present study was to demonstrate the versatility of the catalytic asymmetric strategy in the stereoselective total synthesis of two stereoisomers of Phytophthora mating hormone α1 and to demonstrate biological activity of the two stereoisomers. The construction of three [C(3, 7, 15)] of four stereocenters was achieved in a catalytic enantioselective manner, using CA of MeMgBr to the corresponding thioesters. Biological assays showed that two different synthetically obtained stereoisomers can mimic the activity of MH-α1 and induce the formation of oospores. Our findings demonstrate that synthetic Phytophthora mating hormone α1 can be readily obtained and can be exploited as a powerful tool to further investigate sexual reproduction in an important group of plant pathogens.
Results and Discussion
Catalytic Enantioselective Synthesis of Two Stereoisomers of Mating Hormone α1.
Our retrosynthetic analysis for the stereoisomers of mating hormone α1 (1) is illustrated in Fig. 2. Target molecule 1 may be derived from protected triol 2, in which the carbonyl group is masked as a dithiane. Compound 2 may be accessed via the assembly of two fragments 3 and 4 employing a dithiane coupling strategy (27–31). Both 3 and 4 are available from the α,β-unsaturated thioesters 5 and 6 (11–13), each with a different alcohol protecting group to allow selective deprotection in later stages of the synthesis.
Fig. 2.
Retrosynthesis of mating hormone α1.
In principle, any of the 16 putative stereoisomers can be prepared via this synthetic route by judicious selection of the configuration of the catalysts used to introduce each stereogenic center. Because the absolute stereochemistry of MH-α1 is unknown, we arbitrarily chose the absolute configuration of two stereoisomers of hormone α1 to be synthesized.
The synthesis of the dithiane fragment 3 was accomplished in six steps from the α,β-unsaturated thioester 5 (Scheme 1). Compound 5 itself was obtained in three steps from ethylene glycol on a multigram scale. Enantioselective CA of MeMgBr to 5 in the presence of 2 mol% of the chiral catalyst (R,S)-7 in tBuOMe at −78°C provided 8 with 92% enantiomeric excess (ee) and 75% yield. The absolute configuration of the stereogenic center C(3) was assigned via subsequent derivatization to a known compound (13). A two-step reduction of 8 with diisobutyaluminum hydride (DIBALH) to alcohol 9, followed by silyl protection, provided protected alcohol 10. Selective deprotection of the tert-butyldimethylsilyl (TBS) group followed by iodoxybenzoic acid (IBX) oxidation of the corresponding alcohol 11 provided aldehyde 12. Treatment of 12 with propanedithiol at −78°C in the presence of BF3·OEt2 resulted in the desired dithiane 3 (88% yield) [see supporting information (SI) Text, Remark 1].
Scheme 1.
Synthesis of dithiane 3. Reagents: (a) TBSCl, Et3N, and DMAP in CH2Cl2 at room temperature. (b) IBX in EtOAc at 70°C. (c) PPh3 CH-COSEt in CH2Cl2 at 40°C. (d) MeMgBr and 2 mol% (R, S)-7 in tBuOME at −78°C. (e) DIBALH in CH2Cl2 at −40°C. (f) TBDPSCl, Et3N, and DMAP in CH2Cl2 at room temperature. (g) AcOH, THF, and H2O (3/2/2) at 50°C. (h) IBX in EtOAc at 50°C. (i) HS(CH2)3SH and BF3·OEt2 in CH2 at −78°C.
CH-COSEt in CH2Cl2 at 40°C. (d) MeMgBr and 2 mol% (R, S)-7 in tBuOME at −78°C. (e) DIBALH in CH2Cl2 at −40°C. (f) TBDPSCl, Et3N, and DMAP in CH2Cl2 at room temperature. (g) AcOH, THF, and H2O (3/2/2) at 50°C. (h) IBX in EtOAc at 50°C. (i) HS(CH2)3SH and BF3·OEt2 in CH2 at −78°C.
Preparation of fragment 4 began from α,β-unsaturated thioester 6 (13) (Scheme 2). CA of MeMgBr to 6 catalyzed with 1 mol% of (R,S)-7 resulted in thioester 13 (95% yield and 98% ee). The absolute configuration of the stereogenic center C(15) was assigned in a similar manner as indicated for compound 8. DIBALH reduction of 12 to aldehyde 14, followed by a Wittig reaction provided ketone 15. Hydrogenation of 15 with Pd/C resulted in the saturated ketone 16 (99% yield).
Scheme 2.
Synthesis of iodide 4. Reagents: (a) MeMgBr and 1 mol% (R,S)-7 in tBuOMe at −78°C. (b) DIBALH in CH2Cl2 at −40°C. (c) PPh3 CH-COCH3 in THF at 66°C. (d) H2 and Pd/C in EtOAc at room temperature. (e) CH2
CH-COCH3 in THF at 66°C. (d) H2 and Pd/C in EtOAc at room temperature. (e) CH2 CH-CH
CH-CH CH
CH COTMS(OEt), TBAT, 10 mol% Cu(OTf)2, and 10 mol% (R)-Tol-BINAP in THF at room temperature. (f) H2, Pd/C in EtOAc at room temperature. (g) DIBALH in hexane at −78°C. (h) PPh3
COTMS(OEt), TBAT, 10 mol% Cu(OTf)2, and 10 mol% (R)-Tol-BINAP in THF at room temperature. (f) H2, Pd/C in EtOAc at room temperature. (g) DIBALH in hexane at −78°C. (h) PPh3 CH-COSEt in CH2Cl2 at 40°C. (i) TESOTf and 2,6-lutidine in CH2Cl2 at 0°C to room temperature. (j) MeMgBr and 2 mol% (S,R)-7 in BuOMe at −78°C. (k) DIBALH in CH2Cl2 at −40°C. (l) PPh3, I2, and imidazole in CH2Cl2 at room temperature.
CH-COSEt in CH2Cl2 at 40°C. (i) TESOTf and 2,6-lutidine in CH2Cl2 at 0°C to room temperature. (j) MeMgBr and 2 mol% (S,R)-7 in BuOMe at −78°C. (k) DIBALH in CH2Cl2 at −40°C. (l) PPh3, I2, and imidazole in CH2Cl2 at room temperature.
We anticipated that the tertiary alcohol could be prepared through a catalytic asymmetric vinylogous Mukaiyama aldol condensation of the aliphatic ketone catalyzed by a Cu-complex of Tol-Binap (32). With (R)-Tol-Binap, lactone 17 was obtained from ketone 16 in 81% yield and 92:8 dr. (R)-Tol-Binap leads to the (R)-aldol product in accordance with the proposed stereocontrol by the (R)-Tol-Binap based catalyst (32). We tentatively assigned the (R)-configuration to the newly formed stereogenic center C(11). After hydrogenation of the alkene moiety in 17, lactone 18 was reduced with DIBALH to the corresponding lactol. A Wittig reaction on this lactol yielded the α,β-unsaturated thioester 19 (59% yield). After protection of the tertiary alcohol with a triethylsilyl group, the third stereogenic center C(7) was formed through CA of MeMgBr to 20 with 2 mol% of the chiral catalyst (S,R)-7 in tBuOMe at −78°C with de >90%. In analogy with the results of catalytic CA addition catalyzed by (S,R)-7, we assigned the (R)-configuration to the newly formed center C(7). The thioester 21 was converted efficiently in one step to the corresponding alcohol 22 and subsequently to iodide 4.
With fragments 3 and 4 in hand, the assembly of 2 was undertaken, using a dithiane coupling strategy (Scheme 3) (30). With an equimolar ratio of 3 and 4, lithiated 3 [tBuLi, hexamethylphosphoramide (HMPA)/tetrahydrofuran (THF)] reacted with iodide 4; however, the product 2 was obtained in only 10% yield together with starting materials and traces of side products. To improve the yield of the reaction, the ratio of 3 and 4 was raised to 3:1. The reaction proceeded with 72% yield with nearly complete recovery of excess of dithiane 3. Unmasking of the ketone, using MeI in the presence of CaCO3 (30), furnished the silyl protected triol 23 (78% yield). The final step, conversion to the target molecule 1, proceeded cleanly upon exposure to TBAF, which removed all three silyl protecting groups (92% yield). Another diastereomer 1′, differing from 1 in the configuration at C(7), was prepared in a similar manner (SI Appendix), employing (R,S)-enantiomer of catalyst 7 in the CA of MeMgBr to 20.
Scheme 3.
Synthesis of and stereoisomers. Reagents: (a) tBuLi in 10% HMPA in THF at −78°C. (b) Mel and CaCO3 in MeCN, H2O, and THF (4/1/1) at 50°C. (c) TBAF in THF at room temperature.
The spectroscopic data of the synthetic stereoisomers 1 and 1′ of the hormone α1 (high-resolution mass spectrometry, 1H, 13C NMR, and IR spectroscopy) were identical to those reported for the natural product (33) (see SI Text, Remark 2). Similar to the observations reported by Qi et al. (5), we found traces of an additional compound in the 1H NMR spectrum of 1. We anticipated that the presence of an identical additional component in both, MH-α1 obtained from natural sources and 1 resulting from synthesis, indicates that the two additional minor components are the result of a ketone/hemiacetal equilibrium. This is supported by the presence of the hemiacetal in the structural analog 24 that was independently synthesized (see SI Appendix).
Synthetic Stereoisomers Change the Colony Morphology of A2 Strains.
Biological activity of the synthetic stereoisomers 1 and 1′ of hormone α1 was tested by supplying mycelium cultures of A1 and A2 mating type strains of P. infestans, P. capsici, and P. nicotianae with increasing amounts of 1 and 1′. The amounts tested ranged from 12.5 to 16,000 ng and were applied to each of the two wells adjacent to the colony (Fig. S1). Biological activity was monitored by macroscopic and microscopic examination of the mycelial cultures.
Five days after addition of the synthetic stereoisomers, we observed a clear change in the shape of the colony of the P. infestans A2 strains NL88133 and CN505502B but not of A1 strain NL80029 (Fig. 3 A and B and Fig. S2). The A1 colony continued to grow in a circular shape, whereas, in the presence of synthetic stereoisomers 1 or 1′ (≥ 400 ng), the A2 colony became oval. At higher amounts, the shape of the colony changed even more drastically. Quantification showed that the response was already significant at 200 ng and that stereoisomer 1 was more potent than 1′ (Fig. 3C). P. capsici showed a similar response; the colony shape of the A2 strain LT3241 changed at amounts ≥800 ng, but the A1 strain LT3112 did not show a response, not even at amounts as high as 16,000 ng. In P. nicotianae, there was no difference in colony shape between A1 and A2 strains. The colonies kept their circular shape even in the presence of 16,000 ng of synthetic stereoisomers. The change in colony shape in P. infestans and P. capsici A2 strains could be due to an increase in growth rate or hyphal biomass induced by the synthetic hormone or, alternatively, to a redirection of hyphal tip growth toward the synthetic hormone. Microscopic analysis of mycelium did not show an obvious increase in hyphal biomass but the shape of the hyphae appeared aberrant. In the presence of the synthetic stereoisomers, numerous bulb shaped protrusions were observed that could resemble the initiation of gametangia (Fig. 3 D and E and Fig. S3)
Fig. 3.
Growth deformation in Phytophthora infestans induced by synthetic hormone. (A and B) Mycelium grown for 14 days on clarified V8 medium in the absence (0) or presence (800, 1,600) of stereoisomer 1. Seven days after transfer of a mycelial plug to fresh medium, a 10-μl solution of stereoisomer 1 was added to wells placed on both sides of the mycelial plug along the “a” line. (A) A2 strain NL88133. (B) A1 strain NL80029. For further details on the bioassay, see Materials and Methods. (C) Graphical display of change in colony shape induced by the two stereoisomers, 1 and 1′. The y axis shows the ratio a/b in which “a” and “b” are the diameters of the colony measured along the lines marked by “a” and “b” in A, respectively. The graph shows the result of one experiment. The experiment was repeated three times with the same outcome. (D and E) Morphology of hyphae visualized by light microscopy. Strain NL88133 was grown in the absence (D) or presence (E) of stereoisomer 1 (1,600 ng). The arrow points to one of the many bulbed protrusions observed on the hyphae in the presence of the synthetic hormone.
Synthetic Stereoisomers Induce Oospore Formation in A2 Strains.
Seven days after addition of synthetic stereoisomers, we observed the formation of oospores in A2 strains of all three Phytophthora species even in the P. nicotianae A2 strain that did not show the change in colony shape. The oospores were most abundant at the borders of wells where the synthetic hormone was applied. This was probably the site where the hormone accumulated and the local concentration was the highest (Fig. 4A). For P. infestans and P. capsici, amounts of 800 ng were sufficient, whereas, for P. nicotianae, a 10-fold higher amount was needed to see a response after 7 days (Table 1). At higher amounts, the number of oospores increased, demonstrating that the response is concentration-dependent. Also, the numbers increased over time. After 28 days, the P. nicotianae A2 strain confronted with 2,000 ng of synthetic hormone contained oospores that were not yet observed after 7 days. In a normal mating between an A1 and A2 strain oospore, numbers also increase over time. No oospores were found in any A1 strain.
Fig. 4.
Oospore formation in Phytophthora species induced by synthetic hormone. (A) Mycelium of P. capsici strain LT3241 grown in the presence of 1,600 ng of synthetic hormone. The dark area is the border of the well to which stereoisomer 1 was added. Numerous oospores are formed at the border and right of the border, one of which is marked by a white arrow. The black arrow points to a chlamydospore. Chlamydospores are asexual spores that are also found in P. capsici cultures of single strains so independent of the presence of the opposite mating type or synthetic hormone. At higher magnification, oospores and chlamydospores are clearly distinguishable. (B) Oospore obtained by cocultivation of two P. capsici strains of opposite mating type, LT3112 (A1) and LT3241 (A2). (C–F) Oospores induced by synthetic hormone. (C and D) Oospore of P. capsici strain LT3241. In C, the white arrows point to germination tubes emerging from the oospore. (E and F) Oospore of P. nicotianae strain P582 (E) and P. infestans strain CN505502B (F). (Scale bars: A, 100 μm; B–F, 10 μm.)
Table 1.
Oospore formation in Phytophthora A2 mating type strains induced by synthetic hormone is concentration-dependent
| Phytophthora species | A2 strain | Days* | Dilution series of synthetic hormone† |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 200 | 400 | 800 | 1,600 | 2,000 | 4,000 | 8,000 | 16,000 | |||
| P. infestans | CN505502B | 7 | − | − | − | ++ | ++ | ++ | ++ | nd | nd |
| 28 | − | − | − | ++ | +++ | +++ | +++ | nd | nd | ||
| P. capsici | LT3241 | 7 | − | − | − | + | + | nd | + | ++ | +++ |
| 28 | − | − | − | + | ++ | nd | ++++ | ++++ | ++++ | ||
| P. nicotianae | P582 | 7 | − | − | − | − | − | − | − | + | ++ |
| 28 | − | − | − | − | − | + | ++ | ++ | +++ | ||
*Number of days after addition of synthetic hormone.
†The amount shown is in nanograms per 10 μ l of solution. The approximate number of oospores per 2 cm2: −, none; +, 1–10; ++, 10–50; +++, 100–200; ++++, >500; nd, not determined. The oospore inducing activity of stereoisomer 1 and 1′ was in the same range, but 1 seemed to be slightly more active.
Microscopic analyses revealed that the development of gametangia and oospores induced by the synthetic hormone is similar to that resulting from a normal mating between an A1 and A2 strain. As shown in Fig. 4B, P. capsici has an amphigynous antheridium. A thick oospore wall can be distinguished, which is surrounded by the oogonial wall. P. capsici oospores induced by synthetic hormone look similar (Fig. 4D). The antheridium surrounding the oogonial stalk and the thick-walled oospore are clearly visible (Fig. S4). Also, in P. infestans and P. nicotianae, oospores induced by the synthetic hormone have all of the morphological features of typical oospores (Fig. 4 E and F).
To determine the viability of oospores, we used a vital staining based on tetrazolium bromide (MTT). MTT staining of oospores induced by the synthetic hormone in all three tested species resulted in many pink and red colored oospores indicating that the majority was viable (Fig. S5). Quantification showed that 93% of the oospores obtained from a cross between P. capsici A1 strain LT3112 and A2 strain LT3241 stained pink or red and are thus viable. In oospores induced by synthetic hormone, this percentage is slightly lower (78%). In the case of P. nicotianae, the percentages of viable oospores obtained by a normal cross and by induction with synthetic hormone were comparable (both ≈60%).
The P. capsici oospores induced by synthetic hormone readily germinated and produced one or more germination tubes (Fig. 4C and Fig. S6). Multiple germ tubes are common for P. capsici oospores and are produced by more than half of the oospores resulting from a mating between A1 and A2 P. capsici strains (34). P. capsici is a species that easily gives rise to sexual progeny and has the potential to become an oomycete model species for genetic analysis (35). P. infestans oospores are difficult to germinate under in vitro conditions and the percentage of oospores that gives rise to viable progeny is relatively low (1, 35).
Taken together, we conclude that the synthetic stereoisomers 1 and 1′ both have biological activity. Only A2 mating type strains and not A1 strains respond to the presence of the synthetic stereoisomers and in that respect 1 and 1′ have the same activity spectrum as the natural MH-α1 (5). With respect to sensitivity of the different species, however, the synthetic stereoisomers differ from MH-α1. Qi et al. (5) reported that P. nicotianae, the species from which they purified MH-α1, was the most sensitive of the four tested species, and, in contrast to our observations, they found that P. infestans produced far fewer oospores than P. nicotianae. Theoretically, this difference could be due to the origin of the strains, which differed from the ones we used. Many Phytophthora species are notorious for their phenotypic variability, and this depends highly on culture and storage conditions. The two P. infestans A2 strains tested by us showed a similar change in growth behavior but one of the two produced less oospores in response to the same amounts of synthetic hormone. Still, in our hands, even the least sensitive of the two tested P. infestans A2 strains was more sensitive than P. nicotianae. An alternative explanation is a slight difference in the structure of the synthetic hormone and the natural MH-α1 that cannot be detected by the standard spectroscopic analyses but that influences the biological activity in a species-specific manner. The apparent conservation of mating hormones throughout the Phytophthora genus does not exclude the existence of species-specific variants of these hormones. In this respect, it is worth testing the biological activity of synthetic versions of the 14 remaining stereoisomers of hormone α1.
Conclusions
Total synthesis of two stereoisomers of Phytophthora mating hormone α1 has been completed in a catalytic enantioselective manner with a longest linear sequence of 15 steps with an overall 8.1% yield. Key steps in the synthesis include the CA of MeMgBr to create three of the stereochemical centers, Mukaiyama aldol condensation to generate the chiral tertiary alcohol, and a dithiane coupling. Via this synthetic route, the 16 putative stereoisomers of the hormone can be accessed. The relative and absolute configuration of the natural MH-α1 is currently not known, but, here, we show that at least two stereoisomers obtained by chemical synthesis are biologically active. The oospore inducing activity resembled that of the natural MH-α1 purified by Qi et al. (5), except that the sensitivity of the tested species was slightly different. The synthetic stereoisomers showed a higher activity on P. infestans than on P. nicotianea, whereas MH-α1 was most active on the species from which it was purified, i.e., P. nicotianae (5). Recently, Yajima et al. (33) (see SI Text, Remark 2) reported biological activity of synthetic hormone, but, because their biological assays are very limited, it is not possible to compare it with our assays. They use an undefined racemic mixture of various stereoisomers and only one Phytophthora species without mentioning the strain. Neither Qi et al. (5) nor Yajima et al. (33) noticed the change in colony shape induced by the synthetic hormone, nor did they analyze the viability of the oospores. Our biological assays are thorough and more comprehensive. We give a very precise description of the methodology and how concentrations are defined, tested different strains of three species with strain identities, and included control A1 strains in every experiment. Our results show that (i) the growth behavior of A2 mating type strains changes as a response to increasing amounts of synthetic hormone, (ii) oospores are formed in a concentration-dependent manner, (iii) oospores are viable and can germinate, and (iv) one stereoisomer is more potent than the other.
Some plant disease epidemics are greatly influenced by the ability of a pathogen to reproduce sexually (4). The basic principles of sexual reproduction in Phytophthora are known, and the essential role of volatile mating hormones in the sexual life cycle has long been recognized (1, 18). MH-α1 is the first and, so far, the only mating hormone identified in Phytophthora (5). The structure of a mating hormone produced by A2 mating type strains and the type of receptors involved in perceiving the mating hormones are still unknown. In P. infestans, the mating type locus has been mapped (20, 21), and it is anticipated that comparative genome analysis and the forthcoming annotation of the P. infestans genome will help in identifying the genes that determine the A1 and A2 mating type (16, 35).
Synthetic compounds mimicking the biological activity of a natural Phytophthora mating hormone that, like MH-α1, are produced in only minute quantities will be instrumental for functional studies aimed at unraveling sexual reproduction in these devastating oomycete pathogens. As described here, the methodology to produce unlimited amounts of the desired stereoisomers in a controlled manner is now available. Being able to add defined concentrations of a pure compound and at fixed time points is ideal for experiments aimed at monitoring sexual development and responses to hormones at the transcriptome or metabolome level not only in in vitro cultures but also during growth of the Phytophthora pathogens on plants.
Materials and Methods
Chemical Synthesis of Two Diastereomers of Phytophthora Mating Hormone α1.
Details for the synthesis of two stereoisomers 1 and 1′ are provided in SI Appendix.
Phytophthora Strains.
The activity of the stereoisomers 1 and 1′ was tested on three Phytophthora species. The strains used were P. infestans NL80029 (A1 mating type), NL88133 (A2) and CN505502B (A2), P. capsici LT3112 (A1), LT3241 (A2) and LT3145 (A2), and P. nicotianae P0270797 (A1) and P582 (A2). P. capsici strains and P. nicotianae P0270797 were kindly provided by K. Lamour (University of Tennessee) and the Netherlands Plant Protection Service in Wageningen, respectively. Strains were cultured on rye sucrose agar or V8 agar according to standard procedures (1).
Bioassays.
Stock solutions of synthetic mating hormone were prepared by dissolving the synthesized stereoisomers 1 and 1′ in ethyl acetate (EA) to a final concentration of 160 μg/ml. Dilutions were made in EA and ranged from 1,600 ng/μl to 1.25 ng/μl. Stock solutions and dilutions were stored at −20°C and kept on ice during handling.
The experimental setup of the bioassay is shown in Fig. S1. We prepared Petri dishes [diameter (Ø) = 9 cm] containing 10% clarified-V8 agar with two wells in the agar. A mycelium plug (Ø = 7 mm) was cut from a fresh Phytophthora culture and placed exactly in the middle of the Petri dish. The culture was then incubated at 20°C in the dark. After 3 (P. capsici and P. nicotianae) or 7 days (P. infestans), 10 μl of the diluted solution of stereoisomer 1 or 1′ was added to each of the two wells. Before continuing the incubation at 20°C, the Petri dishes were placed for a few minutes with an open lid in a sterile hood to allow the EA to evaporate. The following days, growth was monitored by measuring the diameters of the colony along the lines marked by “a” and “b” (Fig. S1). After 7 and 28 days, the total number of oospores that were produced within a 2-cm2 area around the well was counted under an inverted microscope (Zeiss; Axiovert 100). Because the solvent evaporates and the synthetic hormone α1 probably diffuses in the agar, we present the data in relation to the absolute amounts of the stereoisomers 1 and 1′ in nanograms instead of a concentration in nanograms per microliter.
Assessment of Oospore Viability.
To assess the viability of oospores, we used 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) staining (Sigma; catalog no. M5655) (1, 36). One volume of 0.1% MTT solution [in 0.1 M phosphate buffer (pH 5.8)] was mixed with an equal volume of oospore suspension. After incubation for 2 days at 36–37°C, staining of the oospores was examined. Pink and red colored oospores were considered to be viable, and unstained or black oospores to be nonviable. As controls for the viability staining we collected oospores from a normal cross of which the majority is viable. Part of this oospore suspension was autoclaved (30 min, 120°C) to kill the oospores for the nonviable control staining.
Supplementary Material
Acknowledgments.
We thank Dr. H.-U. Blaser (Solvias, Basel, Switzerland) for a generous gift of Josiphos ligands and T. D. Tiemersma-Wegman for GC and HPLC support. This research was supported by Chinese State Administration of Foreign Experts Affairs Grant CG2005530006 (to Z.Z.), Yunnan Natural Science Foundation Grant 2004C0024Q (to Z.Z.), an LNV427 grant (“Parapluplan Phytophthora”), from the Dutch Ministry of Agriculture, Nature and Food Quality (to F.G. and K.B.), and a Spinoza grant from the Netherlands Organization for Scientific Research (to B.L.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709289105/DCSupplemental.
References
- 1.Erwin DC, Ribeiro OK. Phytophthora Diseases Worldwide. St. Paul, MN: American Phytopathological Society; 1996. [Google Scholar]
- 2.Fry WE, Goodwin SB. Resurgence of the Irish potato famine fungus. Bioscience. 1997;47:363–371. [Google Scholar]
- 3.Govers F, Latijnhouwers M. In: Encyclopedia of Plant and Crop Science. Goodman RM, editor. New York: Marcel Dekker; 2004. pp. 1–5. [DOI] [Google Scholar]
- 4.McDonald BA, Linde C. Pathogen population genetics, evolutionary potential, and durable resistance. Annu Rev Phytopathol. 2002;40:349–379. doi: 10.1146/annurev.phyto.40.120501.101443. [DOI] [PubMed] [Google Scholar]
- 5.Qi J, et al. Characterization of a Phytophthora mating hormone. Science. 2005;309:1828. doi: 10.1126/science.1114756. [DOI] [PubMed] [Google Scholar]
- 6.López F, Minnaard AJ, Feringa BL. Catalytic enantioselective conjugate addition with Grignard reagents. Acc Chem Res. 2007;40:179–188. doi: 10.1021/ar0501976. [DOI] [PubMed] [Google Scholar]
- 7.Harutyunyan SR, et al. On the mechanism of the copper-catalyzed enantioselective 1,4-addition of Grignard reagents to α,β-unsaturated carbonyl compounds. J Am Chem Soc. 2006;128:9103–9118. doi: 10.1021/ja0585634. [DOI] [PubMed] [Google Scholar]
- 8.Feringa BL, Badorrey R, Peña D, Harutyunyan SR, Minnaard AJ. Copper-catalyzed asymmetric conjugate addition of Grignard reagents to cyclic enones. Proc Natl Acad Sci USA. 2004;101:5834–5838. doi: 10.1073/pnas.0308008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López F, Harutyunyan SR, Minnaard AJ, Feringa BL. Copper-catalyzed enantioselective conjugate addition of Grignard reagents to acyclic enones. J Am Chem Soc. 2004;126:12784–12785. doi: 10.1021/ja046632t. [DOI] [PubMed] [Google Scholar]
- 10.López F, Harutyunyan SR, Meetsma A, Minnaard AJ, Feringa BL. Copper-catalyzed enantioselective conjugate addition of Grignard reagents to α,β-unsaturated esters. Angew Chem Int Ed. 2005;44:2752–2756. doi: 10.1002/anie.200500317. [DOI] [PubMed] [Google Scholar]
- 11.Des Mazery R, et al. An iterative catalytic route to enantiopure deoxypropionate subunits: Asymmetric conjugate addition of Grignard reagents to α,β-unsaturated thioesters. J Am Chem Soc. 2005;127:9966–9967. doi: 10.1021/ja053020f. [DOI] [PubMed] [Google Scholar]
- 12.van Summeren RP, Moody DB, Feringa BL, Minnaard AJ. Total synthesis of enantiopure β-d-mannosyl phosphomycoketides from Mycobacterium tuberculosis. J Am Chem Soc. 2006;128:4546–4547. doi: 10.1021/ja060499i. [DOI] [PubMed] [Google Scholar]
- 13.ter Horst BL, Feringa BL, Minnaard AJ. Catalytic asymmetric synthesis of mycocerosic acid. Chem Commun. 2007;5:489–491. doi: 10.1039/b612593j. [DOI] [PubMed] [Google Scholar]
- 14.Howell GP, Fletcher SP, Geurts K, ter Horst B, Feringa BL. Catalytic asymmetric synthesis of acyclic arrays by tandem 1,4-addition-aldol reactions. J Am Chem Soc. 2006;128:14977–14985. doi: 10.1021/ja0651862. [DOI] [PubMed] [Google Scholar]
- 15.Agrios GN. Plant Pathology. New York: Academic; 2005. [Google Scholar]
- 16.Govers F, Gijzen M. Phytophthora genomics: The plant destroyers' genome decoded. Mol Plant-Microbe Interact. 2006;19:1295–1301. doi: 10.1094/MPMI-19-1295. [DOI] [PubMed] [Google Scholar]
- 17.Keeling PJ, et al. The tree of eukaryotes. Trends in Ecology & Evolution. 2005;20:670–676. doi: 10.1016/j.tree.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Judelson HS. In: Sex in Fungi: Molecular Determination and Evolutionary Implications. Heitman J, Kronstad J, Taylor L, Casselton L, editors. Washington, DC: ASM; 2007. pp. 445–458. [Google Scholar]
- 19.Fabritius AL, Judelson HS. Mating type loci segregate aberrantly in Phytophthora infestans but normally in Phytophthora parasitica: Implications for models of mating type determination. Curr Genet. 1997;32:60–65. doi: 10.1007/s002940050248. [DOI] [PubMed] [Google Scholar]
- 20.Judelson HS, Spielman LJ, Shattock RC. Genetic mapping and non-mendelian segregation of mating type loci in the oomycete. Genetics. 1995;141:503–512. doi: 10.1093/genetics/141.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Lee T, Testa A, Robold A, van 't Klooster J, Govers F. High-density genetic linkage maps of Phytophthora infestans reveal trisomic progeny and chromosomal rearrangements. Genetics. 2004;167:1643–1661. doi: 10.1534/genetics.104.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randall TA, Ah Fong A, Judelson HS. Chromosomal heteromorphism and an apparent translocation detected using a BAC contig spanning the mating type locus of Phytophthora infestans. Fungal Genetics and Biology. 2003;38:75–84. doi: 10.1016/s1087-1845(02)00512-1. [DOI] [PubMed] [Google Scholar]
- 23.Judelson HS. Chromosomal heteromorphism linked to the mating type locus of the oomycete Phytophthora infestans. Molecular and General Genetics. 1996;252:155–161. doi: 10.1007/BF02173215. [DOI] [PubMed] [Google Scholar]
- 24.Judelson HS. Genetic and physical variability at the mating type locus of the oomycete, Phytophthora infestans. Genetics. 1996;144:1005–1013. doi: 10.1093/genetics/144.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajpai R, Yang F, Curran DP. On the structure of the Phytophthora α1 mating hormone: Synthesis and comparison of four candidate stereoisomers. Tetrahedron Lett. 2007;48:7965–7968. doi: 10.1016/j.tetlet.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ojika M, Qi J, Kito Y, Sakagami Y. Stereochemical analysis of α1, a mating hormone of the phytopathogen Phytophthora. Tetrahedron: Asymmetry. 2007;18:1763–1765. [Google Scholar]
- 27.Corey EJ, Seebach D. Carbanions of 1,3-dithianes. Reagents for C–C bond formation by nucleophilic displacement and carbonyl addition. Angew Chem, Int Ed Engl. 1965;4:1075–1077. [Google Scholar]
- 28.Seebach D. Methods of reactivity umpolung. Angew Chem Int Ed Engl. 1979;18:239–258. [Google Scholar]
- 29.Smith AB, III, Condon SM, McCauley JA. Total synthesis of immunosuppressants: Unified strategies exploiting dithiane couplings and σ-bond olefin constructions. Acc Chem Res. 1998;31:35–46. [Google Scholar]
- 30.Smith AB, III, et al. A unified total synthesis of the immunomodulators (−)-rapamycin and (−)-27-demethoxyrapamycin: Construction of the C(21–42) perimeters. J Am Chem Soc. 1997;119:947–962. [Google Scholar]
- 31.Smith AB, III, Lodise SA. Synthesis of tedanolide and 13-deoxytedanolide. Assembly of a common C(1)-C(11) subtarget. Org Lett. 1999;1:1249–1252. doi: 10.1021/ol9909233. [DOI] [PubMed] [Google Scholar]
- 32.Moreau X, Bazan-Tejeda B, Campagne J-M. Catalytic and asymmetric vinylogous Mukaiyama reactions on aliphatic ketones: Formal asymmetric synthesis of taurospongin A. J Am Chem Soc. 2005;127:7288–7290. doi: 10.1021/ja051573k. [DOI] [PubMed] [Google Scholar]
- 33.Yajima A, et al. Synthesis and biological activity of a stereoisomeric mixture of the mating hormone of Phytophthora. Tetrahedron lett. 2007;48:4601–4603. [Google Scholar]
- 34.Timmer LW, Castro J, Erwin DC, Belser WL, Zentmyer GA. Genetic evidence for zygotic meiosis in Phytophthora capsici. Amer J Bot. 1970;57:1211–1218. [Google Scholar]
- 35.Lamour KH, Win J, Kamoun S. Oomycete genomics: New insights and future directions. FEMS Microbiol Lett. 2007;274:1–8. doi: 10.1111/j.1574-6968.2007.00786.x. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland ED, Cohen SD. Evaluation of tetrazolium bromide as a vital stain for fungal oospores. Phytopathology. 1983;73:1532–1535. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.