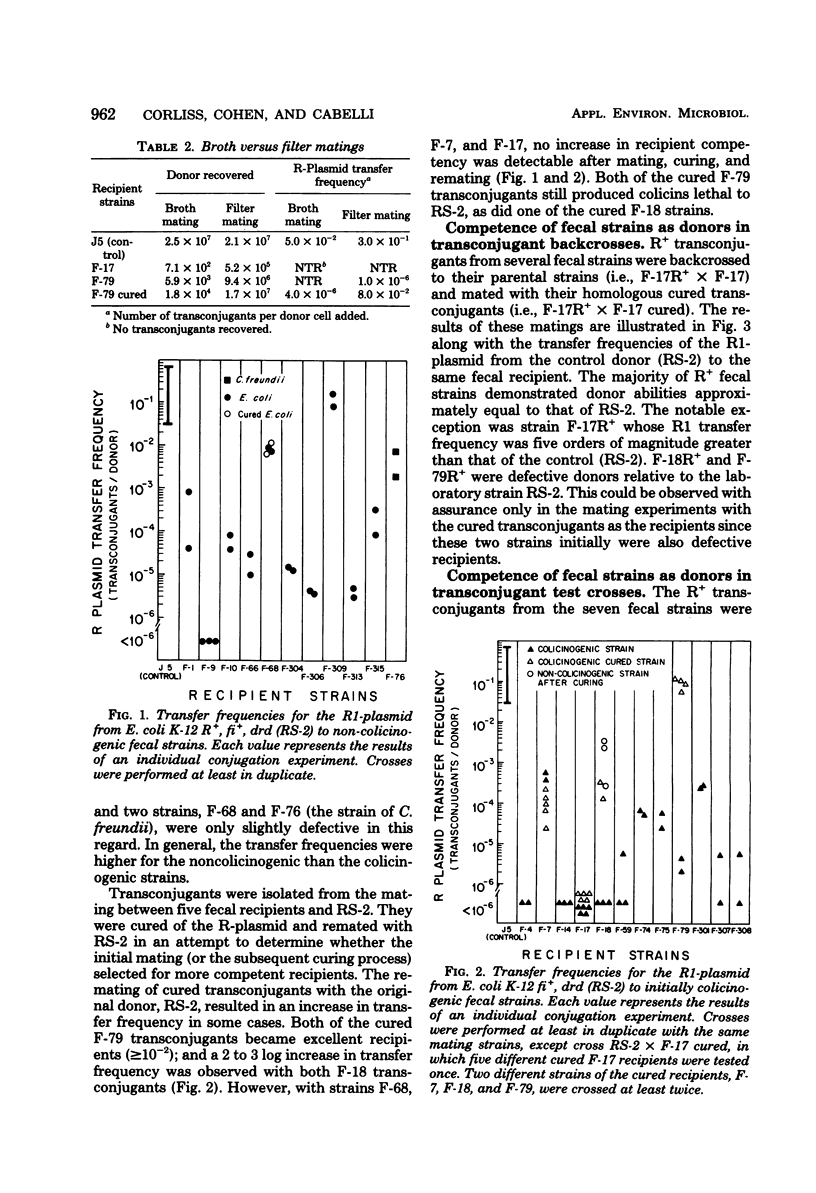
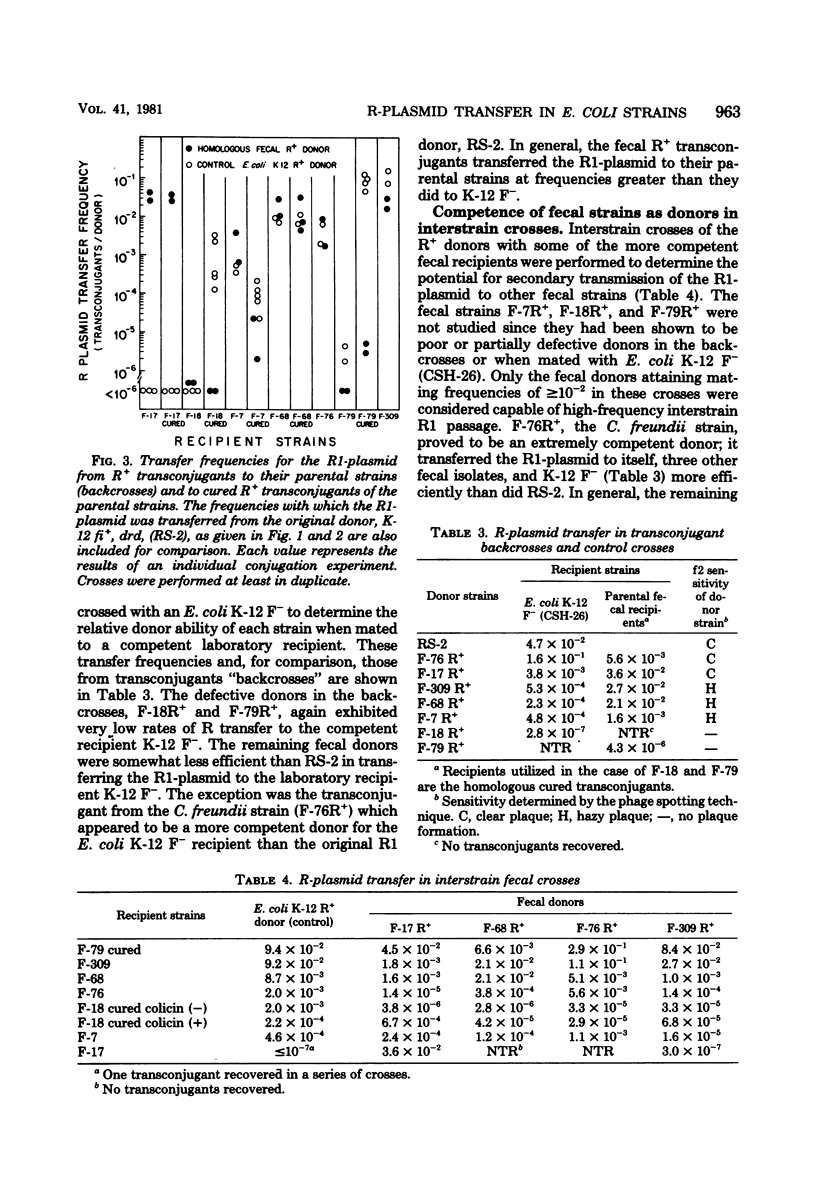
Abstract
Strains of Escherichia coli recently isolated from human feces were examined for the frequency with which they accept an R factor (R1) from a derepressed fi+ strain of E. coli K-12 and transfer it to fecal and laboratory strains. Colicins produced by some of the isolates rapidly killed the other half of the mating pair; therefore, conjugation was conducted by a membrane filtration procedure whereby this effect was minimized. The majority of fecal E. coli isolates accepted the R factor at lower frequencies than K-12 F−, varying from 10−2 per donor cell to undetectable levels. The frequencies with which certain fecal recipients received the R-plasmid were increased when its R+ transconjugant was either cured of the R1-plasmid and remated with the fi+ strain or backcrossed into the parental strain. The former suggests the loss of an incompatibility plasmid, and the latter suggests the modification of the R1-plasmid deoxyribonucleic acid (DNA). In general, the fecal R+E. coli transconjugants were less effective donors for K-12 F− and heterologous fecal strains than was the fi+ K-12 strain, whereas the single strain of Citrobacter freundii examined was generally more competent. Passage of the R1-plasmid to strains of salmonellae reached mating frequencies of 10−1 per donor cell when the recipient was a Salmonella typhi previously cured of its resident R-plasmid. However, two recently isolated strains of Salmonella accepted the R1-plasmid from E. coli K-12 R+ or the R+E. coli transconjugants at frequencies of 5 × 10−7 or less.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S., Smith H. R. Chloramphenicol resistance in the typhoid bacillus. Br Med J. 1972 Aug 5;3(5822):329–331. doi: 10.1136/bmj.3.5822.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Anderson J. D. The effect of R-factor carriage on the survival of Escherichia coli in the human intestine. J Med Microbiol. 1974 Feb;7(1):85–90. doi: 10.1099/00222615-7-1-85. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Cooke E. M., Breaden A. L., Shooter R. A., O'Farrell S. M. Antibiotic sensitivity of Escherichia coli isolated from animals, food, hospital patients, and normal people. Lancet. 1971 Jul 3;2(7714):8–10. doi: 10.1016/s0140-6736(71)90004-3. [DOI] [PubMed] [Google Scholar]
- Diwan N., Sharma K. B. Prevalence of serogroups and resistance plasmids in urinary Escherichia coli encountered in Delhi. Indian J Med Res. 1978 Aug;68:225–233. [PubMed] [Google Scholar]
- Farrar W. E., Jr, Dekle L. C. Tranferable antibiotic resistance associated with an outbreak of shigellosis. Ann Intern Med. 1967 Dec;67(6):1208–1215. doi: 10.7326/0003-4819-67-6-1208. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Fontaine T. D., 3rd, Hoadley A. W. Transferable drug resistance associated with coliforms isolated from hospital and domestic sewage. Health Lab Sci. 1976 Oct;13(4):238–245. [PubMed] [Google Scholar]
- Gangarosa E. J., Bennett J. V., Wyatt C., Pierce P. E., Olarte J., Hernandes P. M., Vázquez V., Bessudo D. An epidemic-associated episome? J Infect Dis. 1972 Aug;126(2):215–218. doi: 10.1093/infdis/126.2.215. [DOI] [PubMed] [Google Scholar]
- Grabow W. O., Prozesky O. W. Drug resistance of coliform bacteria in hospital and city sewage. Antimicrob Agents Chemother. 1973 Feb;3(2):175–180. doi: 10.1128/aac.3.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grüneberg R. N., Shaw E. J. The influence of antibiotic treatment on resistance patterns of coliform bacilli in childhood urinary-tract infection. J Med Microbiol. 1976 May;9(2):233–237. doi: 10.1099/00222615-9-2-233. [DOI] [PubMed] [Google Scholar]
- Guinée P. A. Transfer of multiple drug resistance from Escherichia coli to Salmonella typhi murium in the mouse intestine. Antonie Van Leeuwenhoek. 1965;31(3):314–322. doi: 10.1007/BF02045911. [DOI] [PubMed] [Google Scholar]
- Hartley C. L., Richmond M. H. Antibiotic resistance and survival of E coli in the alimentary tract. Br Med J. 1975 Oct 11;4(5988):71–74. doi: 10.1136/bmj.4.5988.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. A., Cabelli V. J. Membrane filter technique for enumeration of Pseudomonas aeruginosa. Appl Microbiol. 1972 Dec;24(6):864–870. doi: 10.1128/am.24.6.864-870.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton K. B., Richmond M. H., Bevan R., Gillespie W. A. Antibiotic resistance and R factors in coliform bacilli isolated from hospital and domestic sewage. J Med Microbiol. 1974 Feb;7(1):91–103. doi: 10.1099/00222615-7-1-91. [DOI] [PubMed] [Google Scholar]
- MATNEY T. S., ACHENBACH N. E. New uses for membrane filters III. Bacterial mating procedure. J Bacteriol. 1962 Oct;84:874–875. doi: 10.1128/jb.84.4.874-875.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman T. C., Klemm L. Transfer of antibiotic resistance (R factor) in the mouse intestine. Proc Soc Exp Biol Med. 1968 Jun;128(2):392–394. doi: 10.3181/00379727-128-33020. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Sturtevant A. B., Cassell G. H., Feary T. W. Incidence of infectious drug resistance among fecal coliforms isolated from raw sewage. Appl Microbiol. 1971 Mar;21(3):487–491. doi: 10.1128/am.21.3.487-491.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. M., James O. B. Transmission of infectious drug resistance from animals to man. J Hyg (Lond) 1973 Mar;71(1):209–215. doi: 10.1017/s0022172400046374. [DOI] [PMC free article] [PubMed] [Google Scholar]



