Abstract
Cyclin-dependent kinase 5 (Cdk5) is a nontraditional Cdk that is primarily active in postmitotic neurons. Its best known substrates are cytoskeletal proteins. Less appreciated is its role in the maintenance of a postmitotic state. We show here that in cycling cells (NIH 3T3), the localization of Cdk5 changes from predominantly nuclear to cytoplasmic as cells reenter a cell cycle after serum starvation. Similarly, when β-amyloid peptide is used to stimulate cultured primary neurons to reenter a cell cycle, they too show a loss of nuclear Cdk5. Blocking nuclear export pharmacologically abolishes cell cycle reentry in wild-type but not Cdk5−/− neurons, suggesting a Cdk5-specific effect. Cdk5 overexpression targeted to the nucleus of Cdk5−/− neurons effectively blocks the cell cycle, but cytoplasmic targeting is ineffective. Further, in both human Alzheimer's disease as well as in the R1.40 mouse Alzheimer's model and the E2f1−/− mouse, neurons expressing cell cycle markers consistently show reduced nuclear Cdk5. Thus, both in vivo and in vitro, neurons that reenter a cell cycle lose nuclear Cdk5. We propose that the nuclear Cdk5 plays an active role in allowing neurons to remain postmitotic as they mature and that loss of nuclear Cdk5 leads to cell cycle entry.
Keywords: Alzheimer's disease, β-amyloid precursor protein transgenic mice, cell cycle, E2F1, neurodegeneration
Cyclin-dependent kinase 5 (Cdk5) is a proline-directed serine/threonine kinase. Structurally, it is similar to cdc2 (1) but functions differently from traditional cyclin-dependent kinases (2). For example, Cdk5 does not have a cyclin as its activating partner. Instead, one of a unique pair of proteins, p35 or p39, binds to Cdk5 and activates it (3–5). Another example of the atypical nature of Cdk5 is that, despite its membership in the Cdk family, it has no established role in a normal cell cycle. Indeed, its most prominent roles have been linked to developmental processes of cell differentiation and migration (6–9) and to specialized functions in neuronal cell synapses and analogous roles in other differentiated cells (10–13). The paradoxical characteristics of Cdk5 were emphasized yet again by recent observations from our laboratory suggesting that, unlike the cell cycle-promoting functions of its Cdk relatives, Cdk5 functions in normal neocortical neurons to hold the cell cycle in check (6). This finding is intriguing because we and others have shown a close association between neuronal cell death and unscheduled cell cycle events, including DNA replication in several types of neurodegenerative diseases, including Alzheimer's disease (AD) (14–18).
The implications of the return of cell cycle activity to the neurons of the Cdk5−/− brain are clouded somewhat by the cooccurrence of a series of major disturbances in CNS development, including defective migration and incomplete biochemical differentiation. The implications were sufficiently important, however, that we have pursued them both in vivo and in vitro. We have now explored other instances where cell cycle control is lost in “postmitotic” neurons and determined that Cdk5 appears to play a role in each. In AD, neurons in populations that are lost during the course of the disease express a range of cell cycle proteins and replicate their DNA. Similarly, in the AD mouse model known as R1.40, a line of mice that carries the entire human β-amyloid precursor protein (APP) gene on a yeast artificial chromosome, cell cycle events develop in neurons in a pattern that strongly resembles the pattern of cell death in human AD (19–21). We have also examined the neurons of the E2f1−/− mouse brain. Wang et al. (22) have recently reported that large numbers of E2F1-deficient nerve cells begin a cell cycle process and retain high levels of cell cycle proteins in their cytoplasm. We report here that in each of these situations, Cdk5 exits the nucleus of cells before, or concomitant with, the initiation of cell cycle events. Blocking the exit of Cdk5 from the nucleus retains the cell cycle suppression. The findings suggest that Cdk5 has an unexpected but widespread role as a negative cell cycle regulator in postmitotic neurons.
Results
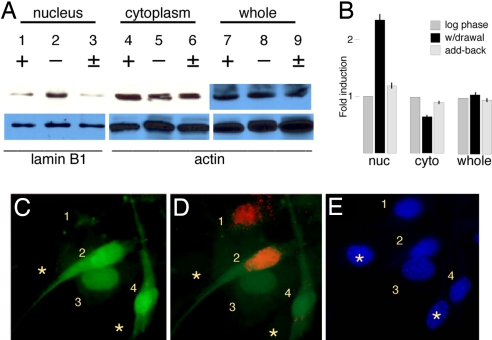
In the Cdk5−/− mutant brain, cell cycle reentry occurs in normally postmitotic neurons (6), implying that Cdk5 acts as a cell cycle suppressor in these cells. To see whether this suppression also occurs in other cell types, we cultured NIH 3T3 cells and used serum withdrawal to manipulate their cell cycle. Log-phase cells have easily detectable quantities of Cdk5 present (Fig. 1A, lane 7) although reportedly not kinase activity (38). When we separate cells into nuclear and cytoplasmic fractions, we find that most of the Cdk5 protein is cytoplasmic (lane 4) rather than nuclear (lane 1). When the cell cycle is arrested by reducing the serum, the total amount of Cdk5 protein barely changes (compare lanes 8 and 7), but the subcellular distribution is significantly altered. The fraction of Cdk5 in the nucleus increases >2-fold (lane 2) whereas the cytoplasmic fraction decreases by ≈one-third (lane 5). When the cell cycle is reinitiated by returning serum to the medium, the initial distribution is restored within hours (lanes 3, 6, and 9). All results are quantified in Fig. 1B and expressed as induction relative to log-phase levels.
Fig. 1.
Nuclear/cytoplasmic distribution of Cdk5 during cell cycle activity in NIH 3T3 cells. (A) Cells were harvested during log phase growth (+), during cell cycle arrest achieved by serum withdrawal (−), or 4 h after resumption of serum add-back (±). Nuclear (lanes 1–3) and cytoplasmic (lanes 4–6) fractions or whole-cell lysates (lanes 7–9) were analyzed on Cdk5 Western blots. Whole-cell lysates (lanes 7–9), as well as the actin and laminB1 loading controls, were analyzed separately. (B) Cdk5:loading control ratio in arbitrary units normalized to log-phase cells. (C–E) GFP:Cdk5 fusion protein location during serum add-back in the presence of BrdU, and Cdk5 fusion protein location during serum add-back in the presence of BrdU. (C) GFP fluorescence shows fusion protein localization. (D) BrdU immunostaining (red) shows that cells with a low nuclear:cytoplasmic ratio of Cdk5 are cycling. (E) DAPI. Numbers are described in Results.
The data in Fig. 1A represent the behavior of the “average” cell in the dish. Examining the cells individually reveals a more nuanced picture. Cells were transfected with a GFP-Cdk5 fusion protein, serum-starved, then restored to cell cycle activity by serum add-back for 4 h in the presence of 10 μM BrdU. The field of cells shown in Fig. 1 C–E reveals that it is the nuclear/cytoplasmic balance of Cdk5 that correlates most closely with cell cycle activity. In cell 1, the nucleus is virtually devoid of Cdk5 protein (Fig. 1C, green), whereas the cytoplasm has modest amounts of (clumped) Cdk5. Cell 1 is also labeled with BrdU (Fig. 1D, red). Cell 2 represents a variant of this situation. The total amount of Cdk5 is high, but the nuclear cytoplasmic balance is nearly uniform or slightly tilted in favor of the cytoplasm. BrdU labeling indicates that this cell, too, is actively dividing. Cells 3 and 4 illustrate the alternative situation. In these cells, the total level of Cdk5 varies, but in both cases, the nuclear component is stronger, and both cells are mitotically inactive (Fig. 1D). DAPI staining reveals two cells in the field that were not transfected (Fig. 1E, asterisks). Following cells over time in culture reveals a dynamic picture that is consistent with these more static results [supporting information (SI) Fig. S1]. Thus, when Cdk5 is higher in the nucleus than in the cytoplasm, cell division ceases; when Cdk5 is higher in the cytoplasm, the cell can reenter a cell cycle. Combined with the Western blot analysis, this finding indicates that, on average, there is a net migration of Cdk5 in and out of the nucleus as the population shifts out of and into the cell cycle.
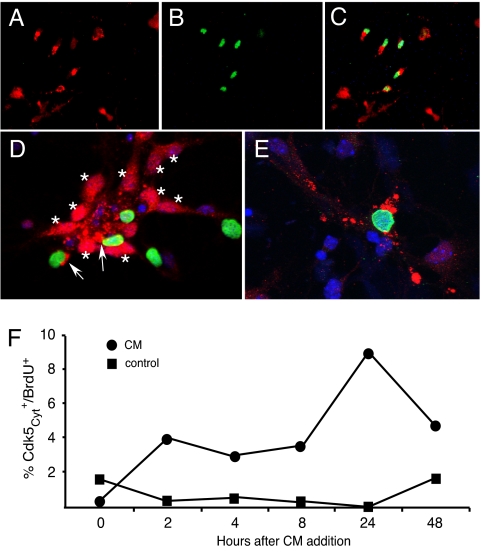
The APP-derived β-amyloid (Aβ) peptide (23, 24), or conditioned medium from Aβ-stimulated THP-1 cells (CM) (25), results in a cell cycle-related neuronal death (CRND), when added to primary cortical neurons. We treated E16.5 cortical neurons with either CM or 10 μM Aβ1–42 for 24 h, by which time many Map2+ neurons had incorporated BrdU. We investigated changes in Cdk5 localization under these conditions. In untreated neurons, we found strong Cdk5 expression visible in the nucleus and perinuclear region of the all neurons (Fig. 2D, asterisks). We then exposed cells to fresh medium with or without Aβ for 24 h, after which many cells displayed Cdk5 immunostaining that was reduced or totally lost in the nucleus (Fig. 2 A, C, and E), and the distribution of the antigen in the perinuclear region appeared clumped. Western blot analysis of whole cultures (data not shown) revealed unaltered levels of total Cdk5 after treatment. The shift of Cdk5 localization was related to cell cycle reentry as shown by double immunolabeling for Cdk5 and proliferating cell nuclear antigen (PCNA) (Fig. 2 A–C), BrdU (Fig. 2 D and E), cyclin A (Fig. S2 A and B) and FISH (Fig. S2 C and D) as indicators of cell cycle and DNA replication. Cell counts of BrdU and nuclear Cdk5 indicated that by 24 h after CM treatment, 9% of Cdk5+ neurons had reduced nuclear Cdk5 and were BrdU+ (Fig. 2F). Confocal imaging clearly shows that the nuclear Cdk5 found in cells in untreated cultures is lost when DNA synthesis begins (Fig. 2 D and E). Thus, loss of Cdk5 from the nucleus occurs concomitantly with loss of cell cycle control in neurons just as it does in 3T3 cells.
Fig. 2.
Cell cycle re-entry in postmitotic neurons is accompanied by the loss of nuclear Cdk5. (A–C) After Aβ treatment, Cdk5 (A) is cytoplasmic in cycling, BrdU-positive (B) cells; (C) merge. (D and E) Confocal micrographs of neurons stained with Cdk5 (red), BrdU (green), and DAPI (blue), before (D) and after (E) Aβ1–42 treatment. (D) Two cycling Cdk5-positive cells (BrdU, green) are indicated by the arrows. Note, however, that in these untreated cells the bulk of the Cdk5 is cytoplasmic. (F) The percentage of total cells that were BrdU-positive and had cytoplasmic Cdk5 as a function of time after CM addition.
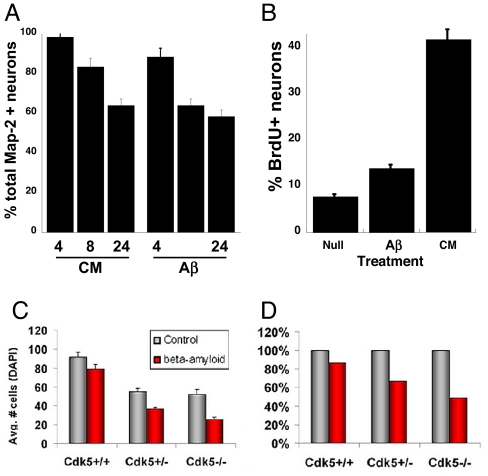
Aβ-induced cell cycle reentry was observed in 15% of the neurons (Fig. 3B); CM was even more effective (38%). Cell cycle reentry was followed by cell death because Map2+ neurons were found that were both BrdU- and TUNEL-positive (ref. 25 and data not shown). This observation is not unexpected because counts indicate a loss of neurons over time with either CM or Aβ treatment (Fig. 3A). The neuronal death in AD has led others to suggest that enhanced Cdk5 activity is pathological and that inhibition of Cdk5 should be protective (26–29). This hypothesis predicts that Cdk5-deficient neurons should be resistant to Aβ-induced death. To test this possibility, we isolated cortical neurons from Cdk5+/+, Cdk5+/−, and Cdk5−/− animals and treated them with Aβ for 24 h. In all genotypes, Aβ induced a fraction of the treated neurons to become positive for cleaved caspase-3 (data not shown), and counts revealed substantial cell loss (Fig. 3C). Indeed, when these data are normalized to the initial number of cells, a greater percentage of cells is lost in Cdk5−/− cultures than in either Cdk5+/− or Cdk5+/+ (Fig. 3D). This result would be unexpected if Cdk5 were pathogenic.
Fig. 3.
The behavior of E16.5 neurons (5 DIV) after Ab1–42 treatment. (A) Both CM and Ab1–42 (Aβ) lead to loss of Map2+ neurons from the culture. Counts are shown at 4, 8, and 24 h after Aβ addition. (B) Proportion of BrdU/Map2 double-positive cells after the indicated treatment. The absence of Cdk5 does not protect from Aβ-induced cell death. (C) Total cells per field in both Aβ-treated (red bars) and untreated (gray bars) cultures. The absence of Cdk5 leads toTuJ1+ cell loss after 24 h (6), but the effect of Aβ can be seen on top of this. (D) When expressed as a percentage of the control cultures, the percentage loss is higher in Cdk5−/− cultures, arguing against a pathogenic role for Cdk5 in neurodegeneration.
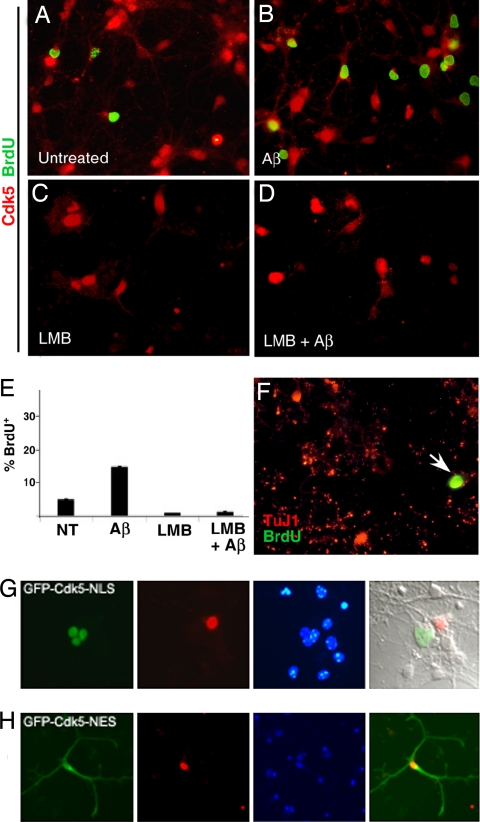
The correlation of Cdk5 translocation with cell cycle activity implies that the two events may be causally linked. To test this hypothesis, we used the nuclear export inhibitor leptomycin B (LMB) to block Cdk5 export from the nucleus. We cultured wild-type E16.5 primary cortical neurons for 5 days in vitro (DIV), after which the medium was changed and 10 μM BrdU, with or without LMB and/or 10 μM Aβ was added. In untreated (BrdU-only) cells, Cdk5 is predominantly nuclear before treatment (Fig. 4A); after Aβ, Cdk5 leaves the nucleus, and BrdU labeling is found (Fig. 4B). LMB treatment leads to Cdk5 retention in the nucleus (Fig. 4 C and D). In this situation, Aβ-induced cycle reentry is blocked (Fig. 4D). There was cell death in all LMB-treated cultures, but the dying (pycnotic) cells were consistently BrdU-negative, demonstrating that no cells died through CRND. Counts of BrdU/Map2 double-labeled cells are shown in Fig. 4E. To ensure that this phenomenon was Cdk5-specific, we cultured Cdk5−/− neurons and treated them with Aβ. In these cultures, substantial numbers of neurons could be labeled with BrdU, even in the presence of LMB (Fig. 4F). As further proof, we transfected two modified GFP-Cdk5 expression vectors into Cdk5−/− neurons. When a nuclear localization signal (NLS) is added to the fusion protein, fluorescence is restricted to the nucleus, and no transfected cell labels with BrdU (Fig. 4G). By contrast, when a nuclear export signal (NES) is added, fluorescence is cytoplasmic, and BrdU-labeled transfected cells are found (Fig. 4H, shown at lower magnification to reveal the entire cell).
Fig. 4.
Nuclear Cdk5 nuclear blocks Aβ-induced cell cycle activity. (A–F ) Cdk5 (red) BrdU (green) double immunostained E16.5 primary neurons after 6 DIV. (A) BrdU labeling of untreated neurons is minimal. (B) Aβ treatment leads to a substantial increase in labeling. (C ) LMB retains Cdk5 protein in the nucleus, and (D) addition of Aβ leads to no BrdU incorporation. (E ) Cell counts of BrdU/TuJ1 double-labeled cells as a fraction of total TuJ1. NT, untreated cultures. (F ) In Cdk5−/− neurons, Aβ stimulates BrdU (green) incorporation (arrow) in TuJ1-positive (red) neurons despite the presence of LMB in the medium. (G) GFP-Cdk5 directed to the nucleus with an NLS is never found in a BrdU-positive (red) neuron. (H) GFP-Cdk5 constrained to the cytoplasm with an NES can be found in a BrdU-positive neuron. Cells in G and H are from Cdk5−/− embryos.
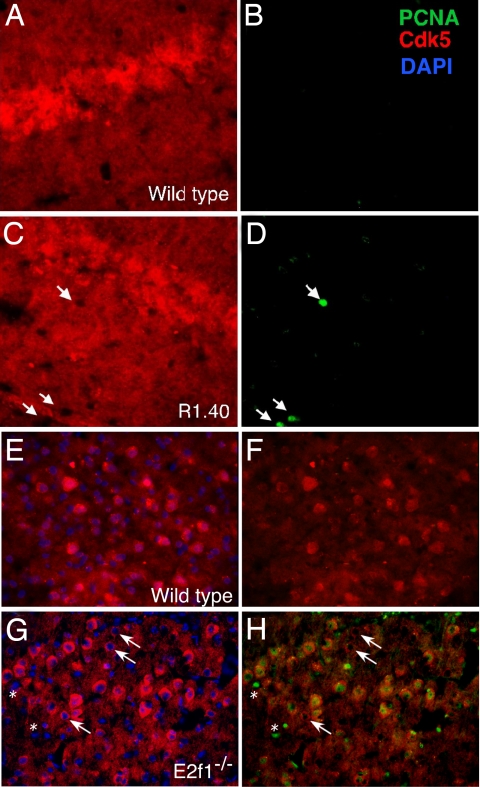
These results have implications for neurodegenerative conditions such as AD and suggest that the role of Cdk5 in disease might be complex, with location as well as activity playing a part in the disease process. These results prompted us to examine the relationship between Cdk5 localization and cell cycle reentry in vivo. As reported, in wild-type mouse brain, Cdk5 immunolabeling is detected throughout the neuropil (7), with protein in both nucleus and cytoplasm (Fig. 5 A and E). We next examined the location of Cdk5 in the AD mouse model, R1.40. Although the wild-type pattern was largely maintained in the transgenics, in brain regions where cell cycle events had begun (hippocampus and frontal neocortex) we found that “cycling” (PCNA-positive) neurons were largely devoid of Cdk5 protein in their nuclei (Fig. 5 C and D, arrows). Such nuclear/cytoplasmic translocation of Cdk5 did not occur in R1.40 cerebellar Purkinje cells, which are unaffected in human AD and do not develop cell cycle events in the R1.40 mouse (data not shown). This finding suggests that Aβ-induced Cdk5 translocation is related to the cell cycle reentry. Confocal images of wild type and R1.40 reveal a similar picture (data not shown).
Fig. 5.
Cdk5 immunostaining (red) is reduced in the nucleus of neurons with cell cycle activity in AD mouse models. (A–D) Immunostained tissue sections of 10-month wild-type (A and B) and R1.40 (C and D) mouse hippocampus. Cycle activity is revealed with PCNA immunostaining (green) (F and H). Cycling cells (arrows) are found only in the R1.40 material. Cdk5 immunostaining is also responsive to cell cycle activity induced by E2F1 deficiency. (E–H) One-month wild-type (E and F ) and E2f1−/− (G and H) neocortex immunostained with Cdk5 (red) and DAPI (blue) (E and G) or Cdk5 and PCNA (green) (F and H). All PCNA-positive cells have low nuclear Cdk5 (asterisks, small BrdU-labeled nonneuronal cells). An occasional cell with low nuclear Cdk5 was also PCNA-negative (arrows).
We further pursued the correlation between Cdk5 location and cell cycling in the cortex of the E2f1−/− mouse. Previous work has shown cell cycle activity in many of the neurons of the CNS, including approximately half of those in the deeper layers of neocortex (22). When we immunostained E2f1−/− cortex for Cdk5, we found that these same regions had many cells with weak nuclear Cdk5 staining (Fig. 5G). Double staining with Cdk5 and PCNA revealed that every neuron that was positive for PCNA had little or no nuclear Cdk5. Curiously, the reverse was not true; a minority of the neurons had low nuclear Cdk5 but did not stain with PCNA (arrows).
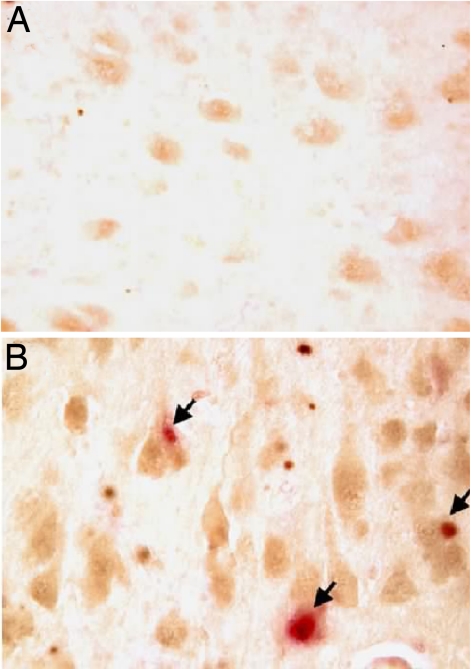
To determine whether these findings with rodent neurons had direct relevance to human disease, we immunostained neurons in the brains of patients who had died with AD. In all stages of AD, neurons at risk for death show cell cycle-related protein expression (18) and DNA replication (16). As in the wild-type mouse, neuronal Cdk5 expression is found ubiquitously in the brain regions we examined. Within most hippocampal and locus coeruleus neurons from control specimens, the protein is distributed nearly uniformly, although in mainly nuclear and perinuclear (cytoplasmic) locations (Fig. 6A). In the AD brain, however, the level of Cdk5 was markedly increased with a large part of that increase found in the nucleus (Fig. 6B). By immunocytochemical criteria, the majority of cells, which are unaffected by cell cycle-induced loss, continue to express high levels of Cdk5. However, we consistently observed that Cdk5 was lost from the nucleus of neurons expressing PCNA (arrows, Fig. 6B). Thus, in all three systems that we examined (cell culture, mouse brain, and human brain), neurons that lose cell cycle control have lost their nuclear Cdk5.
Fig. 6.
Expression of Cdk5 and PCNA in AD brain. Immunostaining of Cdk5 showed substantially darker hippocampal neurons of AD patients (B) compared with age-matched individuals with no dementia (A). Double staining with Cdk5 (brown) and PCNA (red) revealed that in PCNA-positive neurons, Cdk5 was almost exclusively cytoplasmic.
Discussion
We have explored the role of Cdk5 localization as a cell cycle regulator in postmitotic neurons. Our findings suggest first, that Cdk5 acts as a cell cycle suppressor under many different circumstances in many different cell types, and second, that this function requires its presence in the nucleus. In vitro, we exposed 5-day cultures to Aβ1–42 or Aβ-stimulated THP-1 cell CM. This exposure induced both neuronal cell cycle reentry and cell death. We also examined NIH 3T3 cells in both log-phase growth and during cell cycle arrest after serum withdrawal. In both fibroblasts and cycling neurons, we found that most nuclear Cdk5 is lost. The results are consistent with our findings that neurons in the Cdk5−/− mouse continue to cycle in vivo (6), but they suggest that the key to cell cycle arrest is not the total amount of Cdk5, but rather its nuclear location. Our finding that cell cycle reentry is tied to Cdk5 localization is further supported by observations in both human AD and its mouse model (R1.40). In both situations, cycling neurons are characterized by a predominantly cytoplasmic distribution of Cdk5. The finding that this relationship also holds in the E2f1−/− mouse is significant. Presumably, the induction of neuronal cell cycle occurs by a different mechanism in the absence of E2F1 than it does in AD or its models. The consistency of the findings suggests, therefore, that Cdk5 localization plays a general role in neuronal cell cycle inhibition. The reason why a small fraction of neurons in the E2f1−/− brain have cytoplasmic Cdk5 but no cell cycle activity is currently unknown. It may indicate that the shift in Cdk5 location precedes cell cycle initiation but is not sufficient to induce it.
We have used the nuclear export inhibitor, LMB, to determine whether forcing nuclear retention of Cdk5 is able to stop the cell cycle events. The near total absence of BrdU incorporation in the presence of LMB suggests that cell cycling was indeed inhibited. We have confidence in these findings because we have shown both in vitro (25) and in vivo (30) that dying neurons can be labeled with cell cycle markers. The block was not the result of nonspecific inhibition of cell cycle activity because Cdk5−/− neurons are capable of cycling, even in the presence of drug. We have also provided independent support for the conclusions by showing that exogenous Cdk5 forced into the nucleus with an NLS is fully active as a cell cycle suppressor, whereas Cdk5 is inactive when forced into the cytoplasm with an NES.
Previous work has shown that neurons at risk for death in AD exhibit cell cycle protein reexpression and DNA replication (16). Those phenomena are found in both late and early stages of human AD (18). Further, in AD mouse models, neurons begin cell cycle events in the areas that are known to be the most severely affected in the human disease, and these cell cycle events appear much earlier than the formation of the Aβ deposits (20). This finding indicates that the neurons detect the stress of impending AD much earlier than the beginning of plaque deposition. There is little known as to why or how neurons are stimulated to reenter an unscheduled cell cycle. We would speculate that when any toxic insult overwhelms the system, nuclear Cdk5 is transported to the cytoplasm where it might lead to further neuronal stress such as tau hyperphosphorylation. With the loss of nuclear Cdk5, the cell cycle is no longer effectively blocked, and an unscheduled and ultimately lethal cell cycle process begins. We have shown in both the R1.40 mouse model and in human AD tissue that neurons affected by the altered environment of the AD brain reenter a cell cycle and lose their nuclear Cdk5. These data combined with the findings in the E2F1-deficient mouse and our in vitro studies in cell lines and primary neurons all clearly indicate a link between Cdk5 localization and the cell cycle. The exact mechanism by which Cdk5 exerts its cell cycle suppression effect is currently unknown. Possibilities include alterations in cytoskeletal functions and direct action on proteins with cell cycle-specific functions. An example of the latter possibility is the cell cycle inhibitor, p27. Cdk5 can stabilize p27 (31), and the protein has been documented to affect multiple processes in neurons, including cell migration, cell cycle suppression, and differentiation (32–34).
The role of Cdk5 in cell death remains uncertain. Overexpression of the Cdk5 activator, p25, in transgenic mice induces a range of neurodegenerative phenomena (27). These and earlier findings (29) have led to the suggestion that inhibition of Cdk5 should protect against neurodegeneration and, in some cases this has proven to be so. Yet, as shown in Fig. 3, the total absence of Cdk5 (in Cdk5−/− cultures) does not protect neurons against Aβ-induced death as might be predicted. Although the interpretation is confounded somewhat by the requirement of Cdk5 for normal neuronal differentiation (6–8), the heterozygote (Cdk5+/−) data demonstrate that even reducing the levels of Cdk5 by half provides no protection. Thus, Cdk5 may be sufficient, but it is not necessary for nerve cell death. O'Hare and colleagues (35) have shown that in excitotoxic conditions the presence of active Cdk5 in the nucleus is detrimental to cell survival. During DNA damage-mediated cell death, however, dominant-negative Cdk5 with a nuclear localization signal was not sufficient to protect neurons from death, suggesting that blocking nuclear Cdk5 activity is not specific for that cell death pathway. These results appear to conflict with our own. We note, however, that in Drosophila neurons treated with Aβ there is translocation of Cdk5 from the membrane to the perinuclear region that is p25-independent (36). It is likely that different model systems are stressing different aspects of the system. Thus, although the present results make it clear that nuclear Cdk5 acts as a cell cycle suppressor, it must be left to future studies to reconcile different models of the involvement of Cdk5 in neuronal death. At present, because neuronal cell cycle events are tightly correlated with neuronal cell death in a number of neurodegenerative diseases (for a recent review see ref. 37), both our in vitro and in vivo work suggest that inhibiting Cdk5 without regard to its localization might carry risks and promise for a patient.
Methods
Animals.
Cdk5+/−mice were maintained on a mixed (C57BL6/J×129/S1) background (7). Embryos were genotyped by PCR as described in ref. 6. The primers used were Cdk5F1 (ATTGTGGCTCTGAAGCGTGTC), Cdk5R1 (CTTGTCACTATGCAGGACATC), and PGK1 (TCCATCTGCACGAGACTAGT). All animal procedures were carried out in accordance with and Case Western Reserve University and Rutgers University IACUC standards. R1.40 animals were obtained as a congenic C57BL/6 breeding stock from Dr. Bruce Lamb (Cleveland Clinic Foundation) and were maintained by homozygous mating. E2f1−/− animals were obtained as homozygous breeding pairs from The Jackson Laboratory and maintained by homozygous mating.
Primary Neuronal Cultures.
Neuronal cultures were prepared as described in ref. 6. For Cdk5-deficient cultures, all embryos from a Cdk5+/− × Cdk5+/− mating were treated separately. All cultures were grown for a minimum of 5 DIV before any treatment. To assess cell cycle activity, 10 μM BrdU was added for 24 or 48 h. All experiments were performed on a minimum of three litters; each condition was examined in triplicate.
Preparation of Aβ.
The 42-aa isoform of Aβ peptide (Aβ1–42) (American Peptide) was reconstituted in sterile double-distilled H2O, allowed to fibrillarize at 37°C for 5 days, and then vortexed and diluted to the equivalent of 10 μM into culture media. Conditioned medium was prepared as described in ref. 25.
Immunocytochemistry.
Cultured cells were rinsed once with PBS and then fixed in buffered 4% paraformaldehyde in 0.1 M phosphate buffer for 30 min at room temperature followed by three rinses with PBS. For BrdU immunolabeling, cells were exposed to 2 N HCl for 10 min, then neutralized in 0.1 M sodium borate before staining. Antibody concentrations used for cell culture were: 1:1,000 for mouse anti-PCNA (Santa Cruz Biotechnology), 1:100 for rat anti-BrdU (Abcam), 1:500 for mouse anti-Map2a/c (Sigma), 1:1,000 for mouse anti-Tuj1 (Covance), and 1:500 for rabbit anti-Cdk5 (C-18; Santa Cruz Biotechnology). Secondary antibodies used were: goat anti-rat rhodamine (1:300; Jackson Laboratories), goat anti-mouse Alexa Fluor 488 (1:1,000; Molecular Probes), goat anti-rat LRSC (1:500; Jackson Laboratories); goat anti-rabbit FITC (1:500; Jackson Laboratories). Cells were counterstained with 1 μg/ml DAPI.
Human Tissue.
All human tissues were obtained from the Neuropathology Core of the Alzheimer's Disease Research Center of Cleveland (P50 AG08012). Brain tissue from eight pathologically confirmed cases of AD had been fixed in 10% formalin (average of postmortem interval, 12.5 h). Six age-matched controls were verified as free of CNS disease (postmortem interval, 11 h). Specimens were embedded in paraffin, sectioned at 10 μm, stained as described in ref. 18, and coverslipped with Permount. Control sections were treated identically except for the omission of the primary antibody.
Supplementary Material
Acknowledgment.
This work was supported by the National Institutes of Health (NS20591).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0711355105/DCSupplemental.
References
- 1.Hellmich MR, Pant HC, Wada E, Battey JF. Neuronal cdc2-like kinase: A cdc2-related protein kinase with predominantly neuronal expression. Proc Natl Acad Sci USA. 1992;89:10867–10871. doi: 10.1073/pnas.89.22.10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhavan R, Greer PL, Morabito MA, Orlando LR, Tsai LH. The cyclin-dependent kinase 5 activators p35 and p39 interact with the α-subunit of Ca2+/calmodulin-dependent protein kinase II and α-actinin-1 in a calcium-dependent manner. J Neurosci. 2002;22:7879–7891. doi: 10.1523/JNEUROSCI.22-18-07879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee KY, Rosales JL, Tang D, Wang JH. Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J Biol Chem. 1996;271:1538–1543. doi: 10.1074/jbc.271.3.1538. [DOI] [PubMed] [Google Scholar]
- 4.Lew J, Wang JH. Neuronal cdc2-like kinase. Trends Biochem Sci. 1995;20:33–37. doi: 10.1016/s0968-0004(00)88948-3. [DOI] [PubMed] [Google Scholar]
- 5.Lew J, et al. Neuronal cdc2-like kinase is a complex of cyclin-dependent kinase 5 and a novel brain-specific regulatory subunit. Nature. 1994;371:423–426. doi: 10.1038/371423a0. [DOI] [PubMed] [Google Scholar]
- 6.Cicero S, Herrup K. Cyclin-dependent kinase 5 is essential for neuronal cell cycle arrest and differentiation. J Neurosci. 2005;25:9658–9668. doi: 10.1523/JNEUROSCI.1773-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci. 1998;18:6370–6377. doi: 10.1523/JNEUROSCI.18-16-06370.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilmore EC, Herrup K. Neocortical cell migration: GABAergic neurons and cells in layers I and VI move in a cyclin-dependent kinase 5-independent manner. J Neurosci. 2001;21:9690–9700. doi: 10.1523/JNEUROSCI.21-24-09690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohshima T, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH. Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron. 2005;48:825–838. doi: 10.1016/j.neuron.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Lin W, et al. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron. 2005;46:569–579. doi: 10.1016/j.neuron.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Fu AK, et al. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nat Neurosci. 2001;4:374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- 14.Sultana R, Butterfield DA. Regional expression of key cell cycle proteins in brain from subjects with amnestic mild cognitive impairment. Neurochem Res. 2007;32:655–662. doi: 10.1007/s11064-006-9123-x. [DOI] [PubMed] [Google Scholar]
- 15.Vincent I, Jicha G, Rosado M, Dickson DW. Aberrant expression of mitotic cdc2/cyclin B1 kinase in degenerating neurons of Alzheimer's disease brain. J Neurosci. 1997;17:3588–3598. doi: 10.1523/JNEUROSCI.17-10-03588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Geldmacher DS, Herrup K. DNA replication precedes neuronal cell death in Alzheimer's disease. J Neurosci. 2001;21:2661–2668. doi: 10.1523/JNEUROSCI.21-08-02661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Y, Herrup K. Loss of neuronal cell cycle control in ataxia-telangiectasia: A unified disease mechanism. J Neurosci. 2005;25:2522–2529. doi: 10.1523/JNEUROSCI.4946-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer's disease. J Neurosci. 2003;23:2557–2563. doi: 10.1523/JNEUROSCI.23-07-02557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman EJ, Kulnane LS, Lamb BT. Alterations in β-amyloid production and deposition in brain regions of two transgenic models. Neurobiol Aging. 2003;24:645–653. doi: 10.1016/s0197-4580(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Varvel NH, Lamb BT, Herrup K. Ectopic cell cycle events link human Alzheimer's disease and amyloid precursor protein transgenic mouse models. J Neurosci. 2006;26:775–784. doi: 10.1523/JNEUROSCI.3707-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kulnane LS, Lamb BT. Neuropathological characterization of mutant amyloid precursor protein yeast artificial chromosome transgenic mice. Neurobiol Dis. 2001;8:982–992. doi: 10.1006/nbdi.2001.0446. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Wang R, Herrup K. E2F1 works as a cell cycle suppressor in mature neurons. J Neurosci. 2007;27:12555–12564. doi: 10.1523/JNEUROSCI.3681-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Copani A, et al. Mitotic signaling by β-amyloid causes neuronal death. FASEB J. 1999;13:2225–2234. [PubMed] [Google Scholar]
- 24.Copani A, et al. DNA polymerase-β is expressed early in neurons of Alzheimer's disease brain and is loaded into DNA replication forks in neurons challenged with β-amyloid. J Neurosci. 2006;26:10949–10957. doi: 10.1523/JNEUROSCI.2793-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Q, Combs C, Cannady SB, Geldmacher DS, Herrup K. β-Amyloid-activated microglia induce cell cycling and cell death in cultured cortical neurons. Neurobiol Aging. 2000;21:797–806. doi: 10.1016/s0197-4580(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 26.Augustinack JC, Sanders JL, Tsai LH, Hyman BT. Colocalization and fluorescence resonance energy transfer between cdk5 and AT8 suggest a close association in pre-neurofibrillary tangles and neurofibrillary tangles. J Neuropathol Exp Neurol. 2002;61:557–564. doi: 10.1093/jnen/61.6.557. [DOI] [PubMed] [Google Scholar]
- 27.Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. doi: 10.1016/s0896-6273(03)00627-5. [DOI] [PubMed] [Google Scholar]
- 28.Lee MS, et al. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000;405:360–364. doi: 10.1038/35012636. [DOI] [PubMed] [Google Scholar]
- 29.Patrick GN, et al. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999;402:615–622. doi: 10.1038/45159. [DOI] [PubMed] [Google Scholar]
- 30.Herrup K, Busser JC. The induction of multiple cell cycle events precedes target-related neuronal death. Development. 1995;121:2385–2395. doi: 10.1242/dev.121.8.2385. [DOI] [PubMed] [Google Scholar]
- 31.Kawauchi T, Chihama K, Nabeshima Y, Hoshino M. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- 32.Besson A, et al. Discovery of an oncogenic activity in p27Kip1 that causes stem cell expansion and a multiple tumor phenotype. Genes Dev. 2007;21:1731–1746. doi: 10.1101/gad.1556607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen L, et al. p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev. 2006;20:1511–1524. doi: 10.1101/gad.377106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen L, Besson A, Roberts JM, Guillemot F. Coupling cell cycle exit, neuronal differentiation and migration in cortical neurogenesis. Cell Cycle. 2006;5:2314–2318. doi: 10.4161/cc.5.20.3381. [DOI] [PubMed] [Google Scholar]
- 35.O'Hare MJ, et al. Differential roles of nuclear and cytoplasmic cyclin-dependent kinase 5 in apoptotic and excitotoxic neuronal death. J Neurosci. 2005;25:8954–8966. doi: 10.1523/JNEUROSCI.2899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin H, Lin TY, Juang JL. Abl deregulates Cdk5 kinase activity and subcellular localization in Drosophila neurodegeneration. Cell Death Differ. 2007;14:607–615. doi: 10.1038/sj.cdd.4402033. [DOI] [PubMed] [Google Scholar]
- 37.Herrup K, Yang Y. Cell cycle regulation in the postmitotic neuron: Oxymoron or new biology? Nat Rev Neurosci. 2007;8:368–378. doi: 10.1038/nrn2124. [DOI] [PubMed] [Google Scholar]
- 38.Ubeda M, Kemp DM, Habener JF. Glucose-induced expression of the cyclin-dependent protein kinase 5 activator p35 involved in Alzheimer's disease regulates insulin gene transcription in pancreatic beta-cells. Endocrinology. 2004;145:3023–3031. doi: 10.1210/en.2003-1522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.