Abstract
For over half a century, the echolocating bat has served as a valuable model in neuroscience to elucidate mechanisms of auditory processing and adaptive behavior in biological sonar. Our article emphasizes the importance of the bat's vocal-motor system to spatial orientation by sonar, and we present this view in the context of three problems that the echolocating bat must solve: (i) auditory scene analysis, (ii) sensorimotor transformations, and (iii) spatial memory and navigation. We summarize our research findings from behavioral studies of echolocating bats engaged in natural tasks and from neurophysiological studies of the bat superior colliculus and hippocampus, brain structures implicated in sensorimotor integration, orientation, and spatial memory. Our perspective is that studies of neural activity in freely vocalizing bats engaged in natural behaviors will prove essential to advancing a deeper understanding of the mechanisms underlying perception and memory in mammals.
Keywords: active sensing, behavioral neurobiology, echolocation, spatial cognition, navigation
In their seminal 1959 paper, “What the frog's eye tells the frog's brain” (1), Lettvin et al. articulated a key point in neuroscience: Neurobiological experiments should consider the natural context in which biologically relevant sensory information is acquired and behavior is executed. This view, which later became a guiding principle in the field of neuroethology (2), implies that neural activity may change with the animal's behavioral state. This implication has been supported, for example, by findings from the bird song system, where dramatic differences exist between auditory responses to acoustic stimuli in anesthetized and awake animals (3). Similarly, large effects of the animal's behavioral state are observed in the rodent barrel cortex, where neural activity in response to a whisker deflection depends strongly on whether the animal is passive or is actively moving its whiskers (4).
Further, numerous studies (e.g., refs 5–11) demonstrate that a comparative approach to neuroscience yields insights that cannot be obtained by study of a single species. In this article, we consider the behavior and neurobiology of a mammal long studied from a comparative standpoint, the echolocating bat (12–16), and here we stress the additional importance of performing experiments in freely behaving animals. Researchers of bat echolocation have long been inspired by observations of species-specific natural behaviors, but we note that past experimental studies of the bat nervous system have rarely engaged the echolocating bat in these natural behaviors. However, recent research that has explicitly studied neural activity in freely behaving bats has uncovered some surprising discoveries, as detailed below, and we believe future work along these lines is key to a complete understanding of the neurobiology of spatial orientation by echolocation.
We will start with a brief overview of classic studies of bat echolocation and then focus on three topics that, until recently, have received comparatively little attention in the study of echolocating bats: (i) auditory scene analysis, (ii) sensorimotor transformations, and (iii) spatial memory and navigation. We argue that echolocating bats show remarkable performance and are among the “champions” of mammals in these three domains; furthermore, we provide evidence that the bat's active control over its vocalizations plays a key role in its perception, action, and spatial memory.
Bat Echolocation: From Behavior to Neurobiology.
Echolocating bats are small flying mammals (weighing typically <35 g) that emit brief calls through either the mouth or the nostrils and use the returning echoes to orient in the environment and forage for food at night or dusk (13–16). There are >800 species of echolocating bats that occupy a broad range of habitats, and adaptations to habitat and food sources are reflected in their sonar call designs (15). The diversity of bat behavior presents a rich opportunity to understand the adaptive evolution of bats and sonar call design (for comprehensive reviews, we direct the reader to refs. 12 and 16). For this Perspective, we draw from studies of several species and distinguish between specializations and general mechanisms to the extent that data are available.
Most bat species use ultrasonic echolocation calls, but a few species emit sonar calls with components that are audible to humans (17, 18). Bats compute the 3D location of objects from acoustic information carried by echoes of their sonar vocalizations. The bat computes the horizontal and vertical positions of targets from differences in the perceived arrival time, intensity, and spectrum of echoes at the two ears (19), relying on the same acoustic cues as any “standard mammalian auditory system” (19, 20). The bat estimates target range from the time delay between the outgoing vocalization and returning echo (21); some bat species show extraordinary spatial discrimination along the range axis, with thresholds for range changes <0.1 mm (22, 23). Furthermore, the bat's sonar system is used for assessing the detailed properties of the target: the bat perceives the size of an object from the intensity of echoes (24), the target velocity from the Doppler shift of the echoes (25), and the object's shape from the spectrum of the echoes (26). In fact, bats are able to use their sonar for high-level perceptual tasks such as object recognition and classification (26–29) and even for texture discrimination, e.g., the roughness of surfaces (30, 31). Thus, echolocation is an exquisite sensory system that can provide the bat with very detailed information about the environment.
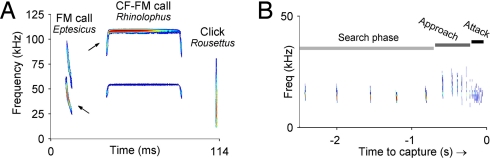
The structure of an individual echolocation call, or “pulse,” follows one of three basic designs. (i) Frequency-modulated (FM) calls, with durations of 0.5–20 ms, often containing harmonics (Fig. 1A Left). Bats that use these calls are known as “FM bats,” and they constitute the majority of echolocating bat species. FM calls can be further subdivided based on finer acoustic properties of their calls and on the signal's adaptations to natural habitats (12). (ii) Constant-frequency (CF) calls are produced by a number of microchiropteran bat species in the Old World (Rhinolophid and Hipposiderid bats) and the New World (Pteronotus parnellii). CF calls can last several tens of milliseconds, and the echo's Doppler shifts allow the bat to estimate target motion. These calls often begin and end with a short FM component (Fig. 1A Center). Bats that produce these calls are known as “CF-FM bats.” (iii) Megachiropteran bats of the genus Rousettus produce very brief ultrasonic clicks (Fig. 1A Right), as short as 40–50 μs (18), similar to the sonar clicks produced by whales and dolphins (32, 33). Unlike FM and CF-FM bats that produce their calls through the larynx, the clicks of Rousettus are produced via the tongue, but like FM and CF-FM bats, Rousettus can use their echolocation signals to orient within complex environments, even in complete darkness (34, 35). It is interesting to note that recent molecular phylogenetic studies have identified all of the Megachiropteran bats, including Rousettus, as the closest relatives of Rhinolophidae and other CF-FM bats (12).
Fig. 1.
Biosonar behavior of echolocating bats. (A) Spectrogram representation (frequency × time) of echolocation calls of three bat species: big brown bat (E. fuscus), which produces FM echolocation calls; lesser horseshoe bat (Rhinolophus hipposideros), which produces CF-FM calls; and Egyptian fruit bat (Rousettus aegyptiacus), which uses clicks for echolocation. Red color indicates maximal intensity. Arrows point to the dominant harmonic: first harmonic in Eptesicus and second harmonic in Rhinolophus. The last two calls were recorded in Israel, courtesy of B. Fenton (University of Western Ontario, London, ON, Canada) and A. Tsoar (Hebrew University of Jerusalem, Jerusalem). (B) Spectrogram of a sequence of FM calls produced by a European free-tailed bat as it chased an insect. Gray bars denote the three echolocation phases of insect-pursuit: search→ approach → terminal phase (attack) (recorded in Israel by N.U. and B. Fenton).
In addition to categorization by their call structure (Fig. 1A), echolocating bat species can be categorized by the duty cycle of their calls, i.e., the percentage of time that their pulses occupy, with most FM bats having a low duty cycle of <15% and most CF-FM bats a high duty cycle of >30% (36, 37)—as well as by the strength of their calls (intense echolocators vs. “whispering bats”); both of these categorizations are related to important ecological and evolutionary aspects of bat echolocation (reviewed in refs. 12 and 15). Bats are often categorized also by their foraging habitat, such as foraging in open space (far from echo-returning objects) or near edges of forests or hunting inside vegetation (15). In fact, the signal structure of each bat species is often suited remarkably well to the bat's foraging habitat. Moreover, as discussed in detail below, the bat's signals may change rapidly and adaptively according to the task at hand, in a manner that can often be well understood from the mathematics of sonar theory.
The bat's echolocation system, or biological sonar, is an active sensing system, sharing characteristics with other active sensing systems in nature (33): the echolocation system of whales and dolphins (32); the electrolocation system of weakly electric fish (38); the whisking-somatosensory system of rodents (4); the olfactory systems of rodents and humans, which strongly rely on active sniffing (39), and of star-nosed moles and water shrews, which actively produce air bubbles underwater to inhale odors (7); and the visual system of primates, which use eye movements to scan the environment (40). The common denominator of all these systems is the importance of the motor component for perception: either production of the signals used to probe the environment (in echolocation and electrolocation) or the movement of the sensor organs (in whisking, sniffing, and vision).
In the case of echolocating bats, the motor repertoire of call production is highly adaptive, both to the environmental context in which the bat operates and to the immediate task demands. A simple example of an adaptive sensory-motor behavior in FM bats is the sequence of changes in echolocation calls produced during hunting (Fig. 1B). As a bat approaches and attacks an insect, the bandwidth of individual calls increases and the rate at which calls are produced also increases, allowing the animal to more accurately and rapidly sample the position and features of a sonar target (32, 41). Similar adaptive changes in echolocation calls occur also in CF-FM and click-producing bats (15, 34) and have been used by researchers to delineate three “phases” of insect pursuit: the “search mode,” “approach mode,” and “terminal phase” (attack mode) (15, 41). Notably, during search mode, the silent intervals between echolocation calls in FM bats can be quite long, often lasting several hundred milliseconds (17, 42) (e.g., Fig. 1B); thus, the FM bat's calls and echoes are similar to the discrete light flashes of a stroboscope. Despite the bat's stroboscopic-like ensonification of the environment, its agile and smooth flight suggests the bat may experience a stable and continuous perceptual world, constructed from the discontinuous arrival of sonar echoes. From an experimental standpoint, the stroboscopic nature of echolocation presents the opportunity for researchers to noninvasively record the timing of sensory inputs in freely moving animals, a powerful tool in neuroscience research, as elaborated below.
At the neurobiological level, the auditory system of echolocating bats has been the focus of intense investigation over the last four decades in several bat species (not all aspects of the neurobiology of echolocation have been studied in all species, and hence the bat species will be mentioned below in every case). We will briefly review here several key findings (for detailed reviews, see refs. 20, 32, and 43). (i) Echo-delay (target range) tuning of neurons, (ii) frequency tuning and “auditory fovea,” (iii) modularity of cortical organization, (iv) dynamic response latency, (v) stimulus-duration tuning, and (vi) object-selective neurons and scale invariance.
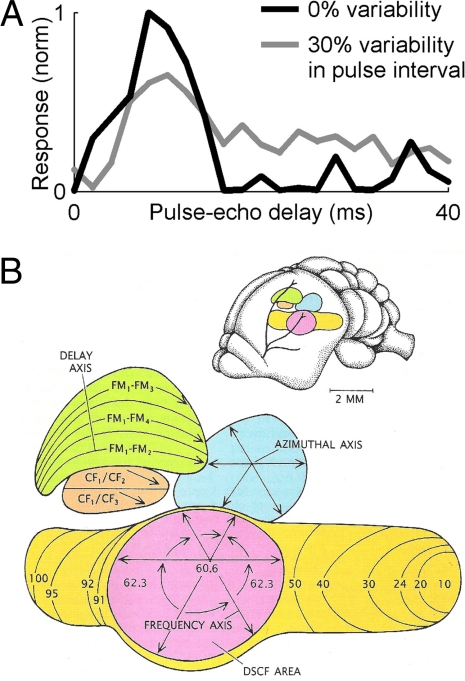
First, the auditory systems of several bat species contain “delay-tuned neurons,” which show facilitated responses when presented with pairs of sounds, a simulated pulse and an echo, separated by a particular time interval (Fig. 2A, black line); this interval (the “best delay”) varies across the neural population, spanning the extent of biologically relevant echo delays encountered by the bat (20, 43–45). These neurons are believed to subserve the computation of target distance by the bat. Delay-tuned neurons have been found in the bat's ascending auditory system (20) and in the midbrain superior colliculus of the big brown bat, Eptesicus fuscus (46), where these neurons are tuned not only to echo delay (target range) but also to echo azimuth and elevation, creating in effect a 3D representation of target location in the bat's midbrain.
Fig. 2.
Neural responses in the auditory system of echolocating bats are suited to behavioral demands. (A) Echo-delay tuning of a “delay-tuned neuron” from the superior colliculus of awake restrained big brown bat, measured using a fixed interval of 100 ms between successively presented calls (black line, 0% variability) or using sequences with 30% variability in the intercall interval (gray line) (data from G. Gifford, University of Maryland, Baltimore). (B) Organization of the auditory cortex of the mustached bat (see Bat Echolocation: From Behavior to Neurobiology for details) (adapted with permission from ref. 10; art courtesy of Patricia J. Wynne).
Second, neurons in the auditory system of CF-FM bats, such as the mustached and horseshoe bats, show extraordinarily sharp frequency tuning, and the frequencies of the bat's dominant CF components are overrepresented throughout the ascending auditory pathway, from the cochlea up to the auditory cortex, forming an “acoustic fovea.” The ultranarrow frequency tuning of these neurons allows the bat to detect Doppler shifts of target echoes, both for computing the velocity of the insect and for detecting the rapid Doppler modulations caused by the fluttering wings of insects (20).
Third, the auditory cortex of the mustached bat contains separate functional modules, which differ in the response properties of their neurons. Doppler-shift constant frequency area (DSCF, Fig. 2B) contains neurons that specialize in detecting rapid Doppler modulations (wing flutter), the CF/CF areas contain neurons that respond selectively to Doppler magnitude (target velocity), and the FM-FM areas contain neurons that respond selectively to echo delay or target range (20, 43). In fact, the discovery of this series of specialized cortical areas during the 1970s and 1980s, with each field processing different aspects of echo information, was an important example that revived the idea of regional specializations in sensory cortices. Interestingly, more recently, it has been demonstrated that these cortical areas are responsive also to the bat's communication calls (47), not just its sonar calls, suggesting some alternative interpretations for the fundamental role of these brain regions. Furthermore, such striking functional organization has not been found in the auditory cortex of FM bats (20). These species differences are not well understood; light on this issue may be shed by using novel optical recording techniques, which allow efficient mapping of responses of all of the neurons in a given cortical area (8).
Fourth, unlike the typical sustained responses in the auditory cortex of monkeys or cats (48), the responses in bat auditory cortex are usually very brief (45), and in the big brown bat, it has been shown that the latency of these brief responses has an important functional property, whereby long-latency neurons exhibit narrow echo-delay tuning and short-latency neurons exhibit wide tuning (45). Thus, after each echolocation call, the population of neurons in this bat's auditory cortex conveys initially coarse-grained information about the target's position, followed by an ultrafast sharpening of the auditory image with the passage of time after each echolocation call, similar to the sharpening of visual images in multiresolution image processing algorithms (45).
Fifth, neurons in the midbrain and cortex of several bat species exhibit tuning to sound duration (49). Such tuning, which was found also in a few other vertebrate species (49), may contribute in bats to processing of the dynamically changing temporal features of bat calls.
Finally, object-selective neurons were recently discovered in bat auditory cortex; these neurons responded to auditory objects in a size-invariant manner (50). Furthermore, as will be discussed below, bats track individual spatial objects by “locking” their sonar beam onto a selected target, suggesting that bats may be an excellent model for studying the neural representation of auditory objects. Taken together, research on the bat's auditory system over the years has demonstrated many principles of good design, in which neural properties show matching to the bat's behavioral requirements (16, 20).
By comparison, neurobiological research over the years has placed considerably less emphasis on the motor side of echolocation; we direct the reader to a comprehensive review of the bat vocal-motor system (Schuller and Moss in ref. 32). More recent research findings that specifically consider the role of the bat's vocal behavior in perception, action, and spatial memory are the focus of this Perspective and are detailed below.
What Is It Like To Be a Bat (51)?
When foraging in real-life situations, the echolocating bat faces multiple complex tasks [see supporting information (SI) Fig. S1], the execution of which requires three key elements, on which we focus in the remainder of this article. (i) Auditory scene analysis is required for separating the echoes that return from multiple targets, clutter, and obstacles and segregating them quickly into distinct auditory objects and for filtering out the calls of other bats. (ii) Sensorimotor transformations are required for rapidly converting sensory information into motor actions, which is necessary for catching prey and for avoiding collisions with obstacles. (iii) Spatial memory is required for navigating the “flyway” from the bat's roost to its hunting grounds, for finding a pup or a roosting spot inside a cave, and in some bat species also for long-range annual migrations (52–54). As we argue below, bats face some of the most difficult neurobiological challenges of any mammal, in the sensory, motor, and memory domains, and solve them with apparent ease and grace. We assert that a deeper understanding of the interactions between these domains can be attained by turning attention to what the bat's voice tells the bat's brain.
Difficult Problem no. 1: Auditory Scene Analysis.
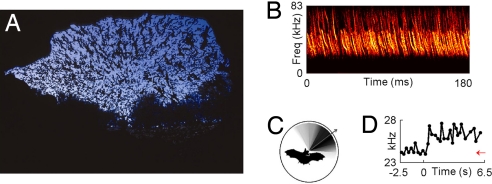
Auditory scene analysis is defined as the ability of animals to separate and group concurrent sounds from several sources into their constituent auditory objects, thus reconstructing the “auditory scene” (55). A well known example is the “cocktail party effect,” whereby humans can listen to one speaker in a cocktail party and ignore others (55). Auditory scene analysis is a very difficult problem in the case of the bat, because each of the bat's vocalizations results in a barrage of echoes from the ground, tree branches, insects, and other objects, and the bat needs to group and segregate all these dynamic auditory sources into a coherent spatial scene—and this must be done extremely rapidly, because the bat flies at speeds up to 10 meters per second. Moreover, the problem is made even more difficult when multiple bats are flying together, requiring the bat to segregate echoes returning from its own call from the calls and echoes of other bats. In some extreme cases, such as the emergence of millions of Brazilian free-tailed bats from caves in Texas and New Mexico, there are thousands of animals in the air simultaneously, nearly brushing wings with each other (Fig. 3A). Under the simplifying assumption that all these bats ensonify the dozens of objects that surround the entrance to the cave, this means that each bat receives a huge number of echoes: nechoes ≈ nbats × nobjects, and this number can reach tens of thousands or even hundreds of thousands of echoes. This multitude of calls and echoes is illustrated in Fig. 3B. We call this problem the “cocktail party nightmare,” and it raises the question: How can the bat solve this sensory processing problem and avoid potentially deadly collisions?
Fig. 3.
The problem of auditory scene analysis in bats. (A) Millions of Brazilian free-tailed bats streaming out of Bracken Cave in Texas at dusk (photo courtesy of Merlin D. Tuttle, Bat Conservation International). (B) Spectrogram of calls produced by Brazilian free-tailed bats emerging from Ney Cave, another large bat colony in Texas. The extreme density of calls and echoes illustrates the problem of the “cocktail-party nightmare” (see Difficult Problem no. 1). Note that the average interval between calls of an individual bat in this species is ≈200 ms (ref. 64), longer than the 180 ms shown here (recording by E. Gillam). (C) The directional emission pattern of big brown bat: black color, high intensity of emissions; white, low intensity. Arrow shows the beam aim, direction of the sonar beam's maximal intensity. Beam shape was measured by K. Ghose, using an array of 16 microphones. (D) Example of jamming avoidance response in a Brazilian free-tailed bat that was suddenly presented at t = 0 with a sequence of echolocation calls at a frequency of 24.3 kHz (red arrow); in response to this stimulus, the bat rapidly shifted the frequency of its calls upward (black line) (data from experiment by E. Gillam (University of Tennessee, Knoxville), G. McCracken (University of Tennessee, Knoxville), and N.U. (64).
There are several possible solutions, which are probably used synergistically. First, the bat could use the directionality of its hearing (19) to perform “spatial filtering” of echoes arriving from different directions. In addition, some bats actively move their external ears (pinna) in synchrony with their echolocation calls, which may further improve spatial filtering; such rhythmic pinna movements have been shown in some CF-FM bats (56) and in the click-producing bat, Rousettus aegyptiacus (57). Most important, spatial filtering is aided also by the directionality of the bat's sonar emissions (Fig. 3C). Further, recent work demonstrates that the free-flying bat scans obstacles and targets by pointing its sonar beam sequentially at different objects, which could facilitate perceptual grouping of dynamic echoes from the same object into streams (58), suggesting the bat may use its beam as a “spotlight of attention.”
Second, the bat could “tag” the timing of its own calls and thus be able to process only its own echoes and ignore the calls and echoes from other bats. This tagging could be achieved, for example, by an “efference copy” signal from the bat's vocal-motor system, and neural recordings from the midbrain of a bat engaged in sonar target tracking provide empirical support for this notion (59). The bat can also use a gating mechanism that allows subsequent echoes to be processed for only a limited period after the call (60). An intriguing mechanism for achieving the required tagging plus gating was demonstrated in studies by Suga et al. (61) based on the finding of “combination-sensitive neurons” in the mustache bat's auditory cortex. This mechanism is detailed in SI Text.
Third, bats could increase the acoustic differences between their own calls and the calls of conspecific bats, to reduce confusion. Such an approach to the jamming problem was shown in the electrolocation system of weakly electric fish, where active changes in the frequency or timing of the electric discharges are exhibited by the fish to avoid jamming; it was termed the “jamming avoidance response” (38). Although several studies provided indirect indications that bats may change the frequency or timing of their calls in the presence of conspecifics (62, 63), there was no direct evidence for this. Recently, however, one of us (N.U.), together with B. Fenton, A. Tsoar, and C. Korine, conducted a field study of European free-tailed bats in the Negev desert of Israel (17) and used analysis techniques borrowed from neurophysiology to demonstrate that pairs of bats flying together maintained a particularly large frequency separation, suggesting a jamming-avoidance response (17). Another demonstration of jamming avoidance was shown very recently by E. Gillam, G. McCracken, and N.U. in a field study of a related bat species, the Brazilian free-tailed bat (64). Sequences of prerecorded bat calls were presented to individual bats as they flew toward a loudspeaker; when the approaching bats were presented with an abrupt change in stimulus frequency, they responded by shifting their own call frequency very rapidly, on average within <200 ms (see example in Fig. 3D). These two studies (17, 64) provided conclusive evidence for a jamming-avoidance response in animals that use biosonar. Interestingly, there are indications that other species of bats that use wideband calls also exhibit jamming avoidance response, whereas bats that use narrowband calls do not (see ref. 64 and discussion therein).
Fourth, note that a jamming-avoidance response would be useful when a few bats are flying together but probably not when thousands of bats are flying together. Under these extreme conditions (e.g., Figs. 3 A and B), a more extreme solution is necessary, such as to simply ignore the barrage of echoes and to rely instead on spatial memory of the familiar underground flight route. And indeed, when a solid door was first placed to block a passageway in the Wyandotte Cave in Indiana during the 19th century, thousands of bats were later found dead after having collided with the door (65); similar incidents occurred in many other caves in the world when solid doors were first placed (13). Clearly, the bats had no sensory limitation in detecting the doors, because they can detect tiny insects using echolocation. Apparently the bats ignored the returning echoes and instead relied on their spatial memory of the familiar route, which was unexpectedly altered by the introduction of a door. Such failures imply that under some conditions, the bat's voice sends no signal to the spatial perceptual system, and instead the bat relies exclusively on spatial memory. By contrast, under other circumstances, the bat's voice does play a key role in spatial memory and navigation, as discussed below (see Difficult Problem no. 3).
Difficult Problem no. 2: Sensorimotor Transformations.
Echolocation is an active sensing system, with the bat itself controlling the rate of echolocation calls, the spectrotemporal structure of each individual call, and the head aim, flight path, and pinna adjustments (13, 16, 66). Thus, there is an inherent behavioral link between the motor behaviors and the auditory images, between action and perception, and vice versa. However, the motor component of echolocation has received comparatively little attention in the neurobiology of echolocation, which has largely focused on the sensory side. Sensorimotor transformation in the case of the bat is a difficult problem, because the echolocation calls and flight path need to be dynamically adjusted by the bat on extremely short timescales, within milliseconds. For example, the entire approach and terminal sequence of a bat, shown in Fig. 1B, lasts typically <1 sec.
The adaptive vocal behaviors exhibited by bats fall broadly into two categories: range-dependent adjustments in the repetition rate and spectro-temporal shape of the call, and velocity-dependent adjustments in sound frequency. Range-dependent adjustments in sonar calls during insect pursuit have been mentioned above (Fig. 1B) and are described in further detail in SI Text 2. A recent study by Sinha and Moss (59) provides evidence that the bat's midbrain superior colliculus may play a role in some of the range-dependent adjustments of sonar call duration.
Range-dependent adaptive changes in echolocation calls occur not only when the bat approaches or tracks an insect but also when approaching obstacles or “clutter” objects such as tree branches or when landing (34, 67, 68). Adaptive increases in call bandwidth are found in individual bats (34, 67, 68), and an interesting parallel was also reported across bat species (69). One way to explain these clutter-related changes in call design comes from classic sonar theory (70), whereby broadband calls enable better range accuracy in estimating the positions of the clutter and of the target (70), which helps in figure-ground segregation (see also ref. 21, which supports this explanation). Another way to explain such changes in the spectrotemporal shape (spectrogram) of echolocation calls is that the bat not only improves accuracy per se but also minimizes the systematic errors introduced into the ranging estimate by its own motion (refs. 70 and 71; see details in SI Text 3). However, because potential ranging errors are typically just a few centimeters in magnitude (70, 71), it is unclear whether compensating for these errors would provide any behavioral benefit. Finally, although increases in call rate in the presence of clutter have been known for many years (67), a recent study has demonstrated a more subtle change in the timing of calls when big brown bats were faced with cluttered conditions (68). In cluttered situations, bats often used groups of several calls with a very stable intercall interval (termed “sonar strobe groups;” ref. 68). To elucidate the possible functional significance of very stable intercall intervals, neurophysiological experiments have been conducted by G. Gifford and C.F.M. in awake restrained bats passively listening to simulated pulse-echo pairs, where the intervals between calls were jittered by varying amounts. Results indicate that the spatial response curves of auditory neurons change with the variability in the intervals between successive sound pairs: response fields of a class of echo-delay tuned neurons in the superior colliculus exhibit facilitated responses and narrow tuning when stable interstimulus intervals are used, but the echo-delay tuning collapses when the stimulus intervals are jittered, even when the jitter is as small as 20–30% (see Fig. 2A). Because neural tuning curves for target range are sharpest under stable signal repetition rates (Fig. 2A, black), we hypothesize that stable repetition rates are important under cluttered situations, when precise ranging accuracy is most needed, which may explain the high incidence of “sonar strobe group” production under cluttered conditions (68). Moreover, the finding that delay tuning collapses under naturalistic jittering of stimulus intervals suggests the need to conduct similar experiments in the auditory cortex and other regions of the bat's ascending auditory system, to test the validity of the common assumption that delay tuning is an invariant property of “delay-tuned neurons.”
Velocity-dependent adjustments in sound frequency are demonstrated by the “Doppler-shift compensation” behavior, exhibited by some species of CF-FM bats, which lower the frequency of their sonar vocalizations to stabilize the frequency of the Doppler-shifted echo during flight (25). The echoes are stabilized at the frequency of the bat's acoustic fovea, where auditory neurons have the narrowest frequency tuning (20), which allows the bat to more easily detect the oscillatory modulations of Doppler shift that arise from fluttering insect prey (27). CF-FM bats can even discriminate different species of insects according to their differing wing-flutter rates (27). Doppler-shift compensation has been studied in detail in bats of the genus Rhinolophus (e.g., refs. 25 and 27); notably, studies of freely vocalizing horseshoe bats have revealed populations of neurons that likely play a role in audiovocal behaviors (72, 73).
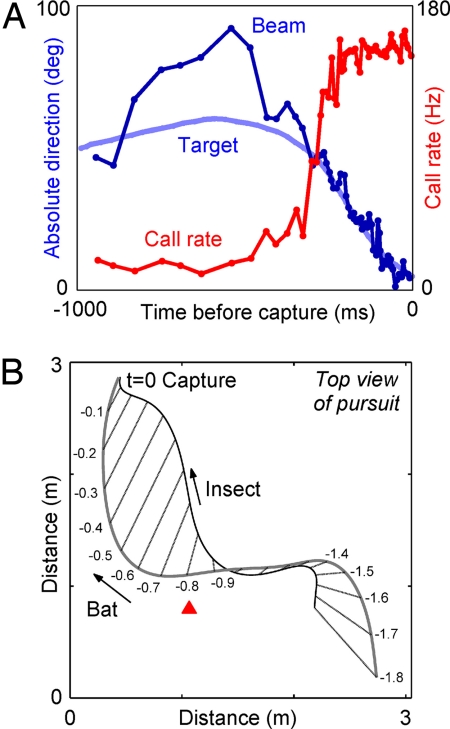
We now turn to another category of adaptive sensory-motor behaviors, which has received attention only in very recent years. This category of behaviors involves adaptive changes in sonar beam aim (head direction) and flight path. These were studied by one of us (C.F.M.) together with K. Ghose, T. Horiuchi, and P. Krishnaprasad, by having big brown bats forage for insects in a large flight room. This room contains two cameras, which allowed reconstruction of the bat's 3D flight path, and an array of 16 microphones, which allowed measurement of the direction where the bat was pointing its ultrasonic emission beam (i.e., measuring the sonar beam axis, or acoustic gaze; Fig. 3C). These experiments showed that when the bat switches from search mode to approach mode of echolocation (i.e., changes the rate of its vocal production), it also points its head toward the target, locking the sonar beam onto the target with an accuracy of ≈2° (66) (see Fig. 4A). When the bat was hunting stationary targets (tethered insects), there was a strong linear relation between the acoustic gaze angle at time t and the flight turn rate at time t + τ. This relation was captured well by a simple linear control law, which may help the bat to simplify the transformation of sensory acoustic information to flight motor commands (66). When the bat was hunting erratically moving targets (flying insects), it still locked its sonar beam onto the target, but in addition, the bat kept a constant absolute direction between its own position and that of the target (Fig. 4B). This constant-absolute direction strategy was shown mathematically to be the optimal strategy for intercepting erratically moving targets, in the sense that it minimizes the time it takes the pursuer to intercept the target (74). These results suggest that the study of flying bats can shed light on pursuit strategies and control laws that may be used generally by locomoting animals.
Fig. 4.
Sensorimotor transformations in echolocating bats. (A) In big brown bats, the pursuit sequence of an insect is composed of an increase in vocal production rate (red), concurrent with changes in the bat's acoustic gaze (blue): the bat “locks” its sonar beam onto the target, seen here by the convergence of the beam direction (dark blue) onto the direction-to-target (light blue) (data by K. Ghose, University of Maryland, Baltimore). (B) Big brown bats use a nearly time-optimal strategy to intercept an erratically flying insect by keeping a constant absolute direction to the target: the bearing lines from the bat to the target (straight lines) are almost parallel to each other during the last 0.8 s of pursuit, starting at the red arrowhead (adapted from ref. 74, with open-access permission from PLoS).
Finally, brain stimulation experiments in the bat provide evidence for a possible involvement of the superior colliculus in production of vocalizations and in controlling the head-aim and pinna movements (75). This raises the possibility that sensorimotor control operates on different time scales through different neural pathways. For example, spatial perception and planning on a time scale of seconds may draw on cortical brain regions, which in turn may modulate the output of lower-level sensorimotor tracking systems that must respond rapidly, on a millisecond time scale, to abrupt changes in the location of targets and obstacles. An assessment of the interaction between these possible mechanisms awaits further experiments with freely behaving animals, which will deepen our understanding of what the bat's voice tells the bat's brain.
Difficult Problem no. 3: Spatial Memory and Navigation.
Echolocating bats rely heavily on spatial memory. The navigational abilities of bats are remarkable in that they show accurate spatial memory on many spatial scales, covering 8 orders of magnitude in space, from the 1,000-km scale (annual migrations) to the 1-cm scale (the accuracy with which bats remember 3D flight paths in laboratory conditions). Moreover, they use spatial memory under very difficult conditions: they fly at night, which makes it more difficult to use visual landmarks, and they fly at high linear and angular velocities, which makes it difficult to integrate self-motion cues (i.e., to use path integration).
Arguably, bats are the best navigators among mammals. On large spatial scales, some bat species exhibit long-range annual migrations (52–54, 76–78), such as the migration of Pipistrellus nathusii in Europe over >1,900 km (Fig. 5A) or Eidolon helvum in Africa over >2,500 km (77), one of the longest migration distances among mammals. Moreover, in some cases, individual bats were shown to return year after year to the same roost, e.g., to the same cave (54, 76, 79, 80), or to the same feeding location, such as Lasiurus cinereus returning annually to feed on insects at the same specific lightpost in Ontario (B. Fenton, personal communication). Homing abilities were also demonstrated in several bat species over distances of up to a few hundred kilometers (52, 81–85).
Fig. 5.
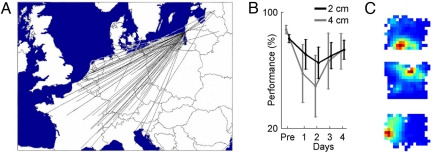
Spatial memory and navigation in echolocating bats. (A) Example of long-range migration by the bat P. nathusii. Straight lines correspond to individual bats that were banded in Eastern Europe and then recaptured as far as Croatia, Italy, and France (adapted with permission from ref. 54). (B) Spatial memory for 3D flight paths, on the centimeter scale: echolocating bats (M. lyra) were trained to fly through an array of wires, and two bats flew through the wires without touching them on >85% of trials (“pre”); when the wires were moved by 4 or even 2 cm, the bats showed a significant drop in performance on days 1–2, followed by slow recovery. Data were measured from ref. 100 and reanalyzed. Error bars, mean ± SEM, computed over all wire-shift trials in these two bats. (C) Place fields of three “place cells,” recorded from the hippocampus of big brown bats, a small bat species weighing ≈15 g, as the animal was crawling in a rectangular arena (103). Blue color, no spiking activity; red, maximum activity of the neuron (data recorded by N.U.).
On medium spatial scales, some bat species were shown to follow fixed routes, or “flyways,” from the roost to the hunting grounds, typically several kilometers in length, and to use the same flyway night after night (71, 83, 86–88). Some bats also use subterranean flyways, navigating out from the depths of large caves, through complex underground passageways that may exceed 3 km in length (65). Further, nectar-feeding bats (“flower bats”) in tropical forests are able to find particular flowers located hundreds of meters apart in the dense rainforest (89). Interestingly, many bat-pollinated flowers possess the unusual property of steady-state flowering, whereby individual flowers secrete nectar for several months consecutively, thus facilitating the use of spatial memory by the bats (89). Equally impressive are the demonstrations, in several forest-dwelling bat species, that individual bats often reuse the same tree across multiple days or weeks (86, 90) and sometimes even across multiple years (90), suggesting that bats can remember the location of a specific tree in the forest.
The year-after-year usage of the same roost, foraging location, or flyway is especially noteworthy, because bats have an unusually long lifespan, sometimes >30 years, 10 times longer than the lifespan of rats or mice (14), indicating that bats might accurately remember spatial locations for dozens of years.
What are the mechanisms underlying bat navigation? Echolocation has a range of no more than ≈100 m for detecting large landmarks (42), because of the strong atmospheric attenuation of ultrasound (91), so echolocation is useful primarily for small- and medium-range navigation (15). Vision also plays a role in bat navigation, especially over long distances. Evidence suggests that bats can navigate toward distal visual beacons such as mountains (84, 92), can use the post-sunset glow in the west (93), or are able to see the starlight of individual stars (94). Magnetic sense was also recently implicated in bat navigation (85). Importantly, in addition to compass-based navigation strategies (93) and navigation toward beacons (84), bats have also been shown to use memory for absolute spatial locations (allocentric navigation) (92, 95, 96), a type of memory believed to depend on the hippo campus (97, 98). We will return below to neurophysiological studies of bat hippocampus.
In small-scale navigation, laboratory studies have shown that echolocation plays a key role. Thus, experiments in complete darkness showed that big brown bats can be trained to fly through a hole in a net, located near a prominent object (a tripod), and they flew unerringly through the hole also when the tripod and hole were moved together. But when the tripod and hole were moved incongruously, the bats crashed into the net at the location adjacent to the tripod, where the bat had learned to expect the hole (99); this suggests the bats used the tripod as an echo-acoustic beacon. The precision and capacity of the bat's spatial memory in 3D are quite remarkable (100, 101). For example, bats of the species Megaderma lyra, trained in wire-avoidance experiments to fly through a grid of wires, developed a preference to fly through particular locations in the grid—but when the grid was unexpectedly moved by several centimeters, the performance of the bats deteriorated, i.e., there was an increase in the rate of collisions with the wires (100). Even when the grid was moved by as little as 2 cm, the performance dropped significantly (see Fig. 5B), suggesting the bat formed a 3D spatial memory on the centimeter scale.
Studies in many animal species have implicated the hippocampus in small-scale (6, 97, 98, 102) and large-scale navigation (11); for example, hippocampal lesions impaired the ability of homing pigeons to navigate over a distance of several tens of kilometers (11). Therefore, it is likely that in bats, many of the navigational abilities described above (although not all of them) depend on the bat's hippocampus. To elucidate how echolocation, the voice of the bat, may affect neural activity and spatial representation in the hippocampus, we conducted a study of hippocampal activity in behaving bats (103). To this end, tetrode-recording techniques (102) were adapted for bats by N.U., which allowed recordings of single-neuron activity from freely crawling bats (103). Big brown bats were trained to forage under small-scale laboratory conditions; the bats foraged for tethered mealworms by crawling in an open-field arena, while we recorded the activity of single neurons in the CA1 area of the hippocampus and local field potentials; the bat's position and echolocation calls were also recorded (103). We found that echolocation calls strongly modulated the neural activity: hippocampal theta oscillation, a 5- to 10-Hz rhythm that in rodents is present primarily when the animal explores the environment or runs at high speeds (97), was present in the bat's hippocampus when the animal did not locomote, but rather when it explored the environment using a high rate of echo-location calls (103). At the single-neuron level, we found place cells in the bat's hippocampus, neurons active only when the animal passed through a restricted region in the arena, termed the place field (Fig. 5C). These place cells in the bat's hippocampus were as common as place cells in the rat, and their place fields were as spatially selective and as stable as in the rat (103), supporting the proposed role of mammalian hippocampus in spatial representation and spatial memory (97). Importantly, preliminary results show a very interesting modulation of bat hippocampal place fields by the timing of individual echolocation calls (N.U., unpublished observations). Much more work still needs to be done on the bat hippocampus to address a range of questions. For example, are there 3D place fields in freely flying bats? How does the use of echolocation vs. vision affect hippocampal neural activity? How do the plastic changes in the bat's sonar signals modulate hippocampal spatial representation? It remains to be seen in further investigations what the bat's voice tells the bat's hippocampus.
Conclusion
In recent years, there has been growing recognition that studies of neural activity in behaving animals are crucial to a more complete understanding of the nervous system. The echolocating bat, a mammal that exhibits an impressive array of well defined and theoretically well understood behaviors, provides a powerful animal model for studies in behavioral neurobiology. We believe that future recordings of neural activity from the free-flying bat, while it engages in the full suite of rich natural behaviors, will yield data that will contribute not only to our understanding of what the bat's voice tells the bat's brain, but also more broadly to our understanding of the behaving brain across species.
Supplementary Material
Acknowledgments.
We thank C. Carr, B. Fenton, J. Fritz, S. Krishnaprasad, L. Las, H.-U. Schnitzler, N. Suga, and A. Tsoar for reading the manuscript. This work was supported by grants from the National Institutes of Health and National Science Foundation, a University of Maryland CNS grant (to C.F.M.), and research grants from the A.M.N. Foundation and from the Chais family program (to N.U.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703550105/DCSupplemental.
References
- 1.Lettvin JY, Maturana HR, McCullough WS, Pitts WH. What the frog's eye tells the frog's brain. Proc Inst Radio Engr. 1959;47:1940–1951. [Google Scholar]
- 2.Pflüger HJ, Menzel R. Neuroethology, its roots and future. J Comp Physiol A. 1999;185:389–392. doi: 10.1007/s003590050399. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nat Neurosci. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- 4.Kleinfeld D, Ahissar E, Diamond ME. Active sensation: Insights from the rodent vibrissa sensorimotor system. Curr Opin Neurobiol. 2006;16:435–444. doi: 10.1016/j.conb.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature. 2004;430:682–686. doi: 10.1038/nature02768. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki WA, Clayton NS. The hippocampus and memory: a comparative and ethological perspective. Curr Opin Neurobiol. 2000;10:768–773. doi: 10.1016/s0959-4388(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 7.Catania KC. Olfaction: Underwater ‘sniffing’ by semi-aquatic mammals. Nature. 2006;444:1024–1025. doi: 10.1038/4441024a. [DOI] [PubMed] [Google Scholar]
- 8.Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature. 2005;433:597–603. doi: 10.1038/nature03274. [DOI] [PubMed] [Google Scholar]
- 9.Van Hooser SD, Heimel JA, Chung S, Nelson SB, Toth LJ. Orientation selectivity without orientation maps in visual cortex of a highly visual mammal. J Neurosci. 2005;25:19–28. doi: 10.1523/JNEUROSCI.4042-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suga N. Biosonar and neural computation in bats. Sci Am. 1990;262:60–68. doi: 10.1038/scientificamerican0690-60. [DOI] [PubMed] [Google Scholar]
- 11.Gagliardo A, Ioale P, Bingman VP. Homing in pigeons: The role of the hippocampal formation in the representation of landmarks used for navigation. J Neurosci. 1999;19:311–315. doi: 10.1523/JNEUROSCI.19-01-00311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones G, Teeling EC. The evolution of echolocation in bats. Trends Ecol Evol. 2006;21:149–156. doi: 10.1016/j.tree.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Griffin DR. Listening in the Dark. New Haven, CT: Yale Univ Press; 1958. [Google Scholar]
- 14.Neuweiler G. The Biology of Bats. Oxford, UK: Oxford Univ Press; 2000. [Google Scholar]
- 15.Schnitzler H-U, Moss CF, Denzinger A. From spatial orientation to food acquisition in echolocating bats. Trends Ecol Evol. 2003;18:386–394. [Google Scholar]
- 16.Jones G, Holderied MW. Bat echolocation calls: Adaptation and convergent evolution. Proc R Soc London Ser B. 2007;274:905–912. doi: 10.1098/rspb.2006.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ulanovsky N, Fenton MB, Tsoar A, Korine C. Dynamics of jamming avoidance in echolocating bats. Proc R Soc London Ser B. 2004;271:1467–1475. doi: 10.1098/rspb.2004.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holland RA, Waters DA, Rayner JM. Echolocation signal structure in the Megachiropteran bat Rousettus aegyptiacus Geoffroy 1810. J Exp Biol. 2004;207:4361–4369. doi: 10.1242/jeb.01288. [DOI] [PubMed] [Google Scholar]
- 19.Aytekin M, Grassi E, Sahota M, Moss CF. The bat head-related transfer function reveals binaural cues for sound localization in azimuth and elevation. J Acoust Soc Am. 2004;116:3594–3605. doi: 10.1121/1.1811412. [DOI] [PubMed] [Google Scholar]
- 20.Popper AN, Fay RR, editors. Hearing by Bats. New York: Springer; 1995. [Google Scholar]
- 21.Simmons JA. The resolution of target range by echolocating bats. J Acoust Soc Am. 1973;54:157–173. doi: 10.1121/1.1913559. [DOI] [PubMed] [Google Scholar]
- 22.Simmons JA. Perception of echo phase information in bat sonar. Science. 1979;204:1336–1338. doi: 10.1126/science.451543. [DOI] [PubMed] [Google Scholar]
- 23.Menne D, Kaipf I, Wagner I, Ostwald J, Schnitzler H-U. Range estimation by echolocation in the bat Eptesicus fuscus: Trading of phase versus time cues. J Acoust Soc Am. 1989;85:2642–2650. doi: 10.1121/1.397758. [DOI] [PubMed] [Google Scholar]
- 24.Simmons JA, Vernon JA. Echolocation: Discrimination of targets by the bat, Eptesicus fuscus. J Exp Zool. 1971;176:315–328. doi: 10.1002/jez.1401760307. [DOI] [PubMed] [Google Scholar]
- 25.Schnitzler H-U. Die ultraschallortungslaute der hufeisennasen-fledermause (Chiroptera, Rhinolophidae) in verschiedenen orientierungssituationen. J Vergl Physiol. 1968;57:376–408. [Google Scholar]
- 26.Simmons JA, et al. Target structure and echo spectral discrimination by echolocating bats. Science. 1974;186:1130–1132. doi: 10.1126/science.186.4169.1130. [DOI] [PubMed] [Google Scholar]
- 27.von der Emde G, Schnitzler H-U. Classification of insects by echolocating greater horseshoe bats. J Comp Physiol A. 1990;167:423–430. [Google Scholar]
- 28.Thiele J, Winter Y. Hierarchical strategy for relocating food targets in flower bats: Spatial memory versus cue-directed search. Anim Behav. 2005;69:315–327. [Google Scholar]
- 29.Holderied MW, von Helversen O. ‘Binaural echo disparity’ as a potential indicator of object orientation and cue for object recognition in echolocating nectar-feeding bats. J Exp Biol. 2006;209:3457–3468. doi: 10.1242/jeb.02386. [DOI] [PubMed] [Google Scholar]
- 30.Zagaeski M, Moss CF. J Acoust Soc Am. 1994;(Suppl 3aAB4):2881. doi: 10.1121/1.409843. [DOI] [PubMed] [Google Scholar]
- 31.Grunwald JE, Schornich S, Wiegrebe L. Classification of natural textures in echolocation. Proc Natl Acad Sci USA. 2004;101:5670–5674. doi: 10.1073/pnas.0308029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas JA, Moss CF, Vater M, editors. Echolocation in Bats and Dolphins. Chicago: Univ of Chicago Press; 2004. [Google Scholar]
- 33.Nelson ME, MacIver MA. Sensory acquisition in active sensing systems. J Comp Physiol A. 2006;192:573–586. doi: 10.1007/s00359-006-0099-4. [DOI] [PubMed] [Google Scholar]
- 34.Herbert H. Echoortungsverhalten des Flughundes Rousettus aegyptiacus (Megachiroptera) Z Säugetierkunde. 1985;50:141–152. [Google Scholar]
- 35.Holland RA, Winter P, Waters DA. Sensory systems and spatial memory in the fruit bat Rousettus aegyptiacus. Ethology. 2005;111:715–725. [Google Scholar]
- 36.Obrist MK, Fenton MB, Eger JL, Schlegel PA. What ears do for bats: A comparative study of pinna sound pressure transformation in chiroptera. J Exp Biol. 1993;180:119–152. doi: 10.1242/jeb.180.1.119. [DOI] [PubMed] [Google Scholar]
- 37.Jones G. Scaling of echolocation call parameters in bats. J Exp Biol. 1999;202:3359–3367. doi: 10.1242/jeb.202.23.3359. [DOI] [PubMed] [Google Scholar]
- 38.Heiligenberg W. Neural Nets in Electric Fish. Boston: MIT Press; 1991. [Google Scholar]
- 39.Mainland J, Sobel N. The sniff is part of the olfactory percept. Chem Senses. 2006;31:181–196. doi: 10.1093/chemse/bjj012. [DOI] [PubMed] [Google Scholar]
- 40.Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. doi: 10.1016/s0896-6273(01)00466-4. [DOI] [PubMed] [Google Scholar]
- 41.Griffin DR, Webster FA, Michael CR. The echolocation of flying insects by bats. Animal Behav. 1960;8:141–154. [Google Scholar]
- 42.Holderied MW, von Helversen O. Echolocation range and wingbeat period match in aerial-hawking bats. Proc R Soc London Ser B. 2003;270:2293–2299. doi: 10.1098/rspb.2003.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suga N. Principles of auditory information-processing derived from neuroethology. J Exp Biol. 1989;146:277–286. doi: 10.1242/jeb.146.1.277. [DOI] [PubMed] [Google Scholar]
- 44.O'Neill WE, Suga N. Target range-sensitive neurons in the auditory cortex of the mustache bat. Science. 1979;203:69–73. doi: 10.1126/science.758681. [DOI] [PubMed] [Google Scholar]
- 45.Dear SP, Simmons JA, Fritz J. A possible neuronal basis for representation of acoustic scenes in auditory cortex of the big brown bat. Nature. 1993;364:620–623. doi: 10.1038/364620a0. [DOI] [PubMed] [Google Scholar]
- 46.Valentine DE, Moss CF. Spatially selective auditory responses in the superior colliculus of the echolocating bat. J Neurosci. 1997;17:1720–1733. doi: 10.1523/JNEUROSCI.17-05-01720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ohlemiller KK, Kanwal JS, Suga N. Facilitative responses to species-specific calls in cortical FM-FM neurons of the mustached bat. NeuroReport. 1996;7:1749–1755. doi: 10.1097/00001756-199607290-00011. [DOI] [PubMed] [Google Scholar]
- 48.Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci. 2003;6:391–398. doi: 10.1038/nn1032. [DOI] [PubMed] [Google Scholar]
- 49.Covey E, Casseday JH. Timing in the auditory system of the bat. Annu Rev Physiol. 1999;61:457–476. doi: 10.1146/annurev.physiol.61.1.457. [DOI] [PubMed] [Google Scholar]
- 50.Firzlaff U, Schuchmann M, Grunwald JE, Schuller G, Wiegrebe L. Object-oriented echo perception and cortical representation in echolocating bats. PLoS Biol. 2007;5:e100. doi: 10.1371/journal.pbio.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagel T. What is it like to be a bat? Philos Rev. 1974;83:435–450. [Google Scholar]
- 52.Griffin DR. In: Biology of Bats. Wimsatt WA, editor. Vol 1. New York: Academic; 1970. pp. 233–64. [Google Scholar]
- 53.Fleming TH, Eby P. In: Bat Ecology. Kunz TH, Fenton MB, editors. Chicago: Univ of Chicago Press; 2003. pp. 156–208. [Google Scholar]
- 54.Hutterer R, Ivanova T, Meyer-Cords C, Rodrigues L. Bat Migrations in Europe. Bonn, Germany: German Agency for Nature Conservation; 2005. [Google Scholar]
- 55.Bregman AS. Auditory Scene Analysis. Boston: MIT Press; 1990. [Google Scholar]
- 56.Mogdans J, Ostwald J, Schnitzler H-U. The role of pinna movement for the localization of vertical and horizontal wire obstacles in the greater horseshoe bat, Rhinolopus ferrumequinum. J Acoust Soc Am. 1988;84:1676–1679. [Google Scholar]
- 57.Holland RA, Waters DA. Echolocation signals and pinnae movements in the fruit bat Rousettus aegyptiacus. Acta Chiropt. 2005;7:83–90. [Google Scholar]
- 58.Moss CF, Surlykke A. Auditory scene analysis by echolocation in bats. J Acoust Soc Am. 2001;110:2207–2226. doi: 10.1121/1.1398051. [DOI] [PubMed] [Google Scholar]
- 59.Sinha SR, Moss CF. Vocal premotor activity in the superior colliculus. J Neurosci. 2007;27:98–110. doi: 10.1523/JNEUROSCI.2683-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roverud RC, Grinnell AD. J Comp Physiol A. 1985;156:457–470. [Google Scholar]
- 61.Suga N, O'Neill WE, Manabe T. Cortical neurons sensitive to combinations of information-bearing elements of biosonar signals in the mustache bat. Science. 1978;200:778–781. doi: 10.1126/science.644320. [DOI] [PubMed] [Google Scholar]
- 62.Habersetzer J. Adaptive echolocation sounds in the bat Rhinopoma hardwickei. J Comp Physiol. 1981;144:559–566. [Google Scholar]
- 63.Obrist MK. Flexible bat echolocation: The influence of individual, habitat and conspecifics on sonar signal design. Behav Ecol Sociobiol. 1995;36:207–219. [Google Scholar]
- 64.Gillam EH, Ulanovsky N, McCracken GF. Rapid jamming avoidance in biosonar. Proc R Soc London Ser B. 2007;274:651–660. doi: 10.1098/rspb.2006.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blatcheley WS. Indiana Caves and Their Fauna. Indianapolis: 21st Annual Report of the Indiana Department of Geology and Natural Resources; 1896. pp. 121–212. [Google Scholar]
- 66.Ghose K, Moss CF. Steering by hearing: A bat's acoustic gaze is linked to its flight motor output by a delayed, adaptive linear law. J Neurosci. 2006;26:1704–1710. doi: 10.1523/JNEUROSCI.4315-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalko EKV, Schnitzler H-U. Plasticity in echolocation signals of European pipistrelle bats in search flight: Implications for habitat use and prey detection. Behav Ecol Sociobiol. 1993;33:415–428. [Google Scholar]
- 68.Moss CF, Bohn K, Gilkenson H, Surlykke A. Active listening for spatial orientation in a complex auditory scene. PLoS Biol. 2006;4:e79. doi: 10.1371/journal.pbio.0040079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Siemers BM, Schnitzler H-U. Echolocation signals reflect niche differentiation in five sympatric congeneric bat species. Nature. 2004;429:657–661. doi: 10.1038/nature02547. [DOI] [PubMed] [Google Scholar]
- 70.Simmons JA, Stein RA. Acoustic imaging in bat sonar: Echolocation signals and the evolution of echolocation. J Comp Physiol A. 1980;135:61–84. [Google Scholar]
- 71.Holderied MW, Jones G, von Helversen O. Flight and echolocation behaviour of whiskered bats commuting along a hedgerow: range-dependent sonar signal design, Doppler tolerance and evidence for ‘acoustic focussing’. J Exp Biol. 2006;209:1816–1826. doi: 10.1242/jeb.02194. [DOI] [PubMed] [Google Scholar]
- 72.Metzner W. A possible neuronal basis for Doppler-shift compensation in echolocating horseshoe bats. Nature. 1989;341:529–532. doi: 10.1038/341529a0. [DOI] [PubMed] [Google Scholar]
- 73.Smotherman M, Zhang S, Metzner W. A neural basis for auditory feedback control of vocal pitch. J Neurosci. 2003;23:1464–1477. doi: 10.1523/JNEUROSCI.23-04-01464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghose K, Horiuchi TK, Krishnaprasad PS, Moss CF. Echolocating bats use a nearly time-optimal strategy to intercept prey. PLoS Biol. 2006;4:e108. doi: 10.1371/journal.pbio.0040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valentine DE, Sinha SR, Moss CF. Orienting responses and vocalizations produced by microstimulation in the superior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol A. 2002;188:89–108. doi: 10.1007/s00359-001-0275-5. [DOI] [PubMed] [Google Scholar]
- 76.Villa BR, Cockrum EL. Migration in the guano bat Tadarida brasiliensis mexicana (Saussure) J Mamm. 1962;43:43–64. [Google Scholar]
- 77.Richter HV, Cumming GS. First application of satellite telemetry to track African straw-coloured fruit bat migration. J Zool. 2008;275:172–176. [Google Scholar]
- 78.Glass BP. Seasonal movements of Mexican freetail bats Tadarida brasiliensis mexicana banded in the Great Plains. Southwest Nat. 1982;27:127–133. [Google Scholar]
- 79.Constantine DG. Activity patterns of the Mexican free-tailed bat. Univ New Mexico Publ Biol. 1967;7:7–79. [Google Scholar]
- 80.Fenton MB. Summer activity of Myotis lucifugus (Chiroptera: Vespertilionidae) at hibernacula in Ontario and Quebec. Can J Zool. 1969;47:597–602. [Google Scholar]
- 81.Williams TC, Williams JM. Radio tracking of homing bats. Science. 1967;155:1435–1436. doi: 10.1126/science.155.3768.1435. [DOI] [PubMed] [Google Scholar]
- 82.Mueller HC, Emlen J. Homing in bats. Science. 1957;126:307–308. doi: 10.1126/science.126.3268.307. [DOI] [PubMed] [Google Scholar]
- 83.Williams TC, Williams JM. Radio tracking of homing and feeding flights of a neotropical bat, Phyllostomus hastatus. Anim Behav. 1970;18:302–309. doi: 10.1016/s0003-3472(66)80047-7. [DOI] [PubMed] [Google Scholar]
- 84.Williams TC, Williams JM, Griffin DR. The homing ability of the neotropical bat Phyllostomus hastatus, with evidence for visual orientation. Anim Behav. 1966;14:468–473. doi: 10.1016/s0003-3472(66)80047-7. [DOI] [PubMed] [Google Scholar]
- 85.Holland RA, Thorup K, Vonhof MJ, Cochran WW, Wikelski M. Navigation: Bat orientation using Earth's magnetic field. Nature. 2006;444:702. doi: 10.1038/444702a. [DOI] [PubMed] [Google Scholar]
- 86.Bernard E, Fenton MB. Bat mobility and roosts in a fragmented landscape in central Amazonia, Brazil. Biotropica. 2003;35:262–277. [Google Scholar]
- 87.Rydell J. Feeding territoriality in female northern bats, Eptesicus nilssoni. Ethology. 1986;72:329–337. [Google Scholar]
- 88.Racey PA, Swift SM. Feeding ecology of Pipistrellus pipistrellus (Ciroptera: Vespertilionidae) during pregnancy and lactation. I. Foraging behaviour. J Anim Ecol. 1985;54:205–215. [Google Scholar]
- 89.von Helversen O, Winter Y. In: Bat Ecology. Kunz TH, Fenton MB, editors. Chicago: Univ of Chicago Press; 2003. pp. 346–397. [Google Scholar]
- 90.Lewis SE. Roost fidelity of bats: A review. J Mamm. 1995;76:481–496. [Google Scholar]
- 91.Lawrence BD, Simmons JA. Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc Am. 1982;71:585–590. doi: 10.1121/1.387529. [DOI] [PubMed] [Google Scholar]
- 92.Gallistel CR. The Organization of Learning. Boston: MIT Press; 1990. [Google Scholar]
- 93.Buchler ER, Childs SB. Use of the post-sunset glow as an orientation cue by big brown bats (Eptesicus fuscus) J Mamm. 1982;63:243–247. [Google Scholar]
- 94.Childs SB, Buchler ER. Perception of simulated stars by Eptesicus fuscus (Vespertilionidae): A potential navigational mechanism. Anim Behav. 1981;29:1028–1035. [Google Scholar]
- 95.Mueller HC, Mueller NS. Sensory basis for spatial memory in bats. J Mamm. 1979;60:198–201. [Google Scholar]
- 96.Gunier WJ, Elder WH. Experimental homing of gray bats to a maternity colony in a Missouri barn. Am Midl Natur. 1971;86:502–506. [Google Scholar]
- 97.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford, UK: Oxford Univ Press; 1978. [Google Scholar]
- 98.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 99.Jensen ME, Moss CF, Surlykke A. Echolocating bats can use acoustic landmarks for spatial orientation. J Exp Biol. 2005;208:4399–4410. doi: 10.1242/jeb.01901. [DOI] [PubMed] [Google Scholar]
- 100.Neuweiler G, Möhres FP. Die rolle des ortsgedachtnisses bei der orientierung der Grossblatt-Fledermaus, Megaderma Lyra. Zeit Ver Physiol. 1967;57:147–171. [Google Scholar]
- 101.Winter Y, Stich KP. Foraging in a complex naturalistic environment: Capacity of spatial working memory in flower bats. J Exp Biol. 2005;208:539–548. doi: 10.1242/jeb.01416. [DOI] [PubMed] [Google Scholar]
- 102.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 103.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.