Abstract
Bacillus anthracis is a spore-forming bacterium that causes anthrax in humans and in other mammals. The glycoprotein BclA (Bacillus collagen-like protein of anthracis) is a major constituent of the exosporium, the outermost surface of B. anthracis spores. The glycosyl part of BclA is an oligosaccharide composed of 2-O-methyl-4-(3-hydroxy-3-methylbutanamido)-4,6-dideoxy-d-glucose, referred to as anthrose, and three rhamnose residues. A structure similar to anthrose, 4-(3-hydroxy-3-methylbutanamido)-4,6-dideoxy-d-glucose is found in the side chain of the capsular polysaccharide (CPS) of Shewanella spp. MR-4. Under certain growth conditions the bacteria produce a variant CPS lacking one methyl group on the hydroxybutyrate, 4-(3-hydroxybutanamido)-4,6-dideoxy-d-glucose. Contrary to anthrose, neither of the Shewanella CPSs is 2-O methylated. Here, we report that both Shewanella CPS variants react with anti-B. anthracis spore sera. We also found that these antisera reacted with flagellae of Pseudomonas syringae, reported to be glycosylated with a similar terminal saccharide, 4-(3-hydroxybutanamido)-4,6-dideoxy-2-O-methyl-d-glucose. Sera produced by immunization with Shewanella or P. syringae cells bound to B. anthracis spores but not to Bacillus cereus spores in a fluorescent microscopy assay. These experiments show that methylation of the anthrose at the O-2 of the sugar ring and at the C-3 of 3-hydroxybutyrate are not essential for induction of cross-reactive antibodies. We report the preparation, characterization, and antibody responses to protein conjugates of the two variants of Shewanella CPS. Both conjugates induced antibodies that bound to both Shewanella CPS variants by ELISA and to B. anthracis spores, as detected by fluorescent microscopy. We propose the use of Shewanella CPS conjugates as a component of an anthrax vaccine.
Keywords: anthrose, capsule, pseudomonas syringae, shewanella, flagellae
Anthrax, a potentially lethal human infection, is a zoonotic disease contracted by humans under natural conditions directly or indirectly from domesticated animals. Disease manifestations occur according to one of three routes of encounter: (i) cutaneous, the most common, by contact with animals or their products; (ii) inhalational, the most serious; or (iii) gastrointestinal (1). The causative organism, Bacillus anthracis, exists in vegetative or in spore form. In its vegetative form, B. anthracis produces two virulence factors that are essential for pathogenesis: the anthrax toxin and the capsule (2). Spores are the infecting agent. They resist extreme heat, dryness, and aggressive chemical conditions and survive for decades in the soil (3).
Spores of B. anthracis are surrounded by a loose layer, the exosporium, composed of a number of proteins. A major protein, called BclA (Bacillus collagen-like protein of anthracis), was shown recently to be a glycoprotein containing short O-linked sugar chains. Its structure (Fig. 1A) was elucidated by NMR and mass spectroscopy (4). It contains three rhamnose residues substituted by an unusual nonreducing terminal sugar, 2-O-methyl-4-(3-hydroxy-3-methylbutanamido)-4,6-dideoxy-d-glucopyranose, named anthrose. The reducing end rhamnose is most likely attached to the protein through a GalNAc moiety. This oligosaccharide (OS) was chemically synthesized in several laboratories and, when covalently attached to a protein, shown to be immunogenic in animals (5–8).
Fig. 1.
Structures of saccharides of Bacillus anthracis BclA glycoprotein (4) (A); Shewanella spp. MR-4 CPS (both structures are present and the ratio depends on the growing conditions) (9) (B and C); and Pseudomonas syringae pv. tabaci 6605 flagellin glycan (10) (D).
The spore OS was reported to be unique for bacilli spores (4, 9). However, carbohydrates of similar structures are present on two other bacteria. The capsular polysaccharide (CPS) of the marine organism Shewanella spp. MR-4 (10) contains side chains with terminal residues of 4-amino-4,6-dideoxy-d-glucopyranose (Qui4N) substituted with 3-hydroxy-3-methylbutyrate or 3-hydroxybutyrate, in different ratios depending on the growth medium (Fig. 1 B and C). Another structure similar to anthrose was found on flagella of Pseudomonas syringae, a plant pathogen (Fig. 1D). It contains two rhamnose residues and a terminal 4-(3-hydroxybutanamido)-4,6-dideoxy-2-O-methyl-d-glucopyranose, thus differing from the anthrax OS by the replacement of 3-hydroxy-3-methylbutyrate with 3-hydroxybutyrate at the amino group of Qui4N (11). In this study we report the serological cross-reactivity of Shewanella and P. syringae saccharides with anthrax spores and the preparation of Shewanella CPS–protein conjugates as a potential component of improved anthrax vaccines.
Results
Characterization of Shewanella spp. MR-4 CPS.
Two types of CPS were purified from Shewanella spp. MR-4: CPSTSB (CPS purified from bacteria grown in Tryptic Soy Broth) and CPSCDM (CPS purified from bacteria grown in chemically defined media). NMR analyses confirmed that the terminal Qui4N was substituted with 3-hydroxy-3-methylbutyrate and 3-hydroxy-butyrate at an approximately 1:1 ratio in CPSTSB and almost entirely (>95%) with 3-hydroxy-butyrate in CPSCDM, in agreement with the reported structures (10). Both CPSs formed viscous solutions and eluted as a single broad peak starting at the void volume of the Sepharose CL-6B column.
Anti-B. anthracis spore and anti-anthrose sera precipitated with both CPSTSB and CPSCDM by immunodiffusion. Table 1 shows that both antisera bound to both forms of Shewanella CSP by ELISA, but the anti-spore serum had a higher titer against CPSCDM than against CPSTSB; the reverse was observed with the anti-anthrose serum.
Table 1.
Binding of rabbit anti-spore and rabbit anti-anthrose sera to Shewanella spp. MR-4 CPS
| Coating antigen | IgG titers* |
|
|---|---|---|
| Anti-spore | Anti-anthrose | |
| CPSTSB | 1600 | 12,800 |
| CPSCDM | 3200 | 500 |
*IgG titer was defined as the serum dilution that gave OD405 of 1 by ELISA. The OD405 of preimmune rabbit serum was <0.08 at the dilution of 1:20.
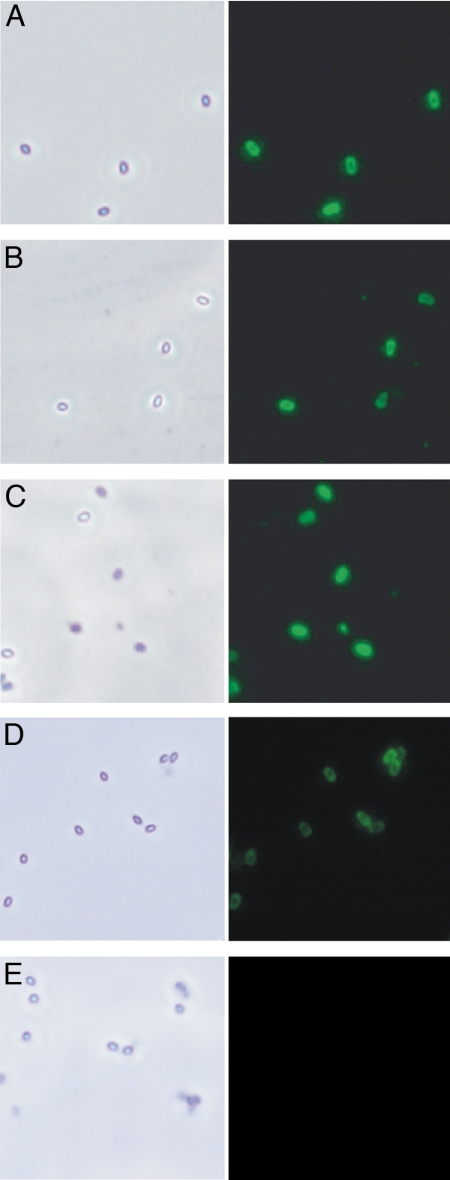
Fluorescence microscopy showed that antiserum induced by Shewanella spp. MR-4 bound to B. anthracis spores (Fig. 2). There was no binding to Bacillus cereus spores (data not shown).
Fig. 2.
Immunofluorescent staining of B. anthracis spores. (Left) Phase-contrast microscopy showing the spores. (Right) Spores treated with hyperimmune anti-Shewanella spp. MR-4 (A); anti-Conjugate no. 1: BSA/CPSTSB (B); anti-Conjugate no. 2: BSA/CPSCDM (C); hyperimmune anti-P. syringae (D); and preimmune control serum (E).
Characterization of P. syringae Flagellae and LPS.
Anti-B. anthracis spore and anti-Shewanella spp. MR-4 sera precipitated with P. syringae flagellae by immunodiffusion, confirming that the common sugar, present on B. anthracis spores, the Shewanella capsule, and the P. syringae flagellae, is cross-reactive. Because P. syringae flagellae could not be isolated free of LPS, the O-specific polysaccharide (O-SP) of P. syringae LPS was isolated, and its structure was analyzed. The results are presented in Table 2: the structure of the O-SP was found to be identical to that of the described P. syringae pv. tabaci 225 serogroup VIII O-SP (12). It did not contain any anthrose-like sugar, and thus the cross-reactivity was ascribed to the glycosylated flagellae. This result was confirmed by Western blot analyses, in which flagellae but not LPS reacted with both anti-spore and anti-anthrose sera (data not shown).
Table 2.
NMR data for P. syringae pv. tabaci 6605 O-SP (δ, ppm; 60°C)
| Residue | Nucleus | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| α-Rha A | H | 4.93 | 4.04 | 4.14 | 3.59 | 4.43 | 1.32 |
| C | 97.1 | 71.1 | 70.1 | 84.0 | 68.2 | 17.9 | |
| α-Rha B | H | 4.96 | 4.15 | 3.96 | 3.73 | 4.02 | 1.29 |
| C | 97.2 | 67.9 | 73.5 | 77.3 | 69.5 | 18.0 | |
| α-Rha C | H | 4.67 | 4.29 | 3.64 | 3.47 | 3.49 | 1.36 |
| C | 101.8 | 68.2 | 78.1 | 71.5 | 73.4 | 18.1 | |
| β-GlcNAc D | H | 4.55 | 3.77 | 3.52 | 3.38 | 3.41 | 3.76/3.94 |
| C | 102.0 | 57.2 | 75.1 | 71.9 | 77.3 | 63.0 | |
| Structure | 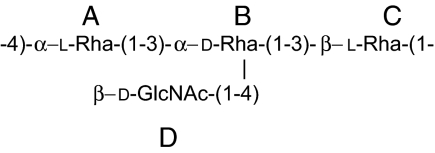 |
||||||
Pseudomonas syringae pv. tabaci 6605 O-SP repeating unit structure is as shown. NAc at E2: C-1 175.9 ppm, H-2/C-2 2.01/23.3 ppm.
Fluorescence microscopy showed that sera induced by P. syringae bound to B. anthracis (Fig. 2) but not to B. cereus spores. The P. syringae anthrose-like sugar contained only 3-hydroxy-butyrate groups, but the antibodies induced by it bound to spores that were reported to carry only 3-hydroxy-3-methylbutyrate groups, indicating that this methyl group is not essential for cross-reactivity.
Characterization of Shewanella spp. MR-4 CSP Conjugates.
Both conjugates, BSA/CPSTSB and BSA/CPSCDM, formed a line of identity with anti-BSA and anti-spore sera by immunodiffusion. Both conjugates had high molecular masses, as shown by their elution at the void volume of the Sepharose CL-6B column. The protein/sugar ratios are shown in Table 3. BSA/CPSTSB had lower sugar content than BSA/CPSCDM, probably because the BSA used for its preparation had a lower hydrazide content (5.2%) than the one used for BSA/CPSCDM (9.8%). Both conjugates had <5 endotoxin units/μg as determined by the limulus amoebocyte lysate assay.
Table 3.
Composition and geometric means (GM) of mouse IgG anti-Shewanella MR-4 CPS induced by BSA conjugates of CPSTSB and CPSCDM
| Conjugate | Protein/saccharide ratio, wt/wt | GM of IgG after 3rd injection, EU |
|
|---|---|---|---|
| Coating antigen CPSTSB | Coating antigen CPSCDM | ||
| BSA/CPSTSB | 2:1 | 54 | 43 |
| BSA/CPSCDM | 0.7:1 | 31 | 32 |
Mice (10 per group) were injected s.c. with 2.5 μg saccharide as a conjugate/mouse 3 times (2 wk apart) and killed 1 wk after the last injection. Antibody levels were calculated relative to a hyperimmune anti-Shewanella spp. MR-4 mouse serum that was assigned a value of 100 EU.
BSA/CPSTSB induced higher antibody levels to CPSTSB than to CPSCDM [54 vs. 43 ELISA units (EU)] but the difference was not statistically significant. There was no difference between antibody levels induced by BSA/CPSCDM and the two CPS (31 vs. 32 EU; Table 2).
Fluorescence microscopy showed that serum induced by both conjugates reacted with B. anthracis spores (Fig. 2). In CPSCDM and in P. syringae flagella, a 3-hydroxy-butyrate group was found in place of the 3-hydroxy-3-methylbutyrate present in B. anthracis spores. Also, both Shewanella CPSs lacked the methyl group at O-2, which was described in anthrose. This suggests that none of these methyl groups is essential for cross-reactivity.
Discussion
The anthrose-containing oligosaccharide present on the exosporium protein BclA was reported to be unique to B. anthracis spores (4, 9); however, the anthrose biosynthesis genes were recently also identified in other bacilli (13). Rabbit antibodies to B. anthracis spores protected mice against B. anthracis infection (14), as did immunization of mice and guinea pigs with live or attenuated spores, improving the protection afforded by the Protective Antigen alone (15, 16). We have shown that saccharides of similar structures in other bacteria cross-react with B. anthracis spores. We describe binding of Shewanella spp. MR-4 CPS and P. syringae flagellae to antibodies induced by live B. anthracis spores or by synthetic anthrose-containing trisaccharide conjugates. Moreover, antibodies induced by whole cells of either of these two bacterial strains or by conjugates prepared with two variants of Shewanella CPS bound to B. anthracis spores but not to B. cereus spores. Our results also show that the presence of methyl groups in anthrose, either on the O-2 of the monosaccharide or the C-3 of butyrate, are not essential for the cross-reactivity.
Antibodies induced by these and possibly other cross-reactive moieties, as well as low levels of anti-toxin and anti-capsule found in adults, may explain the relative resistance of humans to anthrax (1, 17). Similarly, immunity to Haemophilus influenzae type b (Hib) in adults is induced by cross-reactive saccharides containing ribitol-phosphate, including those in Escherichia coli K100 CPS, Staphylococcus aureus teichoic acid, and other bacteria (18). It is therefore possible that the group most susceptible to anthrax is infants and children who have not yet developed such antibodies. This group may be the one most in need of active vaccination were protection against bioterroristic attack deemed essential.
The conjugates of Shewanella CPS described here were prepared and injected in accordance with a clinical protocol and in a similar manner to the preparation of Hib conjugates. We propose to add a Shewanella conjugate to future improved anthrax vaccines. Furthermore, whole-cell vaccines prepared with either Shewanella or P. syringae may be useful in veterinary practice.
Materials and Methods
Growth of Bacteria.
The following bacterial strains were used in the study: B. anthracis Ames 35, an avirulent, Sterne-type strain lacking plasmid pXO2 (19); B. cereus strain 569 (20); Shewanella spp. strain MR-4, a gift from J. A. Gralnick (Department of Microbiology, BioTechnology Institute, University of Minnesota, St. Paul, MN); and P. syringae pv. tabaci 6605, a gift from Yuki Ichinose (Meiji University, Kawasaki, Japan). Shewanella spp. MR-4 was grown in either TSB (Difco Laboratories) or in CDM as described (10). P. syringae were kept on King's medium plates and cultured in LB broth (Difco Laboratories) for 48 h at 25°C as described (11).
Isolation and Purification of Shewanella CPS.
CPS was isolated from the cell surface by vigorous stirring and purified as described (21), followed by ultracentrifugation (Sorvall Discovery 100SE, rotor type 55.2TI, Hitachi, Ltd., Tokyo) at 35,000 rpm for 5 h at 5°C to remove LPS. The supernatant was freeze-dried and passed through a Sepharose CL-6B column (1 × 100 cm) in 0.2 M NaCl. A single high-molecular-mass polysaccharide was obtained for each CPS and designated CPSTSB and CPSCMP according to its culture media. CPS structures were confirmed by NMR (10).
Isolation of P. syringae Flagella, LPS, and O-SP.
Flagella were separated from P. syringae cells by vigorous stirring and purified by ultracentrifugation as described (22). LPS was extracted by hot phenol (23). To obtain the O-SP, LPS was treated with 1% acetic acid at 100°C for 1 h, Lipid A was removed by centrifugation, and the soluble products separated by a Sephadex G-50 column (1 × 100 cm) in pyridine/acetic acid/water buffer (4/8/988). Void volume fractions were freeze-dried and analyzed by NMR.
NMR Spectroscopy.
NMR spectra were recorded at 60°C in D2O on a Varian UNITY INOVA 500 instrument, using acetone as reference for proton (2.225 ppm) and carbon (31.5 ppm) spectra. Varian standard programs COSY, ROESY (mixing time of 400 ms), TOCSY (spinlock time of 120 ms), HSQC, and HMBC (long-range transfer delay of 100 ms) were used. Spectra were assigned by using the Topspin program (Bruker).
Analytical Methods.
Sugar concentration was measured by the phenol/H2SO4 assay (24), protein concentration was measured by the method of Lowry (25), and hydrazide was measured by the trinitrobenzenesulphonic acid assay (26). SDS/PAGE was done using 14% gels, and separated material was electroblotted onto PVDF membranes according to the manufacturer's protocol (Bio-Rad) and reacted with anti-spore and anti-anthrose sera. Double immunodiffusion was performed by using 1% agarose in PBS. Endotoxic activity was measured by the limulus amoebocyte lysate assay as described by the manufacturer (Cambrex).
Antisera.
Hyperimmune sera against Shewanella sp. MR-4 and P. syringae pv. tabaci 6605 were prepared with heat-killed whole bacteria as described (27). Rabbit sera against whole anthrax spores (anti-spore) and against synthetic anthrax spore trisaccharide-keyhole limpet hemocyanin conjugate (anti-anthrose) (5) were supplied by Conrad Quinn (Centers for Disease Control, Atlanta, GA).
Preparation of Conjugates.
Conjugate No. 1.
BSA was derivatized with adipic acid dihydrazide (ADH) as described (26). The product [BSA-adipic acid hydrazide (BSA-AH)] contained 5.2% AH groups (wt %). CPSTSB (10 mg) was dissolved in 1 ml 0.2 M NaCl and the pH was adjusted to 5.5. Next, 10 mg of BSA-AH in 0.5 ml 0.2 M NaCl was added, followed by 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) to a final concentration of 0.1 M EDC. The pH was maintained with an automatic titrator (MeterLab) at pH 5.5 for 4 h at room temperature. Next, the product was dialyzed against 0.2 M NaCl, overnight at 4°C and purified by gel filtration by using a Sepharose CL-6B column (1 × 100 cm) in 0.2 M NaCl. The obtained conjugate was designated BSA/CPSTSB.
Conjugate No. 2.
BSA was derivatized with ADH by using the conditions listed above. The obtained product, BSA-AH, contained 9.8% AH groups. CPSCMP (10 mg) was dissolved in 1 ml 0.2 M NaCl and the pH was adjusted to 5.5, 10 mg of BSA-AH was added, and the reaction proceeded as above. The obtained conjugate was designated BSA/CPSCMP.
Immunization.
Five- to 6-week-old female National Institutes of Health Swiss–Webster mice were injected s.c. three times at 2-wk intervals with 2.5 μg CPS as a conjugate in 0.1 ml PBS. Groups of 10 mice were killed 7 d after the third injections (27). Controls received PBS.
Antibody.
Antibody levels to Shewanella CPS were measured by ELISA by using CovaLink plates (Nalge Nunc Intern) (28). The plates were coated with 100 μl per well CPSTSB or CPSCMP (5 μg/ml, determined to be optimal by checker board titration) dissolved in 10 mM 1-methylimidazole buffer (pH 7.0), and EDC was added to a final concentration of 50 mM and incubated at 37°C overnight. The plates were washed six times with 0.1% Brij 35-saline and blocked with 1% human serum albumin (HSA) in PBS for 1 h at room temperature. Twofold dilutions of the sera were made in 1% HSA-0.1% Brij 35-saline, incubated at 37°C for 4 h, and then washed six times. Goat anti-mouse IgG conjugated to alkaline phosphatase was added, and the mixture was incubated at 37°C for 3 h. Then, 4-nitrophenylphosphate [1 mg/ml in 1 M Tris·HCl buffer (pH 9.8), containing 0.3 mM MgSO4] was added. The OD405 was read after 30 min in an MR600 microplate reader (Dynatech).
Fluorescence Microscopy.
B. anthracis Ames 35 and B. cereus 569 were grown on nutrient sporulation agar at 37°C for 2 d (29). Spores were purified by washing the plates with deionized water, and the spore suspension incubated at 65°C for 30 min. The spore suspension was then washed twice with deionized water and subjected to immunofluorescent staining as described (30) with the following modification. The spore suspensions were applied to coverslips treated with 1% polylysine (Sigma), blocked with 3% milk in PBS for 30 min, and rabbit anti-spore or anti-anthrose, or mouse anti-Shewanella or anti-P. syringae sera added. After three washes in PBS, the coverslips were treated with a secondary antibody, either goat anti-mouse or goat anti-rabbit IgG conjugated to fluorescent dye AF488 (Molecular Probes). After staining, the coverslips were mounted onto slides and examined with a Nikon Eclipse TE2000-U fluorescence microscope.
Acknowledgments.
We thank Chunyan Gou and Chris Mocca for technical assistance. This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Child Health and Human Development, and National Institute of Allergy and Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
References
- 1.Spencer RC. Bacillus anthracis. J Clin Pathol. 2003;56:182–187. doi: 10.1136/jcp.56.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scorpio A, Blank TE, Day WA, Chabot DJ. Anthrax vaccines: Pasteur to the present. Cell Mol Life Sci. 2006;63:2237–2248. doi: 10.1007/s00018-006-6312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RP. Bacillus anthracis and other spore forming bacilli. In: Braude AI, Davis LE, Fierer J, editors. Infectious Disease and Medical Microbiology. Philadelphia: Saunders; 1986. pp. 270–278. [Google Scholar]
- 4.Daubenspeck JM, et al. Novel oligosaccharide side chains of the collagen-like region of BclA, the major glycoprotein of the Bacillus anthracis exosporium. J Biol Chem. 2004;279:30945–30953. doi: 10.1074/jbc.M401613200. [DOI] [PubMed] [Google Scholar]
- 5.Mehta AS, et al. Synthesis and antigenic analysis of the BclA glycoprotein oligosaccharide from the Bacillus anthracis exosporium. Chemistry. 2006;12:9136–9149. doi: 10.1002/chem.200601245. [DOI] [PubMed] [Google Scholar]
- 6.Saksena R, Adamo R, Kovác P. Studies toward a conjugate vaccine for anthrax. Synthesis and characterization of anthrose [4,6-dideoxy-4-(3-hydroxy-3-methylbutanamido)-2-O-methyl-d-glucopyranose] and its methyl glycosides. Carbohydr Res. 2005;340:1591–1600. doi: 10.1016/j.carres.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Werz DB, Seeberger PH. Total synthesis of antigen Bacillus anthracis tetrasaccharide—creation of an anthrax vaccine candidate. Angew Chem Int Ed Engl. 2005;44:6315–6318. doi: 10.1002/anie.200502615. [DOI] [PubMed] [Google Scholar]
- 8.Crich D, Vinigradova O. Synthesis pf the antigenic tetrasaccharide side chain from the major glycoprotein of Bacillus anthracis exosporium. J Org Chem. 2007;72:6513–6520. doi: 10.1021/jo070750s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamborrini M, Werz DB, Frey J, Pluschke G, Seeberger PH. Anti-carbohydrate antibodies for the detection of anthrax spores. Angew Chem Int Ed Engl. 2006;45:6581–6582. doi: 10.1002/anie.200602048. [DOI] [PubMed] [Google Scholar]
- 10.Vinogradov E, Nossova L, Korenevsky A, Beveridge TJ. The structure of the capsular polysaccharide of Shewanella oneidensis strain MR-4. Carbohydr Res. 2005;340:1750–1753. doi: 10.1016/j.carres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi K, et al. Flagellin glycans from two pathovars of Pseudomonas syringae contain rhamnose in D and L configurations in different ratios and modified 4-amino-4,6-dideoxyglucose. J Bacteriol. 2007;189:6945–6956. doi: 10.1128/JB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinogradov E, et al. Somatic antigens of pseudomonads: Structure of the O-specific polysaccharide chain of Pseudomonas syringae pv. tabaci 225 (serogroup VIII) lipopolysaccharide. Carbohydr Res. 1991;212:307–311. doi: 10.1016/0008-6215(91)84071-l. [DOI] [PubMed] [Google Scholar]
- 13.Dong S, et al. Anthrose biosynthetic operon of Bacillus anthracis. J Bacteriol. 2008;190:2350–2359. doi: 10.1128/JB.01899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enkhtuya J, et al. Significant passive protective effect against anthrax by antibody to Bacillus anthracis inactivated spores that lack two virulence plasmids. Microbiology. 2006;152:3103–3110. doi: 10.1099/mic.0.28788-0. [DOI] [PubMed] [Google Scholar]
- 15.Brossier F, Levy M, Mock M. Anthrax spores make an essential contribution to vaccine efficacy. Infect Immun. 2002;70:661–664. doi: 10.1128/iai.70.2.661-664.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen S, et al. Attenuated nontoxinogenic and nonencapsulated recombinant Bacillus anthracis spore vaccines protect against anthrax. Infect Immun. 2000;68:4549–4558. doi: 10.1128/iai.68.8.4549-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer DE, et al. Serum IgG antibody response to the protective antigen (PA) of Bacillus anthracis induced by anthrax vaccine adsorbed (AVA) among U.S. military personnel. Vaccine. 2008;26:869–873. doi: 10.1016/j.vaccine.2007.11.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradshaw MW, Schneerson R, Parke JC, Jr, Robbins JB. Bacterial antigens cross-reactive with the capsular polysaccharide of Haemophilus influenzae type b. Lancet. 1971;1:1095–1096. doi: 10.1016/s0140-6736(71)91837-x. [DOI] [PubMed] [Google Scholar]
- 19.Pomerantsev A, Sitaraman R, Galloway CR, Kivovich V, Leppla SH. Genome engineering in Bacillus anthracis using cre recombinase. Infect Immun. 2006;74:682–693. doi: 10.1128/IAI.74.1.682-693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruhfel RE, Robillard NJ, Thorne CB. Interspecies transduction of plasmids among Bacillus anthracis, B cereus, and B thuringiensis. J Bacteriol. 1984;157:708–711. doi: 10.1128/jb.157.3.708-711.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues LP, Schneerson R, Robbins JB. Immunity to Hemophilus influenzae type b. I. The isolation, and some physicochemical, serologic and biologic properties of the capsular polysaccharide of Hemophilus influenzae type b. J Immunol. 1971;107:1071–1080. [PubMed] [Google Scholar]
- 22.Taguchi F, et al. Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell Microbiol. 2006;8:923–938. doi: 10.1111/j.1462-5822.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 23.Westphal O, Jann K. Extraction with phenol-water and further application of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 24.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 25.Lowry JO, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu CY, et al. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991;59:4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zielen S, et al. Simple determination of polysaccharide specific antibodies by means of chemically modified ELISA plates. J Immunol Methods. 1996;193:1–7. doi: 10.1016/0022-1759(96)00033-6. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer P, Ionesco H, Ryter A, Balassa G. Sporulation of Bacillus subtilis: Genetic and physiological study. Colloq Int CNRS. 1963;124:553–563. [Google Scholar]
- 30.Hu H, Sa Q, Koehler TM, Aronson AI, Zhou D. Inactivation of Bacillus anthracis spores in murine primary macrophages. Cell Microbiol. 2006;8:1634–1642. doi: 10.1111/j.1462-5822.2006.00738.x. [DOI] [PubMed] [Google Scholar]