Abstract
TNF-α is associated with the development of interstitial fibrosis. We have demonstrated that the p38 mitogen-activated protein (MAP) kinase regulates TNF-α expression in monocytes exposed to asbestos. In this report, we asked if extracellular signal–regulated kinase (ERK) was also involved in TNF-α expression in monocytes exposed to asbestos. We found that p38 and ERK were differentially activated in alveolar macrophages obtained from patients with asbestosis compared with normal subjects. More specifically, p38 was constitutively active and ERK activation was suppressed. Since the upstream pathway leading to ERK was intact, we hypothesized that an ERK-specific phosphatase was, in part, responsible for the decreased ERK activity. We evaluated whether the dual specificity phosphatase MAP kinase phosphatase (MKP)-3, which is highly expressed in the lung and specifically dephosphorylates ERK, was increased after exposure to asbestos. We found that MKP-3 increased after exposure to asbestos, and its expression was regulated by p38. We found that p38 and ERK negatively regulated one another, and MKP-3 had a role in this differential activation. We also found that p38 was a positive regulator and ERK was a negative regulator of TNF-α gene expression. Cells overexpressing MKP-3 had a significant increase in TNF-α gene expression, suggesting than an environment favoring p38 MAP kinase activation is necessary for TNF-α production in monocytes exposed to asbestos. Taken together, these data demonstrate that the p38 MAP kinase down-regulates ERK via activation of MKP-3 in human monocytes exposed to asbestos to enhance TNF-α gene expression.
Keywords: asbestos, monocytes, MAP kinase, phosphatase, TNF-α
CLINICAL RELEVANCE
The release of TNF-α by alveolar macrophages in patients with asbestosis plays an integral role in the pathogenesis of the disease. These studies uncover mechanisms that occur in monocytes/macrophages leading to TNF-α gene expression.
Alveolar macrophages are involved in the pathogenesis of immune and inflammatory disorders in the lung. One characteristic feature of alveolar macrophages obtained from patients with chronic lung disorders, such as asbestosis, is that they spontaneously release cytokines (1–4). The release of cytokines contributes to the inflammatory response and is associated with the development of interstitial fibrosis. The regulation of cytokine gene expression in response to most stimuli, such as endotoxin, has been well described. In most of these cases, the second messenger pathways work in a synergistic manner to induce cytokine gene expression (5–12). Although asbestos is known to induce cytokine production, there is limited data on the signaling pathways linking asbestos to cytokine gene expression.
One example of such second messenger pathways includes the mitogen-activated protein (MAP) kinases. The MAP kinases are a family of second messengers that are essential for transferring signals from the cell surface to the nucleus. Multiple studies have demonstrated that MAP kinases are activated by asbestos (13–18). We previously demonstrated that the p38 MAP kinase is essential for regulating TNF-α gene expression in human monocytes exposed to asbestos (13), and others have shown extracellular signal–regulated kinase (ERK) MAP kinase activation in asbestos-exposed epithelial and mesothelial cells (15, 16, 18). Most of these studies have shown that the role of ERK involves regulating cell cycle progression and cellular transformation. To our knowledge, no study has evaluated the role of ERK in regulating asbestos-induced TNF-α gene expression.
The modulation of MAP kinase activity by the dual specificity MAP kinase phosphatases (MKPs) is an important mechanism of regulating the transfer of signals from the cell surface to the nucleus. There are 10 different MKPs, which are a subgroup of the protein tyrosine phosphatases. MKPs have a common structure with a carboxyl-terminal catalytic domain and an amino-terminal noncatalytic domain that is responsible for binding with MAP kinase substrates (19, 20). These phosphatases are capable of dephosphorylating both phosphotyrosine and phosphothreonine residues. MKP-3 is a dual specificity phosphatase that has high expression in the lung, specifically inactivates ERK, and is found primarily in the cytoplasm, which prevents the translocation of ERK to the nucleus (21–24). One study has also shown that MKP-3 is expressed constitutively and is not induced by stress or mitogens (24). Other studies have demonstrated that the catalytic activity of MKP-3 is dependent on ERK binding to the amino-terminal domain, which induces a conformation change that rearranges the active site in the catalytic domain (22, 25–28). This activation of MKP-3 is independent of ERK activity; that is, ERK does not have to be active for MKP-3 to be activated. No study, however, has evaluated the ability of asbestos to regulate MKP-3 expression and activity, or its role in modulating TNF-α gene expression in human monocytes.
Alveolar macrophages obtained from the lungs of patients with chronic lung diseases, including asbestosis, have been shown to resemble monocytes (1, 2, 29, 30). In addition, studies have reported functional differences in cytokine release when normal alveolar macrophages are compared with normal blood monocytes or when compared with alveolar macrophages from patients with chronic lung disorders (3, 13, 31–34). Due to the fact that we have shown previously that the p38 MAP kinase regulates TNF-α gene expression in human monocytes (5–7, 13), we ask in the present study if ERK was also involved in regulating TNF-α expression. We found that ERK was not phosphorylated in alveolar macrophages obtained from patients with asbestosis, as measured by the absence of phosphorylated ERK. In contrast, p38 was constitutively active in these alveolar macrophages. Based on these findings, we hypothesized that p38 regulated the dephosphorylation of ERK in human monocytes exposed to asbestos. The dual specificity phosphatase MKP-3 was increased in human monocytes after exposure to asbestos, and inhibition of p38 decreased its expression. MKP-3 removal by immunoprecipitation resulted in recovery of ERK phosphorylation. To determine the effect of ERK activity on TNF-α gene expression, we overexpressed ERK by transfecting a constitutive active MEK1, which is the upstream kinase that activates ERK, with a reporter vector driven by a TNF-α promoter. Human monocytes expressing the constitutive active MEK1 had a significant decrease in p38 MAP kinase activation and TNF-α promoter activity compared with cells expressing the empty vector. In addition, overexpression of a dominant-negative ERK2 or a wild-type MKP-3 resulted in enhanced TNF-α promoter activity compared with expression of an empty vector. To demonstrate additional biological relevance, we found that alveolar macrophages obtained from the lungs of patients with asbestosis have increased MKP-3 expression compared with cells obtained from normal subjects. Taken together, these data suggest that the p38 MAP kinase down-regulates ERK via activation of MKP-3. These data also suggest that MKP-3 expression augments TNF-α gene expression in human monocytes exposed to asbestos. Furthermore, TNF-α gene expression in human monocytes exposed to asbestos is dependent on a balance of MAP kinase activity that favors an active p38 MAP kinase.
MATERIALS AND METHODS
Cells
The Human Subjects Review Board of the University of Iowa Carver College of Medicine approved the protocol of obtaining alveolar macrophages and blood monocytes by bronchoalveolar lavage and by phlebotomy, respectively, from normal volunteers. Normal volunteers had to meet the following criteria: (1) age between 18 and 55 years; (2) no history of cardiopulmonary disease or other chronic disease; (3) no prescription or nonprescription medication except oral contraceptives; (4) no recent or current evidence of infection; and (5) lifetime nonsmoker. Alveolar macrophages were also obtained from patients with asbestosis. Patients with asbestosis had to meet the following criteria: (1) FEV1 and DlCO at least 60% predicted; (2) current nonsmoker; (3) no recent or current evidence of infection; and (4) evidence of restrictive physiology on pulmonary function tests and interstitial fibrosis on chest computed tomography. Fiberoptic bronchoscopy with bronchoalveolar lavage was performed after subjects received intramuscular atropine, 0.6 mg, and local anesthesia. Each subsegment of the lung was lavaged with five 20-ml aliquots of normal saline, and the first aliquot in each was discarded. The percentage of alveolar macrophages was determined by Wright-Giemsa stain and varied from 90 to 98%. Blood monocytes were obtained by phlebotomy and were isolated by Ficoll-gradient centrifugation. Cells were plated in serum-free RPMI 1640 (Gibco, Carlsbad, CA) and allowed to adhere for 1 hour before experiments. For some of the experiments, a human monocyte cell line, THP-1 cells, was used (American Type Cell Culture, Mannassas, VA). THP-1 cells were maintained in RPMI 1640 containing 10 mM HEPES, 1 mM sodium pyruvate, 4.5 g/L glucose, 1.5 g/L bicarbonate, 2 mM L-glutamine, and 10% fetal bovine serum.
Plasmids, siRNA, and Transfections
The pTL-luc and the pTL-ELK-1 were obtained from the TransLucent in vivo Kinase Assay Kit (Panomics, Redwood City, CA) and were used according to the manufacturer's instructions. In this assay, ELK-1 is fused to a Gal4 DNA-binding domain. The ELK-1–Gal4 fusion protein is constitutively expressed in transfected cells. When ELK-1 is phosphorylated by the ERK MAP kinase, the fusion protein binds upstream of the luciferase gene in the pTL-Luc reporter plasmid and activates transcription of the luciferase. The pTRE-luc and pTET-ATF plasmids were obtained from Clontech and were used according the manufacturer's instructions. TNF-α gene expression was evaluated using a −600 TNF-α–CAT plasmid (a generous gift from Dr. Tom Maniatis, Harvard University, Cambridge, MA) that has been previously described (35). The pCMV-MEK1 and pcDNA-HA-ERK2 (K/A) plasmids (generous gifts from Dr. Roger Davis, University of Massachusetts, Worcester, MA) and the pRKF-MKP3 and pRKF-MKP3 (C293S) plasmids (generous gifts from Dr. Michael Karin, University of San Diego, La Jolla, CA) have been previously described (36, 37). Transfections were performed using the Fugene transfection reagent (Roche, Indianapolis, IN), according to the manufacturer's instructions. Twenty-four hours after transfection, the cells were exposed to crocidolite asbestos (NAIMA Fiber Repository) at a dose of 10 μg/cm2. Chloramphenicol acetyltransferase (CAT) assays, which were normalized to total protein, were performed after 24 hours of exposure to crocidolite asbestos. Cells were harvested in 0.25 M Tris, pH 7.0 buffer and incubated at 60°C for 30 minutes. Cell supernatants were incubated with 0.1 μCi of [14C] chloramphenicol and 1.0 mM acetyl coenzyme A for 2 hours at 37°C. Acetylated derivatives (CM3-AC and CM1-AC) were separated from nonacetylated chloramphenicol (CM) by ascending thin layer chromatography in chloroform/methanol (95:5) solvent. CAT activity was determined by autoradiography.
The p38 (Dharmacon, Lafayette, CO) and the MKP-3 (Santa Cruz Biotechnology, Santa Cruz, CA) siRNA were transfected using DharmaFECT 2, according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were cultured in the presence or absence of crocidolite asbestos for 3 hours.
Western Blot Analysis and In Vitro Kinase Assay
Whole cell lysates were prepared by harvesting the cells after stimulating with 10 μg/cm2 crocidolite asbestos for the indicated amount of time and resuspending in lysis buffer (1% NP-40, 0.15 M NaCl, 0.05 M Tris [pH 7.4]), EDTA-free protease inhibitors (Roche, Indianapolis, IN), and phosphatase inhibitor cocktail (Calbiochem, La Jolla, CA). In vitro kinase assay was performed as previously described (7, 8). Briefly, the p38 MAP kinase was immunoprecipitated from the lysates overnight at 4°C with the p38 rabbit polyclonal antibody (Santa Cruz Biotechnology) bound to Gammabind with sepharose (Pharmacia Biotech, Uppsala, Sweden). In vitro kinase activity was assayed using ATF-2 (Santa Cruz Biotechnology) as a substrate. For Western blot analysis, samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and gels were transferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ). The p-ERK monoclonal, ERK polyclonal, MKP-3 polyclonal, p38 polyclonal, MEK1/2, and Flag polyclonal antibodies were obtained from Santa Cruz and were used at 1:500, 1:2,500, 1:250, 1:1,000, 1:1,000, and 1:500 dilutions, respectively. The p-MEK1/2 polyclonal and p-p38 rabbit monoclonal were obtained from Cell Signaling (Danvers, MA) and were used at 1:500 and 1:250 dilutions, respectively. The β-actin monoclonal antibody was obtained from Sigma (St. Louis, MO) and was used at a dilution of 1:10,000. In certain experiments, SB203580 (Calbiochem), a competitive inhibitor of p38, was used at a concentration of 1 μM.
Phosphatase Activity
Whole cell lysates were prepared by harvesting the cells as above described without the phosphatase inhibitor cocktail. Twenty micrograms of lysate was incubated with 10 ng of active ERK kinase (Stratagene, Cedar Creek, TX) for 20 minutes at 37°C. The active ERK kinase was prepared by phosphorylating ERK in vitro with constitutive active MEK. Samples were separated by SDS-PAGE, and Western analysis was performed using the p-ERK antibody. In some experiments, MKP-3 was immunoprecipitated and removed from the lysate before the incubation with the active ERK kinase.
Real-Time PCR
Total RNA was isolated using the Absolutely RNA RT-PCR Miniprep kit (Stratagene), according to the manufacturer's instructions. Total RNA (1 μg) was reverse transcribed to cDNA using iScript (Bio-Rad, Hercules, CA) or RETROScript (Ambion, Austin, TX), according to the manufacturer's instructions. PCR was performed by adding 2 μl of cDNA with 48 μl of Sybr Green Supermix (Bio-Rad), according to manufacturer's instructions. Amplification was then performed as we have previously described (38). Specific primer sets used for human MKP-3, TNF-α, and the HPRT housekeeping gene are as follows (5′ to 3′): MKP-3 sense, CTTGGTACATTGCTTGGCTGGCAT; MKP-3 antisense, AAGAGAAACTGCTGAAGGGCCAGA; TNF-α sense, CAGCCTCTTCTCCTTCCTGA; TNF-α antisense, AGCCTTGGCCCTTGAAGA; HPRT sense, TTGGAAAGGGTGTTTATTCCTC; HPRT antisense, TCCCCTGTTGACTGGTCATT. Primers were selected based on nucleotide sequences downloaded from the National Center for Biotechnology Information data bank and designed with software by Integrated DNA Technologies (Coralville, IA).
Statistical Analysis
Statistical comparisons were performed using an unpaired, one-tailed t test. Values in figures are expressed as means with standard error, with the probability of P < 0.05 considered to be significant.
RESULTS
The p38 and ERK MAP Kinases Are Differentially Expressed in Alveolar Macrophages Obtained from Patients with Asbestosis
There is evidence that alveolar macrophages from patients with asbestosis function differently than macrophages from the lungs of normal volunteers (39, 40). The cells from patients with asbestosis and other chronic lung diseases have been shown to resemble monocytes (1, 2, 29, 40, 41). In addition, studies have reported functional differences in cytokine release when normal alveolar macrophages are compared with blood monocytes when stimulated or when compared with alveolar macrophages from patients with chronic lung disorders (32, 34, 42–44).
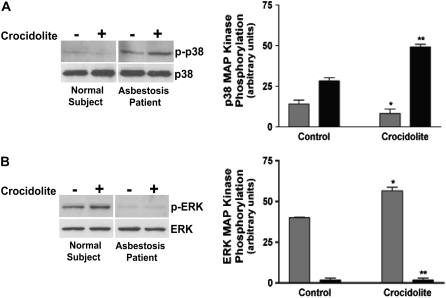
Since we previously demonstrated that the p38 MAP kinase regulates asbestos-induced TNF-α gene expression in human monocytes (13), we first wanted to determine if p38 was active in alveolar macrophages from patients with asbestosis. We obtained alveolar macrophages from three patients with asbestosis and from three normal subjects. The cells obtained from the normal subjects and patients with asbestosis were cultured in the presence or absence of crocidolite asbestos for 1 hour. We found that the p38 MAP kinase was present in the active, phosphorylated form in cells obtained from each patient with asbestosis compared with the cells from normal subjects (P < 0.0052), and it was significantly increased in cells exposed to crocidolite (Figure 1A). In contrast, cells obtained from each normal subject had no significant p38 activity. The sum of three separate experiments is shown graphically by the densitometry of normal subjects and patients with asbestosis with and without in vitro exposure to crocidolite asbestos (Figure 1A).
Figure 1.
Alveolar macrophages from patients with asbestosis have constitutive p38 kinase activity and absent extracellular signal–regulated kinase (ERK) activity compared with normal subjects. Whole cell lysates were prepared from alveolar macrophages obtained from normal subjects (n = 3) or from patients with asbestosis (n = 3) cultured in the presence or absence of crocidolite asbestos. The lysates were separated by SDS-PAGE. Western blot analysis was performed with (A) p-p38 and p38 or (B) p-ERK and ERK monoclonal and polyclonal antibodies to determine activation and confirm equal loading of proteins, respectively. Representative Western blot analyses are shown. In both A and B, densitometry was performed from the three experiments and is expressed graphically in arbitrary units. For statistical comparisons, in A, * denotes a comparison of p-p38 mitogen-activated protein (MAP) kinase in alveolar macrophages exposed to asbestos from patients with asbestosis and normal subjects (P < 0.0001), and ** denotes a comparison of p-p38 in alveolar macrophages from patients with asbestosis with and without exposure to crocidolite asbestos (P < 0.0007); in B, * denotes a comparison of p-ERK in alveolar macrophages from normal subjects with and without exposure to crocidolite asbestos (P < 0.0083), and ** denotes a comparison of p-ERK in asbestos-exposed alveolar macrophages obtained from patients with asbestosis and normal subjects (P < 0.0010). Shaded bars, normal subjects; solid bars, patients with asbestosis.
ERK kinase activity was also determined by performing a Western blot for the phosphorylated form of the kinase. We found that ERK was increased at baseline when compared with cells obtained from patients with asbestosis, and it was further increased with exposure to crocidolite in cells obtained from the three normal subjects. ERK activation, as measured by the presence of phosphorylated ERK, was minimally present in cells obtained from the lungs of each of the three patients with asbestosis with or without exposure to crocidolite asbestos (Figure 1B). The sum of three separate experiments is shown graphically by densitometry of p-ERK (Figure 1B). ERK was, however, present in these cells, as demonstrated by the equal loading of the proteins. These data demonstrate that asbestos-exposed alveolar macrophages have differential activation of MAP kinases, and the activation in normal subjects is functionally opposite to that seen in the cells obtained from patients with asbestosis.
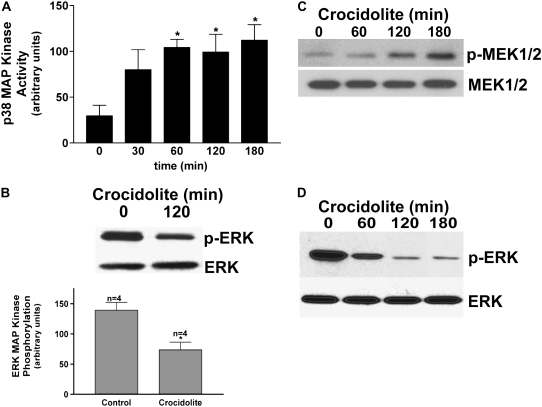
To determine the mechanism of regulation of MAP kinase activation, we performed similar experiments in human monocytes. Human blood monocytes were obtained from three normal subjects and cultured in the presence or absence of crocidolite asbestos. Monocytes exposed to crocidolite had a near-significant increase (P < 0.0570) in p38 activation as early as 30 minutes, and it remained significantly increased over the time course to 3 hours, as determined by in vitro kinase assay using ATF-2 as a substrate (Figure 2A). In contrast, the ERK kinase was decreased after exposure to crocidolite asbestos compared with control cells, as measured by the presence of phosphorylated form of the kinase in four individual normal subjects (Figure 2B). These data demonstrate that human monocytes have differential MAP kinase activation after exposure to asbestos, and this differential activation is similar to that seen in alveolar macrophages obtained from patients with asbestosis.
Figure 2.
Differential MAP kinase activation in human monocytes exposed to crocidolite asbestos. (A) Whole cell lysates were prepared from human monocytes (n = 3) exposed to crocidolite asbestos for the indicated amount of time. The lysates were subjected to immunoprecipitation with the p38 MAP kinase polyclonal antibody. In vitro kinase assays were performed using ATF-2 as the substrate. Western blot analysis for p38 MAP kinase was performed to confirm equal loading of the proteins. *P < 0.019. (B) Human blood monocytes (n = 4) were cultured with or without crocidolite asbestos for 2 hours. Western blot analysis was performed for p-ERK monoclonal and ERK polyclonal antibodies to determine activation and confirm equal loading of the proteins, respectively. Densitometry was performed from the four experiments and is expressed graphically in arbitrary units. For statistical comparisons, * denotes a comparison of p-ERK in blood monocytes cultured with and without crocidolite asbestos (P < 0.0057). (C) Human blood monocytes were exposed to crocidolite asbestos for the indicated amount of time. Western blot analysis was performed with p-MEK1/2 monoclonal and MEK1/2 polyclonal antibodies to determine activation and confirm equal loading of the proteins, respectively. (D) Human blood monocytes were exposed to crocidolite asbestos for the indicated amount of time, and whole cell lysates were incubated with an active ERK kinase for 20 minutes at 37°C, as described in Materials and Methods. Samples were separated by SDS-PAGE and Western analysis was performed with p-ERK monoclonal and ERK polyclonal antibodies.
Two possible mechanisms could be responsible for the absence of ERK MAP kinase activation in human monocytes and alveolar macrophages obtained from patients with asbestosis. The first is that crocidolite asbestos fails to activate the kinases upstream of ERK (Ras → Raf → MEK → ERK). A second possibility is that a phosphatase specifically inactivates ERK downstream of MEK activation. To elucidate the point at which ERK activity is regulated, we first performed a Western blot analysis for the phosphorylated form of the kinase, MEK1/2, which is the upstream kinase that activates ERK. Monocytes were exposed to crocidolite asbestos for the designated amount of time, and a Western blot analysis was performed for p-MEK1/2. We found that active MEK1/2 increased significantly in human monocytes exposed to crocidolite asbestos at 2 hours, and this increased further after three hours of exposure (Figure 2C). This suggests that the pathway upstream of ERK is, at least in part, intact.
To evaluate the second possibility, lysates without phosphatase inhibitors were obtained from cells cultured with crocidolite asbestos for the designated amount of time. These whole cell lysates were incubated with an active, or phosphorylated, ERK kinase for 20 minutes at 37°C before performing the Western analysis for p-ERK. We found that human monocytes exposed to crocidolite had a time-dependent decrease in phosphorylated, or active, ERK (Figure 2D). This time-dependent decrease in phosphorylated ERK started at 1 hour, which was the time that the p38 MAP kinase became significantly activated. Taken together, these results suggest that there is differential MAP kinase activation in alveolar macrophages obtained from the lung of patients with asbestosis and in human monocytes exposed in vitro with crocidolite. In addition, these results suggest that a phosphatase contributes, in part, to the differential MAP kinase activation due to the fact that ERK dephosphorylation occurs before activation of its upstream kinase, MEK1/2.
The p38 MAP Kinase Regulates MKP-3 Expression in Human Monocytes Stimulated with Crocidolite Asbestos
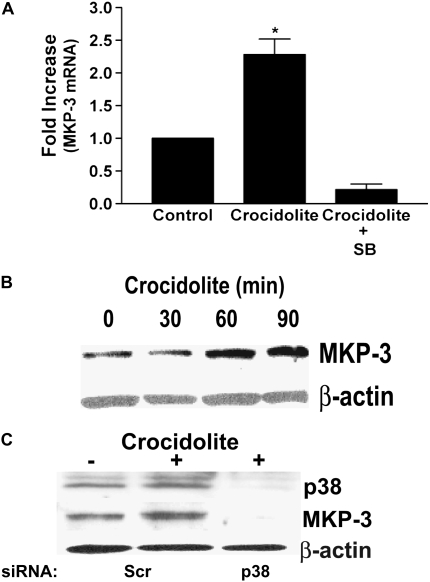
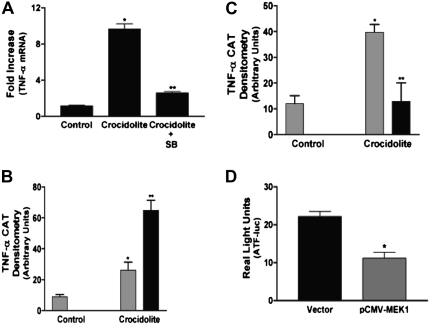
Based on the above results, we chose to evaluate the expression of MKP-3, which is a dual specificity phosphatase that is highly selective for inactivating ERK and has high expression in the lung. Although MKP-3 is transcribed from an immediate-early gene, we first determined the steady-state level of MKP-3 mRNA. Human monocytes were exposed to crocidolite asbestos for 3 hours, and the quantitative steady-state level of MKP-3 mRNA was determined by real-time PCR. In certain experiments, cells were treated with SB203580, a competitive inhibitor of the p38 MAP kinase, for 30 minutes before crocidolite exposure. We found that crocidolite increased MKP-3 expression approximately 2.5-fold, and inhibition of p38 with SB203580 significantly reduced expression below control levels (Figure 3A).
Figure 3.
MAP kinase phosphatase (MKP)-3 expression is regulated by the p38 MAP kinase. (A) Blood monocytes were cultured in the presence or absence of SB203580 (SB) for 30 minutes before exposing to crocidolite asbestos for 3 hours. Total RNA was isolated and reverse transcribed to cDNA. Real-time PCR amplification was performed as described in Materials and Methods. Data are expressed as fold increase of MKP-3 mRNA expression from control. For statistical analysis, * denotes a comparison of crocidolite asbestos to control and to crocidolite + SB (P < 0.025). (B) Blood monocytes were exposed to crocidolite asbestos for the indicated amount of time. Whole cell lysates were separated by SDS-PAGE, and Western blot analysis was performed using the MKP-3 rabbit polyclonal and β-actin monoclonal antibodies to determine expression and equal loading of the proteins. (C) THP-1 cells were transfected with 100 nM of either a scrambled or a p38 siRNA. After 48 hours, the cells were exposed to crocidolite asbestos for 2 hours. Whole cell lysates were separated by SDS-PAGE, and Western blot analysis was performed for p38, MKP-3, or β-actin.
To demonstrate that asbestos increased MKP-3 protein expression since the protein is necessary for phosphatase activity, blood monocytes were exposed to crocidolite asbestos for the designated amount of time, and a Western blot analysis was performed for MKP-3. We found that MKP-3 protein expression increased at 1 hour after exposure to crocidolite asbestos and remained increased for a prolonged period of time in the presence of asbestos (Figure 3B). These data demonstrate that monocytes exposed to asbestos have increased MKP-3 expression, and this increase occurs at a similar time to the dephosphorylation of the ERK MAP kinase.
To avoid the potential nonspecific effects of the pharmacologic inhibitor, we performed a similar experiment with the knockdown of p38 expression by transfection of a p38 siRNA. THP-1 cells were transfected with either a scrambled or a p38 siRNA. After 48 hours, cells were exposed to crocidolite asbestos for 2 hours. Western blot analysis for MKP-3 revealed that cells exposed to crocidolite asbestos had an increase in MKP-3 protein expression in cells transfected with the scrambled siRNA, but cells transfected with the p38 siRNA had a marked reduction in MKP-3 protein expression (Figure 3C). We confirmed that p38 knockdown was present in cells transfected with the p38 siRNA. In aggregate, these data suggest that the p38 MAP kinase modulates the expression of MKP-3 in monocytes exposed to asbestos.
MKP-3 Regulates ERK MAP Kinase Activation in Human Monocytes
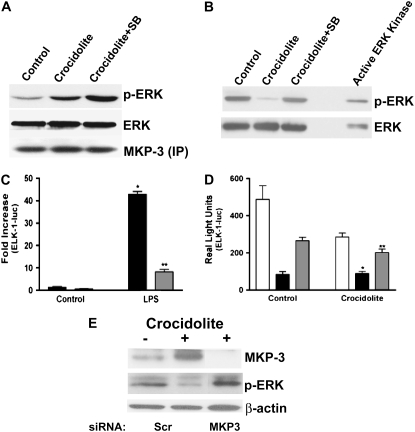
Since crocidolite asbestos increased expression of MKP-3 and ERK is decreased in human monocytes exposed to asbestos, we next determined if immunoprecipitation of MKP-3 before incubating the lysate with an active ERK kinase would prevent dephosphorylation and inactivation of the ERK kinase. Human monocytes were cultured in the presence or absence of the p38 inhibitor, SB203580, before stimulating the cells with crocidolite asbestos. Whole cell lysates were subjected to immunoprecipitation with the MKP-3 rabbit polyclonal antibody. The immunodepleted lysates were incubated with active ERK kinase for 20 minutes, and a Western blot analysis was performed for p-ERK. We found that cells exposed to crocidolite had a significant increase in p-ERK compared with control cells, and cells cultured with SB203580 had an even greater increase in p-ERK (Figure 4A). These data suggest that MKP-3 plays a role in ERK dephosphorylation in human monocytes exposed to asbestos.
Figure 4.
ERK MAP kinase activity is recovered with MKP-3 removal and knockdown and partially recovered with overexpression of a mutant MKP-3. Blood monocytes were cultured in the presence or absence of SB203580 (SB) for 30 minutes before exposing cells to crocidolite asbestos for 2 hours. (A) MKP-3 was immunoprecipitated from whole cell lysates, and the immunodepleted lysates or (B) whole cell lysates were incubated with an active ERK kinase for 20 minutes at 37°C, as described in Materials and Methods. Samples were separated by SDS-PAGE, and Western analysis was performed with the p-ERK monoclonal or ERK polyclonal antibodies to determine activation and confirm equal loading of the proteins, respectively. In A, a Western blot analysis was also performed with the MKP-3 polyclonal antibody to confirm equal loading of the immunoprecipitated proteins. (C) THP-1 cells were transiently transfected with pTL-luc and pTL-ELK-1 and either an empty vector (solid bars) or pRKF-Flag-MKP-3 (shaded bars). (D) THP-1 cells were transiently transfected as in C with the additional plasmid pRKF-Flag-MKP-3 (C293S). Open bars, vector; solid bars, pRKF-MKP3 (WT); shaded bars, pRKF-MKP3 (C293S). After 24 hours, cells were cultured in the presence or absence of (C) 100 μg/ml lipopolysaccharide (LPS) or (D) crocidolite for 6 hours. Luciferase activity, which was normalized to Renilla luciferase, is expressed as (C) fold increase or in (D) real light units. For statistical analysis, in C, * denotes a comparison of LPS to control (vector) (P < 0.0001), and ** denotes a comparison of LPS (vector) to LPS (pRKF-Flag-MKP3) (P < 0.0001); in D, * denotes a comparison of crocidolite (vector) to crocidolite (pRKF-Flag-MKP3) (P < 0.0007), and ** denotes a comparison of crocidolite (pRKF-Flag-MKP3) to crocidolite (pRKF-Flag-MKP3 (C293S)) (P < 0.0037). (E) THP-1 cells were transfected with 100 nM of either a scrambled or a MKP-3 siRNA. After 48 hours, the cells were exposed to crocidolite asbestos for 2 hours. Whole cell lysates were separated by SDS-PAGE, and Western blot analysis was performed for MKP-3, p-ERK, or β-actin.
Due to the fact that MKP-3 expression is, in part, modulated by p38, we questioned whether inhibition of p38 alone would prevent ERK dephosphorylation. Human monocytes were cultured in the presence or absence of SB203580 for 30 minutes before exposure to crocidolite asbestos. Whole cell lysates were incubated with the active ERK kinase for 20 minutes at 37°C. A Western blot analysis for p-ERK showed that p-ERK was significantly reduced in cells exposed to crocidolite alone, but cells cultured with the p38 inhibitor, SB203580, had a recovery of ERK activation to control levels (Figure 4B).
To avoid the possibility of nonspecific effects using a pharmacologic inhibitor, we used a wild-type MKP-3 expression vector to evaluate kinase activity in vivo. Since crocidolite did not activate ERK, we first determined if the MKP-3 expression vector was effective in our system by evaluating if MKP-3 would inhibit ERK activation by endotoxin (lipopolysaccharide [LPS]), which is a strong activator of ERK kinase activity (8–10, 45). Cells were transfected with pTL-luc and pTL-ELK-1 and either an empty vector or the wild-type MKP-3 expression vector. After 24 hours, the cells were harvested after being stimulated with LPS for 6 hours, and a luciferase assay was performed. Cells expressing the empty vector had a robust (> 40-fold) increase in ERK kinase activity when stimulated by LPS, as measured by the ELK-1 luciferase. In contrast, cells expressing the MKP-3 expression vector had a significant reduction in ELK-1 luciferase activity (Figure 4C). These data ensured that the MKP-3 expression vector is effective in inhibiting ERK activity in THP-1 monocytes.
We next evaluated the effect of MKP-3 regulating ERK MAP kinase in vivo after crocidolite asbestos exposure. THP-1 cells were transfected with pTL-luc and pTL–ELK-1 and either an empty vector, the wild-type MKP-3, or a mutant MKP-3 expression vector, in which the catalytic cysteine at 293 converted to a serine. After 24 hours, the cells were harvested after being exposed to crocidolite asbestos for 6 hours, and a luciferase assay was performed. As expected, cells expressing the empty vector had a significant reduction in ELK-1 luciferase activity after exposure to crocidolite. Cells expressing the wild-type MKP-3 expression vector had a significant decrease in luciferase activity, while cells expressing the mutant MKP-3 (C293S) had a partial recovery of the ELK-1 luciferase activity that remained below baseline (Figure 4D). The fact that the mutant MKP-3 did not salvage ERK activation is not surprising, considering the fact that the amino-terminal, noncatalytic domain binds to the ERK and retains it in the cytoplasm regardless of whether ERK is phosphorylated (21, 46, 47). In particular, one study has demonstrated that overexpression of a catalytic mutant MKP-3 vector prevents downstream ERK-mediated gene expression (21). Both the wild-type (pRKF-MKP-3) and the mutant (pRKF-MKP-3 [C293S]) expression vectors have intact amino-terminal domains.
Since the catalytic mutant MKP-3 did not fully recover ERK activation in monocytes, we used a different approach to investigate the effect of MKP-3 regulation of the ERK MAP kinase in cells exposed to crocidolite asbestos. THP-1 monocytes were transfected with either a scrambled or a MKP-3 siRNA. After 48 hours, cells were exposed to crocidolite asbestos for 2 hours. Western blot analysis for p-ERK revealed that cells exposed to crocidolite asbestos had a reduction in the phosphorylated form of ERK in cells transfected with the scrambled siRNA. In contrast, cells transfected with the MKP-3 siRNA had a significant increase in ERK phosphorylation in cells exposed to asbestos (Figure 4E). We confirmed that MKP-3 knockdown was present in cells transfected with the MKP-3 siRNA. These data demonstrate that MKP-3 modulates ERK activation in human monocytes exposed to crocidolite asbestos.
The p38 and ERK MAP Kinases Have Differential Effects on TNF-α Gene Expression in Human Monocytes
To explain the physiologic relevance of the differential MAP kinase activation and MKP-3 expression, we determined if human monocytes expressed TNF-α after exposure to crocidolite asbestos. Blood monocytes were exposed to crocidolite asbestos for 3 hours in the presence or absence of SB203580, which is the competitive inhibitor of the p38 MAP kinase, and the quantitative level of TNF-α mRNA was determined by real-time PCR. Crocidolite induced approximately a 10-fold increase in TNF-α mRNA, while inhibition of p38 with SB203580 significantly reduced mRNA levels to near-control levels (Figure 5A).
Figure 5.
Differential MAP kinase activation is necessary for optimal TNF-α gene expression. (A) Blood monocytes were cultured in the presence or absence of SB203580 (SB) for 30 minutes before exposing to crocidolite asbestos for 3 hours. Total RNA was reverse transcribed to cDNA. Real-time PCR amplification was performed as described in Materials and Methods. Data are expressed as fold induction of TNF-α mRNA expression from control. For statistical analysis, * denotes a comparison of crocidolite asbestos to control (P < 0.0037), and ** denotes a comparison of crocidolite to crocidolite + SB203580 (P < 0.0046). (B) THP-1 cells were transiently transfected with the -600 TNF-α-CAT reporter plasmid and either an empty vector (shaded bars) or pcDNA-HA-ERK2 (K/A) (solid bars) expression vector. After 24 hours, cells were exposed to crocidolite asbestos for 24 hours. Whole cell lysates, which were normalized to protein, were incubated with 0.1 μCi of [14C] chloramphenicol and 1.0 mM acetyl coenzyme A for 2 hours at 37°C. Acetylated derivatives (CM3-AC and CM1-AC) were separated from nonacetylated chloramphenicol (CM) by ascending thin layer chromatography in chloroform/methanol (95:5) solvent. *P < 0.0175, **P < 0.0050. (C) THP-1 cells were transiently transfected with the −600 TNF-α-CAT reporter plasmid and either an empty vector (shaded bars) or the constitutive active pCMV-MEK1 (solid bars) expression vector. After 24 hours, cells were exposed to crocidolite asbestos for 24 hours, and the CAT assay was performed as described in B. *P < 0.012, **P < 0.038. In B and C, densitometry of three separate experiments was performed and is expressed graphically in arbitrary units. For statistical analysis, * denotes a comparison of control to crocidolite (vector); and ** denotes a comparison of crocidolite (vector) to (B) crocidolite (pcDNA-HA-ERK2 [K/A]) and (C) crocidolite (pCMV-MEK1). (D) THP-1 cells were transiently transfected with pTRE-luc and pTET-ATF and either an empty vector (solid bars) or the constitutive active MEK1 (shaded bars). After 24 hours, cells were exposed to crocidolite asbestos for 6 hours. Luciferase activity, which was normalized to Renilla luciferase, is expressed in real light units. For statistical comparisons, * denotes a comparison of vector to pCMV-MEK1 (P < 0.0031).
We next investigated the direct effect of ERK activity on TNF-α expression using a CAT reporter driven by a full-length TNF-α promoter (−600 TNF-α–CAT). THP-1 cells were transiently transfected with the CAT reporter plasmid and either an empty vector or a dominant-negative ERK2 expression vector. After 24 hours, the cells were exposed to crocidolite asbestos for 24 hours. Crocidolite asbestos significantly increased CAT activity in cells expressing the empty vector, but this activity was significantly enhanced in cells expressing the dominant-negative ERK2 (Figure 5B). In addition, CAT activity was significantly increased in cells expressing the dominant-negative ERK without exposure to asbestos (data not shown). In aggregate, these data suggest that the p38 and ERK MAP kinases have differential roles in regulating TNF-α gene expression in human monocytes exposed to asbestos, and part of the regulation is dependent on MKP-3 expression and activity.
Although inhibition of ERK activity with the dominant-negative expression vector increased CAT activity, we wanted to determine the effect of constitutive ERK activity on TNF-α gene expression. We transiently transfected THP-1 cells with the −600 TNF-α–CAT and either an empty vector or a constitutive active MEK-1, which is the upstream kinase that activates ERK. As expected, crocidolite asbestos increased CAT activity in cells expressing the empty vector. In contrast, we found that the constitutive active MEK1→ERK pathway significantly inhibited gene expression driven by a TNF-α promoter (Figure 5C). In aggregate, these data suggest that the p38 and ERK MAP kinases have opposing roles in regulating TNF-α gene expression in human monocytes exposed to asbestos; that is, p38 is a positive regulator and ERK is a negative regulator of TNF-α expression.
Although the ERK MAP kinase could negatively regulate TNF-α gene expression by multiple mechanisms, we evaluated the effect of constitutive MEK1→ERK activation on p38 MAP kinase activity. Using an in vivo kinase assay, THP-1 cells were transiently transfected with the pTRE-luc and pTET-ATF plasmids with either an empty vector or the constitutive active MEK1 expression vector. After 24 hours, the cells were exposed to crocidolite asbestos for 6 hours. The cells were harvested, lysed, and luciferase activity was determined. We found that cells expressing the constitutive active MEK1 had a significant reduction in p38 MAP kinase activity compared with cells expressing the empty vector (Figure 5D). These results suggest that these MAP kinases negatively regulate one another. In addition, these results demonstrate that ERK negatively regulates gene expression driven by a TNF-α promoter, in part, by inhibiting p38 MAP kinase activity.
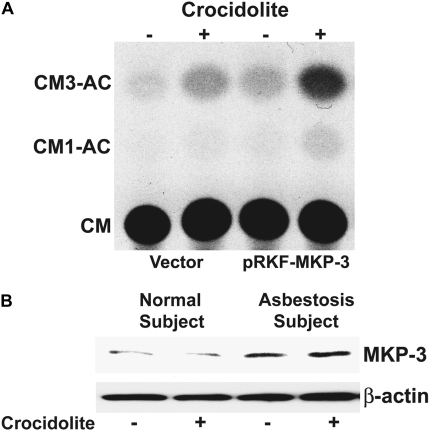
Since MKP-3 expression has a role in modulating differential MAP kinase activity, we overexpressed MKP-3 in cells to determine the effect of MKP-3 in regulating TNF-α gene expression. THP-1 cells were transiently transfected with the −600 TNF-α–CAT and either an empty vector or the wild-type MKP-3 expression vector. We found that crocidolite increased CAT activity in cells expressing the empty vector, and this activity was significantly augmented in cells expressing MKP-3 (Figure 6A). Taken together, these data suggest that the p38 MAP kinase regulates TNF-α gene expression in human monocytes and that over expression of MKP-3, which specifically inhibits ERK, significantly enhances gene expression driven by a TNF-α promoter.
Figure 6.
MKP-3 augments TNF-α gene expression in monocytes exposed to asbestos. (A) THP-1 cells were transiently transfected with the −600 TNF-α-CAT reporter plasmid and either an empty vector or pRKF-Flag-MKP-3 expression vector. After 24 hours, cells were exposed to crocidolite asbestos for 24 hours. Whole cell lysates, which were normalized to protein, were incubated with 0.1 μCi of [14C] chloramphenicol and 1.0 mM acetyl coenzyme A for 2 hours at 37°C. Acetylated derivatives (CM3-AC and CM1-AC) were separated from nonacetylated chloramphenicol (CM) by ascending thin layer chromatography in chloroform/methanol (95:5) solvent. (B) Whole cell lysates were prepared from alveolar macrophages obtained from normal subjects (n = 2) or from patients with asbestosis (n = 2) cultured for 1 hour in the presence or absence of crocidolite asbestos. Lysates were separated by SDS-PAGE. Western blot analysis was performed using the MKP-3 polyclonal and β-actin monoclonal antibodies to determine expression and equal loading of the proteins, respectively. A representative Western blot analysis is shown.
To provide further physiologic relevance for MKP-3, we obtained alveolar macrophages from two patients with asbestosis and two normal subjects. The cells were cultured in the presence or absence of crocidolite for 1 hour, and a Western blot analysis was performed for MKP-3. We found that the MKP-3 expression was high in cells obtained from the patients with asbestosis, and cells obtained from normal subjects had minimal expression of MKP-3 (Figure 6B). Equal loading of the proteins was confirmed with a Western blot for β-actin. In aggregate, these data demonstrate that alveolar macrophages obtained from the lungs of patients with asbestosis and normal blood monocytes have differential MAP kinase activity, and this differential activity is, at least in part, modulated by MKP-3. These data also suggest that the p38 and ERK MAP kinases negatively regulate one another and differentially modulate TNF-α production in response to asbestos. Furthermore, these data corroborate previous studies showing that alveolar macrophages from patients with asbestosis function differently than macrophages obtained from the lungs of normal subjects and that these cells from patients with asbestosis and other chronic lung diseases resemble monocytes.
DISCUSSION
We have previously shown that the p38 MAP kinase is essential for cytokine gene expression in monocytes and macrophages (7, 8, 13, 38). In this study, we asked if the ERK MAP kinase had a role in regulating TNF-α production in human monocytes exposed to asbestos. Interestingly, we found that p38 and ERK were differentially activated in alveolar macrophages obtained from patients with asbestosis and in human monocytes exposed in vitro to asbestos. More specifically, p38 was constitutively active and ERK activity was suppressed. We have evidence that human monocytes exposed to crocidolite asbestos have an active MKK3/6 (data not shown), which is the upstream kinase that activates the p38 MAP kinase via the classic mechanism (48–51). Since the upstream pathway leading (MEK1) to ERK was also intact, we hypothesized that an ERK-specific phosphatase was, in part, responsible for the decreased ERK activity. We evaluated if the dual specificity phosphatase MKP-3, which is highly expressed in the lung and specifically dephosphorylates ERK, was increased after exposure to asbestos and found that MKP-3 was increased in monocytes, and its expression was regulated by p38. In addition, our data demonstrated that these MAP kinases negatively regulate one another, and MKP-3 had an essential role in the differential activation in human monocytes exposed to asbestos. To demonstrate that this differential MAP kinase activation had biological relevance, we assessed the effect of p38, ERK, and MKP-3 on expression of TNF-α. We found that p38 was a positive regulator and ERK was a negative regulator of TNF-α gene expression. Furthermore, cells overexpressing MKP-3 had a significant increase in TNF-α gene expression, suggesting that an environment favoring p38 MAP kinase activation is necessary for TNF-α production in monocytes exposed to asbestos. This interaction of MAP kinases, MKP-3, and TNF-α in normal human monocytes and alveolar macrophages from patients with asbestosis, as verified by our data, is depicted schematically in Figure 7. This is in contrast to the interaction in normal alveolar macrophages, which have increased ERK and decreased p38 activity, low expression of MKP-3, and no TNF-α production after being exposed to asbestos in vitro.
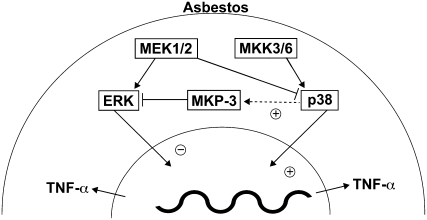
Figure 7.
Schematic diagram of the interaction of p38 and ERK MAP kinases, MKP-3, and TNF-α gene expression in monocytes exposed to crocidolite asbestos. The p38 MAP kinase is a positive regulator, and the ERK MAP kinase is a negative regulator of TNF-α gene expression. The differential activation of the p38 and ERK MAP kinases is due, in part, to asbestos-induced MKP-3 expression. The dynamic balance of MAP kinase signaling in cells overexpressing MKP-3 suggests that an environment favoring p38 MAP kinase activation is necessary for TNF-α production in monocytes exposed to asbestos.
One novel aspect of this study is that human monocytes exposed to asbestos have differential MAP kinase activation and the fact that this differential activation has a critical role in regulating TNF-α gene expression. This differential activation of MAP kinases appears to be unique in human monocytes exposed to asbestos because other studies have demonstrated that activation of multiple MAP kinases has a synergistic effect on cytokine gene expression using other stimuli (5–8, 10–12, 52). In fact, we have previously demonstrated that ERK activity is necessary for LPS-induced cytokine gene expression in both monocytes and macrophages (8, 10).
In certain instances, however, MAP kinases negatively regulate or potentiate activity of one another. We previously demonstrated that constitutive activation of the MEK→ERK pathway inhibits p38 activation and NF-κB–dependent gene expression (45). Other studies have shown that activation of the p38 MAP kinase with anisomycin in mast cells inhibits the activation of ERK2 and that inhibition of the p38 MAP kinase with SB202190 resulted in increased ERK MAP kinase activity (53, 54). Additional studies have also shown that these MAP kinases have opposing effects on apoptosis and induction of nitric oxide synthase (55, 56). None of these studies, however, determined the mechanism by which the MAP kinases were regulating one another. The novel aspect of the current study is that the p38 MAP kinase indirectly down-regulates ERK via modulating expression of MKP-3.
Another novel aspect of the current study is that cells expressing a constitutive active MEK1 have decreased p38 MAP kinase activity after exposure to asbestos. Our data demonstrate, however, that MEK1 is activated in monocytes exposed to asbestos, but it is not active in monocytes until 2 hours after exposure to crocidolite. The primary difference is that the native MEK1 is not constitutively active like the plasmid. Thus, the effect of a transiently active MEK1 may have different consequences than a constitutive active MEK1 as far its role in regulating gene expression. Our data demonstrate that MEK1, when constitutive active, decreases p38 MAP kinase activation in monocytes exposed to asbestos, while p38 MAP kinase is strongly activated by asbestos in cells expressing the native MEK1. Although we did not investigate the mechanism of p38 inhibition by over expression of MEK1, one plausible explanation may simply be an increase in the activity of the MEK1→ERK pathway, which has been linked to the regulation of MKP-1 gene expression (57). This mechanism would corroborate our previous study demonstrating that MKP-1 inhibits TNF-α gene expression, in part, by regulating p38 MAP kinase activity (38). Current studies in our laboratory are investigating the inhibitory effect of constitutive active MEK1 on asbestos-induced TNF-α gene expression.
While other studies have demonstrated that ERK is necessary for inflammatory gene expression (8–10, 58–60), the data in the current study are the first to show that ERK negatively regulates TNF-α gene expression. In fact, we have previously shown that ERK inhibition results in decreased cytokine gene expression in monocytes and macrophages stimulated with endotoxin (8–10). The data presented in the current study, however, are the first to show that ERK negatively regulates TNF-α gene expression. There is one study that showed ERK inhibited IL-12 expression in LPS/IFN-γ–stimulated murine macrophages, but the role of ERK was only studied with the use of the MEK chemical inhibitor, PD98059 (56). In contrast to our study, ERK was activated in the macrophages to promote survival of an intracellular parasite.
Our data suggest that the mechanism(s) by which ERK inhibits, or negatively regulates, TNF-α gene expression in human monocytes is, in part, by decreasing p38 MAP kinase activation. This mechanism is consistent with our previous study in endotoxin-stimulated macrophages (45). This explanation does not necessarily explain the mechanism by which MKP-3 augments TNF-α gene expression unless MKP-3 was constitutively active. One study has shown, however, that a catalytically inactive mutant MKP-3 can bind to ERK and retain it in the cytoplasm, thus preventing ERK translocation to the nucleus (46). Although MKP-3 can be present in nonstimulated cells, we do not believe this to be the case in human monocytes because our data demonstrate that MKP-3 expression increases in a time-dependent manner after exposure to asbestos.
The dual specificity phosphatases, such as MKP-3, have an important role in dephosphorylating and inactivating MAP kinases. The dual phosphorylation of Thr-183 and Tyr-185 is required for ERK MAP kinase activation (61–64), and dephosphorylation of either residue is sufficient for loss of activity. We focused the current study on MKP-3 secondary to the novel finding that ERK phosphorylation was decreased in blood monocytes exposed to asbestos and was absent in alveolar macrophages obtained from patients with asbestosis. The absence of ERK phosphorylation in alveolar macrophages obtained from the lungs of patients with asbestosis and in human monocytes exposed to asbestos could result from either failure of asbestos to activate the kinases upstream of ERK or the presence of a phosphatase that specifically inactivates ERK downstream of MEK activation. We confirmed that MEK1/2, which is the kinase upstream of ERK, was activated in human monocytes exposed to asbestos, so we investigated the latter mechanism in more detail. Studies have shown that the catalytic activity of MKP-3 is regulated by its physical interaction with ERK independent of ERK kinase activity (22, 25–28). Although we did not evaluate the direct interaction of MKP-3 and ERK, we demonstrated that total ERK protein was present in the cells, indicating that the conformational rearrangement of the active site in MKP-3 may occur, since this interaction is independent of ERK activity (28). MKP-3, however, also functions as an immediate-early gene (22), so the presence of the protein, in part, is indicative of its activity, and we found that its mRNA and protein expression was increased in human monocytes exposed to asbestos. In aggregate, our data suggest that differential MAP kinase activity is, at least in part, modulated by MKP-3 in human monocytes exposed to asbestos. The data also suggest potential therapeutic targets to consider to avert the inflammatory response and the development of interstitial fibrosis in response to asbestos exposure.
This is the first study to demonstrate that the p38 MAP kinase is mechanistically linked to MKP-3 expression. In fact, our novel data suggests that the differential activation of the p38 and ERK MAP kinases is due, in part, to asbestos-induced MKP-3 expression. Additional novel data demonstrated that MKP-3 expression was induced in human monocytes exposed to crocidolite asbestos, and alveolar macrophages from patients with asbestosis had a higher level of MKP-3 expression compared with those from normal subjects. Taken together, these data demonstrate that the p38 MAP kinase down-regulates ERK via activation of MKP-3. Furthermore, these data suggest that this differential activation of MAP kinases is necessary for optimal TNF-α gene expression in human monocytes exposed to crocidolite asbestos.
Acknowledgments
The authors thank Drs. Maniatis, Kuprash, Davis, and Karin for providing their respective plasmids; Kevin Orcutt for technical assistance; and Gary Hunninghake for review of the manuscript.
This work was supported by National Institutes of Health grants ES-015981 and ES-014871 (to A.B.C.) RR00059 from the General Clinical Research Centers Program, a Veterans Affairs Merit Review Grant, and an American Lung Association Career Investigator Award.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0356OC on February 28, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hance AJ, Douches S, Winchester RJ, Ferrans VJ, Crystal RG. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol 1985;134:284–292. [PubMed] [Google Scholar]
- 2.Peters-Golden M, McNish RW, Brieland JK, Fantone JC. Diminished protein kinase C-activated arachidonate metabolism accompanies rat macrophage differentiation in the lung. J Immunol 1990;144:4320–4326. [PubMed] [Google Scholar]
- 3.Schwartz DA, Galvin JR, Frees KL, Dayton CS, Burmeister LF, Merchant JA, Hunninghake GW. Clinical relevance of cellular mediators of inflammation in workers exposed to asbestos. Am Rev Respir Dis 1993;148:68–74. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Lee TC, Guillemin B, Yu MC, Rom WN. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol 1993;150:4188–4196. [PubMed] [Google Scholar]
- 5.Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 1994;372:739–746. [DOI] [PubMed] [Google Scholar]
- 6.Lee JC, Young PR. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol 1996;59:152–157. [DOI] [PubMed] [Google Scholar]
- 7.Carter AB, Knudtson KL, Monick MM, Hunninghake GW. The p38 mitogen-activated protein kinase is required for NF-κB-dependent gene expression: the role of TATA-binding protein (TBP). J Biol Chem 1999;274:30858–30863. [DOI] [PubMed] [Google Scholar]
- 8.Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am J Respir Cell Mol Biol 1999;20:751–758. [DOI] [PubMed] [Google Scholar]
- 9.Monick MM, Carter AB, Flaherty DM, Peterson MW, Hunninghake GW. Protein kinase Cζ plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J Immunol 2000;165:4632–4639. [DOI] [PubMed] [Google Scholar]
- 10.Monick MM, Carter AB, Gudmundsson G, Mallampalli R, Powers LS, Hunninghake GW. A phosphatidylcholine-specific phospholipase C regulates activation of p42/44 mitogen-activated protein kinases in lipopolysaccharide-stimulated human alveolar macrophages. J Immunol 1999;162:3005–3012. [PubMed] [Google Scholar]
- 11.Hoffmeyer A, Grosse-Wilde A, Flory E, Neufeld B, Kunz M, Rapp UR, Ludwig S. Different mitogen-activated protein kinase signaling pathways cooperate to regulate tumor necrosis factor alpha gene expression in T lymphocytes. J Biol Chem 1999;274:4319–4327. [DOI] [PubMed] [Google Scholar]
- 12.Rawadi G, Ramez V, Lemercier B, Roman-Roman S. Activation of mitogen-activated protein kinase pathways by Mycoplasma fermentans membrane lipoproteins in murine macrophages: involvement in cytokine synthesis. J Immunol 1998;160:1330–1339. [PubMed] [Google Scholar]
- 13.Carter AB, Tephly LA, Venkataraman S, Oberley LW, Zhang Y, Buettner GR, Spitz DR, Hunninghake GW. High levels of catalase and glutathione peroxidase activity dampen H2O2 signaling in human alveolar macrophages. Am J Respir Cell Mol Biol 2004;31:43–53. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez LA, Zanella C, Fung H, Janssen YM, Vacek P, Charland C, Goldberg J, Mossman BT. Role of extracellular signal-regulated protein kinases in apoptosis by asbestos and H2O2. Am J Physiol 1997;273:L1029–L1035. [DOI] [PubMed] [Google Scholar]
- 15.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Different accumulation of activated extracellular signal-regulated kinases (ERK 1/2) and role in cell-cycle alterations by epidermal growth factor, hydrogen peroxide, or asbestos in pulmonary epithelial cells. Am J Respir Cell Mol Biol 2001;24:405–413. [DOI] [PubMed] [Google Scholar]
- 16.Ramos-Nino ME, Timblin CR, Mossman BT. Mesothelial cell transformation requires increased AP-1 binding activity and ERK-dependent Fra-1 expression. Cancer Res 2002;62:6065–6069. [PubMed] [Google Scholar]
- 17.Swain WA, O'Byrne KJ, Faux SP. Activation of p38 MAP kinase by asbestos in rat mesothelial cells is mediated by oxidative stress. Am J Physiol Lung Cell Mol Physiol 2004;286:L859–L865. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Z, Taatjes DJ, Mossman BT, Heintz NH. The duration of nuclear extracellular signal-regulated kinase 1 and 2 signaling during cell cycle reentry distinguishes proliferation from apoptosis in response to asbestos. Cancer Res 2004;64:6530–6536. [DOI] [PubMed] [Google Scholar]
- 19.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J 2000;14:6–16. [PubMed] [Google Scholar]
- 20.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 2000;12:186–192. [DOI] [PubMed] [Google Scholar]
- 21.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev 1999;13:2905–2927. [DOI] [PubMed] [Google Scholar]
- 22.Camps M, Nichols A, Gillieron C, Antonsson B, Muda M, Chabert C, Boschert U, Arkinstall S. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 1998;280:1262–1265. [DOI] [PubMed] [Google Scholar]
- 23.Castelli M, Camps M, Gillieron C, Leroy D, Arkinstall S, Rommel C, Nichols A. MAP kinase phosphatase 3 (MKP3) interacts with and is phosphorylated by protein kinase CK2α. J Biol Chem 2004;279:44731–44739. [DOI] [PubMed] [Google Scholar]
- 24.Groom LA, Sneddon AA, Alessi DR, Dowd S, Keyse SM. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 25.Fjeld CC, Rice AE, Kim Y, Gee KR, Denu JM. Mechanistic basis for catalytic activation of mitogen-activated protein kinase phosphatase 3 by extracellular signal-regulated kinase. J Biol Chem 2000;275:6749–6757. [DOI] [PubMed] [Google Scholar]
- 26.Nichols A, Camps M, Gillieron C, Chabert C, Brunet A, Wilsbacher J, Cobb M, Pouyssegur J, Shaw JP, Arkinstall S. Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J Biol Chem 2000;275:24613–24621. [DOI] [PubMed] [Google Scholar]
- 27.Zhou B, Wu L, Shen K, Zhang J, Lawrence DS, Zhang ZY. Multiple regions of MAP kinase phosphatase 3 are involved in its recognition and activation by ERK2. J Biol Chem 2001;276:6506–6515. [DOI] [PubMed] [Google Scholar]
- 28.Zhou B, Zhang ZY. Mechanism of mitogen-activated protein kinase phosphatase-3 activation by ERK2. J Biol Chem 1999;274:35526–35534. [DOI] [PubMed] [Google Scholar]
- 29.Krombach F, Gerlach JT, Padovan C, Burges A, Behr J, Beinert T, Vogelmeier C. Characterization and quantification of alveolar monocyte-like cells in human chronic inflammatory lung disease. Eur Respir J 1996;9:984–991. [DOI] [PubMed] [Google Scholar]
- 30.Striz I, Wang YM, Svarcova I, Trnka L, Sorg C, Costabel U. The phenotype of alveolar macrophages and its correlation with immune cells in bronchoalveolar lavage. Eur Respir J 1993;6:1287–1294. [PubMed] [Google Scholar]
- 31.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol 1997;159:3034–3043. [PubMed] [Google Scholar]
- 32.Bernaudin JF, Yamauchi K, Wewers MD, Tocci MJ, Ferrans VJ, Crystal RG. Demonstration by in situ hybridization of dissimilar IL-1β gene expression in human alveolar macrophages and blood monocytes in response to lipopolysaccharide. J Immunol 1988;140:3822–3829. [PubMed] [Google Scholar]
- 33.Bitterman PB, Wewers MD, Rennard SI, Adelberg S, Crystal RG. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest 1986;77:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strieter RM, Remick DG, Lynch JPD, Genord M, Raiford C, Spengler R, Kunkel SL. Differential regulation of tumor necrosis factor-alpha in human alveolar macrophages and peripheral blood monocytes: a cellular and molecular analysis. Am J Respir Cell Mol Biol 1989;1:57–63. [DOI] [PubMed] [Google Scholar]
- 35.Goldfeld AE, Doyle C, Maniatis T. Human tumor necrosis factor alpha gene regulation by virus and lipopolysaccharide. Proc Natl Acad Sci USA 1990;87:9769–9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wartmann M, Hofer P, Turowski P, Saltiel AR, Hynes NE. Negative modulation of membrane localization of the Raf-1 protein kinase by hyperphosphorylation. J Biol Chem 1997;272:3915–3923. [DOI] [PubMed] [Google Scholar]
- 37.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 2005;120:649–661. [DOI] [PubMed] [Google Scholar]
- 38.Tephly LA, Carter AB. Differential expression and oxidation of MKP-1 modulates TNF-α gene expression. Am J Respir Cell Mol Biol 2007;37:366–374. [DOI] [PubMed] [Google Scholar]
- 39.Rom WN, Bitterman PB, Rennard SI, Cantin A, Crystal RG. Characterization of the lower respiratory tract inflammation of nonsmoking individuals with interstitial lung disease associated with chronic inhalation of inorganic dusts. Am Rev Respir Dis 1987;136:1429–1434. [DOI] [PubMed] [Google Scholar]
- 40.Spurzem JR, Saltini C, Rom W, Winchester RJ, Crystal RG. Mechanisms of macrophage accumulation in the lungs of asbestos-exposed subjects. Am Rev Respir Dis 1987;136:276–280. [DOI] [PubMed] [Google Scholar]
- 41.Kiemle-Kallee J, Kreipe H, Radzun HJ, Parwaresch MR, Auerswald U, Magnussen H, Barth J. Alveolar macrophages in idiopathic pulmonary fibrosis display a more monocyte-like immunophenotype and an increased release of free oxygen radicals. Eur Respir J 1991;4:400–406. [PubMed] [Google Scholar]
- 42.Iwamoto GK, Monick MM, Burmeister LF, Hunninghake GW. Interleukin 1 release by human alveolar macrophages and blood monocytes. Am J Physiol 1989;256:C1012–C1015. [DOI] [PubMed] [Google Scholar]
- 43.Standiford TJ, Kunkel SL, Rolfe MW, Evanoff HL, Allen RM, Strieter RM. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol 1992;6:75–81. [DOI] [PubMed] [Google Scholar]
- 44.Hancock A, Armstrong L, Gama R, Millar A. Production of interleukin 13 by alveolar macrophages from normal and fibrotic lung. Am J Respir Cell Mol Biol 1998;18:60–65. [DOI] [PubMed] [Google Scholar]
- 45.Carter AB, Hunninghake GW. A constitutive active MEK→ERK pathway negatively regulates NF-κB-dependent gene expression by modulating TATA-binding protein phosphorylation. J Biol Chem 2000;275:27858–27864. [DOI] [PubMed] [Google Scholar]
- 46.Colucci-D'Amato GL, D'Alessio A, Califano D, Cali G, Rizzo C, Nitsch L, Santelli G, de Franciscis V. Abrogation of nerve growth factor-induced terminal differentiation by ret oncogene involves perturbation of nuclear translocation of ERK. J Biol Chem 2000;275:19306–19314. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson M, Mathers J, Dickinson RJ, Mandl M, Keyse SM. Both nuclear-cytoplasmic shuttling of the dual specificity phosphatase MKP-3 and its ability to anchor MAP kinase in the cytoplasm are mediated by a conserved nuclear export signal. J Biol Chem 2004;279:41882–41891. [DOI] [PubMed] [Google Scholar]
- 48.Cheung PY, Zhang Y, Long J, Lin S, Zhang M, Jiang Y, Wu Z. p150(Glued), Dynein, and microtubules are specifically required for activation of MKK3/6 and p38 MAPKs. J Biol Chem 2004;279:45308–45311. [DOI] [PubMed] [Google Scholar]
- 49.Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38α mitogen-activated protein kinase. J Biol Chem 2006;281:26225–26234. [DOI] [PubMed] [Google Scholar]
- 50.Perchonock CE, Fernando MC, Quinn WJ III, Nguyen CT, Sun J, Shapiro MJ, Shapiro VS. Negative regulation of interleukin-2 and p38 mitogen-activated protein kinase during T-cell activation by the adaptor ALX. Mol Cell Biol 2006;26:6005–6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 1996;16:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunninghake GW. Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am J Physiol 1998;275:L389–L397. [DOI] [PubMed] [Google Scholar]
- 53.Zhang C, Baumgartner RA, Yamada K, Beaven MA. Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-alpha and release of arachidonic acid in mast cells. Indications of communication between p38 and p42 MAP kinases. J Biol Chem 1997;272:13397–13402. [DOI] [PubMed] [Google Scholar]
- 54.Singh RP, Dhawan P, Golden C, Kapoor GS, Mehta KD. One-way cross-talk between p38(MAPK) and p42/44(MAPK): inhibition of p38(MAPK) induces low density lipoprotein receptor expression through activation of the p42/44(MAPK) cascade. J Biol Chem 1999;274:19593–19600. [DOI] [PubMed] [Google Scholar]
- 55.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 1995;270:1326–1331. [DOI] [PubMed] [Google Scholar]
- 56.Feng GJ, Goodridge HS, Harnett MM, Wei XQ, Nikolaev AV, Higson AP, Liew FY. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J Immunol 1999;163:6403–6412. [PubMed] [Google Scholar]
- 57.Cook SJ, Beltman J, Cadwallader KA, McMahon M, McCormick F. Regulation of mitogen-activated protein kinase phosphatase-1 expression by extracellular signal-related kinase-dependent and Ca2+-dependent signal pathways in Rat-1 cells. J Biol Chem 1997;272:13309–13319. [DOI] [PubMed] [Google Scholar]
- 58.DeSilva DR, Feeser WS, Tancula EJ, Scherle PA. Anergic T cells are defective in both Jun NH2-terminal kinase and mitogen-activated protein kinase signaling pathways. J Exp Med 1996;183:2017–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minden A, Lin A, Smeal T, Derijard B, Cobb M, Davis R, Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol 1994;14:6683–6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL6. Proc Natl Acad Sci USA 1993;90:2207–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahn NG, Seger R, Bratlien RL, Diltz CD, Tonks NK, Krebs EG. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem 1991;266:4220–4227. [PubMed] [Google Scholar]
- 62.Payne DM, Rossomando AJ, Martino P, Erickson AK, Her JH, Shabanowitz J, Hunt DF, Weber MJ, Sturgill TW. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J 1991;10:885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robbins DJ, Cobb MH. Extracellular signal-regulated kinases 2 autophosphorylates on a subset of peptides phosphorylated in intact cells in response to insulin and nerve growth factor: analysis by peptide mapping. Mol Biol Cell 1992;3:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins DJ, Zhen E, Owaki H, Vanderbilt CA, Ebert D, Geppert TD, Cobb MH. Regulation and properties of extracellular signal-regulated protein kinases 1 and 2 in vitro. J Biol Chem 1993;268:5097–5106. [PubMed] [Google Scholar]