Abstract
Human endothelial cells (EC) are typically resistant to the apoptotic effects of stimuli associated with lung disease. The determinants of this resistance remain incompletely understood. Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine produced by human pulmonary artery EC (HPAEC). Its expression increases in response to various death-inducing stimuli, including lipopolysaccharide (LPS). We show here that silencing MIF expression by RNA interference (MIF siRNA) dramatically reduces MIF mRNA expression and the LPS-induced increase in MIF protein levels, thereby sensitizing HPAECs to LPS-induced cell death. Addition of recombinant human MIF (rhMIF) protein prevents the death-sensitizing effect of MIF siRNA. A common mediator of apoptosis resistance in ECs is the death effector domain (DED)-containing protein, FLIP (FLICE-like inhibitory protein). We show that LPS induces a transcription-independent increase in the short isoform of FLIP (FLIPs). This increase is blocked by MIF siRNA but restored with the addition of recombinant MIF protein (rHMIF). While FLIPs siRNA also sensitizes HPAECs to LPS-induced death, the addition of rhMIF does not affect this sensitization, placing MIF upstream of FLIPs in preventing HPAEC death. These studies demonstrate that MIF is an endogenous pro-survival factor in HPAECs and identify a novel mechanism for its role in apoptosis resistance through the regulation of FLIPs. These results show that MIF can protect vascular endothelial cells from inflammation-associated cell damage.
Keywords: endothelial cells, macrophage migration inhibitory factor, FLICE-like inhibitory protein, apoptosis
CLINICAL RELEVANCE
Our study shows that macrophage migration inhibitory factor acts to protect vascular endothelial cells from sepsis-associated cell damage through its novel regulation of the short isoform of FLICE-like inhibitory protein.
Sepsis is a life-threatening condition associated with hyperactivity of the inflammatory response to infection leading to systemic vascular collapse, disseminated intravascular coagulation, acute lung injury, and multisystem organ failure (1). Vascular endothelial cells (EC) constitute the immediate cellular interface between the systemic circulation and organ parenchyma, and are directly exposed to numerous proinflammatory and cytotoxic stimuli during sepsis. EC are key regulators of vascular tone, coagulation, and permeability, and their dysfunction or destruction are suspected to be key contributors to additional pathologic complications associated with sepsis, such as acute lung injury/acute respiratory distress syndrome (ALI/ARDS) (2–4).
Studies in animals and humans indicate that EC apoptosis likely contributes to the dysfunction of the vascular endothelium associated with sepsis and sepsis-induced organ failure. Apoptosis is the programmed disassembly and elimination of cells and is a critical component of the normal development and homeostatic regulation of cell number. The induction of apoptosis, however, also contributes to the cellular injury and tissue destruction seen in a number of disease states and disorders (5). Biopsy and autopsy samples from humans with ALI/ARDS secondary to sepsis demonstrate increased endothelial cell apoptosis compared with control lung tissue. Studies in mice show that endotoxemia and gram-negative sepsis result in apoptosis in the gut, lymphoid tissue, and lung (6). More specifically, pathologic examination of the lungs from such animals reveals apoptosis of pulmonary vascular EC (7). Systemic administration of apoptosis inhibitors improves survival in animal models of endotoxin-induced injury (8), suggesting that apoptotic cell death contributes causally to disease-related mortality (9).
Many stimuli associated with sepsis, including TNF-α, the gram-negative endotoxin, lipopolysaccharide (LPS), reactive oxygen species, and hypoxia, activate the apoptotic machinery in a cell- and species-specific manner (10). Studies of cultured bovine or ovine EC have shown that these cells undergo apoptosis in response to these stimuli (11, 12), while cultured human-derived umbilical vein endothelial cells (HUVEC) and dermal microvascular endothelial cells (HDMEC) are relatively resistant to TNF-α and LPS (13, 14). Human ECs are sensitized to TNF-α– and LPS-induced apoptosis when treated with inhibitors of protein synthesis (13, 14), suggesting that short-lived or newly synthesized proteins influence the capacity of such stimuli to induce death. In the case of TNF-α, studies on isolated human EC show that it induces NF-κB activation and increases the transcription of a number of inhibitor of apoptosis (IAP) proteins that blunt TNF-dependent death signaling (13, 15). In contrast, suppressing NF-κB activity does not sensitize EC to LPS-induced cell death (13), although the application of the protein synthesis inhibitor cycloheximide (CHX) does, suggesting that resistance to LPS is dependent on a short-lived protein that is not linked to NF-κB activation. Anti-apoptosis proteins known to undergo rapid turnover in the cell include XIAP, Mcl-1, and FLICE-like inhibitor protein or FLIP (16, 17). Treatment of HUVEC with CHX leads to a rapid decrease in protein levels of the long isoform of FLIP (FLIPL), coinciding with increased sensitivity to LPS (15). FLIP is a death effector domain (DED)-containing protein that is homologous to caspase 8 (a.k.a. FLICE) but possesses little or no caspase activity. As such, it has been proposed that it may act in a dominant-negative manner to block caspase 8 activation in response to death receptor activation (17). Forced expression of the long isoform of FLIP (FLIPL) in HUVEC prevents apoptosis caused by combined LPS and CHX treatment (18), suggesting that FLIPL may be responsible for the resistance of these cells to LPS-induced cell death. Additional factors that control the ability of sepsis-associated pro-inflammatory stressors to induce apoptosis of human EC, however, have not been systematically examined.
We chose primary cultures of human pulmonary artery endothelial cells (HPAEC) as an in vitro system to further define the effects of proinflammatory and cytotoxic stimuli on EC survival in the lung. Similar to what has been reported in the literature for HUVEC (18) and HDMEC (19), our data show that HPAEC exhibit an inherent resistance to multiple potential apoptotic stimuli. We investigated the role that the proinflammatory cytokine, macrophage migration inhibitory factor (MIF), which is constitutively expressed and secreted by HPAEC in culture and is present in vascular EC in vivo (1), might play in governing this resistance, as there is growing evidence for both cytoprotective and pro-growth effects of MIF (20–24), although a role for MIF in the apoptosis of EC has not been reported. To test the hypothesis that MIF promotes survival of HPAEC and thus contributes to their resistance to proinflammatory stressors, we suppressed MIF protein expression in HPAEC by RNA interference (RNAi) and then evaluated changes in basal cell death and sensitivity to apoptotic stimuli caused by this suppression. We show here that suppression of MIF expression sensitizes HPAEC to apoptosis triggered by LPS and that MIF is normally required for the LPS-mediated induction of the short isoform of FLIP (FLIPs). This induction occurs independently of changes in FLIP transcription, is mimicked by proteosome inhibitors, and is essential in conferring resistance on HPAEC to LPS-induced cell death. These studies support a role for MIF in the natural resistance of human pulmonary EC to apoptosis in general and the pro-apoptotic effects of LPS, in particular, through its regulation of FLIPs and suggest that MIF may act to protect vascular endothelial cells from sepsis-associated cell damage.
MATERIALS AND METHODS
Reagents and Cell Lines
HPAEC derived from individual donors (Clonetics Corporation, Walkersville, MD) were maintained according to the manufacturer's recommendations. Cells were used between passages 5 and 8 and were cultured, unless otherwise indicated, in complete EGM media (Clonetics). For each experiment, cells were matched for donor and passage number. Results from at least four different donors were pooled. Where indicated, cells were incubated with TNF-α (final concentration: 100ng/ml), LPS (100 ng/ml) (Sigma Chemical Co, St. Louis, MO), or recombinant human MIF (1 nM) (R&D Systems, Minneapolis, MN). Hypoxia was achieved using an airtight Plexiglas chamber placed inside a 37°C incubator. A humidified mixture of 95% nitrogen and 5% CO2 was continually passed through the chamber. Duplex RNAs encoding control (GFP) siRNA (sense strand: GGCTACGTCCAGG AGCGCACC), human MIF siRNA (sense strand: ACAGGGUCUACAUCAAUAdTdT), caspase 8 (sense strand: GGACAAAGUUUACCAAAUGUU) and FLIPs (sense strand: CACCCUAUGCC CAUUGUCCdTdT) were used for RNAi and were manufactured by Dharmacon Inc. (Lafayette, CO). A pool of four duplex siRNAs that targets the common sequences between FLIPL and FLIPs were used to suppress total FLIP expression (SiGENOME SMARTpool reagent M-003772-06-0005; Dharmacon, LaFayette, CO). Transfection of the duplex RNA was performed using Geneporter B reagent (Genlantis, San Diego, CA) according to the manufacturer's recommendations. Briefly, 1.5 × 106 HPAECs were plated into a 35-mm dish. The next morning, the medium was replaced with 900 μl of basal EGM (Clonetics). RNAi duplexes were diluted into a total of 50 μl of Geneporter buffer B. Geneporter transfection reagent (2 μl) was diluted into a total of 50 μl of basal EGM. The diluted RNA duplexes were mixed with the diluted transfection reagent and then added drop-wise to the cells. Four hours later, 1 ml of growth medium was added to the cells. Final concentration of RNA duplexes was 100 nM. The protein synthesis inhibitor CHX was used at a final concentration of 10 μM, and the proteosome inhibitor MG132 was used at a final concentration of 1 μM.
Apoptosis
Apoptotic cells were identified on the basis of altered nuclear morphology after staining with Hoechst dye 33342. Necrotic cell death was evaluated by uptake of the normally cell-impermeable DNA dye, propidium iodide (PI). A total of 200 to 300 cells/sample were evaluated by fluorescence microscopy. The caspase 8 inhibitor, zIETD-fmk (MP Biochemicals, Solon, OH) was used at a final concentration of 50 μM. Additional assays of cell death are described in the online supplement.
Western Blotting and Immunoprecipitation
For Western blotting, cells were washed in cold phosphate-buffered saline and protein extracted in lysis buffer (60 mm Tris [pH 6.8], 1% SDS, 20% glycerol, containing 1× protease inhibitor cocktail [Cat # P8340; Sigma Chemical]), sonicated, boiled, and then cleared by centrifugation. Proteins were separated by SDS-PAGE and the gel then electrophorectically transferred to a PVDF membrane (Millipore Corporation, Bedford, MA). Specific proteins were detected with the following antibodies; anti-Bax (Ab-N20; Santa Cruz Biotechnology, Santa Cruz, CA), anti-MIF (R&D Systems), anti-FLIP (NF6); anti-actin (Santa Cruz Biotechnology), anti-cleaved caspase 3 (Cat #9664; Cell Signaling), anti-caspase 9 (Cat #9508; Cell Signaling), and anti-human BID (horseradish peroxidase–conjugated secondary antibodies were then incubated with the blot and the antibody complexes detected by chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ).
Quantitative RT-PCR
Total RNA was isolated from cell cultures using the Trizol reagent per the manusfacturer's suggestions (InVitrogen, Carlsbad, CA). The RNA was precipitated in isopropanol and then subjected to additional purification using the RNeasy RNA isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. RNA was reverse transcribed to generate cDNA using a First-Strand Synthesis kit (Amersham) and random hexamers as primers. The cDNA was then used as the template for quantitative PCR using an iCycler Thermal Cycler Real-Time PCR machine (Bio-Rad, Hercules, CA). The products of PCR amplification were detected with Syber green fluorescent dye and the relative expression of each gene of interest expressed with reference to that of glyceraldehyde phosphate dehydrogenase (GAPDH). The PCR products were analyzed on Tris-Acetate-EDTA agarose gels to confirm its correct size. The sequence of the primers used for PCR are as follows. MIF-F: 5′-CATCATGCCGATGTTCATCG-3′; MIF-R: 5′-AGCAGCTT-GCTGTAGGAGCG-3′; FLIPL F: 5′-TTGGCCAATTTGCCTGTATG-3′; FLIPL R-: 5′-TCGGCTCACCAGGACACA-3′; FLIPs F-:5′-GCAGGGACAAGTTACAGGAATG T-3′; FLIPs R-: 5′-GGACAATGGGCATAGGGTGT-3′. GAPDH control primers were obtained from Applied Biosystems (Foster City, CA).
Statistical Evaluation of Data
All data are expressed as the mean ± SE. Unpaired or paired Student t test was used for statistical comparisons when appropriate. Differences were considered significant at P < 0.05.
RESULTS
HPAEC Are Resistant to Apoptotic Stimuli and Produce Increased Intracellular MIF Protein Levels in Response to These Stressors
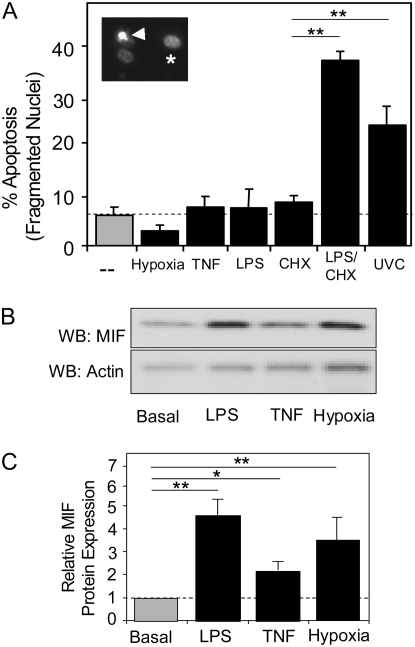
The sensitivity of HPAEC to multiple pro-apoptotic stimuli was evaluated in cell culture. HPAEC were exposed to prolonged hypoxia (<1% FiO2), the inflammatory cytokine TNF-α (10 ng/ml), the bacterial endotoxin LPS, or ultraviolet irradiation (100 J/m2 UVC) for 24 hours. Apoptotic and necrotic cells were identified by nuclear condensation and fragmentation after staining with the nuclear dye Hoechst 33342 and by uptake of the normally cell-impermeable DNA dye, propidium iodide (PI), respectively. The white arrow in the inset to Figure 1A shows normal (asterisks) and fragmented nuclear staining (white arrow) by Hoechst 33342 in cells treated with LPS and the protein synthesis inhibitor CHX. In culture, basal rates of HPAEC apoptosis were low (5.5% ± 3%), with no significant increase above basal levels resulting from exposure to hypoxia, TNF-α, or LPS (Figure 1A). Necrosis in untreated cells was minimal (0.5 ± 0.2%) and did not change with any of the treatments shown in Figure 1A. HPAECs did undergo increased apoptosis after exposure to UVC, demonstrating that the basic apoptotic machinery was intact in these cells. As has been shown previously for other EC (13, 14), apoptosis in response to LPS could be induced after pre-treatment with the protein synthesis inhibitor CHX. CHX pre-treatment alone did not affect basal cell death (Figure 1A).
Figure 1.
The response of human pulmonary artery endothelial cells (HPAEC) to potential apoptotic stimuli in culture. (A) Multiple cytotoxic stressors were evaluated for their ability to induce apoptosis in HPAEC. Basal apoptosis (dotted line) is minimal, representing less than 5% of the total cells evaluated under the culture conditions detailed in Materials and Methods. To induce hypoxia, a mixture of nitrogen and carbon dioxide (95%/5%) gases were used to purge cultures of oxygen for 48 hours before quantification of apoptotic cells. Additional cultures were incubated in the presence of TNF (100 ng/ml), lipopolysaccharide (LPS; 100 ng/ml), cycloheximide (CHX; 50 μg/ml), or LPS/CHX (100 ng/ml and 50 μg/ml, respectively) for 24 hours before analysis. Insert shows Hoechst 3342 staining of normal (asterisk) and fragmented (arrowhead) nuclei. (B) Macrophage migration inhibitory factor (MIF) protein and β-actin were identified by Western blotting in total cell lysates of untreated HPAEC and LPS-, TNF-α–, and hypoxia-treated HPAEC. (C) Graphical representation of intracellular MIF protein levels normalized to β-actin expression. Results from at least three independent experiments. (*P > 0.01; **P > 0.005).
Others have demonstrated that LPS, TNF-α, and hypoxia increase intracellular MIF protein levels in HUVEC, fibroblasts, and tumor cell lines, respectively (25–28). We show here that all three stimuli cause a significant increase in MIF intracellular protein levels in HPAEC (Figure 1B).
Suppression of MIF Gene Expression Sensitizes HPAEC to Cell Death Caused by LPS
The increase in MIF expression caused by hypoxia, LPS, or TNF-α could be (i) a death signaling event that is counteracted by the concurrent activation of pro-survival pathways, (ii) a pro-survival event activated by apoptotic stimuli that counteracts the death program, or (iii) irrelevant to either the survival or death of HPAEC. To distinguish between these possibilities, we examined the effect of acute suppression of MIF mRNA and protein levels in HPAEC using RNA interference (RNAi). A chemically synthesized double-stranded RNA (siRNA) was introduced into HPAEC by liposome-facilitated transfection. Parallel cultures transfected with fluorochrome-tagged double stranded RNA of similar size revealed a transfection efficiency of greater than 95% and persistence of fluorescence for greater than 5 days in culture.
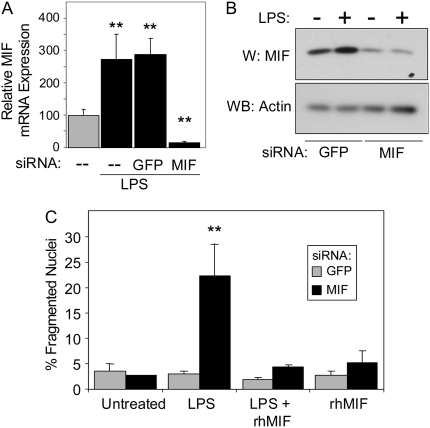
We first examined the effect of MIF suppression on LPS-stimulated cell death. Figure 2A shows that 16 hours of LPS exposure induced an approximate 2.6-fold increase in MIF mRNA levels. Transfection with a control siRNA directed against the mRNA for green fluorescent protein (GFP) (negative control) begun 8 hours before the addition of LPS had no significant effect on the induction of MIF mRNA by LPS. Transfection with the MIF siRNA, however, not only prevented the increase in MIF mRNA expression caused by LPS but reduced basal levels to less than 5% of untreated control samples. Analysis of intracellular MIF protein levels in control siGFP-transfected cells revealed a 3.4-fold increase in MIF expression after 18 hours of exposure to LPS (Figure 2B). This LPS-induced increase was similar in magnitude to that seen in untransfected cells in Figure 1B. Exposure of HPAEC to MIF siRNA for 24 hours reduced basal MIF protein levels to approximately 20% of basal (GFP siRNA/no LPS) levels and prevented the LPS-induced increase in MIF protein. Figure 2C shows that neither the control/GFP siRNA nor the MIF siRNA had any significant effect on basal cell death 24 hours after transfection. LPS treatment combined with MIF siRNA transfection, however, resulted in a marked increase in cell death. There was no change in cell death over basal levels in control/GFP siRNA–transfected cells treated with LPS.
Figure 2.
Selective suppression of MIF enhances the sensitivity of HPAEC to LPS-induced apoptosis. (A) Quantitative RT-PCR measurements of MIF mRNA levels in untreated HPAEC and in response to LPS. Results are normalized to GAPDH mRNA expression. LPS caused a 2.6-fold increase in MIF mRNA that was unaffected by control (GFP) siRNA but markedly suppressed to less than 10% of control levels by MIF-selective siRNA. (B) Protein expression in HPAEC transfected for 8 hours with control/GFP- or MIF siRNA and then exposed to 100 ng/ml LPS for an additional 16 hours. LPS induced a 3.4-fold increase in MIF protein levels. Both basal and LPS-induced levels were suppressed approximately 20% of unstimulated basal levels by MIF siRNA. (C). HPAEC were transfected with GFP siRNA (solid bars) or MIF siRNA (shaded bars) at a final concentration of 100 nM. Six hours after transfection, cells were challenged with PBS, 100 ng/ml LPS in PBS, 100 ng/ml LPS, and 10 ng/ml recombinant human MIF protein (rHMIF), or rHMIF alone and incubated for an additional 18 hours before quantification of apoptotic cell death by fluorescent microscopy as described in Materials and Methods. **P > 0.005.
To further characterize the specificity of MIF siRNA and exclude the possibility that nontargeted gene effects led to the sensitization of these cells to LPS, both control/GFP siRNA– and MIF siRNA–transfected cells were treated with recombinant human MIF, exposed to LPS, and then analyzed for changes in cell death. The addition of recombinant MIF protein prevented LPS-induced cell death in the MIF siRNA–transfected HPAEC, demonstrating that the effect of the MIF siRNA on HPAEC survival could be bypassed by directly supplying the protein to the cell, which strongly suggests that the effect of the MIF siRNA was due alone to suppression of MIF (Figure 2C).
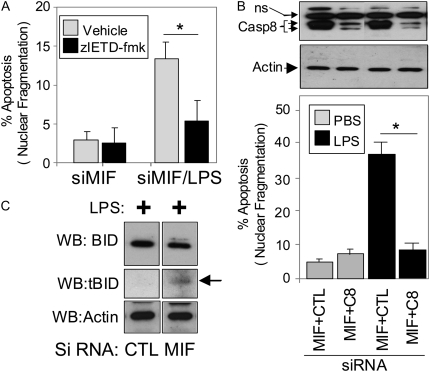
Previous work by others has implicated activation of caspase 8 in LPS-induced EC death (15–17). In HPAEC, the caspase 8–specific inhibitor, zIETD-fmk, blocked cell death caused by the combination of LPS and MIF siRNA (Figure 3A), indicating that caspase 8 activity was necessary for cell death. To substantiate this conclusion, we transfected HPAEC with individual siRNAs for MIF and either caspase 8 or control (GFP) siRNA, such that the total siRNA load on the cell was kept at 100 nM. The caspase 8 siRNA markedly reduced caspase 8 protein levels within 24 hours of transfection (Figure 3B, upper panels) and markedly attenuated LPS/MIF siRNA cell death.
Figure 3.
Role of caspase 8 in LPS-induced cell death in HPAEC. (A) The caspase 8 inhibitor, zIETD-fmk, inhibits LPS/MIF siRNA cell death. HPAEC were transfected with MIF siRNA, exposed to 50 μM zIETD-fmk 7 hours later, then stimulated with LPS 1 hour later and assessed for cell death 24 hours after the addition of LPS. *P < 0.005. (B) Suppression of caspase 8 expression by siRNA prevents LPS/MIF siRNA cell death. Western blotting results in upper two panels for caspase 8 (Casp 8) and actin are aligned with bar graphs below. NS, nonspecific interaction of antibody with unknown cellular protein in HPAEC. *P < 0.005. (C) LPS plus MIF siRNA, but not LPS alone, simulates BID cleavage. Cleavage product is indicated by the arrow.
One of the downstream targets of caspase 8 cleavage is the cytosolic BH3-only protein, Bid. Cleavage results in a truncated from of Bid (tBID) that promotes mitochondrial death signaling initated by the tBID-dependent activation of BAX/BAK. Figure 3C shows that treatment of HPAEC with LPS and MIF siRNA causes significant tBID expression, while treatment with LPS alone did not. These results suggest that LPS and MIF siRNA activate caspase 8, resulting in BID cleavage and increased cell death.
LPS Up-Regulates Expression of FLIPs in an MIF-Dependent Manner
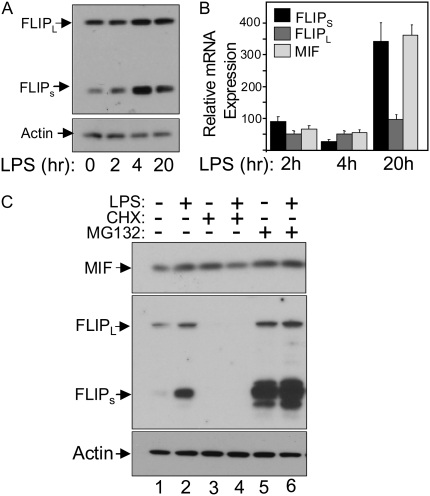
A number of studies on isolated ECs have implicated the pro-survival protein known as FLIP as an important regulator of cell death caused by TNF-α, FasL, ischemia-reperfusion, and LPS (14, 17, 29, 30). Data in Figures 4A and 4B show that LPS causes a rapid induction in FLIPs expression, resulting in an approximate 5.4-fold increase in protein levels 4 hours after LPS treatment. In contrast, FLIPL protein levels increased only by 1.4-fold on average over the same period. The up-regulation of FLIPs occurred in a transcription-independent manner as quantitative RT-PCR showed that FLIPs and FLIPL, mRNA levels actually fell over the time period in which protein levels increased (Figure 3B). This demonstrates that changes in FLIPs protein levels occur independently of changes in mRNA levels and, therefore, were likely to be mediated by protein turnover. Figure 4C shows that inhibition of protein synthesis caused by a 4-hour incubation with the protein synthesis inhibitor CHX resulted in complete loss of FLIPs and FLIPL protein levels, while MIF protein levels were virtually unchanged after normalizing the data to β-actin protein levels. On the other hand, incubation with the proteosomal inhibitor MG132 for 4 hours markedly increased protein levels of FLIPs, but had a minimal effect on FLIPL protein levels. This differential effect of proteasome inhibition on FLIPs versus FLIPL protein levels has been previously reported and shown to be due to destabilizing sequences in the unique C-terminus of FLIPs (32). Together, these results show that the changes observed in FLIPs in response to LPS are transcription-independent and may be due to increased protein stabilization caused by selective prevention of proteasomal-mediated degradation of FLIPs.
Figure 4.
LPS induces a rapid increase in FLIPs protein levels. (A) Changes in FLIPL, FLIPs, and β-actin protein levels in HPAEC in response to LPS. HPAEC were exposed to 100 ng/ml LPS and then harvested at the indicated times and processed for Western blotting. LPS induced a rapid increase in FLIPs protein levels without significantly affecting FLIPL levels. (B) HPAEC were treated with LPS as described above and harvested at the indicated times for total RNA isolation followed by quantitative RT-PCR for FLIPL, FLIPs, MIF, and β-actin mRNA. Results are presented relative to unstimulated cells and normalized to β-actin levels. (C) Changes in FLIPL, FLIPs, and MIF protein levels in HPAEC 4 hours after the addition of the protein synthesis inhibitor CHX (10 μM) or the proteasome inhibitor MG-132 (1 μM) with and without addition of 100 ng/ml LPS. Lanes 1 and 2 show the increase in FLIPs protein caused by 4 hours of LPS exposure. Lanes 3 and 4 show that both FLIPs and FLIPL protein levels are markedly reduced once protein synthesis is inhibited, while lanes 5 and 6 show a disproportionate effect of inhibiting proteasomal-mediated degradation on FLIPs, compared with FLIPL.
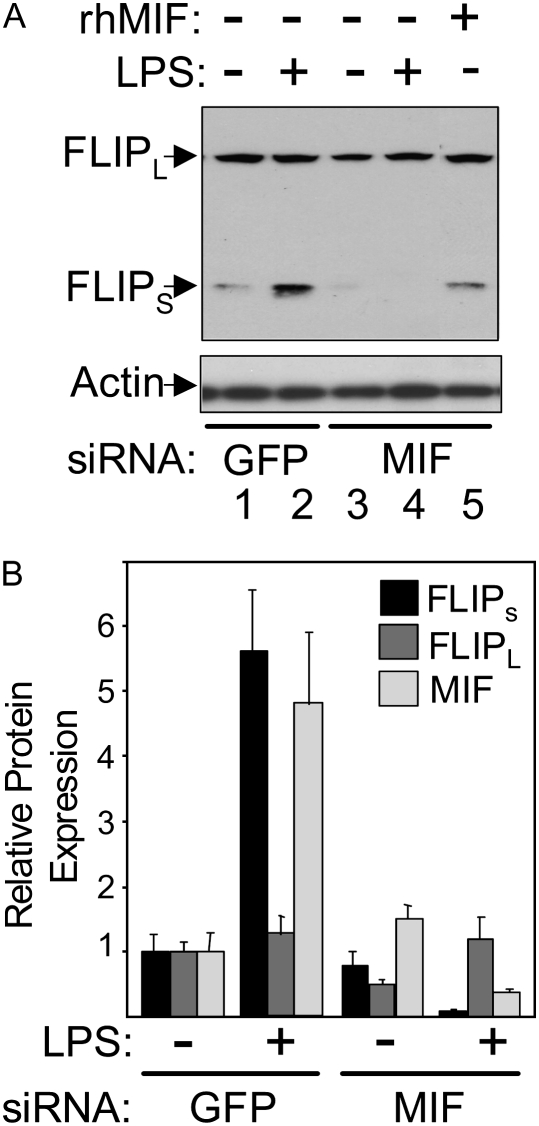
The Western blot shown in Figure 5A and the averaged results in Figure 5B show that in cells treated with MIF siRNA, the transient increase in FLIPs expression in response to LPS is prevented, actually falling below unstimulated levels. In contrast, FLIPL levels are only slightly affected by these treatments. To determine if the suppression of MIF expression was alone responsible for the effects of MIF siRNA treatment on FLIPs protein expression, rHMIF was added along with MIF siRNA in a separate experiment. The addition of rHMIF protein in MIF siRNA–treated HPAEC restored FLIPs protein levels to normal, allowed FLIPs protein levels to increase in response to LPS, and prevented the LPS-induced cell death in MIF siRNA–transfected cells (Figure 2C). These results show that MIF is required for the rapid increase in FLIPs protein levels in response to LPS.
Figure 5.
Changes in FLIPs protein levels in response to LPS are dependent on MIF. (A) FLIPs and FLIPL protein levels were measured in control/GFP siRNA– and MIF siRNA–transfected HPAEC before and 4 hours after LPS stimulation. Lanes 1 and 2 show the increase in FLIPs expression seen with LPS, while the comparison of lanes 3 and 4 shows that this increase is not only prevented by suppression of MIF but that FLIPs levels fall below basal. Lane 5 shows that addition of rHMIF prevents the decline in FLIPs protein levels caused by MIF siRNA. (B) Graphical representation of relative changes in FLIPs, FLIPL, and MIF protein levels after normalization to β-actin expression from three independent experiments. The normalized value for each protein in unstimulated control/GFP siRNA–transfected cells was arbitrarily set at 1, with all changes in expression referenced to this point.
Reducing FLIPs Protein Levels Is Alone Sufficient to Sensitize HPAEC to LPS-Induced Death
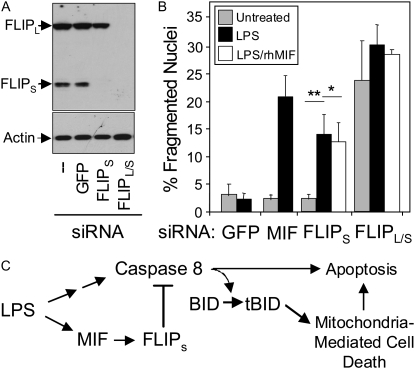
To assess the significance of the LPS-induced increase in FLIPs protein expression in the HPAEC resistance to LPS-induced cell death, FLIPs expression was selectively suppressed by a target-specific siRNA. Figure 6A shows that the FLIPs-selective siRNA suppressed only FLIPs protein levels, with no effect on FLIPL or actin. Suppression of FLIPs was virtually complete within 24 hours of application. Figure 6B shows that the FLIPs siRNA had no effect on basal cell death in the absence of LPS, but caused significant cell death in its presence, an effect identical to that seen with short-term application of MIF siRNA. In contrast to MIF siRNA–mediated sensitization, which was bypassed by adding recombinant MIF (rHMIF) protein to the HPAEC (Figure 2C), the increased LPS-induced cell death caused by FLIPs siRNA was not prevented by the addition of rHMIF to the cells. These results are, therefore, consistent with a model in which MIF lies upstream of FLIP and acts to suppress LPS-induced cell death through regulation of FLIPs protein levels. In contrast to the effect of silencing FLIPs alone, application of a siRNA that suppresses both FLIPL and FLIPs (FLIPL/s in Figure 5A) caused a marked increase in basal cell death within 24 hours of application (Figure 5B). As was the case for FLIPs siRNA treatment, the effects of FLIPL/s siRNA were not reversed by the addition of rhMIF, suggesting that the constitutive protection by FLIP as revealed by FLIPL/S siRNA treatment may lie distal to or independent of MIF.
Figure 6.
Effect of FLIPs-specific siRNA transfection on the sensitivity of HPAEC to LPS-induced cell death. (A) Analysis of FLIP and actin protein expression in HPAEC transfected with control (GFP) siRNA, FLIPs-specific siRNA, and siRNA that targets both FLIPs and FLIPL by Western blotting demonstrates the specificity of the reagents used to manipulate FLIP levels. (B) Results of apoptosis analysis of HPAEC pretreated with control, MIF, or FLIP siRNAs and then stimulated with LPS. Some cells were pre-treated with rHMIF 6 hours before LPS stimulation. **P < 0.005. (C) Schematic diagram summarizing the role of MIF in apoptosis resistance to LPS. Stimulation of HPAEC activates both pro-death and pro-survival pathways. The latter requires the presence of MIF and involves a MIF-dependent increase in FLIPs protein levels, which acts to suppress caspase 8 activity/activation and cell death.
Taken together, these data suggest the following sequence of events occurring in HPAECs in response to LPS (Figure 6C). LPS activates both a death-promoting as well as a death-inhibitory pathway in HPAEC. The death-promoting pathway is mediated through caspase 8 and involves engagement of the mitochondrial death pathway, possibly through activation/cleavage of BID. The death-inhibiting pathway involves up regulation of MIF expression which, in turn, is linked to the up-regulation of FLIPs protein levels. Increased FLIPs protein interacts directly with caspase 8 to suppress caspase 8–dependent death signaling events.
Prolonged Suppression of MIF Protein Levels Increases Basal Cell Death via the Instrinsic/Mitochondrial Death Pathway
The data in Figure 2 demonstrating the sensitization of HPAEC to LPS-induced death by MIF siRNA were obtained after only 24 hours of exposure to siRNA (8 h pre-LPS + 16 h in LPS). At that time, MIF mRNA levels were suppressed to less than 5% of untreated or control siRNA–treated cells, while basal levels of MIF protein were reduced by approximately 80%. We therefore extended the time from transfection to harvest to determine if MIF protein levels would continue to decrease. The data in Figure E1 in the online supplement show a representative Western blot of the time course of MIF suppression. Treatment of HPAEC for 72 hours with MIF siRNA resulted in suppression of MIF protein expression to almost undetectable levels (2.7 ± 0.8% of control siRNA–treated cells) and, contrary to what had been observed at earlier time-points, markedly increased basal cell death, which could be further increased by the addition of LPS. Control/GFP siRNA treatment had no effect on MIF mRNA and protein levels over the same time period and did not affect basal cell death. Importantly, the addition of recombinant human MIF protein at 48 hours after the addition of MIF siRNA prevented the cell death caused by MIF siRNA at 72 hours, ruling out a nonspecific effect of the siRNA treatment (Figure 6C).
Cells treated with MIF siRNA alone for 72 hours exhibited morphologic changes characteristic of apoptotic cell death. Specifically, they displayed cytoplasmic blebbing (zeiosis) and nuclear condensation or nuclear fragmentation (Figures E2A and E2B). Caspases were activated in association with MIF loss, as demonstrated by the appearance of cleaved caspase 3 products in lysates from MIF siRNA– but not in control/GFP siRNA–transfected cells (Figure E2C) and by the ability of the broad-spectrum caspase inhibitor z-VAD-fmk to block cell death (Figure E2D). Data described in detail in the online supplement show that prolonged MIF suppression led to activation of the mitochondrial death-signaling pathway, resulting in increased BAX activation and cytochrome c release into the cytosol, which others have shown to activate caspase 9 (35). Accordingly, apoptosis caused by prolonged MIF deficiency was blocked by overexpression of either Bcl-xL, which blocks BAX activation (34, 35), or expression of caspase 9 containing an inactive catalytic domain, which acts as a dominant-negative mutant (Figure E3).
DISCUSSION
We have shown in this study that human endothelial cells isolated from the pulmonary artery (HPAEC) and maintained in culture at low passage are resistant to multiple apoptotic stressors. Resistance to apoptosis is a common feature of many other human endothelial cells (13, 14, 16, 18). This resistance is not absolute, as demonstrated by the sensitivity of HPAEC to UVC- and STS-induced apoptosis and the ability of the protein synthesis inhibitor CHX to sensitize HPAEC to cell death induced by the bacterial endotoxin, LPS (Figure 1). These observations suggest that it is unlikely that there is a deficiency in the cellular machinery needed to execute cell death, but rather support the notion that these cells possess endogenous inhibitor(s) that de-sensitize them to certain death stimuli. While the nature of the resistance is likely to be multifactorial, a number of characteristics suggest that the cytokine MIF could be an important contributor to this resistance. Endothelial cells contain intracellular stores of MIF protein in vivo and in culture, and both the intracellular accumulation and secretion of MIF is increased by apoptogenic stimuli (25–28) (Figure 1). Treatment of animals with neutralizing antibodies to MIF decreases capillary density at the site of tumor implantation (20, 21, 36), suggesting that MIF normally plays a role in promoting angiogenesis, perhaps through increased EC proliferation and survival. More directly, recombinant MIF protein has been shown to protect cells from apoptosis induced by oxidative stress (22, 37), and both macrophages and fibroblasts derived from MIF-deficient mice exhibit accelerated apoptosis in culture (38).
In this context, we have shown that when challenged with potential apoptotic stressors, such as the bacterial endotoxin LPS, MIF intracellular protein levels increase in HPAEC, but no additional cell death over that of basal levels occurs. Blocking the increase in MIF expression caused by LPS through siRNA-mediated post-transcriptional gene silencing (PTGS), however, leads to increased cell death, suggesting that MIF sensitizes the cells to the death-inducing effects of LPS. Importantly, this sensitization to cell death in MIF siRNA–transfected HPAEC is prevented by supplying recombinant human MIF protein to the culture, strongly suggesting that the effect of transfecting the MIF siRNA into the cells was related to the loss of MIF protein and not to nonspecific effects on other genes that may influence apoptosis resistance. Cell death caused by LPS + MIF siRNA is inhibited by the relatively specific inhibitor of caspase 8 activity, z-IETD-fmk, as well as by siRNA-mediated of suppression of caspase 8 expression (Figures 3A and 3B). Cell death is also accompanied by increased appearance of tBID, a caspase 8–dependent truncation of the BH3-only protein, BID, that activates the mitochondrial death signaling pathway. These data suggest that cell death caused by the combination of LPS and MIF siRNA involves activation of the intrinsic death pathway in the cell, and they implicate activation of caspase 8 as a possible regulatory step that is normally suppressed in MIF-expressing HPAEC.
The identification of factors responsible for resistance and sensitivity to LPS-induced apoptosis has been an area of active investigation (10, 13, 14, 17–19). LPS has the capacity to activate the transcription factor NF-κB, which has been linked to both pro-death and pro-survival signaling pathways (10) but increased NF-κB activity does not appear to be required for the resistance of some human EC to LPS-induced apoptosis (13, 14, 39). However, enforced overexpression of the cell death regulatory protein, FLIP has been shown to protect human EC from LPS-induced apoptosis, suggesting that FLIP expression is one component of resistance to LPS-induced cell death (13, 17, 29). In addition, FLIP protein expression is rapidly suppressed by the protein synthesis inhibitor CHX (13) (Figure 3C), which sensitizes all human EC examined to date to LPS-induced cell death (including HPAEC in this article). A possible involvement of FLIP in the resistance of HPAEC to LPS is also suggested by our data demonstrating the importance of caspase 8 in LPS/MIF siRNA–associated cell death (Figure 3).
FLIP is highly homologous to caspase 8 (a.k.a., FLICE or FADD-like IL-1β–converting enzyme) and is likely to have resulted from duplication of the caspase 8/FLICE gene, as it resides on the same chromosomal fragment as caspase 8 (for a review, see Ref. 31). Caspase 8 is an initiator caspase that is recruited to the death-induced signaling complex (DISC) that forms upon ligand activation of death receptors (CD95/Fas, TNFR1, DR5) through homotypic protein interactions with the death receptor adaptor protein, FADD (Fas-associated death domain–containing protein) mediated by the protein–protein interaction motif known as the death effector domain (DED). Two splicing variants of FLIP have been identified. The long isoform of FLIP (FLIPL) is similar in overall length and domain structure to caspase 8. It contains two N-terminal DEDs, but the region in FLIPL that corresponds to the catalytic region in caspase 8 differs so that proteolytic activity is markedly suppressed, if not totally abolished. The short form of FLIP (FLIPs) contains only the two DEDs and has no proteolytic activity at all. Recruitment of either FLIP isoform to the DISC is thought to modulate or block death receptor–stimulated cell death by competing with caspase 8 for binding to FADD (40). Both FLIP isoforms are transcribed in an NF-κB–dependent manner (44), but differential expression of FLIPL and FLIPS proteins in various cell types suggests the existence of additional mechanisms to regulate individual isoform accumulation. For example, p53 overexpression has been reported to enhance proteasome-mediated degradation of FLIPL (45), while in Hela cells, TNF-α induces the specific up-regulation of FLIPS without affecting the levels of FLIPL (46). Interestingly, expression of the viral transactivator E1A in Hela cells confers increased sensitivity to TNF-α–mediated cell death by preventing not only the induction of FLIPs mRNA but also by promoting its selective degradation is a proteasomal-mediated manner (46).
Our data show that in response to LPS, FLIPs protein levels in HPAEC are rapidly and markedly increased, while FLIPL protein levels are only slightly increased (Figure 4). When MIF siRNA–treated HPAEC are stimulated with LPS, FLIPs levels not only fail to increase but fall below pre-stimulation levels. This change in FLIPs protein levels is likely to be important for mediating MIF-associated apoptosis resistance in these cells because selective suppression of FLIPs by siRNA alone sensitized HPAEC to LPS (Figure 6). Interestingly, the increase in FLIPs protein levels in response to LPS occurs in the complete absence of any increase in FLIPs mRNA (Figure 3) and, therefore, must be due to either increased translation of existing mRNA or suppression of FLIPs protein turnover. While the application of a protein synthesis inhibitor did result in complete loss of both FLIPs and FLIPL, the proteasome inhibitor, MG132, had a disproportionately greater effect on increasing FLIPs protein levels compared to FLIPL (Figure 3C). Together, these observations suggest that MIF selectively regulates FLIPs protein stabilization. The molecular mechanism through which this is achieved is unknown, but may involve the reported interaction of MIF with Jab1/CSN5 (47). Jab-1 is a transcriptional co-activator and essential component of the COP9 signalosome. It is involved in ubiquitin/26S proteasome-mediated protein degradation of several cell cycle regulators and intracellular signaling intermediates (48). Alterations of Jab-1 function through its interactions with MIF contribute to the reported differential turnover of other proteins including p27 (32) (Figure 4B) and could account for the changes in FLIPs expression that we have described here.
The marked selectivity of MIF siRNA in suppressing FLIPs but not FLIPL protein expression in HPAEC is particularly noteworthy, since previous studies have suggested that FLIPL provides constitutive protection against LPS-induced cell death (13, 14, 16, 18). FLIPL protein levels in FLIPs-deficient HPAEC, however, are virtually identical to those of untreated HPAEC, yet the cells still undergo cell death. While our data indicate that both FLIPs and FLIPL normally inhibit cell death, their effects are not redundant. Selective suppression of FLIPs reveals that it controls the sensitivity of HPAEC to apoptosis induced by LPS (and perhaps other stimuli, as studies in other cell types would suggest [46, 49, 50]), without affecting basal levels of cell death, while combined FLIPs and FLIPL suppression increases basal cell death. We speculate that the latter effect is due to the suppression of FLIPL alone and not to synergistic effects caused by suppression of both splice variants (51), but this possibility needs to be formally tested and the relative contributions of FLIPs and FLIPL to EC death more precisely defined.
In contrast to short-term suppression of MIF, which had no effect on basal cell death, long-term suppression of MIF (72–96 h) results in markedly increased basal cell death (Figure E1). Is this effect of prolonged suppression of MIF linked to changes FLIPs or FLIPL as well? While combined FLIPL/FLIPs suppression produced increased basal cell death in the absence of any other apparent stimulus just as prolonged MIF siRNA treatment did, no consistent reduction in FLIPL expression was detected in association with prolonged MIF suppression alone (data not shown). The nature of increased cell death caused by long-term suppression of MIF was shown to be apoptotic and mediated through the intrinsic/mitochondrial death pathway (Figures E2 and E3). It is unlikely that engagement of the mitochondrial death pathway due to long-term MIF suppression is due to cleavage of BID, since FLIP levels do not change and suppression of FLIPs does not activate cell death on its own. A related but different mechanism, however, may involve the ability of intracellular MIF to directly or indirectly antagonize the mitochondrial apoptotic pathway through binding of another BH3-only protein family, known as Bnip3L or NIX (53). Bnip3L is a class of pro-apoptotic proteins, which induce apoptosis via the mitochondrial death pathway (54). Interestingly, Bnip3L/NIX is expressed at high levels in the lung (53). Our preliminary data show that Bnip3L/NIX is expressed in HPAECs and that MIF can functionally antagonize the apoptotic effects of overexpressed Bnip3L (R. L. Damico and M. T. Crow, unpublished observations). Current investigations are underway to further define the role, if any, of Bnip3L/NIX in apoptosis after MIF suppression.
In conclusion, the present study shows that MIF provides an essential pro-survival function in HPAEC and contributes to their resistance to LPS-induced cell death through post-transcriptional regulation of FLIPs expression.
Acknowledgments
The authors thank Dr. Gabriel Nunez (University of Michigan) for the caspase 9 vector. The adenoviral vector encoding human Bcl-xL was obtained from the NHLB1 Gene Transfer Core at the University of Pittsburgh.
This research was supported by National Institutes of Health grants HL078113 (R.D.) and HL073935 (M.T.C.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2007-0248OC on January 31, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Clark I., Awburn M. Migration inhibitory factor in the cerebral and systemic endothelium in sepsis and malaria. Crit Care Med 2002;30:S263–S267. [DOI] [PubMed] [Google Scholar]
- 2.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med 2001;29:S28–S34; discussion S34–S35. [DOI] [PubMed] [Google Scholar]
- 3.Bochud PY, Calandra T. Pathogenesis of sepsis: new concepts and implications for future treatment. BMJ 2003;326:262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkis RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003;348:138–150. [DOI] [PubMed] [Google Scholar]
- 5.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science 1995;1456–1462. [DOI] [PubMed]
- 6.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock 1997;7:247–253. [DOI] [PubMed] [Google Scholar]
- 7.Fujita M, Kunitake R, Hagimoto N, Miyazaki H, Kaneko Y, Kawasaki M, Maeyama T, Hara N. Endothelial cell apoptosis in lipopolysaccharide-induced lung injury in mice. Int Arch Allergy Immunology 1998;117:202–208. [DOI] [PubMed] [Google Scholar]
- 8.Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, Tanaka T, Maeyama T, Hara N. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol 2000;157:597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papathanassoglou ED, Moynihan JA, Ackerman MH. Does programmed cell death (apoptosis) play a role in the development of multiple organ dysfunction in critically ill patients? A review and a theoretical framework. Crit Care Med 2000;28:537–549. [DOI] [PubMed] [Google Scholar]
- 10.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol 2003;284:L899–L914. [DOI] [PubMed] [Google Scholar]
- 11.Hoyt DG, Mannix RJ, Rusnak JM, Pitt BR, Lazo, JS. Collagen is a survival factor against LPS-induced apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Physiol 1995;269:L171–L177. [DOI] [PubMed] [Google Scholar]
- 12.Petrache I., Birokuv K, Zaiman AL, Crow MT, Deng H, Wadaonkar R, Romer LH, Garcia JGN. Caspase-dependent cleavage of myosin light chain kinase (MLCK) is involved in TNF-{alpha}-mediated bovine pulmonary endothelial cell apoptosis. FASEB J 2003;17:407–416. [DOI] [PubMed] [Google Scholar]
- 13.Bannerman DD, Tupper JC, Ricketts WA, Bennett CF, Winn RK, Harlan JM.. A constitutive cytoprotective pathway protects endothelial cells from lipopolysaccharide-induced apoptosis. J Biol Chem 2001;276:14924–14932. [DOI] [PubMed] [Google Scholar]
- 14.Zen K, Karsan A, Stempien-Otero A, Yee E, Tupper J, Li X, Eunson T, M. Kay MA, Wilson CB, Winn RK, Harlan JM. NF-kappa B activation is required for human endothelial survival during exposure to tumor necrosis factor-alpha but not to interleukin-1beta or lipopolysaccharide. J Biol Chem 1999;274:28808–28815. [DOI] [PubMed] [Google Scholar]
- 15.Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: a question of life or death. J Biochem Mol Biol 2002;35:28–40. [DOI] [PubMed] [Google Scholar]
- 16.Stehlik C, de Marti R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med 1998;188:211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bannerman DD, Eiting KT, Winn RK, Harlan JM. FLICE-like inhibitory protein (FLIP) protects against apoptosis and suppresses NF-kappaB activation induced by bacterial lipopolysaccharide. Am J Pathol 2004;165:1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi KB, Wong F, Harlan JM, Chaudhary, PM, Hood L, Karsan A. Lipopolysaccharide mediates endothelial apoptosis by a FADD-dependent pathway. J Biol Chem 1998;273:20185–20188. [DOI] [PubMed] [Google Scholar]
- 19.Hu X, Yee E, Harlan JM, Wong F, Karsan A. Lipopolysaccharide induces the antiapoptotic molecules, A1 and A20, in microvascular endothelial cells. Blood 1998;92:2759–2765. [PubMed] [Google Scholar]
- 20.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med 1991;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y, Degrandpre P, Kharfi A, Aloum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endrocrinol Metab 2000;85:4721–4727. [DOI] [PubMed] [Google Scholar]
- 22.Baumann R., Casaultra C, Simon D, Conus S, Yousefi S, Simon HU. Macrophage migration inhibitory factor delays apoptosis in neutrophils by inhibiting the mitochondria-dependent death pathway. FASEB J 2003;17:2221–2230. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Lu C, Xing G, Zhu Y, He F. Macrophage migration inhibitory factor directly interacts with hepatopoietin and regulates the proliferation of hepatoma cell. Exp Cell Res 2004;300:379–387. [DOI] [PubMed] [Google Scholar]
- 24.Ren Y, Tsui HT, Poon RT, Ng IO, Li Z, Chen Y, Jiang G, Lau C, Yu WC, Bache M, Fan ST. Macrophage migration inhibitory factor: roles in regulating tumor cell migration and expression of angiogenic factors in hepatocellular carcinoma. Int J Cancer 2003;107:22–29. [DOI] [PubMed] [Google Scholar]
- 25.Koong AC, Denko NC, Hudson KM, Schindler C, Swiersz L, Koch, C, Evans S, Ibrahim H, Le QT, Terris DT, Giaccia AJ. Candidate genes for the hypoxic tumor phenotype. Cancer Res 2000;60:883–887. [PubMed] [Google Scholar]
- 26.Froidevaux C, Roger T, Martin C, Glauser MP, Calandra T. Macrophage migration inhibitory factor and innate immune responses to bacterial infections. Crit Care Med 2001;29:S13–S15. [DOI] [PubMed] [Google Scholar]
- 27.Munaut C, Boniver J, Foidart JM, Deprez M. Macrophage migration inhibitory factor (MIF) expression in human glioblastomas correlates with vascular endothelial growth factor (VEGF) expression. Neuropathol Appl Neurobiol 2002;28:452–460. [DOI] [PubMed] [Google Scholar]
- 28.Bacher M, Schrader J, Thompson N, Kuschela K, Gemsa D, Waeber G, Schlegel J. Up-regulation of macrophage migration inhibitory factor gene and protein expression in glial tumor cells during hypoxic and hypoglycemic stress indicates a critical role for angiogenesis in glioblastoma multiforme. Am J Pathol 2003;162:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erwert RD, Winn RK, Harlan JM, Bannerman DD. Shiga-like toxin inhibition of FLICE-like inhibitory protein expression sensitizes endothelial cells to bacterial lipopolysaccharide-induced apoptosis. J Biol Chem 2002;277:40567–40574. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Wang Y, Zhang J, Kim HP, Ryter SW, Choi AM. FLIP protects against hypoxia/reoxygenation-induced endothelial cell apoptosis by inhibiting Bax activation. Mol Cell Biol 2005;25:4742–4751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Budd RC, Yeh WC, Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat Rev Immunol 2006;6:196–204. [DOI] [PubMed] [Google Scholar]
- 32.Poukkula M, Kaunisto A, Hietakangas V, Denessiouk K, Katajamaki T, Johnson, MS, Sistonen L, Eriksson JE. Rapid turnover of c-FLIPshort is determined by its unique C-terminal tail. J Biol Chem 2005;280:27345–27355. [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Leng L, Wang T, Wang, W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 2006;25:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 1999;18:2330–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res 2004;95:957–970. [DOI] [PubMed] [Google Scholar]
- 36.Amin, MA, Volpert OV, Woods JM, Kumar P, Harlow LA, Koch AE . Migration inhibitory factor mediates angiogenesis via mitogen-activated protein kinase and phosphatidylinositol kinase. Circ Res 2003;93:321–329. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen MT, Lue H, Kleemann R, Thiele M, Tolle G, Finkelmeier D, Wagner E, Braun A, Bernhagen J. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J Immunol 2003;170:3337–3347. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell RA, Liao H, Chesney J, Fingerle-Rowson G, Baugh J, David J, Bucala R. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA 2002;99:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bannerman DD, Tupper JC, Erwert RD, Winn RK, Harlan JM. Divergence of bacterial lipopolysaccharide pro-apoptotic signaling downstream of IRAK-1. J Biol Chem 2002;277:8048–8053. [DOI] [PubMed] [Google Scholar]
- 40.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, V Steiner, Bodmer JL, Schroter M, Burns K, Mattmann C, Rimoldi D, French LE, Tschopp J. Inhibition of death receptor signals by cellular FLIP. Nature 1997;388:190–195. [DOI] [PubMed] [Google Scholar]
- 41.Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT1 is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor-induced apoptosis. J Biol Chem 1996;271:4961–4965. [DOI] [PubMed] [Google Scholar]
- 42.Nam YJ, Mani K, ashton AW, Peng CF, Krishamurthy B, Hayakawa Y, Lee P. Korsmeyer SJ, Kitsis RN. Inhibition of both the extrinsic and intrinsic death pathways through nonhomotypic death-fold interactions. Mol Cell, 2004. l 15:901–912. [DOI] [PubMed] [Google Scholar]
- 43.Kataoka T, Budd RC, Holer N, Thome M Martinous R, Irmler M, Burns K, Hahna M, Kennedy N, Kovacsovics M, Tschopp J. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol, 2000;10:640–648. [DOI] [PubMed] [Google Scholar]
- 44.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol, 2001;21:3964–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukazawa T, Fujiwara T, Uno F, Teraishi Y, Kadowaki T, Itoshima T., Takata Y, Kagawa S, Roth JA, Tschopp J, Tanaka N. Accelerated degradation of cellular FLIP protein through the ubiquitin-proteasome pathway in p53-mediated apoptosis of human cancer cells. Oncogene 2001;20:5225–5231. [DOI] [PubMed] [Google Scholar]
- 46.Perez D, White E. E1A sensitizes cells to tumor necrosis factor alpha by downregulating c-FLIP S. J Virol 2003;77:2651–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleemann R Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, Johanees FJ, Roger T, Calandra T, Kapurniotu A, Grell M, Finkelmeier D, Btunner H, Bernhagen J. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000;408:211–216. [DOI] [PubMed] [Google Scholar]
- 48.Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature, 1990;398:160–165. [DOI] [PubMed] [Google Scholar]
- 49.Bin L, Xiaoyan L, Xu L-G, Shu HB. The short splice form of Casper/c-FLIP is a major cellular inhibitor of TRAIL-induced apoptosis. FEBS Lett 2002;510:37–40. [DOI] [PubMed] [Google Scholar]
- 50.Salon C, Eymin B, Micheau O, Chaoerot L, Plumas J, Brambilla C, Brambillam E, Gazzeri S. E2F1 induces apoptosis and sensitizes human lung adenocarcinoma cells to death-receptor-mediated apoptosis through specific downregulation of c-FLIP(short). Cell Death Differ 2006;13:260–272. [DOI] [PubMed] [Google Scholar]
- 51.Sharp DA, Lawrence DA, Ashkenazi A. Selective knockdown of the long variant of cellular FLICE inhibitory protein augments death receptor-mediated caspase-8 activation and apoptosis. J Biol Chem 2005;280:19401–19409. [DOI] [PubMed] [Google Scholar]
- 52.Lue H, Kapurniotu A, Fingerle-Rowson G, Roger T, Leng L, Thiele M, Calandra T, Bucala R, Bernhagen J. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal 2006:18:688–703. [DOI] [PubMed] [Google Scholar]
- 53.Shen L, Hu J, Lu H, Wu M, Qin W, Wan D, Li YY, Gu J. The apoptosis-associated protein BNIPL interacts with two cell proliferation-related proteins, MIF and GFER. FEBS Lett 2003;540:86–90. [DOI] [PubMed] [Google Scholar]
- 54.Crow MT. Hypoxia, BNip3 proteins, and the mitochondrial death pathway in cardiomyocytes. Circ Res 2002;91:183–185. [DOI] [PubMed] [Google Scholar]