Abstract
4-Hydroxy-2-nonenal (HNE), a major lipid peroxidation product, is toxic at high concentrations, but at near-physiological concentrations it induces detoxifying enzymes. Previous data established that in human bronchial epithelial (HBE1) cells, both genes for glutamate cysteine ligase (GCL) are induced by HNE through the c-Jun N-terminal kinase (JNK) pathway. The protein-tyrosine phosphatase SH2 domain containing phosphatase-1 (SHP-1) is thought to play a role as a negative regulator of cell signaling, and has been implicated as such in the JNK pathway. In the present study, SHP-1 was demonstrated to contribute to HNE-induced-gclc expression via regulation of the JNK pathway in HBE1 cells. Treatment of HBE1 cells with HNE induced phosphorylation of mitogen-activated protein kinase kinase 4 (MKK4), JNK, and c-Jun. HNE was able to inhibit protein tyrosine phosphatase activity of SHP-1 through increased degradation of the protein. Furthermore, transfection with small interference RNA SHP-1 showed an enhancement of JNK and c-Jun phosphorylation, but not of MKK4, leading to increased gclc expression. These results demonstrate that SHP-1 plays a role as a negative regulator of the JNK pathway and that HNE activated the JNK pathway by inhibiting SHP-1. Thus, SHP-1 acts as a sensor for HNE and is responsible for an important adaptive response to oxidative stress.
Keywords: SHP-1, 4-hydroxynonenal, glutamate cysteine ligase, protein degradation, protein tyrosine phosphatase
CLINICAL RELEVANCE
4-hydroxynonenal (HNE), a lipid peroxidation product, mediates adaptation to oxidative stress. HNE caused degradation of the protein tyrosine phosphatase SHP-1, leading to activation of c-Jun N-terminal kinase and expression of the first enzyme in glutathione synthesis.
4-Hydroxynonenal (HNE) is one of the numerous products of lipid peroxidation, mainly of ω-6 polyunsaturated acid such as arachidonic and linoleic acids (1, 2). HNE has high bioreactivity due to the electrophilic nature of the α,β-unsaturated bond that readily reacts with cellular nucleophiles, such as cysteine, histidine, or lysine residues in proteins to form Michael adducts and/or Schiff bases (1). It may also form cross links if it reacts first in a Michael addition and then forms a Schiff base. One of the consequences of oxidative stress in tissues is lipid peroxidation, primarily involving polyunsaturated fatty acids. There is increasing evidence that aldehydes, such as HNE, generated endogenously during the process of lipid peroxidation, are involved in many of the pathophysiological effects associated with oxidative stress in cells and tissues. Whereas HNE exhibits cytotoxic properties at high (generally > 25 μM) concentrations, nontoxic concentrations are able to induce cell proliferation and gene expression, inhibit synthesis of proteins, and increase protein degradation, and thus potentially activate a stress response mechanism (3–5). How HNE acts as an intracellular mediator leading to such cellular responses is incompletely understood.
Glutamate-cysteine ligase (GCL) catalyzes the first and rate-limiting step in de novo glutathione synthesis. The enzyme is composed of two subunits: GCLC, the catalytic subunit; and GCLM, the modulatory subunit that alters kinetic properties of GCLC (6). The transcription of both GCL genes is regulated by many cellular stresses, of which HNE is one of the more potent inducers (7–10). Several enhancer elements, including the electrophile response element (EpRE, also called the antioxidant response element), an activator protein (AP)-1–binding site (TRE element), and an NF-κB–binding site have been claimed to be involved in the control of GCL expression dependent upon the stimulant and cell type (11). Previous studies found that, in human bronchial epithelial (HBE1) cells, GCL expression is TRE and EpRE mediated (12). One of the transcription factors established as being able to bind EpRE is Nrf2, which in resting cells is located in the cytosol bound to Keap1 (also called iNrf2). Upon stimulation, Nrf2 is translocated into the nucleus after dissociation from Keap1 (13). In the nucleus Nrf2 heterodimerizes with proteins such as c-Jun and then binds to EpRE to up-regulate gene expression (14). The role of different upstream signal transduction pathways involved in HNE-induced GCL expression has been characterized and found to be cell type and species dependent. In fact, HNE can cause an immediate increase in tyrosine phosphorylation, which is accompanied by activation of all three major members of mitogen-activated protein (MAP) kinase family that play important roles in transducing many extracellular signals into intracellular responses through protein phosphorylation cascades (15). Previous work on HBE1 cells showed that c-Jun N-terminal kinase (JNK), but neither p38 nor extracellular signal–regulated kinase (ERK), is involved in GCLC and GCLM induction. JNK is characterized by its strong response to cellular stresses, targets of which include several transcription factors, including c-Jun, one of the proteins of Jun family able to bind to the TRE element and also potential partner of Nrf2 (14).
The mechanism by which HNE activates JNK is not completely clear; in fact, it is also activated by alternative mechanisms depending on the cell line. For example, HNE activates JNK by direct binding in hepatic stellate cells but by activation of the upstream kinase, SEK1/MAP kinase kinase 4 (MKK4) in human neuroblastoma cells (16, 17).
Phosphorylation of tyrosine residues in proteins is known as a key element for regulating eukaryotic cellular signaling pathways involved in the control of cell growth, proliferation, differentiation, and metabolism (18–20). This process is precisely regulated by the balanced action of two types of enzymes, protein tyrosine kinases (PTK) and protein tyrosine phosphatases (PTP). It is known that PTP activity can be regulated by oxidation (21–23). In addition to the catalytic cysteine in the active site, which has a high susceptibility to oxidants due to its low pKa, other conserved residues that also play a role in the catalytic process can be oxidatively modified. PTP inactivation may occur not only by reactive oxygen species generation, but also by aldehydes produced during lipid peroxidation, such as HNE as the active site cysteine, which in the thiolate (S−) form, is a strong nucleophile (24).
Biochemical and genetic studies indicate that protein phosphatases can play a role as both positive and negative regulator in signaling pathways, and exert crucial physiological roles in a variety of mammalian tissues and cells (25). For example, MAP kinases are activated by MAP kinase kinases, which phosphorylate the conserved Tyr and Thr located within the TXY motif in the activation loop of MAP kinases (26). Dephosphorylation of either pThr by protein phosphatase 2A, pTyr by hematopoietic PTP, or both residues by MAP kinase phosphatases (MKPs) results in loss of MAP kinase activity and thus in the regulation of signal transduction (27, 28).
One of the PTP of particular interest is SH2 domain containing phosphatase-1 (SHP-1) because of its role in the regulation of the JNK and ERK pathways (29). The SHP-1 protein-tyrosine phosphatase is thought to play a role as a negative regulator of cell signaling in cells of hematopoietic origin (30). Its involvement has been implicated in colony-stimulating factor 1 receptor signaling pathways, B cell receptor–induced apoptosis and signaling, Fcγ receptor–mediated phagocytosis, HoxA-mediated transcriptional repression, the Abl-induced DNA damage response, and T cell receptor signaling (30–36).
The aim of the current study was to provide a better understanding of the mechanism of HNE induction of the JNK pathway in HBE1 cells. In particular, the role of SHP-1 on the regulation of GCLc induction by HNE via the JNK pathway was analyzed by knocking down the expression of SHP-1 by small interference RNA (siRNA). Treatment of HBE1 cells exerts an inhibitory effect on SHP-1, due to an increase of protein degradation. In addition, siSHP-1 enhances GCLC expression and phosphorylation of JNK and c-Jun induced by HNE. The results suggest that inhibition of SHP-1 by HNE contributes to the activation of GCLC induction via JNK pathway.
MATERIALS AND METHODS
Chemical and Reagents
All the chemicals and reagents were obtained form Sigma Chemical (St. Louis, MO) unless stated otherwise. HNE was purchased from Cayman Chemical (Ann Arbor, MI). TaqMan reverse transcription reagent and SYBR Green Master Mix were form Applied Biosystems (Foster City, CA). FuGENE 6 transfection reagent was from Roche (Indianapolis, IN). siRNA was purchased from Ambion (Austin, TX). Anti–SHP-1, anti-actin antibodies were from BD transduction laboratories (San Jose, CA). Anti–p-cJun and anti-laminin were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-pMKK4 and RediPlate 96 EnzChek tyrosine phosphatase assay kit (Molecular Probes, Invitrogen) was purchased from Invitrogen (Carlsbad, CA).
Cell Culture
HBE1 cell were obtained from Dr. James Yankaskas of the University of North Carolina. The cells were maintained at 37°C in a humidified 5% CO2 incubator and grown in serum-free F12 Ham media supplemented with six hormones (5 μg/ml human insulin, 1 × 10−6 M hydrocortisone, 3.7 μg/ml endothelial cell growth supplement, 25 ng/ml epidermal growth factor, 3 × 10−8 M tri-iodothyronine, 5 μg/ml transferrin), 100 U/ml penicillin, 100 μg/ml streptomycin, and 40 μg/ml gentamicinin on collagen-coated dishes. HNE was dissolved in ethanol. HBE1 cells close to confluence were treated with vehicle control (0.05% ethanol) or different concentrations of HNE as indicated in Results.
Cell Viability Assay
Cells at 80% confluence were plated on 12-well plates in 1 ml F12 medium. Cell viability was evaluated using the conventional MTT reduction assays, in which cells of each well were incubated with 100 μl 0.5% MTT for 2 hours at 37°C, and the reaction was stopped by adding 1 ml of MTT solubilization solution (10% Triton X-100, 0.1 N HCl in anhydrous isopropanol). The amount of MTT formazan product was determined by measuring absorbance using a plate reader (Molecular Devices, Sunnyvale, CA) at a test wavelength of 570 nm and a reference wavelength of 690 nm.
PTP Activity Assay
PTP activity was measured with a RediPlate 96 EnzChek tyrosine phosphatase assay kit (Molecular Probes) in accordance with the manufacturer's recommendations. In brief, cells were suspended in a buffer containing 10 mM Tris HCl, 0.15 M NaCl, and 2 mM EDTA and then subjected to four cycles of freezing/thawing in liquid nitrogen. Supernatant, containing the whole protein extract, was placed into RediPlate wells and incubated for 25 minutes at 20 to 22°C before reading for fluorescence.
Immunoprecipitation and SHP-1 Activity
Phosphatase activity of SHP-1 was performed by using the RediPlate 96 EnzChek Tyrosine Phosphatase Assay kit (Molecular Probes). HBE1 were seeded in 60-mm wells and, when close to confluence, were treated with 15 μM HNE or vehicle for several times of exposure before lysis by 30 seconds of sonification in 1 ml buffer containing 150 mM NaCl, 1% Triton X-100, 0.5% NP40, and 2 mM EDTA. The protein concentration of whole cell lysates was measured using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) and adjusted to 500 μg before immunoprecipitation with protein A–sepharose-conjugated antibodies to SHP-1 at 4°C overnight. The antibodies had been previously crosslinked to protein A–sepharose beads using dimethyl pimelimidate (DMP) as described by Schneider and coworkers (37), and stored at 4°C until used. The immunocomplexes were washed four times with the cell lysis buffer and used for the phosphatase assay into RediPlate wells, and were incubated for 30 minutes at 20 to 22°C before reading the fluorescence.
Transfection of SHP-1 siRNA
Transfection of siRNA was performed using FuGENE 6 transfection reagent following the procedure provided with the transfection reagent. For SHP-1, p-cJun, pJNK, p-MKK4 protein assay, HBE1 cells at approximately 60 to 80% confluence were exposed to 50 nM SHP-1 siRNA (Ambion) in 60-mm plates. Twenty hours after the transfection, media were replaced and cells were treated with 15 μM HNE or vehicle for different time of exposure as indicated in Results. For SHP-1, p-cJun, pJNK, and p-MKK4 protein assay, the cells were collected and the whole cell proteins or the cytosol and nuclear protein were extracted using M-PER mammalian protein extraction reagent NE-PER and C-PER, nuclear and cytosolic extraction reagents (Pierce, Rockford, IL), respectively. For the effect of SHP-1 siRNA on GCLC expression, transfection was performed in 6-well dishes and the cells at 60 to 80% confluence were transfected with 50 nM SHP-1 siRNA or control siRNA before exposing the cells to HNE for 3 hours.
Real-Time PCR Assay of mRNA Levels
Total RNA was extracted using TRIzol reagent and treated with DNA-free reagent according to the manufacturer's protocol (Ambion). RNA samples were reverse-transcribed using the TaqMan random hexamers (Applied Biosystems) and the contents of GCLC mRNA were measured by real-time PCR polymerase chain reaction (RT-PCR) with a Cephedi 1.2 real-time PCR machine. In brief, 5 μl of reverse transcription reaction product was added to a reaction tube containing 12.5 μl SYBR green PCR Master Mix and primers specific for GCLC mRNA. The total PCR sample reaction was 25 μl. GAPDH was used as an internal control. The primer sequences used are as follows. GCLc: sense 5′-ATGGAGGTGCAATTAACAGAC-3′, antisense 5′-ACTGCATTGCCACCTTTGCA-3′; GAPDH: sense 5′-TGGGTGTGAACCATGAGAAG-3′, antisense 5′-CCATCACGACACAGTTTCC-3′.
Western Blot
Proteins were resolved on a 4 to 20% Tris-glycine acrylamide gel (Invitrogen, Carlsbad, CA) under denaturing conditions before being transferred electrophoretically onto a polyvinylidene difluoride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA). Membranes were blocked with 5% nonfat dry milk (NFDM) at room temperature for 1 hour and then incubated overnight at 4°C with primary antibody diluted in 5% NFDM in Tris buffer saline (TBS) as indicated (1:500 anti-p cJun; 1:5,000 anti-actin; 1:1,000 anti–phospho JNK; 1:500 JNK1; 1:500 anti–SHP-1; 1:1,000 anti-pMKK4; 1:500 anti-laminin). After being washed with TBS containing 0.05% Tween 20, the membrane was incubated with goat anti-mouse IgG or goat anti-rabbit conjugated to horseradish peroxidase (1:2,000) at room temperature for 2 hours. The blots were developed by the enhanced chemiluminescence technique (ECL Plus; Amersham, Arlington Heights, IL) according to the manufacturer's instructions. The bands of interest were imaged with and quantified by photon counting using the charged-coupled device camera of a Kodak Image Station 2000R (Kodak, Rochester, NY) and Kodak 1D 3.6 Image Analysis Software. Photon counting was used for graphing and statistical analysis.
Statistical Analysis
For the relative GCLC mRNA quantification, the threshold cycle value (Ct) for GCLC and reference gene (GAPDH) were obtained and the difference ΔCt was calculated. Primer efficiencies for the test genes were comparable to those for GAPDH. Ct values were converted to absolute values and the results expressed in fold of increase compared with control. Each experiment was performed at least three times. All data were expressed as the mean ± SD. Sigma Stat software was used for statistical analysis and statistical significance was accepted when P < 0.05. ANOVA and the Tukey's test were performed to compare the variants between experimental groups.
RESULTS
HNE Does Not Induce Cytotoxycity in HBE1 Cells
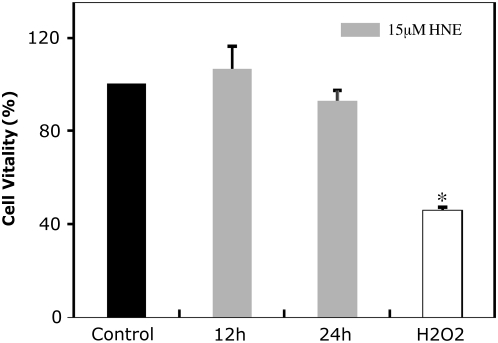
To show that the highest concentration of HNE used in our experiments was not cytotoxic, we performed an MTT reduction assay to determine the effect of HNE on cell viability (Figure 1). As shown in Figure 1, there was not a statistically significant decrease of cell viability in cells exposed to 15 μM HNE (12 h and 24 h). Thus, this concentration was subtoxic and has been used in our experimental model to mimic mild oxidative stress in the lungs. To verify that this negative result was not due to a failure of the assay, cells exposed to a high concentration of H2O2. The figure shows that cells exposed for 2 hours to a concentration of 1 mM H2O2 decrease viability to 50%.
Figure 1.
Viability of human bronchial epithelial (HBE)1 cells after the addition of 4-hydroxy-2-nonenal (HNE) and H2O2. Cells were exposed to 15 μM HNE for 12 and 24 hours, vehicle for 24 hours, or 1 mM H2O2 for 2 hours. The formation of purple formazan crystals were measured after 2 hours of incubation at 37°C. Values were reported as percentage of viability of the control and expressed as mean ± SD of four experiments in triplicate. *P < 0.05 compared with the control.
HNE Inhibits Protein Tyrosine Phosphatase Family in HBE1 Cells
Previous work suggested that the α, β-unsaturated aldehyde derived from lipid peroxidation, HNE, induces de novo synthesis of GSH through the increase of GCLC and GClM expression in human lung bronchial epithelial cells via the JNK pathway. How this mechanism can be achieved by HNE is still not fully understood. In this study we tested the hypothesis that protein tyrosine phosphatases are involved in the mechanism of JNK pathway activation, either acting on the upstream or downstream proteins or on JNK itself.
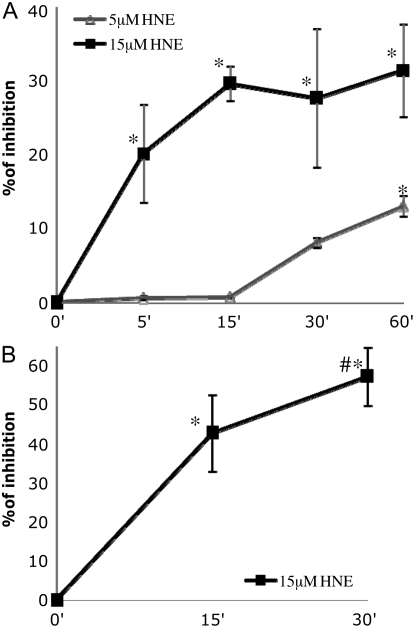
We measured the total PTP activity in HBE1 cells after exposure to HNE (Figure 2A). We treated the cells under time and HNE concentration conditions (15 μM) used in previous work that showed maximal GCL induction via the JNK pathway. The results revealed that HNE at 15 μM, which is not cytotoxic and actually induces GCL and glutathione biosynthesis (12), has an inhibitory effect on total PTP activity. After 5 minutes of treatment the inhibition was already detectable (20% inhibition). The inhibition increased with the time until a maximum of 30 minutes (∼ 30% inhibition), after which HNE did not produce a further inhibition. At a concentration of 5 μM HNE, inhibition of PTP activity was not detectable before 30 minutes (∼ 10% inhibition) and reached the maximum at 60 minutes (∼ 15% inhibition). It appears clear that there is a dependency between the inhibition of total PTP activity by HNE and the time and dose of exposure. SHP-1 is a member of the PTP family that has been shown to be involved in the regulation of the JNK activation pathway. In fact SHP-1 overexpression was able to inhibit H2O2-induced JNK phosphorylation (29). Because we hypothesized that SHP-1 also could be a target of HNE, we decided to focus our attention on this protein. SHP-1 activity was measured after treatment with HNE. Figure 2B shows that after 15 minutes HNE was able to inhibit SHP-1 activity (∼ 40% inhibition) and that inhibition reached 60% after 30 minutes. These results are in line with the data related to the inhibition of total PTP activity, and actually indicate that SHP-1 has a higher susceptibility to HNE than PTP in general.
Figure 2.
Inhibitory effect of HNE on the activity of protein tyrosine phosphatases (PTP). (A) HBE1 cells were exposed to either 5 μM or 15 μM 4HNE or vehicle for indicated times. The activity was measured by a fluorescence-based assay using as a substrate the 6,8-difluoro-4-metylumbelliferyl phosphate. (B) Five hundred micrograms of proteins were immunoprecipitated with a SHP-1 monoclonal antibody prebound to protein A sepharose beads. The SH2 domain containing phosphatase-1 (SHP-1) activity was measured using the bead-immunocomplex. Values plotted are reported as percent inhibition compared with the value of the control and were expressed as mean ± SD of three experiments in triplicate. *P < 0.05 compared with control, #P < 0.05 compared with other samples.
HNE Decreases SHP-1 Protein in HBE1 Cells
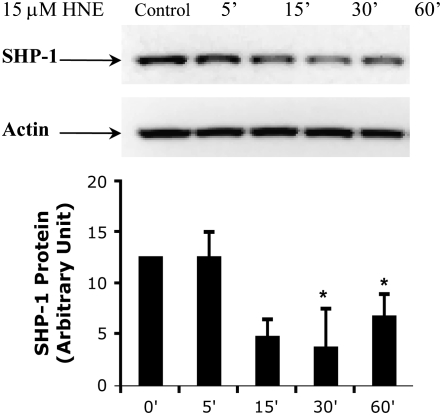
The mechanism underlying the inhibition of SHP-1 caused by HNE exposure was examined. Exposure to HNE can cause formation of HNE adducts. It has been reported that HNE-modified proteins are degraded at a considerably higher rate than the other proteins as an essential defense mechanism to oxidative stress (38). We examined whether the SHP-1 protein was similarly decreased after exposure to HNE. Western blot analysis using monoclonal antibody specific for SHP-1 showed that the presence of protein in HBE1 cells changed during the time of HNE exposure. There was a detectable decrease of the protein after 15 minutes that reached a maximum after 30 minutes but without a further decrease after 60 minutes (Figure 3). Reprobing the same membrane with an anti-actin antibody indicated similar protein loading into each lane. Interestingly, these data show that there was a correlation between the time point at which the inhibition of SHP-1 activity by HNE reached the maximum and the decrease of SHP-1 protein. This suggests that the SHP-1 activity is regulated by HNE through increased degradation of the protein.
Figure 3.
HNE induces decrease of SHP-1 protein expression in HBE1 cells. HBE1 were treated with 15μM 4HNE or vehicle for the indicated exposure time. The SHP-1 protein expression was determined by Western analysis with a monoclonal antibody. The membranes were then blotted with the actin antibody for loading control. Data are expressed in arbitrary units and indicate mean ± SD of three separate experiments (*P < 0.05).
SHP-1 Knockdown Enhances HNE-Induced c-Jun and JNK Phopshorylation but Does Not Enhance MKK4 Phosphorylation
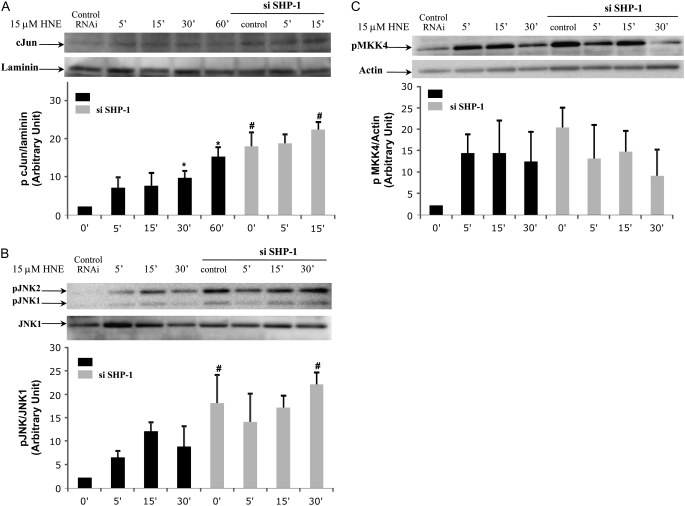
After confirming that HNE was able to inhibit SHP-1 activity in HBE1 cells, the next important step was to examine whether SHP-1 was involved in the JNK pathway in our experimental model. Expression of SHP-1 was decreased by siRNA and then the effect of HNE on c-Jun and JNK phosphorylation was measured. Nuclear extract and total extract from HBE1 cells transfected with siRNA for SHP-1 and nontransfected HBE1 cells exposed to HNE for several lengths of exposure were subjected to Western blot analysis using a monoclonal antibody to the phosphorylated form of c-Jun and JNK1/2. As shown in Figures 4A and 4B, transfection of HBE1 cells with SHP-1 siRNA enhanced the phosphorylation of c-Jun and JNK1/2 induced by HNE. Interestingly, SHP-1 had an effect on the basal phosphorylation of c-Jun and JNK 1/2, since the phosphorylation of the protein was detectable with SHP-1 siRNA in the absence of HNE. This suggests that a decline in SHP-1 activity is sufficient for partial activation of JNK.
Figure 4.
HNE induces activation of c-Jun, c-Jun N-terminal kinase (JNK), and mitogen-activated protein kinase kinase 4 (MKK4), and SHP-1 knockdown enhances HNE-induced c-Jun and JNK phosphorylation but not MKK4. Cells were transfected with SHP-1 small interference RNA (siRNA) and control siRNA. After 20 hours of transfection the media were replaced and HBE1, near confluence, were treated with 15 μM HNE or vehicle for the indicated time, and c-Jun, JNK, and MKK4 phosphorylation levels were determined by Western blot with the appropriate antibody (A–C). The blots were reprobed with (A) laminin, (B) JNK1, and (C) actin antibody to show that the equivalent amounts of protein were in each lane. Shown are representative blots, and bar graphs represent the mean and SD of photon counts obtained as described in Materials and Methods (n = 3; *P < 0.05 compared with control, #P < 0.05 compared with SHP-1 siRNA sample).
MKK4 is one of a member of a tyrosine/threonine protein kinase family that is able to activate JNK protein. We therefore tested whether, in our experimental system, MKK4 was involved in activation of the JNK pathway by HNE and whether SHP-1 played a role in the JNK pathway acting upstream of MKK4. Following the same procedure described above, we measured (using a polyclonal antibody for pSer 257 and pThr 261) the phosphorylation and therefore the activation of MKK4. HNE induced phosphorylation of MKK4 after 5 minutes of exposure, and this started to decrease only after 30 minutes (Figure 4C). Although unlike JNK and c-Jun, phosphorylation of MKK4 induced by HNE was not enhanced by siRNA for SHP-1, knocking down SHP-1 was sufficient to induce phosphorylation of MKK4 in cells without stimulation, suggesting the possibility that the protein target for SHP-1 might be upstream of MKK4 activation.
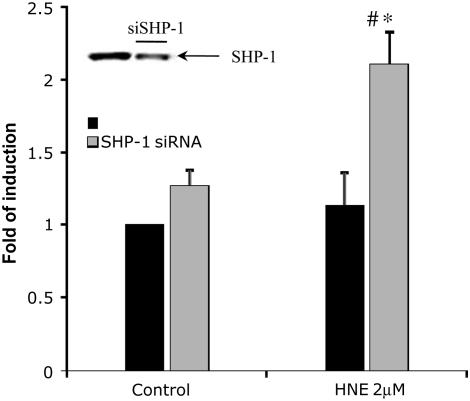
SHP-1 Knockdown Increases HNE-Induced GCLC Expression
As the data above showed that SHP-1 plays a role in the regulation of JNK pathway activation induced by exposure to HNE, we examined whether SHP-1 affected downstream signaling by the JNK pathway, in particular the regulation of GCLC expression. To establish the role of SHP-1 in HNE-induced GCLC expression, SHP-1 was knocked down by siRNA and then the effect of HNE on GCLC mRNA expression was examined using real-time RT-PCR. Total RNA extracts, from siRNA-transfected and nontransfected cells exposed to HNE for 3 hours, were subjected to reverse transcriptase to obtain cDNA used as template for the real-time PCR reaction. As the evidence above showed that both HNE and siRNA for SHP-1 both decreased SHP-1, we used a submaximally GCL-inducing concentration of HNE, 2 μM, to emphasize a possible enhancing effect of knocking down SHP-1 on GCLC expression. As shown in Figure 5, exposure to 2 μM HNE resulted in a low induction of GCLC expression. The figure shows that knockdown of SHP-1 expression enhanced the HNE-induced GCLC expression approximately 2.5-fold compared with the control sample and approximately 1.5-fold compared with the sample exposed to HNE, suggesting a role of SHP-1 as a negative regulator in GCLC induction. Furthermore, the results suggested that SHP-1 also might have a role in controlling the basal level of GCLC expression, as knockdown of SHP-1 alone resulted in an increase of 1.5-fold of gene induction compared with the control.
Figure 5.
Effect of SHP-1 siRNA on the glutamate-cysteine ligase (GCLc) expression induced by HNE in HBE1cells. HBE1 were transfected with SHP-1 siRNA for 20 hours and then treated in fresh media with 2 μM HNE for 3 hours. The values plotted represent the fold GCLC induction in control and treated samples of transfected and nontransfected cells. mRNA levels were measured with real-time PCR methods (n = 4; *P < 0.001 compared with control, #P < 0.001 compared with HNE untransfected samples.) Inset: Western blot of SHP-1 in total extract from HBE1 cells transfected with SHP-1 siRNA for 18 hours.
DISCUSSION
HNE is one of the major products of lipid peroxidation. Under physiological conditions the concentration of HNE in the plasma has been estimated to be in the 0.1- to 1.4-μM range (1, 39); however, under conditions of oxidative stress it can reach millimolar concentration (2). High concentrations of HNE have been associated with the pathogenesis of a number of degenerative diseases such as atherosclerosis, Parkinson's, and Alzheimer's diseases (1, 2). A potential role for high concentrations of HNE has been implicated in the signaling events involved in lung inflammation leading to the development of chronic obstructive pulmonary disease (40). However, at concentrations slightly above the endogenous level, HNE can activate cell signaling pathways rather than cause cytoxicity (5, 41). For example, HNE is able to induce, through activation of different signal pathways, antioxidant and detoxifying enzymes such as GCL as an adaptive mechanism to a subsequent oxidative stress (11, 42). Previous data have demonstrated that the transcription of the GCL mRNA is dependent upon activation of both Nrf2 and the JNK signaling pathway in human bronchial epithelial cells, but the signaling mechanisms inducing JNK activation are not completely characterized in this cell line (12, 43). Moreover, whether HNE is capable of inducing GCL expression via conjugation through direct interaction with JNK or by signaling upstream seems to be controversial due to a clear cell type dependence of the mechanisms (16, 17).
To extend our knowledge of how the HNE activation of the JNK pathway is involved in downstream signaling, we examined the regulation of the gclc gene in human bronchial epithelial cells. Our experimental model used a noncytotoxic concentration of HNE that could be generated during a mild oxidative stress caused by an external stressor such as air pollutants and cigarette smoke. It is known that oxidative stresses cause increased expression of proinflammatory genes through oxidant-mediated activation of transcription factors such as NF-κB and AP-1, as well as activation of stress-response protective genes such as GCL in lungs (7, 44). An imbalance of an array of redox-regulated antioxidant versus pro-inflammatory genes might be associated with the susceptibility or tolerance to pulmonary diseases (45). Therefore a better understanding of the pathway through which GCL and other genes are expressed may be important to be able to push the balance toward anti-inflammatory gene expression.
We examined whether SEK1/MKK4, an upstream kinase of JNK, and SHP-1, a member of the PTP family, contribute to the regulation of GCLC induction via the JNK pathway in response to low concentrations of HNE in this specific experimental model. After first verifying the effect of HNE on the activity of the PTP family, in general, we focused on SHP-1, and its role in GCLC expression in relation to proteins involved in the JNK pathway.
Oxidation has emerged as an important regulatory mechanism for the PTP family (46). While most of studies in this area have focused on the mechanism by which reactive oxygen species, particularly H2O2, inhibit PTP reversibly, little is known about the mechanisms by which other electrophilic stimuli, such as HNE inactivate PTP. A recent article demonstrated that PTP present on the membrane of platelet cells can be inhibited by 2-nonenal, which is closely related to HNE (24). They hypothesized that this inhibition was due to the formation of adducts with cysteine, lysine, and histidine by a Michael type reaction, as would be predicted from the well-established mechanism for reaction of α,β-unsaturated aldehydes with various proteins (47, 48). Nonetheless, which residues are modified by addition of aldehydes and if such residues are essential for the activity of the enzyme family has not been determined (24). In accordance with these data, our results showed that PTP family members can be inhibited in HBE1 by a concentration of HNE that mimics a mild oxidative stress in which adaptive responses, such as induction of Phase II genes, occurs. Interestingly, SHP-1 was more susceptible to inhibition by HNE compared with PTP in general. This inhibition suited perfectly in the picture of our data showing that in the experimental model used, SHP-1 protein seemed to be regulated by HNE treatment through an increased rate of degradation. The greatest percent inhibition, in fact, was reached at 30 minutes, the same time point that the presence of the protein in the cells reached the lowest amount.
HNE can form adducts with various intracellular proteins as do other α,β-unsaturated aldehydes as described above. Proteins modified by HNE may alter their structures and functions. If a sufficient percentage of such modified proteins are inhibited in their function and/or accumulate, the resulting alterations in cellular structure and function may be toxic. Nonetheless, it has been suggested that conjugation of HNE to proteins may act as a signal that leads cells to removal of these HNE-modified proteins to avoid disruption of the cellular functions (38, 49). The modification/removal mechanism may also be used for regulation of activity of proteins such as the PTP, as protein degradation and resynthesis is another mechanism that cells use for signaling; for example, cyclin synthesis, ubiquitination, and degradation in the cell cycle (50, 51). Biochemical and structural data regarding PTP regulation revealed that the Cys residue in this family of enzyme might act as a redox sensor in response to proximal reactive oxygen/nitrogen species (21, 52). Likewise, SHP-1 might act as an oxidative sensor in response to increase of HNE occurring during oxidative stress. In support of this, our results also revealed that SHP-1 acts as a negative regulator in the pathway of JNK responsible for GCLC induction. We found, in fact, that c-Jun—a transcription factor downstream of JNK and critical in gclc expression (12)—was phosphorylated, and thus activated, in response to HNE, and that SHP-1 knockdown enhanced the phosphorylation of both c-Jun and JNK, suggesting a functional role for SHP-1 in JNK activation.
SHP-1 likely works as a brake on the activation of the pathway that is removed by degradation when an oxidative stress occurs. This event allows the signal to be transmitted and leads the cell to increase the oxidant defenses by the expression of gclc. The observation that other phosphatases involved in the JNK pathway can be regulated by increasing the rate of their degradation by oxidation has been reported by Chen and colleagues (53). In their system, JNK activation by H2O2 plus pyrrolidine dithiocarbamate (PDTC) was mainly due to down-regulation of M3/6 phosphatase, a dual-specificity phosphatase and a negative regulator of JNK, rather than to activation of kinases upstream of JNK (54). This was, however, a specific effect of H2O2 and PDTC, as UV-C irradiation did not cause the down-regulation of this phosphatase (53). Although the mechanism underlying the regulation seems to have some similarity, our results suggested that MKK4, which is upstream of JNK, is involved in HNE-dependent JNK activation. A main difference is that the H2O2 plus PDTC system will irreversibly oxidize not only cys, his, and lys but many other amino acids because it generates hydroxyl radical, whereas an α,β-unsaturated aldehyde such as HNE conjugates with cys, his, and lys by Michael addition and forms Schiff base with amines.
The observation that SHP-1 was involved in the regulation of the signal transduction was first demonstrated using mice lacking functional SHP-1 (55). These mice develop systemic autoimmune disease and are affected with numerous anomalies of the hemopoietic system, such as an increase in the number of erythroid progenitor cells, monocytes, macrophages, and neutrophils. In agreement with these phenotypes, SHP-1 has been reported to function in the negative regulation of signaling pathways in a number of hematopoietic systems (56). Furthermore, it was reported that SHP-1 plays a crucial role as a negative regulator in H2O2-induced ERK and JNK phosphorylation (29).
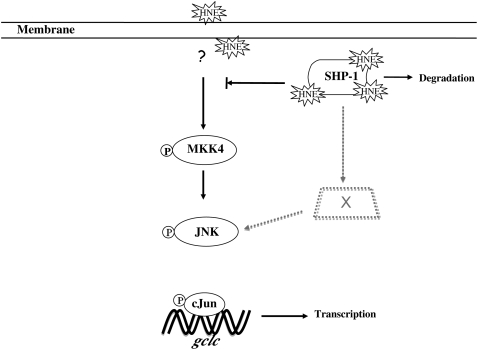
The mechanism underlying JNK pathway activation triggered by HNE is controversial. Our results clearly suggest the dependence of HNE-induced JNK activation on upstream kinases. In contrast with our observations, Parola and coworkers showed that, in hepatic stellate cells, upstream kinases are not involved in JNK activation by HNE, and instead result from direct conjugation of a His residue with HNE (16). Our findings are, however, consistent with a report published by Song and coworkers in which, in PC12 cells, the JNK pathway was activated by HNE through MKK4 (17). The mechanisms underlying the apparent difference in JNK activation may be due to the variation in cellular capacity to remove HNE by enzymes responsible for its metabolism and/or differences in organization of signaling proteins among cell types. The new finding here is that SHP-1 inactivation by HNE is upstream of the activation of MKK4, although we do not as yet know the intervening steps. Nonetheless, activation of MKK4 may not be the only mode of action by HNE on the JNK pathway. SHP-1 siRNA caused phosphorylation of c-Jun and JNK protein without the presence of HNE; however, unlike the phosphorylation of c-Jun and JNK, SHP-1 siRNA did not further increase MKK4 phosphorylation in the presence of HNE. This implies that the MKK4 phosphorylation was sufficient for downstream JNK activation but that some additional action of HNE resulted in greater phosphorylation of JNK and c-Jun. Together, previous results and the new information here suggest that two different pathways for HNE activation of JNK are possible. Finally, our findings showed that the induction of GCLC in HBE1 cells by HNE occurred, partially through increased degradation of the negative regulator, SHP-1 protein. In addition, the activation of MKK4, and the increased phosphorylation of JNK and c-Jun resulting from SHP-1 inactivation by HNE, lead to increased gclc expression (Figure 6).
Figure 6.
SHP-1 inhibition by HNE regulates the activation of JNK pathway. The black solid arrows indicate the activation of JNK pathway by MKK4 dependent from SHP-1, whereas the gray dashed lines indicate the role of SHP-1 on a possible coexisting pathway for the activation of JNK.
This work was supported by National Institutes of Health grant ES 05511 to H.J.F.
Originally Published in Press as DOI: 10.1165/rcmb.2007-0371OC on February 14, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 1991;11:81–128. [DOI] [PubMed] [Google Scholar]
- 2.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res 2003;42:318–343. [DOI] [PubMed] [Google Scholar]
- 3.Botzen D, Grune T. Degradation of HNE-modified proteins–possible role of ubiquitin. Redox Rep 2007;12:63–67. [DOI] [PubMed] [Google Scholar]
- 4.Cerbone A, Toaldo C, Laurora S, Briatore F, Pizzimenti S, Dianzani MU, Ferretti C, Barrera G. 4-Hydroxynonenal and PPARgamma ligands affect proliferation, differentiation, and apoptosis in colon cancer cells. Free Radic Biol Med 2007;42:1661–1670. [DOI] [PubMed] [Google Scholar]
- 5.Leonarduzzi G, Robbesyn F, Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic Biol Med 2004;37:1694–1702. [DOI] [PubMed] [Google Scholar]
- 6.Huang CS, Anderson ME, Meister A. Amino acid sequence and function of the light subunit of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem 1993;268:20578–20583. [PubMed] [Google Scholar]
- 7.Liu RM, Borok Z, Forman HJ. 4-Hydroxy-2-nonenal increases gamma-glutamylcysteine synthetase gene expression in alveolar epithelial cells. Am J Respir Cell Mol Biol 2001;24:499–505. [DOI] [PubMed] [Google Scholar]
- 8.Morales A, Miranda M, Sanchez-Reyes A, Colell A, Biete A, Fernandez-Checa JC. Transcriptional regulation of the heavy subunit chain of gamma-glutamylcysteine synthetase by ionizing radiation. FEBS Lett 1998;427:15–20. [DOI] [PubMed] [Google Scholar]
- 9.Rahman I, Antonicelli F, MacNee W. Molecular mechanism of the regulation of glutathione synthesis by tumor necrosis factor-alpha and dexamethasone in human alveolar epithelial cells. J Biol Chem 1999;274:5088–5096. [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Sevanian A. OxLDL induces macrophage gamma-GCS-HS protein expression: a role for oxLDL-associated lipid hydroperoxide in GSH synthesis. J Lipid Res 2001;42:813–823. [PubMed] [Google Scholar]
- 11.Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med 2005;38:547–556. [DOI] [PubMed] [Google Scholar]
- 12.Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang H, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic Biol Med 2002;33:974. [DOI] [PubMed] [Google Scholar]
- 13.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 1999;13:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickinson DA, Iles KE, Zhang H, Blank V, Forman HJ. Curcumin alters EpRE and AP-1 binding complexes and elevates glutamate-cysteine ligase gene expression. FASEB J 2003;17:473–475. [DOI] [PubMed] [Google Scholar]
- 15.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation: 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem 1999;274:2234–2242. [DOI] [PubMed] [Google Scholar]
- 16.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest 1998;102:1942–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song BJ, Soh Y, Bae M, Pie J, Wan J, Jeong K. Apoptosis of PC12 cells by 4-hydroxy-2-nonenal is mediated through selective activation of the c-Jun N-terminal protein kinase pathway. Chem Biol Interact 2001;130–132:943–954. [DOI] [PubMed] [Google Scholar]
- 18.Bittorf T, Seiler J, Zhang Z, Jaster R, Brock J. SHP1 protein tyrosine phosphatase negatively modulates erythroid differentiation and suppression of apoptosis in J2E erythroleukemic cells. Biol Chem 1999;380:1201–1209. [DOI] [PubMed] [Google Scholar]
- 19.Hunter T. The role of tyrosine phosphorylation in cell growth and disease. Harvey Lect 1998;94:81–119. [PubMed] [Google Scholar]
- 20.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 2000;20:5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr Opin Struct Biol 2004;14:679–686. [DOI] [PubMed] [Google Scholar]
- 22.Hao Q, Rutherford SA, Low B, Tang H. Selective regulation of hydrogen peroxide signaling by receptor tyrosine phosphatase-alpha. Free Radic Biol Med 2006;41:302–310. [DOI] [PubMed] [Google Scholar]
- 23.Weibrecht I, Bohmer SA, Dagnell M, Kappert K, Ostman A, Bohmer FD. Oxidation sensitivity of the catalytic cysteine of the protein-tyrosine phosphatases SHP-1 and SHP-2. Free Radic Biol Med 2007;43:100–110. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Hernandez A, Garabatos MN, Rodriguez MC, Vidal ML, Lopez-Revuelta A, Sanchez-Gallego JI, Llanillo M, Sanchez-Yague J. Structural characteristics of a lipid peroxidation product, trans-2-nonenal, that favour inhibition of membrane-associated phosphotyrosine phosphatase activity. Biochim Biophys Acta 2005;1726:317–325. [DOI] [PubMed] [Google Scholar]
- 25.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol 2000;12:186–192. [DOI] [PubMed] [Google Scholar]
- 26.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001;22:153–183. [DOI] [PubMed] [Google Scholar]
- 27.Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol 1995;5:283–295. [DOI] [PubMed] [Google Scholar]
- 28.Sun H, Charles CH, Lau LF, Tonks NK. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 1993;75:487–493. [DOI] [PubMed] [Google Scholar]
- 29.Lee K, Esselman WJ. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic Biol Med 2002;33:1121–1132. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Somani AK, Siminovitch KA. Roles of the SHP-1 tyrosine phosphatase in the negative regulation of cell signalling. Semin Immunol 2000;12:361–378. [DOI] [PubMed] [Google Scholar]
- 31.Chen HE, Chang S, Trub T, Neel BG. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol 1996;16:3685–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eklund EA, Goldenberg I, Lu Y, Andrejic J, Kakar R. SHP1 protein-tyrosine phosphatase regulates HoxA10 DNA binding and transcriptional repression activity in undifferentiated myeloid cells. J Biol Chem 2002;277:36878–36888. [DOI] [PubMed] [Google Scholar]
- 33.Kant AM, De P, Peng X, Yi T, Rawlings DJ, Kim JS, Durden DL. SHP-1 regulates Fcgamma receptor-mediated phagocytosis and the activation of RAC. Blood 2002;100:1852–1859. [PubMed] [Google Scholar]
- 34.Kharbanda S, Bharti A, Pei D, Wang J, Pandey P, Ren R, Weichselbaum R, Walsh CT, Kufe D. The stress response to ionizing radiation involoves c-Abl-dependent phosphorylation of SHPTP1. Proc Natl Acad Sci USA 1996;93:6898–6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizuno K, Tagawa Y, Mitomo K, Watanabe N, Katagiri T, Ogimoto M, Yakura H. Src homology region 2 domain-containing phosphatase 1 positively regulates B cell receptor-induced apoptosis by modulating association of B cell linker protein with Nck and activation of c-Jun NH2-terminal kinase. J Immunol 2002;169:778–786. [DOI] [PubMed] [Google Scholar]
- 36.Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem 2001;276:44315–44322. [DOI] [PubMed] [Google Scholar]
- 37.Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem 1982;257:10766–10769. [PubMed] [Google Scholar]
- 38.Marques C, Pereira P, Taylor A, Liang JN, Reddy VN, Szweda LI, Shang F. Ubiquitin-dependent lysosomal degradation of the HNE-modified proteins in lens epithelial cells. FASEB J 2004;18:1424–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strohmaier H, Hinghofer-Szalkay H, Schaur RJ. Detection of 4-hydroxynonenal (HNE) as a physiological component in human plasma. J Lipid Mediat Cell Signal 1995;11:51–61. [DOI] [PubMed] [Google Scholar]
- 40.Rahman I, van Schadewijk AA, Crowther AJ, Hiemstra PS, Stolk J, MacNee W, De Boer WI. 4-Hydroxy-2-nonenal, a specific lipid peroxidation product, is elevated in lungs of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:490–495. [DOI] [PubMed] [Google Scholar]
- 41.Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med 2004;37:607–619. [DOI] [PubMed] [Google Scholar]
- 42.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med 2005;39:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep 2007;12:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagi O, Aoshiba K, Nagai A. Activation of nuclear factor-kappaB in airway epithelial cells in patients with chronic obstructive pulmonary disease. Respiration 2006;73:610–616. [DOI] [PubMed] [Google Scholar]
- 45.Macnee W, Rahman I. Oxidants and antioxidants as therapeutic targets in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;160:S58–S65. [DOI] [PubMed] [Google Scholar]
- 46.Ross SH, Lindsay Y, Safrany ST, Lorenzo O, Villa F, Toth R, Clague MJ, Downes CP, Leslie NR. Differential redox regulation within the PTP superfamily. Cell Signal 2007;19:1521–1530. [DOI] [PubMed] [Google Scholar]
- 47.Ishii T, Tatsuda E, Kumazawa S, Nakayama T, Uchida K. Molecular basis of enzyme inactivation by an endogenous electrophile 4-hydroxy-2-nonenal: identification of modification sites in glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 2003;42:3474–3480. [DOI] [PubMed] [Google Scholar]
- 48.Siems WG, Hapner SJ, van Kuijk FJ. 4-hydroxynonenal inhibits Na(+)-K(+)-ATPase. Free Radic Biol Med 1996;20:215–223. [DOI] [PubMed] [Google Scholar]
- 49.Grune T, Davies KJ. The proteasomal system and HNE-modified proteins. Mol Aspects Med 2003;24:195–204. [DOI] [PubMed] [Google Scholar]
- 50.Bassermann F, Peschel C, Duyster J. Mitotic entry: a matter of oscillating destruction. Cell Cycle 2005;4:1515–1517. [DOI] [PubMed] [Google Scholar]
- 51.Devoy A, Soane T, Welchman R, Mayer RJ. The ubiquitin-proteasome system and cancer. Essays Biochem 2005;41:187–203. [DOI] [PubMed] [Google Scholar]
- 52.Rhee SG, Chang TS, Bae YS, Lee SR, Kang SW. Cellular regulation by hydrogen peroxide. J Am Soc Nephrol 2003;14:S211–S215. [DOI] [PubMed] [Google Scholar]
- 53.Chen YR, Shrivastava A, Tan TH. Down-regulation of the c-Jun N-terminal kinase (JNK) phosphatase M3/6 and activation of JNK by hydrogen peroxide and pyrrolidine dithiocarbamate. Oncogene 2001;20:367–374. [DOI] [PubMed] [Google Scholar]
- 54.Muda M, Theodosiou A, Rodrigues N, Boschert U, Camps M, Gillieron C, Davies K, Ashworth A, Arkinstall S. The dual specificity phosphatases M3/6 and MKP-3 are highly selective for inactivation of distinct mitogen-activated protein kinases. J Biol Chem 1996;271:27205–27208. [DOI] [PubMed] [Google Scholar]
- 55.Bignon JS, Siminovitch KA. Identification of PTP1C mutation as the genetic defect in motheaten and viable motheaten mice: a step toward defining the roles of protein tyrosine phosphatases in the regulation of hemopoietic cell differentiation and function. Clin Immunol Immunopathol 1994;73:168–179. [DOI] [PubMed] [Google Scholar]
- 56.Krautwald S, Buscher D, Kummer V, Buder S, Baccarini M. Involvement of the protein tyrosine phosphatase SHP-1 in Ras-mediated activation of the mitogen-activated protein kinase pathway. Mol Cell Biol 1996;16:5955–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]