Abstract
Arbuscular mycorrhizas (AM) are widespread, ancient endosymbiotic associations that contribute significantly to soil nutrient uptake in plants. We have previously shown that initial fungal penetration of the host root is mediated via a specialized cytoplasmic assembly called the prepenetration apparatus (PPA), which directs AM hyphae through the epidermis (Genre et al., 2005). In vivo confocal microscopy studies performed on Medicago truncatula and Daucus carota, host plants with different patterns of AM colonization, now reveal that subsequent intracellular growth across the root outer cortex is also PPA dependent. On the other hand, inner root cortical colonization leading to arbuscule development involves more varied and complex PPA-related mechanisms. In particular, a striking alignment of polarized PPAs can be observed in adjacent inner cortical cells of D. carota, correlating with the intracellular root colonization strategy of this plant. Ultrastructural analysis of these PPA-containing cells reveals intense membrane trafficking coupled with nuclear enlargement and remodeling, typical features of arbusculated cells. Taken together, these findings imply that prepenetration responses are both conserved and modulated throughout the AM symbiosis as a function of the different stages of fungal accommodation and the host-specific pattern of root colonization. We propose a model for intracellular AM fungal accommodation integrating peri-arbuscular interface formation and the regulation of functional arbuscule development.
INTRODUCTION
Arbuscular mycorrhizal (AM) fungi are the most widespread mutualistic symbionts of terrestrial plants and are considered to be essential for optimal plant growth, especially in nutrient-poor soils. AM fungi enhance the capacity of the root to scavenge water and mineral nutrients from the rhizosphere, while receiving in return photosynthates that are essential for the completion of their life cycle (Harrison, 2005). Nutrient exchange takes place within inner root tissues via intracellular branched fungal structures called arbuscules (Bonfante, 1984). The so-called accommodation of the microsymbiont involves invagination of the host plasma membrane around the fungus, thus creating an apoplastic space bordered by a specialized symbiotic interface (Bonfante, 2001; Parniske, 2004; Harrison, 2005). The intracellular accommodation of AM fungi represents an important paradigm for plant biotrophic interactions. Although the ultrastructural features of mature arbusculated cells have been relatively well documented, the cellular and molecular mechanisms responsible for the codifferentiation of the symbiotic partners within the root cortex are still poorly understood.
To reach the inner cortex, the AM fungus first needs to cross epidermal and outer cortical cell layers. Recent in vivo confocal imaging studies making use of fluorescent cellular markers have revealed that AM fungal penetration across epidermal cells of the model legume Medicago truncatula is immediately preceded by the transient formation of a novel intracellular assembly comprising cytoskeletal and endoplasmic reticulum (ER) components (Genre et al., 2005). This assembly, which we have named the prepenetration apparatus (PPA), includes a broad cytoplasmic bridge linking the site of fungal adhesion on the outer cell surface to the cell nucleus and defines the future intracellular path of hyphal penetration. This process also involves complex nuclear dynamics, including initial repositioning of the nucleus at the site of fungal adhesion and subsequent transcellular migration leading to the assembly of the PPA (Genre et al., 2005). We have hypothesized that the PPA is directly involved in the construction of the membrane/matrix interface surrounding the AM infection hypha. Together, these observations highlight the major role of the host plant during epidermal penetration by the fungal partner.
This discovery raises several important questions. First, is the PPA response observed in M. truncatula roots specific to legumes? This particular plant family possesses the unique capacity to form endosymbiotic relationships with both AM fungi and nitrogen-fixing rhizobia, and in both cases the microbe enters the root through an intracellular apoplastic compartment (Parniske, 2004). The characterization of mutants that block epidermal infection by both AM fungi and nitrogen-fixing rhizobia argues that, at least for this plant family, there is an active regulation of initial root entry for these symbiotic microbes (Parniske, 2004). Secondly, whereas AM hyphae simply traverse the epidermis to reach the root cortex, it is within inner cortical cells that the extensive fungal colonization of the root takes place, leading to the complex codifferentiation that generates the highly convoluted interface surrounding the ramified fungal branches of the functional arbuscule (Bonfante and Perotto, 1995; Gianinazzi-Pearson, 1996). It is therefore essential to determine whether prepenetration mechanisms similar to those described in epidermal cells also operate during the next stages of AM cortical colonization and peri-arbuscular interface formation (Smith et al., 2006).
Finally, it is now well established that cortical colonization patterns for AM fungi depend on the identity of the symbiotic partners, particularly the plant host. At one end of the so-called Arum-Paris continuum (Dickson, 2004), root cortical colonization occurs essentially via intercellular hyphal development, which is followed by intracellular infection and the formation of arbuscules within individual cortical cells. This type of colonization is typical of the M. truncatula/Gigaspora spp association. By contrast, Paris-type colonization involves the direct passage of intracellular hyphae from one inner cortical cell to the next, with the progressive formation of coils and intercalary arbuscules within adjacent cells. This pattern characterizes the interaction between Daucus carota and Gigaspora spp. Since the origin and biological significance of these differences in AM fungal colonization patterns are subjects of considerable debate, it is important to establish whether the underlying molecular and cellular mechanisms are related.
To address these questions, we have performed a detailed investigation of host cellular responses throughout AM fungal colonization for both M. truncatula and D. carota using an in vivo confocal microscopy approach similar to that described in Genre et al. (2005). This strategy has provided important information on the cellular dynamics that precede and accompany the two distinct modes of fungal colonization in both outer and inner root cortical tissues. In particular, we have been able to show that the formation of PPA-like intracellular assemblies not only precedes epidermal and outer cortical cell infection in both hosts, but that similar basic mechanisms also operate during arbuscule formation in the inner cortex. In addition, this in vivo cellular approach has allowed us to identify key stages during arbuscule development for both plant species and to reveal fundamental differences between colonization mechanisms in terms of cell-to-cell signaling within the inner cortex. Finally, advanced transmission electron microscopy (TEM) analysis of PPA-like structures formed during cortical colonization has provided strong evidence supporting the central role of this cytoplasmic assembly in symbiotic interface synthesis and has also revealed a striking remodeling of the pre-infection host cell nucleus. Based on these findings, we propose a model for intracellular fungal development and peri-arbuscular interface assembly that includes the regulation of functional arbuscule differentiation by the host cell.
RESULTS
Earlier studies of the host cellular dynamics associated with AM infection through the root epidermis used green fluorescent protein (GFP) cytoskeletal and ER cellular markers expressed in transformed roots of M. truncatula (Genre et al., 2005). To acquire confocal images deeper in the root and to follow subsequent cortical cell infection and colonization by the AM fungus in both M. truncatula and D. carota, we used the highly fluorescent ER marker GFP-HDEL (Haseloff et al., 1997). This marker labels the ER throughout the cytoplasm as well as at the nuclear periphery and hence allows precise nuclear localization within the cell. This is important since PPA formation is characterized by specific nuclear dynamics associated with strong ER labeling of the nuclear-linked cytoplasmic bridge that directs subsequent intracellular fungal infection. To further facilitate the identification of potential PPAs within the M. truncatula root, we used transgenic roots expressing GFP-HDEL driven by the Mt ENOD11 promoter (Genre et al., 2005). This promoter is rapidly and specifically induced during all stages of AM colonization (Chabaud et al., 2002), and its activation precedes PPA formation (Genre et al., 2005). Thus, PMtENOD11:GFP-HDEL serves as an excellent reporter for facilitating the identification of the earliest stages of cell activation preceding fungal infection within M. truncatula root tissues.
Colonization of the Root Outer Cortex Is PPA Dependent in M. truncatula
Different stages of outer cortical root infection for M. truncatula are illustrated in the in vivo confocal images presented in Figures 1A to 1F, where the GFP-labeled ER is visualized in green and the endogenous fluorescence of the Gigaspora hyphae in yellow/red. These images reveal that prepenetration responses in the outer cortical cells of M. truncatula closely resemble those previously described for epidermal cells (Genre et al., 2005). In Figures 1A and 1B, the adhesion of a fungal hyphopodium (also referred to as appressorium; Genre and Bonfante, 2007) on the root surface triggered the formation of a PPA, visualized as a broad ER bridge traversing the epidermal cell, and linking the hyphopodium contact point to the cell nucleus positioned at the opposite side of the cell. Note that the nucleus of the underlying outer cortical cell has migrated to a position opposite the epidermal cell nucleus and the terminal end of the PPA. Once the fungus entered and fully traversed the epidermal cell (Figures 1C and 1D), a second PPA was seen crossing the outer cortical cell and linking the hyphal tip to the nucleus that was then positioned at the opposite (inner) side of the outer cortical cell. Finally, as for epidermal infection, the outer cortical PPA disassembled once the fungus had traversed the cell, leaving a thin layer of residual ER around the intracellular hypha (Figures 1E and 1F). The image in Figure 1F also shows that hyphal ramification can occur in the outer cortex. Taken together, these results reveal that the cellular dynamics characteristic of epidermal infection involving nuclear migration and associated PPA formation and disassembly are conserved during subsequent fungal penetration of the outer cortical cell layer.
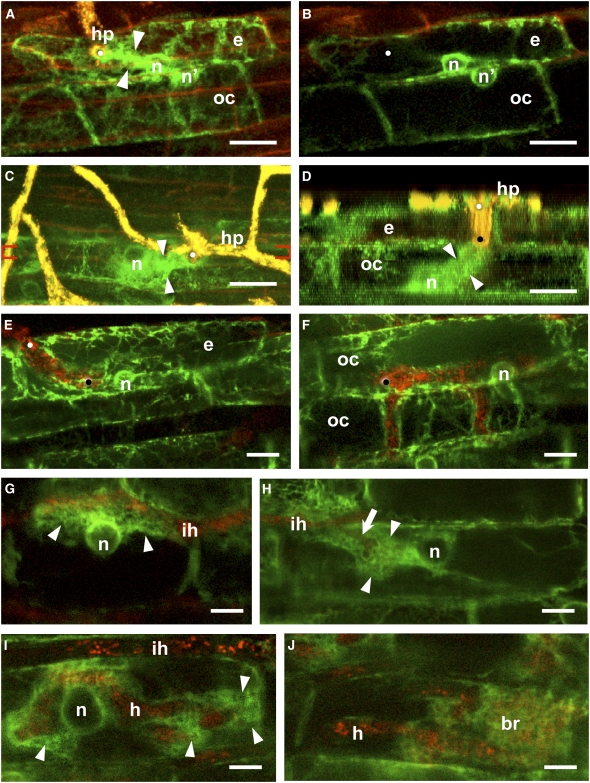
Figure 1.
Cellular Dynamics in the Root Cortex of M. truncatula during AM Colonization.
The plant ER is fluorescently labeled with GFP-HDEL (green), and the autofluorescence of the fungal partner G. gigantea is visualized in yellow-red depending on the depth of the optical section within the root. The GFP reporter was expressed under either the Mt Enod11 ([A] to [D]) or cauliflower mosaic virus 35S promoter ([E] to [J]). Bars = 10 μm.
(A) and (B) Nuclear repositioning (n') in an outer cortical cell (oc) in response to PPA formation in the overlying epidermal cell (e). (A) is a z axis projection of serial optical sections through the epidermis and outer cortex, and (B) is a single optical section that shows the relative positioning of the two cell nuclei. The hyphopodium (hp) on the surface of the epidermal cell elicited a transcellular PPA (arrowheads) that connected the contact site (white dot) with the epidermal nucleus (n).
(C) and (D) PPA development in an outer cortical cell. (C) is a z axis projection of serial optical sections through the epidermis and outer cortex, and (D) is an orthogonal section (x–z side view) through the epidermal hyphal infection site between the red brackets indicated in (C). Fungal penetration (white dot) in the epidermal cell elicited a transcellular PPA (arrowheads) within the outer cortical cell, which linked the tip of the penetrating hypha (black dot) with the cortical cell nucleus at the base of the cell.
(E) and (F) Intracellular fungal growth through epidermal and outer cortical cells. In both cell types, the PPA disassembled after the fungus traversed the cell (the epidermal and cortical cell penetration sites are marked by white and black dots, respectively). Simple hyphal ramification within the outer cortex (upper cell) is shown in (F).
(G) Inner cortical cell response to contact with an intercellular hypha (ih). The enlarged nucleus (n) and a large cytoplasmic aggregation of ER (arrowheads) were positioned along the cell wall contacted by the hypha.
(H) In the lumen of an inner cortical cell, a broad PPA (arrowheads) was positioned between the nucleus and the penetrating branch (arrow) of an intercellular hypha (ih).
(I) Prebranching cytoplasmic aggregations (arrowheads) were visible as localized ER accumulating at distinct sites along a large hypha (h) within an inner cortical cell. The lack of fungal autofluorescence within the ER aggregations indicated that hyphal branches had not yet developed. The host cell nucleus (n) was enlarged and centrally positioned. An intercellular hypha (ih) was visible above the cell.
(J) Mature arbuscule in an inner cortical cell. ER surrounded the hyphal branches (br) as an extended fine mesh and was also visible as a thin layer around the main trunk hypha (h).
AM hyphae very occasionally penetrated the M. truncatula root by growing down through spaces between adjacent epidermal cells. In such cases, hyphae were observed in direct contact with the underlying outer cortical cells, subsequently leading to PPA-dependent intracellular infection (see Supplemental Figure 1A online). In no case did we observe intercellular infection through both the epidermis and outer cortex, suggesting that at least one step of intracellular infection is required for successful colonization of the inner root cortex.
PPA-Related Dynamics Are Associated with Arbuscule Development in M. truncatula
Once hyphae reach the M. truncatula inner root cortex, there is a switch to intercellular hyphal growth through the apoplastic spaces of the cortical parenchyma before hyphae penetrate individual cells to initiate arbuscule development. Although confocal images are of lower resolution at this depth, the intensity of the GFP-HDEL ER marker proved sufficient for following intracellular dynamics throughout colonization and arbuscule differentiation (Figures 1G to 1J). Our results reveal a localized accumulation of ER within inner cortical cells contacted by intercellular hyphae (Figure 1G; see Supplemental Figures 2A and 2B online). This ER accumulation is indicative of cytoplasmic aggregation on the side of the cell contacted by the hypha and was always associated with nuclear repositioning as previously observed in epidermal cells following initial hyphopodium contact (Genre et al., 2005). However, in the case of the inner cortex, such repositioned nuclei were significantly enlarged in size compared with normal cortical cell nuclei (Figure 1G; see Supplemental Figure 2B online).
When intracellular infection occurred in the M. truncatula inner cortex, the enlarged nucleus had moved to a central position within the cell (Figure 1H) and there was a broad PPA-like cytoplasmic bridge enveloping the hypha and linking it to the nucleus. Initial hyphal growth into the inner cortical cell took place through this PPA-like bridge. However, in contrast with epidermal and outer cortical cell infection, once the initial phase of arbuscule development was completed with the formation of broad intracellular hyphae, localized aggregations of ER-rich cytoplasm organized at distinct sites along these so-called trunk hyphae (Figure 1I). Our observations suggest that these cytoplasmic aggregations predict the emergence of lateral hyphal branches. Finally, once arbuscule differentiation was complete, only a thin layer of ER-fluorescing cytoplasm remained around the initial hyphal trunk (Figure 1J). On the other hand, a dense three-dimensional meshwork of ER cisternae was present between the fine secondary branches of the arbuscule. Significantly, this pattern of ER distribution closely mirrors the organization of the peri-arbuscular actin cytoskeleton observed by Genre and Bonfante (1998) using immunofluorescence labeling.
In summary, these in vivo confocal studies have revealed four distinct stages of root inner cortical AM colonization for M. truncatula. Initially, intercellular hyphae trigger nuclear enlargement and repositioning, associated with cytoplasmic aggregation, in all contacted cells. Secondly, intracellular hyphal infection is preceded by nuclear movement from the fungal entry site toward the cell center and the assembly of a cytoplasmic bridge resembling the epidermal/outer cortical PPA. After hyphal infection along this PPA, the subsequent development of the arbuscular branches is preceded by the formation of prebranching cytoplasmic aggregations along the intracellular hypha. Finally, a layer of ER-rich cytoplasm surrounds the arbuscule branches. We therefore conclude that PPA-related intracellular accommodation mechanisms are associated with all the key stages of M. truncatula root colonization by AM fungi.
Long-Distance PPA Formation Prefigures Intracellular AM Colonization of D. carota
To investigate whether similar cellular mechanisms operate during AM infection of other host plants, we focused on the nonlegume D. carota. As stated earlier, the colonization pattern for carrot is radically different than that for M. truncatula. This is illustrated in Figure 2, where the intercellular hyphal growth responsible for AM inner root colonization in M. truncatula is replaced by an exclusively intracellular cell-to-cell colonization pattern in carrot roots. Using the identical GFP-HDEL ER marker and a similar Agrobacterium rhizogenes–based root transformation approach, we initially studied Gigaspora infection across the outer cell layers of the D. carota root.
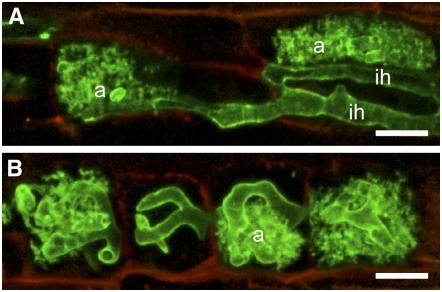
Figure 2.
Different Patterns of G. gigantea Colonization in the Inner Cortex of M. truncatula and D. carota Roots.
The AM fungal cell wall was stained using tetramethylrhodamine-conjugated wheat germ agglutinin and false colored in green to maximize the contrast with the weak red autofluorescence of the plant cell walls. Bars = 20 μm.
(A) In M. truncatula roots, terminal arbuscules (a) were formed within inner cortical cells following infection by intercellular hyphae (ih).
(B) In D. carota, intercalary arbuscules (a) were formed within adjacent intracellularly infected inner cortical cell files, and intercellular hyphae were absent. Both images are z axis projections of serial optical sections acquired from vibratome longitudinal root sections.
The image in Figure 3A shows that transient cytoplasmic assemblies resembling the M. truncatula root PPAs were also formed in carrot epidermal cells directly contacted by AM hyphopodia. As in M. truncatula, initial nuclear repositioning at the hyphal contact site is followed by nuclear migration across the cell and the progressive formation of the PPA cytoplasmic bridge that predicts the intracellular path subsequently taken by the penetration hypha. Similar pre-infection responses in the carrot outer cortex are shown in Figure 3B, where a transcellular PPA is positioned between the cell nucleus and the infecting hypha that had just penetrated across the overlying epidermal cell. Such observations lead us to conclude that pre-infection responses involving transient PPA formation in outer root tissues of D. carota are very similar to those observed for M. truncatula. Interestingly, AM hyphal penetration through spaces between root epidermal cells appeared to be more frequent for carrot compared with M. truncatula. However, as for M. truncatula, this always led to intracellular infection of the exposed outer cortical cell (see Supplemental Figure 1B online). Therefore, for both plant species, access of AM hyphae to inner root tissues always involves the intracellular crossing of at least one outer root cell layer.
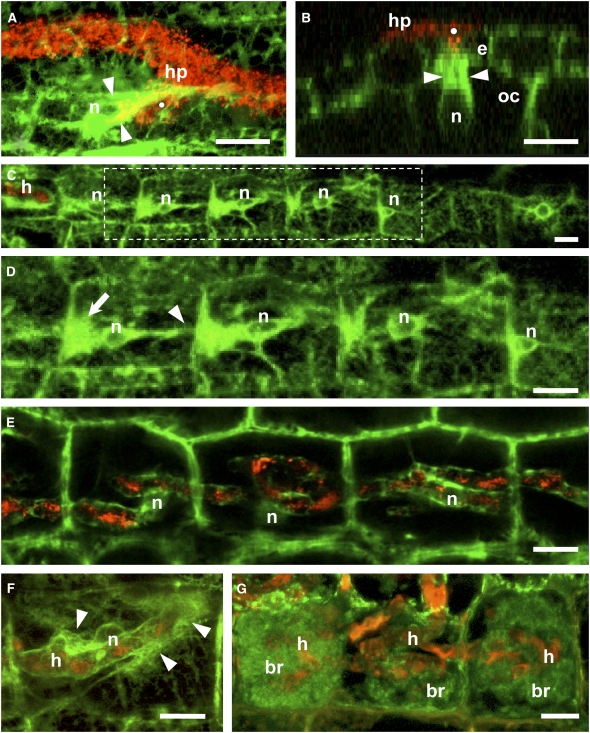
Figure 3.
Cellular Dynamics Associated with AM Colonization of D. carota Root Tissues.
Identical fluorescent ER labeling and color coding as in Figure 1. Bars = 10 μm.
(A) A PPA (arrowheads) was assembled in an epidermal cell underneath the fungal hyphopodium (hp) contact point (white dot). The position of the nucleus (n) is indicated.
(B) Orthogonal section (x–z side view) perpendicular to the root axis through a fungal penetration site in the epidermis (white dot). The hypha produced by the hyphopodium (hp) penetrated into the epidermal (e) cell lumen, and the underlying outer cortical (oc) cell assembled a broad PPA (arrowheads) connected to the nucleus.
(C) and (D) Aligned PPAs in a file of inner cortical cells ahead of an intracellular hypha (h) growing from left to right. The area outlined in (C) is magnified in (D), where progressive stages in PPA development can be observed from right to left. These polarized PPAs were characterized by a large aggregation of ER (arrow) directed toward the incoming fungus, a central enlarged nucleus and a narrow ER bridge (arrowhead) directed toward the following cell in the file.
(E) Sixteen hours later, the inner cortical cells imaged in (D) were colonized by broad coiled hyphae. The nuclei maintained their central position, and the hyphae were surrounded by a thin layer of ER.
(F) An inner cortical cell colonized by a broad hypha with localized prebranching aggregations of ER (arrowheads). One of these aggregations was associated with a budding hyphal branch. The host cell nucleus was in a central position, partly hidden by the hypha.
(G) Mature intercalary arbuscules inside three adjacent inner cortical cells from a longitudinal root section. The large main hyphae (h) were surrounded by fine branches (br) covered by a dense network of ER.
Once infection hyphae reached the inner cortex, major differences in the cell prepenetration responses between M. truncatula and D. carota were revealed by these confocal microscopy studies. The images in Figures 3C and 3D illustrate the highly polarized intracellular organization observed within a linear file of inner cortical cells lying ahead of an advancing AM hypha. This polarization was characterized by an enlarged centrally located nucleus, flanked on one side by a broad, highly fluorescent cytoplasmic bridge directed toward the approaching fungal hypha. The nucleus was bordered on the other side by a narrow bridge linked to the opposite face of the cell and aligned with the cytoplasmic bridge in the adjacent cell (see Supplemental Figures 2C and 2D online). The cells furthest from the fungus had smaller aggregations, and the nucleus was often less enlarged and positioned close to the wall facing the fungus. This suggests that cell activation at a distance from the fungus is progressive and involves nuclear migration toward the center of the cell with the associated formation of broad and narrow cytoplasmic bridges. When the colonization site shown in Figures 3C and 3D was observed 16 h later, fungal infection had progressed along the entire row of adjacent activated cells, generating a series of hyphal coils (Figure 3E). The intracellular remodeling that predicted the fungal trajectory in these linear inner cortical cell files is again reminiscent of the PPA as defined by specific nuclear dynamics and the formation of a cytoplasmic bridge that determines the fungal intracellular pathway. However, PPA-dependent intracellular infection in the carrot inner cortex was not identical to that observed in M. truncatula due to the fact that in D. carota, both the PPA and the hypha fully traverse the cell.
Finally, as observed during arbuscule development in M. truncatula, dense ER aggregations also assembled at distinct sites along the intracellular hyphae within infected carrot inner cortical cells (Figure 3F; see Supplemental Figures 2E and 2F online), and these localized cytoplasmic aggregations were often associated with incipient lateral branches. In mature arbusculated coils, a network of ER surrounded both hyphal branches (Figure 3G) and the centrally located host cell nucleus (see Supplemental Figure 2F online). In conclusion, despite major differences in the strategies of inner root colonization, the basic host cellular mechanisms associated with AM infection appear to be conserved between M. truncatula and D. carota. For both colonization modes, PPA-related cellular responses precede and accompany inner cortical infection and arbuscule differentiation. However, whereas inner cortical PPA formation in M. truncatula depends on direct fungal contact, progressive intracellular colonization in D. carota is characterized by long-distance coordinated PPA development.
Ultrastructural Evidence for Intense Membrane Trafficking in PPA-Like Structures
For more detailed ultrastructural analysis, we focused on the PPAs formed during inner cortical infection of carrot roots because of the relative ease of initially identifying these linearly aligned cytoplasmic bridges using confocal microscopy. Thus, carrot root segments containing inner cortical PPAs were visualized using the fluorescent ER label and then prepared for TEM using a flat-embedding procedure. This approach is illustrated in Supplemental Figure 3 online, where aligned PPA-containing cells first observed in the confocal microscope (see Supplemental Figure 3A online) were then identified in embedded 0.5-μm-thick resin sections (see Supplemental Figure 3B online) prior to the cutting of 70-nm ultrathin sections for TEM observation (see Supplemental Figure 3C online).
TEM images revealed the cytoplasmic composition of the PPA (Figure 4A; see Supplemental Figure 3C online). As expected from the strong GFP-HDEL fluorescent signal, large quantities of ER membranes were present within the cytoplasmic aggregation, particularly in the vicinity of the tonoplast and the nucleus (Figure 4B). In addition, Golgi stacks with electron-dense cisternae and either small (30 to 50 nm) dark-staining vesicles (Figures 4C and 4E) or larger (60 to 70 nm) light-staining vesicles (Figures 4D and 4F) were particularly abundant compared with control cortical cells (see Supplemental Figure 4 online). Furthermore, budding Golgi vesicles, often associated with microtubules (Figure 4C), fused into extensive tubular-vesicular trans-Golgi networks (TGNs; Figures 4E and 4F). The ubiquitous presence of such TGNs within the PPA argues that Golgi-derived membranes are one of the main components involved in perifungal membrane assembly. Multivesicular bodies (MVBs), most likely involved in membrane recycling (Tse et al., 2004; An et al., 2006), were also abundant, often in the vicinity of the tonoplast. These MVBs were either small with electron-dense vesicles (Figure 4G) or larger structures containing electron-transparent vesicles (Figure 4H). Similar MVBs were also present in colonized cells ahead of the developing hypha (Figure 4A). In conclusion, the simultaneous presence of all the main organelles associated with membrane trafficking in the PPA is a clear indicator of major endo- and exocytotic activities within inner cortical cells preparing for fungal penetration.
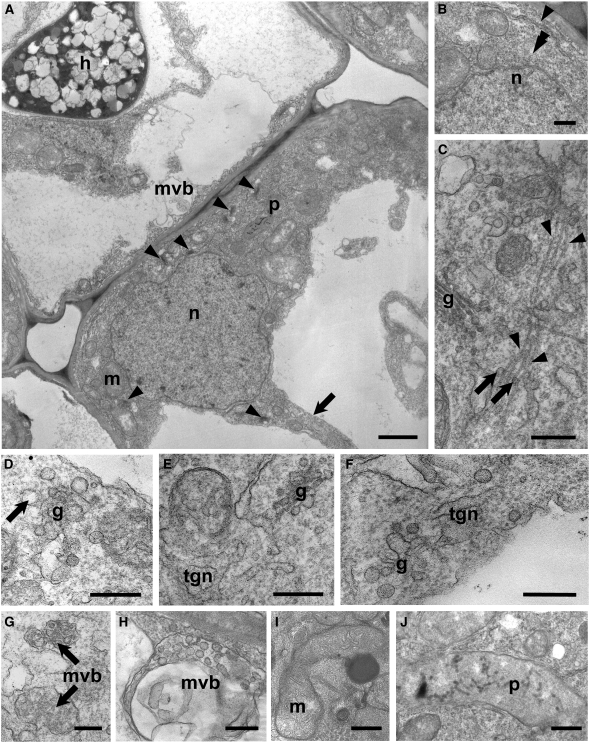
Figure 4.
TEM Imaging of PPA-Containing Inner Cortical Cells of D. carota.
(A) A typical PPA (extensive region of dense gray cytoplasm in the lower right-hand cell) associated with an enlarged nucleus (n) was positioned close to the junction with an adjacent inner cortical cell (above left) containing an AM hypha (h). Several Golgi stacks (arrowheads), mitochondria (m), and plastids (p) were visible within the broad cytoplasmic aggregation. Decondensed chromatin was present in the nucleus, and a narrow cytoplasmic bridge (arrow) was directed away from the approaching fungus. A smaller aggregation of cytoplasm and a multivesicular body (mvb) were visible in the adjacent colonized cell, ahead of the advancing hypha.
(B) to (J) High-magnification images of distinctive features of the PPA.
(B) Part of the nuclear envelope (double arrowhead) and an ER cisterna (arrowhead) in the vicinity of the nucleus (n).
(C) Vesicles (arrows) budding from a Golgi stack (g) and associated with bundled microtubules (arrowheads) oriented along the cytoplasmic bridge axis.
(D) Front view of a Golgi stack (g) with large, electron transparent budding vesicles (arrow).
(E) and (F) TGN membranes originating from the fusion of large vesicles budding from Golgi stacks.
(G) MVBs filled with small dense vesicles.
(H) Large multivesicular body, containing large-size electron-transparent vesicles.
(I) Dividing mitochondrion (m), rich in cristae and associated with an electron-dense body.
(J) Chromoplast-like plastid (p) devoid of starch granules, containing several electron-dense membranes and globules.
Bars = 2 μm in (A), 0.5 μm in (B) and (D), and 0.25 μm in (C) and (E) to (J).
Additional striking features of the inner cortical PPA included polymorphic mitochondria, often elongated and dividing (Figure 4I), as well as elongated and chromoplast-like plastids without starch granules and rich in electron-dense membranes and globules (Figures 4A and 4J). Such plastids, most likely involved in apocarotenoid synthesis (Schliemann et al., 2006), have been described in arbusculated cells (Scannerini and Bonfante, 1977; Fester et al., 2007). Finally, bundles of microtubules were clearly visible (Figure 4C), mostly oriented along the axis of the cytoplasmic bridge as previously observed for the M. truncatula epidermal PPA using the microtubule-specific fluorescent marker GFP:Map4-MBD (Genre et al., 2005). Together, these ultrastructural data on organelle remodeling highlight the dramatic change in metabolic activity of the host cell associated with the formation of the PPA and in particular the development of all the cellular machinery required for intense membrane trafficking.
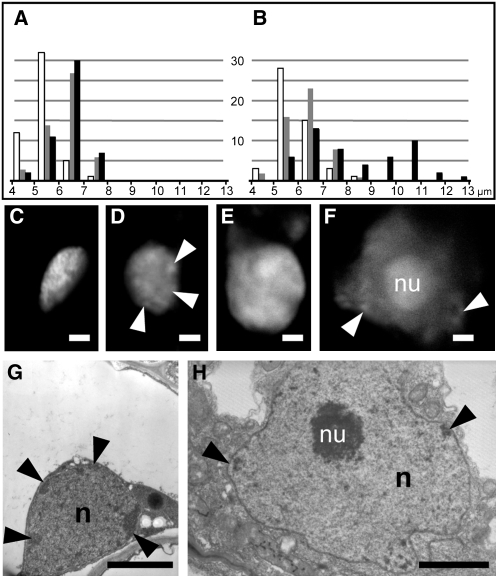
Inner Cortical Cells Preparing for AM Infection Are Characterized by an Increase in Nuclear Size and Nuclear Remodeling
As indicated earlier in both M. truncatula and D. carota, there is a marked increase in nuclear dimensions associated with the inner cortical cells exhibiting prepenetration responses. We used confocal microscopy imaging to make a statistical study of the changes in nuclear size during AM root colonization of M. truncatula. The data presented in Figures 5A and 5B show that when the inner cortex is colonized only by intercellular hyphae (see Supplemental Figures 2A and 2B online) there is an additional population of large nuclei with an average diameter of 10 to 11 μm, compared with the 6- to 7-μm diameter typical of inner and outer cortical cell nuclei in uncolonized roots. This additional population of large nuclei corresponds to responding cells in direct contact with the intercellular hyphae (see Supplemental Figure 2B online).
Figure 5.
Nuclear Enlargement and Remodeling in Inner Cortical Cells Associated with Prepenetration Responses.
(A) and (B) Histograms showing the distribution of nuclear sizes in epidermal (white), outer cortical (gray), and inner cortical cells (black) in M. truncatula control (A) and colonized roots (B). The plots represent 50 independent measurements for each cell type. The population of significantly enlarged nuclei in colonized roots corresponded to inner cortical cells with prepenetration responses.
(C) to (F) Propidium iodide fluorescent staining of DNA to visualize changes in nuclear morphology. Control inner cortical cells of D. carota (C) and M. truncatula (E) had densely stained nuclei without visible nucleoli. By contrast, enlarged nuclei in D. carota (D) and M. truncatula (F) cells with prepenetration responses contained only isolated patches of condensed chromatin (arrowheads) at the nuclear periphery, and the nucleolus (nu) was often very large (F).
(G) and (H) TEM images of the nucleus (n) in a control (G) and AM-responding cell (H) of D. carota at the same magnification. In addition to the obvious size difference, the prepenetration-responsive nucleus (H) had lower chromatin density, fewer dark peripheral patches of heterochromatin (arrowheads), and a very large and granular nucleolus (nu). Bars = 2 μm.
In addition to a change in dimension, fluorescent propidium iodide staining of nuclei from sectioned root segments of both plants indicated that there is also a general remodeling of chromatin architecture. Figures 5C to 5F show that decondensed chromatin and large nucleoli were typical characteristics of the larger-size nuclei of PPA-containing inner cortical cells, in contrast with the denser nuclear content and small nucleoli in control inner cortical cells. Furthermore, TEM analysis showed that the dense heterochromatic regions present at the periphery of control nuclei (Figure 5G) disappear in the enlarged nuclei (Figure 5H; see Supplemental Figure 5A online) and that the nucleolus becomes highly organized with visible granular regions at the periphery (Figure 5H). A very similar nuclear morphology was also present in AM-infected inner cortical cells (see Supplemental Figure 5B online), in line with previous observations (Balestrini et al., 1992). In conclusion, the PPAs that developed in inner cortical cells of both M. truncatula and D. carota during AM colonization and prior to fungal penetration were associated with enlarged and remodeled nuclei, indicating intense transcriptional activity.
DISCUSSION
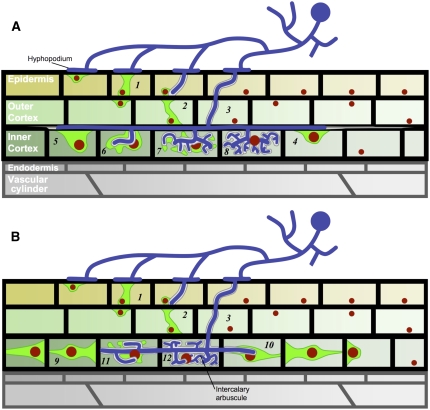
Comparative in vivo confocal microscopy studies performed on a legume (M. truncatula) and a nonlegume species (D. carota) displaying different patterns of AM root colonization (schematically outlined in Figure 6) have revealed that there is a common cellular mechanism underlying all phases of intracellular fungal accommodation. Prior to penetration, the host cell undergoes a major reorganization characterized by complex nuclear dynamics leading to the formation of a polarized cytoplasmic bridge termed the PPA, which defines the future hyphal path. Our results have shown that nuclear movement and PPA organization are modulated as a function of the root cell type and the nature of the subsequent intracellular fungal development. The initial stages of AM root colonization requires that the symbiotic fungus traverses both the epidermal and outer cortical tissues to reach the root inner cortex where the arbuscular symbiotic association is established during the subsequent growth and development of the fungus within and along the root (Figures 2 and 6). In this article, we have shown that prepenetration responses in the outer cortex of M. truncatula and D. carota are very similar to those described for the epidermis and are characterized by the formation of transcellular cytoplasmic bridges oriented toward the inner root tissues (Figures 1A, 1D, 3A, and 3B). Our studies further suggest that access to the inner root cortex requires at least one intracellular infection step, even in those cases where AM hyphae have bypassed the epidermal cell layer. Since intracellular accommodation via a PPA-dependent mechanism allows strict regulation by the host, it is therefore likely that the epidermal and outer cortical cell layers represent essential checkpoints for initial fungal entry (Novero et al., 2002; Demchenko et al., 2004).
Figure 6.
Schematic Summarizing the Different AM Colonization Patterns and Associated Cellular Dynamics for M. truncatula and D. carota.
In both plants, the adhesion of a fungal hyphopodium to the root surface triggers the assembly of a PPA in the contacted epidermal cell (1). This, in turn, initiates PPA formation in the underlying outer cortical cell (2). After radially oriented fungal crossing of the outer root layers (3), the subsequent longitudinal colonization patterns and associated inner cortical cell responses differ between the two plants. In M. truncatula (A), intercellular hyphae grow along and between the cells. Upon direct hyphal contact, these cells respond with nuclear repositioning and localized cytoplasmic aggregations (4). Inner cortical cell penetration is preceded by the assembly of large PPAs (5 and 6). Prebranching cytoplasmic aggregations organize at sites along the intracellular trunk hyphae (6 and 7), preceding the development of the arbuscular fine branches (8). In D. carota (B), inner cortical cell files are progressively colonized by individual intracellular hyphae that move from one cell to the next. A number of adjacent cells ahead of the hyphal tip respond concomitantly by organizing polarized funnel-shaped PPAs that predict the direction of fungal colonization (9 and 10). As for M. truncatula, prebranching cytoplasmic aggregations (11) precede the formation of arbuscular side branches (12). Structures are represented with the following color code: red, nucleus; green, cytoplasmic aggregation; white, perifungal interface; blue, fungus.
When AM hyphae reach the root inner cortex, there is a fundamental change in the pattern of root colonization since in both plants the main direction of fungal growth has switched from radial to longitudinal. In the case of M. truncatula, inner cortical growth is essentially intercellular, whereas colonization of the carrot inner cortex remains intracellular (Figures 2 and 6). Nevertheless, despite these differences, intracellular infection leading to arbuscule development in both plant species was always preceded by cellular reorganization and the formation of cytoplasmic bridges that predict hyphal entry. The PPA assembled in the M. truncatula inner cortical cell results from direct contact with intercellular hyphae (Figures 1G and 1H; see Supplemental Figures 2A and 2B online), whereas prepenetration responses in the carrot inner cortex are characterized by aligned, polarized PPAs that anticipate the path of fungal growth along and within files of adjacent cells (Figures 3C and 3D; see Supplemental Figures 2C and 2D online). Thus, PPA formation and structure in the inner cortex clearly reflected the different AM colonization strategies adopted in the two species, which are examples of alternative colonization patterns from within the Arum-Paris continuum (Dickson, 2004).
The remarkable series of linear polarized PPAs formed in the D. carota inner cortex can extend over at least four to five adjacent cells ahead of the advancing fungus (Figure 3C). The alignment of cytoplasmic bridges at cell junctions (Figure 3D; see Supplemental Figure 2D online) argues in favor of sequential cell-to-cell signaling. Discovering the nature of this long-distance signaling mechanism that coordinates the cellular differentiation of PPAs and could involve both fungal and plant signals will be a major challenge for future studies.
Finally, it is very tempting to draw an analogy between these longitudinally aligned PPAs observed in the D. carota inner cortex and the radially aligned cytoplasmic bridges called pre-infection threads, which are formed across the width of the legume cortex prior to rhizobial infection of the root hair (van Brussel et al., 1992). Despite the different orientations within the root, in both cases the aligned bridges predict the intracellular apoplastic path taken by the respective symbiotic microorganisms. However, it remains to be determined whether this structural analogy is reflected by similar molecular and cellular mechanisms.
The Role of Nuclear Dynamics and Remodeling before Intracellular Accommodation of AM fungi
In vivo confocal studies have revealed that the host cell nucleus is a central player throughout AM root colonization, during both PPA formation and arbuscule differentiation. In the case of epidermal cell infection, we have previously shown that the earliest visible response to fungal adhesion is the repositioning of the nucleus directly opposite the contact site (Genre et al., 2005). Interestingly, the nuclear repositioning that precedes PPA formation in the M. truncatula outer cortex shown in Figure 1B clearly did not require direct physical contact with the AM fungus, since the hypha had not yet traversed the overlying epidermal cell. Furthermore, since the nucleus was positioned directly opposite the epidermal nucleus/PPA, it is likely that this early pre-infection response also resulted from direct cell-to-cell signaling between adjacent cells and suggests that the intracellular crossing of outer root tissues is a progressive cell-by-cell process. This also would be consistent with the proposed checkpoint role for the outer root tissues discussed earlier. Finally, it should be noted that nuclear repositioning is a well-known feature of several plant cell responses to both biotic and abiotic stimuli (Genre and Bonfante, 2007).
Our confocal studies have shown that when AM hyphae reach the M. truncatula inner cortex, intercellular hyphal progression along the root axis is systematically accompanied by nuclear repositioning opposite the site of fungal contact (Figure 1G; see Supplemental Figure 2A online). Furthermore, this migration of the nucleus toward the fungal contact site is accompanied by nuclear enlargement (see Supplemental Figure 2B online) and localized cytoplasmic aggregation. It should be noted that significant nuclear enlargement was not observed during PPA formation in the outer root tissues (cf. Figures 1B and 1F). On the other hand, an increase in nuclear dimensions is a well-documented feature of arbusculated cells (Balestrini et al., 1992); indeed, increases in nuclear ploidy have been reported in mycorrhizal roots, which could explain nuclear enlargement (Fusconi et al., 2005). Although we have no information on cell ploidy from our studies, our ultrastructural data show that the increase in nuclear size is associated with chromatin decondensation and the appearance of large, highly organized nucleoli, both suggesting intense transcriptional activity. Since only a proportion of the inner cortical cells contacted by the intercellular hypha will subsequently be infected and differentiate arbuscules, we consider that this particular cell activation status is an indicator of competence for fungal accommodation and arbuscule formation.
Enlarged nuclei with structural remodeling are also characteristic of PPA-containing inner cortical cells in carrot roots prior to fungal infection (Figures 4A and 5H; see Supplemental Figure 5A online), and these nuclei are similar to those of arbusculated cells (see Supplemental Figure 5B online). Thus, data from two host plants with different patterns of AM colonization clearly indicate that the preparation of inner cortical cells for arbuscule development involves major nuclear remodeling prior to fungal entry. This certainly is consistent with the intensive membrane and protein synthesis that necessarily accompanies PPA formation and subsequent arbuscule differentiation (see below).
A Cellular Model for the Coordinated Development of the Peri-Arbuscular Interface and Functional Arbuscule
An acknowledged hallmark of AM accommodation in host inner cortical root cells is the de novo membrane synthesis related to the formation of the extensive perifungal interface. The structures of the various organelles that could be visualized in the TEM images of inner cortical PPAs are clear indicators of significant exo- and endocytotic activities (Figure 4), presumably related to the preparation for fungal accommodation. Interestingly, plasma membrane synthesis involving a vesicular-tubular stage is also associated with plant cell division and in particular the development of the cell plate during cytokinesis (Otegui et al., 2001; Bednarek and Falbel, 2002). It is therefore conceivable that perifungal interface development involves a similar mechanism, with Golgi vesicles and TGN tubules generating the membranous components that are later assembled through membrane fusion to create the apoplastic tunnel ahead of the advancing hyphal tip. If so, then the adaptation of essential cellular processes, such as cell division (Van Damme et al., 2007), could be responsible for the ancient evolutionary development of AM fungal accommodation dating back at least 400 million years. In addition to the major proliferation of the cell endomembranous systems, TEM analysis of PPAs in the carrot inner cortex also revealed extensive plastid modifications as well as mitochondrial enlargement and proliferation. The ability to use in vivo fluorescent markers, such as GFP-HDEL, to identify responding host cells in the root cortex will be invaluable for studying this intense metabolic activation in more detail using single-cell approaches such as laser microdissection (Balestrini et al., 2007).
Based on the results presented in this article, we propose a two-step model for the concerted development of the peri-arbuscular interface and the functional arbuscule. In this model, the PPA response that we have observed in inner cortical cells anticipates fungal entry and constructs the interface compartment around the broad trunk hyphae that initially develop in the colonized cell. Observations in both M. truncatula and D. carota roots revealed that the ER-rich cytoplasm surrounding these large hyphae was subsequently reassembled at localized sites in the form of small cytoplasmic aggregations (Figures 1I and 3F). Since these aggregations anticipated sites of lateral branch initiation, it is likely that they play a role in extending the periarbuscular interface, analogous to the functioning of the PPA. Interestingly, Javot et al. (2007) have shown that arbuscule development is prematurely aborted at the initial stage of trunk formation in AM-colonized M. truncatula plants defective in the host phosphate transporter gene Mt PT4. The authors deduce that functional arbuscule development, which involves additional hyphal branching, is dependent upon the capacity of the microsymbiont to supply phosphate to the host. Since our data suggest that the lateral branching of trunk hyphae depends upon the formation of the prebranching cytoplasmic aggregations, we propose that this critical step leading to major interface expansion could be the target for host regulation of functional arbuscule development. Furthermore, these prebranching aggregations might also be responsible for targeting host phosphate transporters to the perifungal membrane, since Mt PT4 is expressed only in arbusculated cells and localized at the peri-arbuscular interface. In this context, it is interesting to note that Drissner et al. (2007) have identified a potential membrane-associated lysophosphatidylcholine signal that can activate host phosphate transporter genes in potato roots.
The PPA therefore appears to be a highly versatile mechanism that can be modulated by the plant according to the specific needs of each cell. In epidermal and outer cortical cells, the transcellular radially oriented PPA is involved in the assembly of the interface compartment that surrounds the intracellular hypha. During longitudinal colonization of the inner cortex, a similar mechanism operates, but with the additional requirement of assembling the extensive periarbuscular interface around the complex three-dimensional branched structure of the arbuscule. Future research will establish whether this generalized host cellular mechanism that has evolved to accommodate AM fungi can be extended to other important plant–microbe biotrophic interactions.
METHODS
Plant and Fungal Material
Experiments were performed with Agrobacterium rhizogenes–transformed root cultures derived from either Medicago truncatula Jemalong A17 or Daucus carota var Sativus. The AM fungal partner used in this study was Gigaspora gigantea. This particular AM fungus can be observed using confocal microscopy because of its yellowish cytoplasmic autofluorescence (Séjalon-Delmas et al., 1998). Spores of G. gigantea were produced using sand-grown clover that was surface-sterilized and stored at 4°C according to Bécard and Fortin (1988).
GFP-Tagged Cellular Markers Expressed in M. truncatula and D. carota Roots
The GFP tag used to monitor ER and nuclear cellular dynamics during AM fungal infection was GFP-HDEL, expressed using either the cauliflower mosaic virus 35S or Mt ENOD11 promoters as described by Genre et al. (2005). Only the 35S:GFP-HDEL construct was used in D. carota since the Mt ENOD11 promoter is not expressed during AM colonization in carrot roots. Vectors were transformed into the A. rhizogenes strain Arqua-1, which was then used to transform M. truncatula Jemalong A17 and D. carota using the protocols described by Boisson-Dernier et al. (2001) and Bécard and Fortin (1988), respectively. For both species, transformed roots with high levels of GFP fluorescence were excised, decontaminated, and then propagated in vitro on M medium (Boisson-Dernier et al., 2001). In each case, a single representative clone was chosen for further studies. Transgene expression in cloned roots was stable throughout in vitro root subculturing, and the presence of the transgenic constructs had no observable effects on root growth or on the interaction with the AM fungus.
In Vivo Microscopy Observation of AM Root Infection
The targeted AM inoculation technique for studying early stages of the symbiotic association between Gigaspora species and transformed root cultures, developed by Chabaud et al. (2002) and adapted for confocal observation by Genre et al. (2005), was applied to both plant species. Germinated spores of G. gigantea were transferred to vertically oriented Petri dishes containing a growing transgenic root explant. The root and fungus were covered with a thin (25 μm) gas-permeable plastic film (bioFOLIE 25; Sartorius) that allowed repeated and prolonged microscopic observation as well as minimizing potential contamination of the coculture.
Confocal Microscopy
Fungal colonization of the transformed root was monitored using a stereomicroscope. Potential infection sites were identified and followed in detail using a Leica TCS SP2 confocal microscope fitted with a long-distance ×40 water-immersion objective (HCX Apo 0.80). The argon laser band of 488 nm was used to excite both the GFP and the G. gigantea autofluorescence. The two signals were distinguished via specific emission windows: 500 to 525 nm for GFP and 590 to 630 nm for fungal autofluorescence. The latter channel was then false-colored in red to maximize the contrast in overlapping images.
Fungal cell wall staining with wheat germ agglutinin was performed on 100-μm-thick vibratome sections of colonized root segments. Sections were treated for 5 min in 1% NaClO, thoroughly washed in phosphate buffer, pH 6.8, incubated with tetramethylrhodamine-conjugated wheat germ agglutinin (Invitrogen) at a final concentration of 20 μg/mL (Vierheilig et al., 2005), and imaged with an excitation of 543 nm (argon laser) and a 590- to 630-nm emission window. Tetramethylrhodamine fluorescence was false-colored in green to maximize contrast with the weak plant cell wall autofluorescence, visualized using the same excitation wavelength and a 630- to 700-nm emission window. Vibratome sections were also used for staining plant nuclei. Sections were incubated with 50 ng/mL propidium iodide (Invitrogen) in phosphate buffer, pH 6.8, and imaged with an excitation of 488 nm and an emission window of 600 to 700 nm.
Nuclear diameters were estimated in living roots using the Leica LCS software to measure a transect across the nucleus based on the GFP-labeled nuclear periphery. The data in Figures 5A and 5B represent a total of 50 nuclei for each cell type (epidermis, outer cortex, and inner cortex) and for each condition (control and colonized roots).
Electron Microscopy
Carrot root segments with aligned PPAs in the inner cortex were initially identified under the confocal microscope. They were then excised under a stereomicroscope and rapidly fixed at room temperature for 2 h in 0.1 M phosphate-saline buffer, pH 6.8, containing 2% glutaraldehyde, 0.1 mM EGTA, 2 mg/mL tannic acid, and 0.1 mM MgCl2. Samples were then rinsed three times with phosphate buffer for 30 min and postfixed in 1% OsO4 in phosphate buffer for 2 h at 4°C. After several washes with phosphate buffer, followed by 1 h in distilled water, en-bloc staining with aqueous 0.5% uranyl acetate was performed for 2 h at room temperature. Samples were then washed in distilled water, dehydrated progressively in an ethanol series, incubated two times in absolute acetone, infiltrated in Epon-Araldite resin (Hoch, 1986), and flat-embedded in a thin resin layer between Teflon-coated glass slides (Howard and O'Donnell, 1987). The resin was polymerized for 24 h at 60°C.
Fungal colonization sites within flat-embedded samples were selected under an optical microscope, excised using a razor blade, and mounted on resin stubs prior to ultramicrotomy. Semithin sections of 0.5 μm were stained with 1% toluidine blue to check the sample contents using optical microscopy. Based on these observations, ultrathin (70 nm) sections were cut, counterstained with uranyl acetate and lead citrate (Reynolds, 1963), and then viewed with a Philips CM10 transmission electron microscope.
Accession Number
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number AJ297721 (M. truncatula ENOD11 gene).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Intercellular Colonization of the Root Epidermis by G. gigantea.
Supplemental Figure 2. Prepenetration Responses in the Inner Cortex of M. truncatula and D. carota during AM Colonization.
Supplemental Figure 3. Ultrastructural Studies of D. carota Inner Cortical PPAs: From Confocal Microscopy to TEM.
Supplemental Figure 4. Ultrastructural Organization of a Control Cortical Cell from D. carota.
Supplemental Figure 5. Comparison of Nuclear Architecture in Inner Cortical Cells of D. carota
Supplementary Material
Acknowledgments
We thank Robert W. Roberson (Arizona State University, Tempe, AZ) for sharing his fixation protocol and for his advice concerning TEM sample preparation, Ton Timmers (Laboratory of Plant-Microbe Interactions) for advice and discussion about the use of GFP cellular markers, Alain Jauneau (Microscopy Platform, Institut Fédératif de Recherche 40) for his technical assistance concerning the confocal microscopy in Castanet Tolosan, and Joëlle Fournier (Laboratory of Plant-Microbe Interactions) for useful discussions. This research was supported by grants to P.B. from the Italian Ministry of Education (Progetti di Rilevanza Nazionale 2006), from the University of Torino (60%), Consiglio Nazionale delle Ricerche (Biodiversity Project), and the EU INTEGRAL Project. The San Paolo Company (Torino, Italy) is acknowledged for contributing to the acquisition of a Leica confocal microscope at the Laboratory of Advanced Microscopy in Torino.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Paola Bonfante (p.bonfante@ipp.cnr.it).
Online version contains Web-only data.
References
- An, Q., Hückelhoven, R., Kogel, K., and van Bel, A. (2006). Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell. Microbiol. 8 1009–1019. [DOI] [PubMed] [Google Scholar]
- Balestrini, R., Berta, G., and Bonfante, P. (1992). The plant nucleus in mycorrhizal roots: Positional and structural modifications. Biol. Cell 75 235–243. [Google Scholar]
- Balestrini, R., Gómez-Ariza, J., Lanfranco, L., and Bonfante, P. (2007). Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol. Plant Microbe Interact. 20 1055–1062. [DOI] [PubMed] [Google Scholar]
- Bécard, G., and Fortin, J.A. (1988). Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol. 108 211–218. [DOI] [PubMed] [Google Scholar]
- Bednarek, S.Y., and Falbel, T.G. (2002). Membrane trafficking during plant cytokinesis. Traffic 3 621–629. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier, A., Chabaud, M., Garcia, F., Bécard, G., Rosenberg, C., and Barker, D.G. (2001). Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol. Plant Microbe Interact. 14 695–700. [DOI] [PubMed] [Google Scholar]
- Bonfante, P. (1984). Anatomy and morphology. In V. A. Mycorrhizas, C.L. Powell and D.J. Bagyaraj, eds (Boca Raton, FL: CRC Press), pp. 5–33.
- Bonfante, P. (2001). At the interface between mycorrhizal fungi and plants: The structural organization of cell wall, plasma membrane and cytoskeleton. In The Mycota IX: Fungal Associations, B. Hock, ed (Berlin: Springer), pp. 45–61.
- Bonfante, P., and Perotto, S. (1995). Tansley Review No. 82. Strategies of arbuscular mycorrhizal fungi when infecting host plants. New Phytol. 130 3–21. [Google Scholar]
- Chabaud, M., Venard, C., Defaux-Petras, A., Bécard, G., and Barker, D.G. (2002). Targeted inoculation of Medicago truncatula in vitro root cultures reveals MtENOD11 expression during early stages of infection by arbuscular mycorrhizal fungi. New Phytol. 156 265–273. [DOI] [PubMed] [Google Scholar]
- Demchenko, K., Winzer, T., Stougaard, J., Parniske, M., and Pawlowski, K. (2004). Distinct roles of Lotus japonicus SYMRK and SYM15 in root colonization and arbuscule formation. New Phytol. 163 381–392. [DOI] [PubMed] [Google Scholar]
- Dickson, S. (2004). The Arum-Paris continuum of mycorrhizal symbioses. New Phytol. 163 187–200. [DOI] [PubMed] [Google Scholar]
- Drissner, D., Kunze, G., Callewaert, N., Gehrig, P., Tamasloukht, M., Boller, T., Felix, G., Amrhein, N., and Bucher, M. (2007). Lyso-phosphatidylcholine is a signal in the arbuscular mycorrhizal symbiosis. Science 318 265–268. [DOI] [PubMed] [Google Scholar]
- Fester, T., Lohse, S., and Halfmann, K. (2007). “Chromoplast” development in arbuscular mycorrhizal roots. Phytochemistry 68 92–100. [DOI] [PubMed] [Google Scholar]
- Fusconi, A., Lingua, G., Trotta, A., and Berta, G. (2005). Effects of arbuscular mycorrhizal colonization and phosphorus application on nuclear ploidy in Allium porrum plants. Mycorrhiza 15 313–321. [DOI] [PubMed] [Google Scholar]
- Genre, A., and Bonfante, P. (1998). Actin versus tubulin configuration in arbuscule-containing cells. New Phytol. 140 745–752. [DOI] [PubMed] [Google Scholar]
- Genre, A., and Bonfante, P. (2007). Check-in procedures for plant cell entry by biotrophic microbes. Mol. Plant Microbe Interact. 9 1023–1030. [DOI] [PubMed] [Google Scholar]
- Genre, A., Chabaud, M., Timmers, T., Bonfante, P., and Barker, D.G. (2005). Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17 3489–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi-Pearson, V. (1996). Plant cell responses to arbuscular mycorrhizal fungi: Getting to the roots of the symbiosis. Plant Cell 8 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, M.J. (2005). Signaling in the arbuscular mycorrhizal symbiosis. Annu. Rev. Microbiol. 59 19–42. [DOI] [PubMed] [Google Scholar]
- Haseloff, J., Siemering, R.K., Prasher, D.C., and Hodge, S. (1997). Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 94 2122–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch, H.C. (1986). Freeze-substitution of fungi. In Ultrastructure Techniques of Microorganisms, H.C. Aldrich and W.J. Todd, eds (New York: Plenum Press), pp. 183–211.
- Howard, R.J., and O'Donnell, K.L. (1987). Freeze substitution of fungi for cytological analysis. Exp. Mycol. 11 250–269. [Google Scholar]
- Javot, H., Penmetsa, V.R., Terzaghi, N., Cook, D.R., and Harrison, M.J. (2007). A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 104 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novero, M., Faccio, A., Genre, A., Stougaard, J., Webb, K.J., Mulder, L., Parniske, M., and Bonfante, P. (2002). Dual requirement of the LjSym4 gene for mycorrhizal development in epidermal and cortical cells of Lotus japonicus roots. New Phytol. 154 741–749. [DOI] [PubMed] [Google Scholar]
- Otegui, M.S., Mastronarde, D.N., Kang, B., Bednarek, S.Y., and Staehelin, L.A. (2001). Three-dimensional analysis of syncytial-type cell plates during endosperm cellularization visualized by high resolution electron tomography. Plant Cell 13 2033–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske, M. (2004). Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr. Opin. Plant Biol. 7 414–421. [DOI] [PubMed] [Google Scholar]
- Reynolds, E.W. (1963). The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol. 17 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannerini, S., and Bonfante, P. (1977). Unusual plastids in an endomycorrhizal root. Can. J. Bot. 55 2471–2474. [Google Scholar]
- Schliemann, W., Schmidt, J., Nimtz, M., Wray, V., Fester, T., and Strack, D. (2006). Accumulation of apocarotenoids in mycorrhizal roots of Ornithogalum umbellatum. Phytochemistry 67 1196–1205. [DOI] [PubMed] [Google Scholar]
- Séjalon-Delmas, N., Magnier, A., Douds, D.D., Jr., and Bécard, G. (1998). Cytoplasmic autofluorescence of an arbuscular mycorrhizal fungus Gigaspora gigantea and nondestructive fungal observations in planta. Mycologia 90 921–926. [Google Scholar]
- Smith, S.E., Barker, S.J., and Zhu, Y.-G. (2006). Fast moves in arbuscular mycorrhizal symbiotic signalling. Trends Plant Sci. 11 369–371. [DOI] [PubMed] [Google Scholar]
- Tse, Y.C., Mo, B.X., Hillmer, S., Zhao, M., Lo, S.W., Robinson, D.G., and Jiang, L.W. (2004). Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 16 672–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brussel, A.A.N., Bakhuizen, R., van Spronsen, P.C., Spaink, H.P., Tak, T., Lugtenberg, B.J.J., and Kijne, J.W. (1992). Induction of pre-infection thread structures in the leguminous host plant by mitogenic lipooligosaccharides of Rhizobium. Science 257 70–72. [DOI] [PubMed] [Google Scholar]
- Van Damme, D., Vanstraelen, M., and Geleen, D. (2007). Cortical division zone establishment in plant cells. Trends Plant Sci. 12 458–464. [DOI] [PubMed] [Google Scholar]
- Vierheilig, H., Schweiger, P., and Brundrett, M. (2005). An overview of methods for the detection and observation of arbuscular mycorrhizal fungi in roots. Physiol. Plant. 125 393–404. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.