Abstract
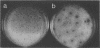
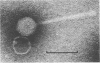
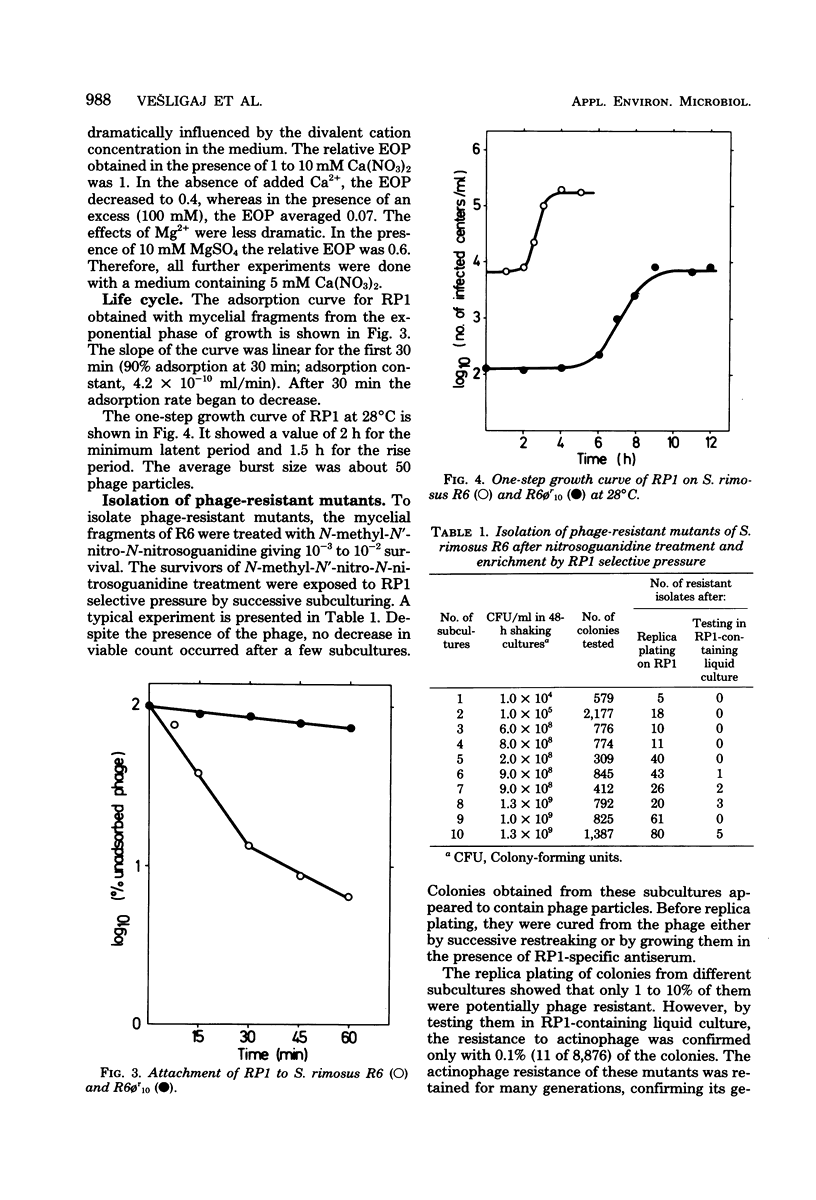
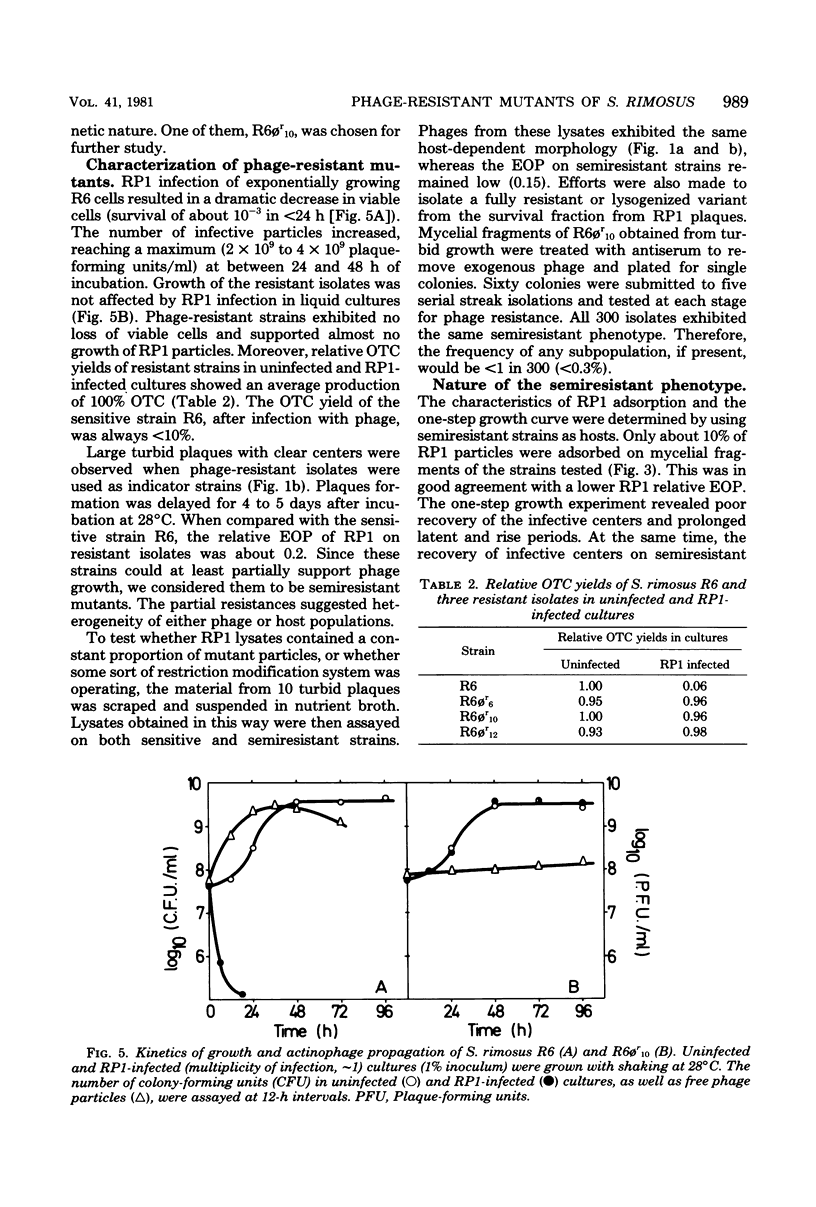
The infection of Streptomyces rimosus by the virulent actinophage RP1 was partially characterized. RP1 infection of the host cells results in a dramatic decrease in viable cell count, followed by reduced antibiotic production. Phage-resistant mutants were isolated after mutagenic treatment and RP1 selective pressure. Characterization of the isolated mutants has revealed that RP1 infection had no influence on their growth and antibiotic production. However, multiplication of the phage particles in the lawns of resistant mutants was detected. Since these strains differ from the wild type in RP1 relative efficiency of plating, plaque morphology, and the time necessary for plaque appearance, they are considered to be semiresistant mutants. The propagation of RP1 on semiresistant strains is characterized by lower adsorption of phage particles and longer latent and rise periods. As a consequence, the multiplication of the phage is slower than that of their host, which consequently reduces the ratio of phage to its host, thus diluting out the phage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carta G. R., Bryson V. Mutants of Escherichia coli variably resistant to bacteriophage T1. J Bacteriol. 1966 Oct;92(4):1055–1061. doi: 10.1128/jb.92.4.1055-1061.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delić V., Hopwood D. A., Friend E. J. Mutangenesis by N-methyl-N'-nitro-N-nitrosoguanidine (NTG) in Streptomyces coelicolor. Mutat Res. 1970 Feb;9(2):167–182. doi: 10.1016/0027-5107(70)90055-2. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Reeves P. Bacteriophage resistance in Escherichia coli K-12: general pattern of resistance. J Bacteriol. 1975 Mar;121(3):983–993. doi: 10.1128/jb.121.3.983-993.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hranueli D., Pigac J., Vesligaj M. Characterization and persistence of actinophage RP2 isolated from Streptomyces rimosus ATCC 10970. J Gen Microbiol. 1979 Oct;114(2):295–303. doi: 10.1099/00221287-114-2-295. [DOI] [PubMed] [Google Scholar]
- KOERBER W. L., GREENSPAN G., LANGLYKKE A. F. Observations on the multiplication of phages affecting Streptomyces griseus. J Bacteriol. 1950 Jul;60(1):29–37. doi: 10.1128/jb.60.1.29-37.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONASTERO F., MEANS J. A., GRENFELL T. C., HEDGER F. H. Terramycin; chemical methods of assay and identification. J Am Pharm Assoc Am Pharm Assoc. 1951 May;40(5):241–245. doi: 10.1002/jps.3030400507. [DOI] [PubMed] [Google Scholar]
- WAHL R. La semi-résistance aux bactériophages. Ann Inst Pasteur (Paris) 1953 Jan;84(1):51–59. [PubMed] [Google Scholar]
- WELSCH M. The behaviour towards actinophage of mutants surviving its lytic action. Antonie Van Leeuwenhoek. 1957;23(1):59–80. doi: 10.1007/BF02545859. [DOI] [PubMed] [Google Scholar]