Abstract
Two distinct biosynthetic pathways for Phe in plants have been proposed: conversion of prephenate to Phe via phenylpyruvate or arogenate. The reactions catalyzed by prephenate dehydratase (PDT) and arogenate dehydratase (ADT) contribute to these respective pathways. The Mtr1 mutant of rice (Oryza sativa) manifests accumulation of Phe, Trp, and several phenylpropanoids, suggesting a link between the synthesis of Phe and Trp. Here, we show that the Mtr1 mutant gene (mtr1-D) encodes a form of rice PDT with a point mutation in the putative allosteric regulatory region of the protein. Transformed callus lines expressing mtr1-D exhibited all the characteristics of Mtr1 callus tissue. Biochemical analysis revealed that rice PDT possesses both PDT and ADT activities, with a preference for arogenate as substrate, suggesting that it functions primarily as an ADT. The wild-type enzyme is feedback regulated by Phe, whereas the mutant enzyme showed a reduced feedback sensitivity, resulting in Phe accumulation. In addition, these observations indicate that rice PDT is critical for regulating the size of the Phe pool in plant cells. Feeding external Phe to wild-type callus tissue and seedlings resulted in Trp accumulation, demonstrating a connection between Phe accumulation and Trp pool size.
INTRODUCTION
Aromatic amino acids, such as Phe, Trp, and Tyr, are not only important components of proteins but also sources of numerous aromatic secondary metabolites, including indole alkaloids (Kutchan, 1995), phenylpropanoids (Dixon and Paiva, 1995), and lignin (Whetten and Sederoff, 1995). Thus, they play an important role in plant development and in defense systems of land plants (Frey et al., 1997; Zhao et al., 2001). Aromatic amino acids are also essential as nutrients for human health and as raw materials for pharmaceutical development and industry (Bongaerts et al., 2001). Knowledge of the biosynthetic pathways for these amino acids may provide a basis for manipulation of their levels or those of aromatic secondary metabolites in land plants.
These three aromatic amino acids are biosynthesized via the shikimate pathway. The biosynthetic pathway for Trp branches off from this pathway at the level of the precursor chorismate. The regulation of Trp biosynthesis is relatively well characterized as a result of the identification of several mutants that accumulate intermediates in the biosynthetic pathway (Last and Fink, 1988; Niyogi et al., 1993) or exhibit resistance to toxic analogs of Trp, such as 5-methyltryptophan (5MT) or α-methyltryptophan (Widholm, 1972; Ranch et al., 1983; Kreps and Town, 1992). The genes for all of the enzymes that catalyze the steps in the conversion of chorismate to Trp, including anthranilate synthase (AS), phosphoribosylanthranilate transferase, phosphoribosylanthranilate isomerase, indole-3-glycerol phosphate synthase, and Trp synthase, have been identified in the Trp biosynthetic pathway (Last et al., 1991; Niyogi and Fink, 1992; Rose et al., 1992; Niyogi et al., 1993; Li et al., 1995a, 1995b; Radwanski et al., 1995). Mutations of the gene for an α-subunit of AS, which catalyzes the conversion of chorismate to anthranilate and is feedback regulated by Trp, have been shown to generate a feedback-insensitive form of the enzyme that results in the accumulation of Trp in Arabidopsis thaliana (Li and Last, 1996). The gene for a feedback-insensitive ASα subunit has also been used to generate transgenic rice (Oryza sativa), potato (Solanum tuberosum), and Arabidopsis that accumulate Trp (Tozawa et al., 2001; Matsuda et al., 2005; Ishihara et al., 2006; Wakasa et al., 2006).
Chorismate mutase (CM), which catalyzes the conversion of chorismate to prephenate (Figure 1), is well characterized as the enzyme responsible for catalyzing the committed step in Phe and Tyr biosynthesis. At least two isoforms of CM are present in plants, one of which is activated by Trp and subject to feedback inhibition by Phe and Tyr (d'Amato et al., 1984; Eberhard et al., 1996; Mobley et al., 1999). Given that no mutants with altered CM activity have been identified on the basis of accumulation of Phe or Tyr, the biosynthetic pathways of Phe and Tyr may be regulated at the level of other enzymes in land plants. Indeed, arogenate dehydrogenase (ADH), which catalyzes the last step of Tyr biosynthesis, has been shown to be feedback regulated by Tyr in Arabidopsis (Rippert and Matringe, 2002). Phe is considered to be biosynthesized through a dual route in land plants, and two pathways from prephenate to Phe have been proposed, one via arogenate and the other via phenylpyruvate (Figure 1).
Figure 1.
Schematic Diagram of Presumed Dual Pathways for Phe Synthesis from Chorismate.
The enzymes that catalyze each conversion are shown in italics.
The presence of arogenate dehydratase (ADT) activity in sorghum (Sorghum bicolor) seedlings (Siehl and Conn, 1988), spinach (Spinacia oleracea) chloroplasts, and cultured cells of Nicotiana sylvestris (Jung et al., 1986) supports the notion that arogenate is the final substrate for Phe biosynthesis. On the other hand, the detection of prephenate dehydratase (PDT) activity in etiolated Arabidopsis seedlings exposed to blue light (Warpeha et al., 2006) is consistent with Phe biosynthesis via phenylpyruvate. Six ADT genes with homology to bacterial PDT were recently identified in Arabidopsis (Cho et al., 2007). All six gene products showed ADT activity, whereas three of them also catalyzed the PDT reaction. The preference of these enzymes for arogenate suggested that they functioned as ADTs in Phe biosynthesis. However, it has remained unknown which enzyme catalyzes this final step of Phe biosynthesis.
The activity of this critical enzyme in plants is likely feedback regulated by Phe because the enzymes that mediate Phe biosynthesis in microorganisms, such as PDT and P-protein, are subject to such feedback regulation (Nester and Jensen, 1966; Liberles et al., 2005). Indeed, the inhibition of plant ADT activity by Phe also has been described (Jung et al., 1986; Siehl and Conn, 1988). Plant mutants with feedback-insensitive forms of the committing enzyme may thus be expected to accumulate Phe, analogous to the mutant or transgenic plants expressing a feedback-insensitive ASα subunit that accumulate Trp. However, to our knowledge, no plant mutants with such a phenotype have been identified to date. However, a few mutants that accumulate both Phe and Trp have been detected. Mutant cell lines (V6 and V7) of maize (Zea mays), for example, showed increased amounts of Phe and Trp and smaller amounts of Tyr, His, and Val compared with the wild type (Miao et al., 1988).
A rice mutant, 5MT resistant 1 (Mtr1), also accumulates Phe and Trp at high levels in both callus tissue and leaves (previously referred to as MTR1 in Wakasa and Widholm, 1987). To date, Mtr1 is the only mutant known to transmit the ability to accumulate more than one aromatic amino acid. Given that Mtr1 was originally established from callus tissue resistant to the Trp analog 5MT and that the resistance was shown to be controlled by a dominant nuclear gene(s) (Wakasa and Widholm, 1991), the responsible mutation was initially suspected to be in AS of the Trp biosynthetic pathway. However, the observation that transgenic rice expressing a feedback-insensitive ASα subunit accumulated a large amount of Trp but not Phe (Tozawa et al., 2001; Wakasa et al., 2006) suggested that a mutation in AS is not responsible for the Mtr1 phenotype. It was also possible that the causative mutation was located within the synthetic pathways for shikimate or other aromatic amino acids.
We have now undertaken map-based cloning of the gene responsible for the mutant phenotype of Mtr1 (mtr1-D), which is closely linked to 5MT resistance. We have also performed metabolic profiling to clarify the mechanism responsible for the increased production of Phe and Trp in the Mtr1 mutant. Here, we describe both the identification of the gene responsible for the Mtr1 phenotype and the characterization of the encoded enzyme. Our results provide important insight into Phe biosynthesis in land plants.
RESULTS
Characterization of the Mtr1 Mutant
Consistent with previous observations (Wakasa and Widholm, 1987), the rice mutant Mtr1 was shown to be resistant to the Trp analog 5MT as demonstrated by the ability to grow on medium containing 300 μM 5MT (see Supplemental Figure 1 online). Analysis of aromatic amino acids by HPLC coupled with electrospray ionization–mass spectroscopy (ESI-MS) revealed increased levels of free Phe and Trp in progeny callus tissue of Mtr1 (Figure 2A); the concentrations of these amino acids in Mtr1 were 55 and 27 times those in Norin 8, respectively. The increased levels of Trp and 5MT resistance in Mtr1 suggested that the responsible mutation might occur in a key enzyme of the Trp biosynthetic pathway, given that a feedback-insensitive form of AS has been shown to be responsible for Trp accumulation and 5MT resistance in several plant mutants (Kreps et al., 1996; Li and Last, 1996). However, feedback inhibition of AS activity by Trp did not differ between Mtr1 and Norin 8 (Figure 2B). The range of sensitivity to Trp was 20 to 100 μM for AS from both Mtr1 and Norin 8, with 100 μM Trp resulting in inhibition of AS activity by ∼80% in both lines. The AS of Mtr1 was thus as sensitive to Trp as was the AS of control rice.
Figure 2.
Characteristics of Mtr1.
(A) Levels of Phe and Trp in calli of Norin 8 and Mtr1. Data are expressed as nanomoles of amino acid per gram of fresh weight (FW) and are means ± sd from three independent experiments.
(B) Activity of AS in callus extracts of Norin 8 and Mtr1. Activity was measured in the presence of the indicated concentrations of Trp. Data are expressed as picomoles of anthranilate formed per minute per milligram of protein and are means ± sd from three independent experiments.
We next compared the metabolic phenotypes of calli of Norin 8 and Mtr1 using liquid chromatography and photodiode array detection (LC-PDA) at wavelengths of 254 and 280 nm for monitoring indole and phenylpropanoid metabolites, respectively. Our analysis revealed that the amounts of many compounds, including Trp and Phe, were increased in Mtr1 (Figure 3). The structures of eight metabolites that showed marked increases in abundance in Mtr1 were identified by LC-MS/MS and nuclear magnetic resonance (NMR) as γ-glutamylphenylalanine (1), benzyl glucoside (2), O-feruloylquinate (3), 2′-O-β-glucosylrosine (4), rosine (5), indole alkaloid glycoside (6), 2′-O-β-glucosyl-6′-O-malonylrosine (7), and 6′-O-malonylrosine (8) (Figure 4). With the exception of 6, these compounds are all phenylpropanoids derived from Phe; 6 is identical to an indole derivative detected in transgenic rice that accumulates Trp (Morino et al., 2005). Mtr1 was thus shown to accumulate many phenylpropanoid compounds at high levels relative to Norin 8, likely as a result of an altered regulation of Phe biosynthesis.
Figure 3.
Metabolic Profiling of Calli of Norin 8 and Mtr1.
The top two and bottom two chromatograms were obtained with detection at 280 and 254 nm, respectively. The peaks for Trp and Phe are indicated. Chemical structures were determined for the numbered peaks.
Figure 4.
Chemical Structures of the Numbered Peaks in Figure 3.
MW, molecular weight.
Isolation of a Candidate Gene for mtr1-D
To localize the candidate gene for mtr1-D on the genetic map, we generated an F2 population from Mtr1 crossed with the indica rice variety Kasalath. Initially, the mtr1-D locus was roughly mapped to rice chromosome 7. Detailed analysis of 578 F2 plants and 375 F3 plants with simple sequence repeat (SSR) and cleaved amplified polymorphic sequence markers resulted in localization of mtr1-D to the end of the long arm of chromosome 7 (Figure 5A), specifically to an ∼150-kb region between the SSR marker RM172 in the P1 phage artificial chromosome (PAC) clone P0627E10 and the chromosome end in the fosmid clone OSJNOa136M23 (which contains the telomeric region). The rice database indicated the presence of 33 putative open reading frames (ORFs) in this region (http://ricegaas.dna.affrc.go.jp). We focused on one (AK066428) of these ORFs that had been annotated as a putative gene for PDT, a key enzyme in the Phe biosynthetic pathway in bacteria, as an mtr1-D candidate gene, given that our metabolic profiling suggested that Phe biosynthesis was altered in the Mtr1 mutant.
Figure 5.
Genetic Map of mtr1-D and Alignment of Nucleotide and Predicted Amino Acid Sequences Surrounding the Mutation Site in a Candidate mtr1-D Gene of Rice.
(A) RM420 and RM172 (SSR markers) as well as C213 (cleaved amplified polymorphic sequence marker) delineate the position of mtr1-D on the linkage and physical maps. P0034A01 and P0627E10 are PAC clones, and OSJNOa136M23 is a fosmid clone. Gray and black boxes indicate putative ORFs. cM, centimorgan.
(B) Alignment of the nucleotide sequence surrounding a mutation site in the ORF of a putative PDT gene from Mtr1. The sequence derived from Mtr1 is compared with those of Norin 8 and Nipponbare. Asterisks indicate identical residues. Nucleotide numbers correspond to the cDNA sequence relative to the first ATG codon.
(C) Domain organization of the 364–amino acid protein encoded by the putative PDT gene of rice. The protein contains a plastid-targeting signal peptide (TP, residues 1 to 42), a PDT domain (residues 78 to 256), and an ACT domain (residues 266 to 354). S298I indicates the Ser-to-Ile substitution at position 298 in the MTR1 protein.
(D) Alignment of sequences of the ACT domain in P-proteins or PDTs from various species of plants and microorganisms. The amino acid sequence of Norin 8 was identical to that of Nipponbare (AK066428). Shaded regions indicate two highly conserved motifs, GALV and ESRP.
Comparison of cDNA sequences corresponding to the putative PDT gene of Mtr1, Norin 8, and Nipponbare revealed a single base difference at nucleotide position 893 (relative to the first ATG codon) between Mtr1 and the other two rice varieties (Figure 5B). This base change results in the replacement of Ser with Ile at amino acid position 298 (Figure 5C), which is located in a region corresponding to the feedback-regulated site in the ACT domain of bacterial PDT (Figures 5C and 5D). The ACT domain is present in a wide range of metabolic enzymes that are subject to allosteric regulation, and the name originates from aspartokinase, chorismate mutase, and TyrA (prephenate dehydrogenase) (Liberles et al., 2005). We designated the ORFs of the mtr1-D candidate gene (AB300404) and the corresponding wild-type gene (AK066428) as PDTS298I and PDTWT, respectively. Sequencing of the genomic regions of PDTS298I revealed the absence of another mutation in ∼1.2 kb of the 5′ flanking region (relative to the first ATG codon) and ∼2.0 kb of the 3′ flanking region (relative to the final TGA codon) in Mtr1 (data not shown). Rice genomic sequences containing PDT gene corresponded to those in PAC clone P0627E10.
Reproduction of Mtr1 Characteristics in Transformed Callus Lines
To determine experimentally the effects of the single mutation detected in the putative PDT gene of Mtr1, we subjected Nipponbare callus tissue to Agrobacterium tumefaciens–mediated transformation with constructs comprising the maize ubiquitin gene promoter (Ubipro) linked to either PDTS298I or PDTWT cDNA. Representatives of the resulting M (harboring Ubipro:PDTS298I) and wild-type (harboring Ubipro:PDTWT) lines shown to harbor and express an intact transgene (see Supplemental Figure 2 online) were analyzed for 5MT resistance, Phe and Trp content, and metabolic profiles. All calli of the M lines tested grew on 2N6 medium containing 150 μM 5MT, whereas calli of the wild-type lines did not (Figure 6A). The Phe and Trp contents of calli of the M lines were up to 87.3 and 18.6 times those of wild-type callus lines, respectively (Figures 6B and 6C). Analysis by LC-PDA at wavelengths of 254 and 280 nm for monitoring of indole and phenylpropanoid metabolites revealed that M callus lines also showed metabolic profiles similar to that of Mtr1 (Figure 7A). The content of 8 (6′-O-malonylrosine) in M lines was up to 10.3 times that in wild-type lines (Figure 7B). Together, these results thus showed that expression of PDTS298I both conferred 5MT resistance and resulted in the accumulation of Phe, Trp, and various phenylpropanoids, confirming that a single mutated enzyme causes the multiple characteristics of Mtr1.
Figure 6.
Expression of Mtr1 Characteristics in Transformed Callus Lines.
(A) Growth of transformed callus lines on 2N6 medium containing 150 μM 5MT. Wild-type and mutant (M) lines are transformed callus lines of Nipponbare harboring wild-type (PDTWT) or mutant (PDTS298I) versions of the putative rice PDT gene, respectively. Each of the numbered quadrants contains three pieces of callus tissue from an independent line (lines numbered 1 to 4).
(B) Levels of Phe in transformed callus lines.
(C) Levels of Trp in transformed callus lines. Data in (B) and (C) are means ± sd from three independent experiments.
Figure 7.
Metabolic Characteristics of Transformed Callus Lines.
(A) Metabolic profiling of calli of Norin 8 and Mtr1 as well as of transformed callus lines expressing PDTWT (WT24) or PDTS298I (M47). The top four and bottom four chromatograms were obtained with detection at 280 and 254 nm, respectively. The peaks for Trp and Phe are indicated. The numbered peaks correspond to the compounds shown in Figure 4.
(B) Content of 6′-malonylrosine (peak 8 in [A]) in transformed callus lines. Data are means ± sd from three independent experiments.
Characterization of PDTWT and PDTS298I
The deduced amino acid sequence of rice PDT is predicted to contain a 42-residue chloroplast-targeting signal at its NH2 terminus (http://www.cbs.dtu.dk/services/targetP). To confirm the localization of rice PDTWT, we synthesized the recombinant protein with the use of a wheat embryo cell-free translation system and performed an in vitro import assay with intact pea (Pisum sativum) chloroplasts (Kasai et al., 2005). SDS-PAGE and autoradiography revealed that the full-length PDTWT protein (40 kD) was processed to a product of 35 kD during the incubation with pea chloroplasts and that the 35-kD product was protected from degradation by thermolysin (Figure 8). These results thus indicated that PDTWT was imported into chloroplasts and processed to the mature form of the protein, indicative of chloroplast localization of the rice protein.
Figure 8.
Chloroplast Import Assay of Rice PDT.
The full-length (precursor) form of rice PDT was synthesized and labeled with 35S using a cell-free translation system. It was then subjected to a chloroplast import assay as described previously (Kasai et al., 2005). The reaction mixture was subsequently incubated in the absence (–) or presence (+) of thermolysin before analysis by SDS-PAGE and autoradiography.
If the putative rice PDT has a function similar to that of bacterial PDT, then Phe would be expected to be produced via phenylpyruvate in plant cells. However, ADT activity, which mediates the conversion of arogenate to Phe, has been detected in many land plants (Jung et al., 1986; Bonner and Jensen, 1987; Siehl and Conn, 1988). Dual pathways for Phe biosynthesis, such as conversion of prephenate to phenylpyruvate catalyzed by PDT and that of arogenate to Phe catalyzed by ADT, might therefore operate in land plants (Figure 1). We next performed functional characterization of rice PDT to clarify the main route of Phe biosynthesis in this plant species. We synthesized the mature forms of the wild-type (PDTWT) and MTR1 (PDTS298I) proteins (each lacking the NH2-terminal 42 residues) with the use of the cell-free system and then examined their enzymatic activities with two candidate substrates, prephenate and arogenate.
Both PDTWT and PDTS298I possessed PDT activity with prephenate and ADT activity with arogenate. However, the catalytic properties of the two proteins depended on the substrate: the Michaelis constant (Km) values were 0.12 and 0.17 mM and the maximal velocity (Vmax) values were 5.32 and 6.05 μmol min−1 mg−1 for PDTWT and PDTS298I, respectively, with arogenate as the substrate, whereas the Km values were 2.29 and 4.88 mM and the Vmax values were 9.10 and 15.1 μmol min−1 mg−1, respectively, with prephenate as the substrate (Table 1; see Supplemental Figure 3 online). The catalytic efficiencies (kcat/Km) of these proteins with arogenate as substrate were ∼10 times those with prephenate as substrate (Table 1). These results revealed that rice PDT is a bifunctional enzyme similar to cyclohexadienyl dehydratase (CDT) of several microorganisms, which was classified as an ADT.
Table 1.
Kinetic Properties of Rice PDT
| Enzyme-Substratea | Km (mM) | Vmax (μmol min−1 mg−1) | kcat/Km (M−1 s−1) | Ki or IC50 (μM)b |
|---|---|---|---|---|
| WT-arogenate | 0.12 ± 0.03 | 5.32 ± 0.55 | (2.67 ± 0.49) × 104 | 7.36 |
| S298I-arogenate | 0.17 ± 0.04 | 6.05 ± 0.73 | (2.20 ± 0.28) × 104 | 61.5 |
| WT-prephenate | 2.29 ± 0.51 | 9.10 ± 1.49 | (2.44 ± 0.16) × 103 | 16 |
| S298I-prephenate | 4.88 ± 2.82 | 15.1 ± 6.67 | (1.97 ± 0.27) × 103 | >500 |
Data are means ± sd of triplicates from a representative experiment.
WT (wild type) and S298I indicate PDTWT and PDTS298I, respectively.
Ki or IC50 of Phe for ADT and PDT activities, respectively.
Given that the point mutation in the gene encoding PDTS298I is located in a region corresponding to the feedback-regulated site in the ACT domain of bacterial PDT (Figures 5C and 5D), we next examined whether rice PDT is subject to feedback regulation by performing the ADT and PDT assays for PDTWT and PDTS298I in the absence or presence of Phe. Determination of the inhibition constant (Ki) for the ADT assay and the median inhibitory concentration (IC50) for the PDT assay revealed that PDTWT was more sensitive to inhibition by Phe than was PDTS298I with respect to both ADT and PDT activities (Table 1). Whereas the activities of PDTWT were abolished by low concentrations of Phe (Ki and IC50 values of 7.36 and 16 μM, respectively), the Ki and IC50 values for PDTS298I were 61.5 and >500 μM, respectively (Table 1; see Supplemental Figure 4 online). These results indicated that PDTS298I possesses Phe-insensitive ADT activity and that its Phe insensitivity is attributable to the S298I mutation. Our biochemical data were therefore consistent with the high level of Phe accumulation observed in the Mtr1 mutant and in transformed callus lines expressing PDTS298I.
Effects of Exogenous Phe on Trp Synthesis and 5MT Resistance
Given that the mutation in Mtr1 was shown to reside in an enzyme of the Phe biosynthetic pathway, the associated increase in Phe content is likely a direct consequence of this mutation. However, whereas the observed changes in phenylpropanoid synthesis in Mtr1 are readily reconcilable with the increased Phe content, it was difficult to explain how the accumulation of Phe resulted in both Trp accumulation and 5MT resistance. To examine the relation of Phe accumulation to Trp content and 5MT resistance, we determined the effects of feeding callus tissue and seedlings with external Phe.
Although the callus of Mtr1 showed a high level of resistance to 100 μM 5MT in the absence or presence of external Phe (Figure 9A), the presence of 100 μM 5MT resulted in marked inhibition of the growth of Norin 8 callus in the absence of Phe (Figure 9B). Feeding with exogenous 300 μM Phe resulted in recovery of the growth of Norin 8 callus tissue in the presence of 100 μM 5MT (Figure 9B). The Trp content of Nipponbare callus was decreased in the presence of 25 μM 5MT (Figure 9C) that did not cause serious damage for callus growth. Feeding with 600 μM Phe in the presence of 25 μM 5MT increased the Trp content of Nipponbare callus by a factor of 3.8 compared with that observed in the presence of 25 μM 5MT without Phe feeding (Figure 9C). The uptake of 5MT into callus tissue was not affected by external Phe (Figure 9D). These results thus suggested that overproduction of Phe is responsible for both the accumulation of Trp and the resistance to 5MT in Mtr1.
Figure 9.
Effects of Feeding with External Phe in Calli of Norin 8, Mtr1, or Nipponbare.
(A) Growth of callus tissue of Mtr1 on 2N6 medium in the absence or presence of 100 μM 5MT or 300 μM Phe, as indicated.
(B) Growth of callus tissue of Norin 8 on 2N6 medium in the absence or presence of 100 μM 5MT or 300 μM Phe, as indicated. Data in (A) and (B) are means ± sd from 24 pieces of callus.
(C) Quantification of free Trp in the callus of Nipponbare grown on 2N6 medium in the absence or presence of 25 μM 5MT or 300 or 600 μM Phe, as indicated. Data are means ± sd from three independent experiments.
(D) Effect of Phe on 5MT uptake into Nipponbare callus tissue. Calli were grown on 2N6 medium in the absence or presence of 300 or 600 μM Phe or 25 μM 5MT, as indicated. Data are means ± sd from three independent experiments.
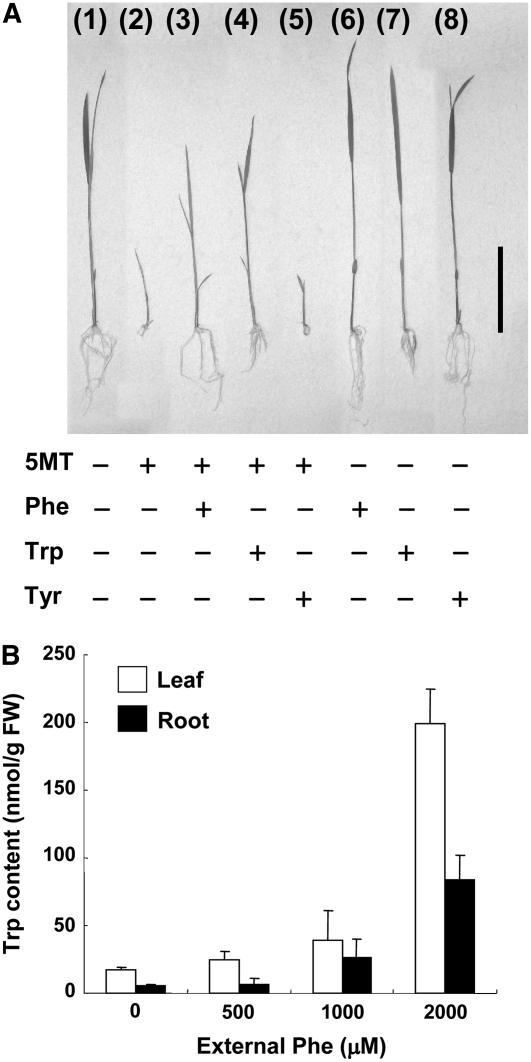
Feeding with Phe or Trp at 300 μM also conferred similar levels of 5MT resistance in a germination test of Nipponbare; however, the presence of external Tyr at the same concentration did not confer 5MT resistance (Figure 10A). Such feeding with Phe also resulted in the accumulation of Trp in the rice seedlings, with a Trp content as high as 198.9 nmol per gram of fresh weight in leaves and 83.9 nmol/g in roots being detected in the presence of 2000 μM Phe; these values represent 11.4- and 16.1-fold increases compared with the corresponding values for seedlings grown without Phe feeding (Figure 10B). Excess uptake of Phe by roots thus resulted in the accumulation of Trp not only in roots but also in leaves.
Figure 10.
Effects of Feeding with External Phe on Seedlings of Nipponbare.
(A) Effects of aromatic amino acids on the sensitivity of Nipponbare seedlings to 5MT. Seedlings (1) to (8) were cultured on MS medium in the absence or presence of 300 μM 5MT, 300 μM Phe, 300 μM Trp, or 300 μM Tyr, as indicated. Bar = 50 mm.
(B) Quantification of free Trp in the leaves and roots of Nipponbare seedlings grown on MS medium containing 0, 500, 1000, or 2000 μM Phe. Data are means ± sd from three independent experiments.
DISCUSSION
The Mtr1 mutant was initially thought to express a variant form of AS, similar to other mutants that are resistant to 5MT and accumulate Trp (Widholm, 1972; Carlson and Widholm, 1978; Li and Last, 1996). However, our observations that AS of Mtr1 has a normal sensitivity to Trp and that Phe and phenylpropanoids accumulate in Mtr1 callus tissue suggested that Mtr1 has an altered biosynthetic pathway for Phe rather than for Trp. We identified a putative PDT gene as a candidate for mtr1-D by positional cloning. Overexpression of this gene in rice calli conferred the manifold characteristics of the Mtr1 phenotype, including the accumulation of Phe and Trp, the production of phenylpropanoids, and 5MT resistance, providing further evidence that this putative PDT gene was indeed the mtr1-D gene.
Rice PDT possesses catalytic activities of both ADT and PDT; that is, the dehydration coupled with decarboxylation of arogenate or prephenate. Each reaction is a step in one of the two proposed biosynthetic pathways for Phe (Figure 1). Both catalytic activities of wild-type rice PDT were inhibited by Phe, whereas the extent of inhibition of the MTR1 (PDTS298I) enzyme by Phe was markedly reduced. These findings indicate that rice PDT participates directly in Phe biosynthesis and that its activity is controlled by feedback regulation. The relaxed feedback sensitivity of the mutant enzyme also explains the accumulation of Phe in Mtr1. Feedback regulation has been shown to be an important determinant of pool size for aromatic amino acids. Relaxed feedback regulation of AS thus results in the accumulation of Trp (Widholm, 1972; Carlson and Widholm, 1978; Li and Last, 1996), and ADH, which catalyzes the formation of Tyr, has also been shown to be sensitive to feedback inhibition by Tyr (Rippert and Matringe, 2002). The accumulation of Phe at a high concentration in Mtr1 indicates the importance of feedback inhibition of PDT by Phe in the regulation of Phe pool size.
Phe is synthesized via phenylpyruvate by PDT or P-protein in many microorganisms, with P-protein possessing both CM and PDT activities (Nester and Jensen, 1966; Im and Pittard, 1971; Hall et al., 1982). However, several microorganisms, such as Pseudomonas aeruginosa and Xanthomonas campestris, possess dual routes for Phe biosynthesis from the intermediates prephenate and arogenate. The committed enzyme for these biosynthetic pathways is CDT, which catalyzes the conversions of prephenate to phenylpyruvate and of arogenate to Phe (Patel et al., 1977; Hall et al., 1982; Ahmad et al., 1990; Zhao et al., 1992). By contrast, the primary biosynthetic pathway of Phe in land plants has remained unclear, given that both activities of ADT and PDT have been detected. ADT activity has thus been identified in several plant species (Jung et al., 1986; Siehl and Conn, 1988), whereas PDT activity was detected in etiolated Arabidopsis seedlings (Warpeha et al., 2006), with the corresponding protein shown to be located in the cytosol.
Our in vitro enzymatic assays have now shown that rice PDT is a bifunctional enzyme exhibiting both PDT and ADT activities. The preference of both PDTWT and PDTS298I proteins for arogenate over prephenate as a substrate suggests, however, that rice PDT should be classified primarily as an ADT. Although this finding suggests the possible existence of dual pathways to Phe in plant cells, consistent with the previous detection of both ADT and PDT activities, the ∼10-fold higher catalytic efficiency of rice PDT observed with arogenate suggests the almost exclusive operation of the arogenate route. Arogenate is known to exist in plant cells because it is the substrate of ADH, which catalyzes its dehydrogenation in the biosynthesis of Tyr (Rippert and Matringe, 2002). Consistent with the preference of rice PDT for arogenate, six PDT homologs in Arabidopsis were recently found to catalyze the ADT reaction (Cho et al., 2007). Three of these homologs showed no activity with prephenate, whereas the other three catalyzed the PDT reaction, although even these latter enzymes preferred arogenate over prephenate as substrate. These observations suggested that these enzymes function as ADTs in Phe biosynthesis.
On the basis of amino acid sequence similarity, the six ADTs of Arabidopsis were classified into three subgroups (Cho et al., 2007). To examine the relations among four rice PDTs, six Arabidopsis ADTs, four P-proteins, and six PDTs of microorganisms, we generated a phylogenetic tree (see Supplemental Figure 5 and Supplemental Data Set 1 online). Rice (AK066428) is most closely related to At3g07630 (ADT2) of subgroup II in Arabidopsis (see Supplemental Figure 5 online). Although rice PDT accepts arogenate and prephenate as substrates similar to CDT of bacteria, it was found to be closely related to P-protein and PDT from the viewpoint of phylogeny based on amino acid sequence. The substrate specificities of P-protein and PDT of enteric bacteria and cyanobacteria are narrower than that of CDT, with the enzymes accepting only prephenate as substrate (Dopheide et al., 1972; Hall et al., 1982). Whether dehydratase activity for cyclodiene compounds prefers arogenate or prephenate might depend on structural differences in regions of the enzymes that are not reflected in the sequence homology.
Rice PDT contains the ACT domain (Figure 5C), which mediates allosteric regulation by amino acids in P-protein and PDT (Liberles et al., 2005). Several motifs within the ACT domain of enzymes involved in Phe biosynthesis have been implicated in allosteric regulation by Phe. A hydrophobic sequence (GALV) and a hydrophilic sequence (ESRP) are thus thought to contribute to feedback regulation by Phe in bacterial P-protein and PDT (Pohnert et al., 1999; Jimenez et al., 2000). Functional analysis of P-protein by site-directed mutagenesis revealed that the GALV motif is more sensitive to feedback inhibition by Phe than is the ESRP motif (Pohnert et al., 1999). The GALV motif is changed to GQLF, whereas the ESRP motif is conserved in rice PDT (Figure 5D). The latter motif is mutated to EIRP in the MTR1 protein, and this single mutation is sufficient to relax feedback regulation by Phe. The ESRP motif is conserved in all the listed P-proteins and PDTs (Figure 5D) and in the six Arabidopsis ADTs, suggesting the importance of this sequence in the feedback regulation of enzyme activity in plants and microorganisms, although the activity of the Arabidopsis enzymes has not been assayed in the presence of Phe. In silico prediction based on the ESRP motif also revealed the existence of at least four homologs (AK066427, AK066428, AK066644, and AK103606) of rice PDT in rice (http://ricegaas.dna.affrc.go.jp/). The importance of the ESRP motif in the binding of Phe was also indicated in mammalian Phe hydroxylase, which catalyzes the conversion of Phe to Tyr and is activated by Phe (Kobe et al., 1999; Gjetting et al., 2001; Liberles et al., 2005).
The expected function of rice PDT appeared inconsistent with some of the characteristics of the Mtr1 mutant. Mtr1 shows accumulation of Trp and a high level of resistance to 5MT, but it was not clear how the relaxed feedback regulation of Phe biosynthesis might affect Trp metabolism in Mtr1. To clarify the relation between Phe accumulation on the one hand and 5MT resistance accompanied by Trp accumulation on the other, we fed exogenous Phe to wild-type rice calli and seedlings. Such feeding of Phe induced Trp accumulation and 5MT resistance in both calli and seedlings, consistent with the observed characteristics of Mtr1. The overproduction of Phe in Mtr1 is thus likely responsible for the increase in Trp content, which in turn gives rise to 5MT resistance.
The large increase in Trp content in transgenic Arabidopsis and potato overexpressing a feedback-insensitive form of AS has been shown to be accompanied by smaller increases in the levels of Phe and Tyr (Matsuda et al., 2005; Ishihara et al., 2006). In this instance, it is likely that CM, which is a committing enzyme for Phe and Tyr biosynthesis (Eberhard et al., 1996), is activated by the increased level of Trp. By analogy, Phe accumulation in Mtr1 may activate an enzyme (or enzymes) in the Trp biosynthetic pathway. A candidate for such an enzyme is Trp synthase, given that overexpression of the gene for the β1 subunit of this enzyme was shown to result in the accumulation of Trp and insensitivity to 5MT in Arabidopsis (Hsiao et al., 2007); however, no marked upregulation of the expression of the genes for the α- or β-subunits of Trp synthase was detected in Mtr1 in comparison with Norin 8 (see Supplemental Figure 6 online). Alternatively, given the importance of feedback regulation of AS in the control of Trp pool size, the presence of excess Phe may induce relaxation of feedback regulation of AS. Although its precise nature remains unknown, our results suggest the existence of a connection between Trp and Phe biosynthesis in land plants.
Phe is a precursor for a variety of important secondary metabolites, including phenylpropanoids (Dixon and Paiva, 1995), lignin (Whetten and Sederoff, 1995), anthocyanins, and other flavonoids (Holton and Cornish, 1995), in land plants. Metabolic profiling revealed that Mtr1 accumulates various phenylpropanoid compounds in addition to Phe. Three such metabolites, 2′-O-β-glucosylrosine (4), 2′-O-β-glucosyl-6′-O-malonylrosine (7), and 6′-O-malonylrosine (8), identified in Mtr1 have not, to our knowledge, previously been detected in rice. This suggests that overproduction of Phe as a result of a point mutation in the rice PDT gene may reveal currently unknown metabolic pathways in land plants. Furthermore, although the synthetic fluxes of secondary metabolites derived from Phe are usually controlled by several enzymes, such as Phe ammonia-lyase, cinnamate 4-hydroxylase, and 4-coumarate:CoA ligase (Bate et al., 1994; Howles et al., 1996; Kao et al., 2002; Achnine et al., 2004), the content of biosynthesized Phe also might be closely associated with the biosynthesis of phenylpropanoid compounds. The expression of a rice PDT mutant enzyme with relaxed feedback sensitivity in combination with manipulation of other genes might prove advantageous biotechnologically for the production of various beneficial compounds in land plants.
METHODS
Culture Conditions and Determination of 5MT Sensitivity
The Mtr1 mutant of rice (Oryza sativa var Norin 8) was established as described (Wakasa and Widholm, 1987). Seed calli of Mtr1 progeny were induced and maintained as described (Tozawa et al., 2001). The 5MT sensitivity of calli was tested as described (Wakasa and Widholm, 1987; Tozawa et al., 2001).
Quantification of Free Aromatic Amino Acids
Rice calli (∼0.9 g fresh weight) grown on 2N6 medium for 2 weeks were homogenized with a Polytron disrupter (Kinematica) in 5 volumes of ice-cold methanol:water (80:20, v/v) containing 0.2% acetic acid, and the resulting extracts were treated as described previously (Morino et al., 2005) before determination of the concentrations of Phe, Trp, and Tyr by HPLC coupled with ESI-MS (LCMS-2010; Shimadzu) as described (Matsuda et al., 2005).
Assay of AS Activity
AS activity was measured in rice calli grown for 2 weeks on 2N6 medium as described (Tozawa et al., 2001). The anthranilate produced in the reaction mixture was quantified with a spectrofluorometer (EP-777; JASCO). The protein concentration of extracts was measured with a protein assay kit (Bio-Rad) for calculation of the specific activity of AS.
Nontargeted Metabolic Profiling by LC-PDA Analysis
Callus extracts (5 μL) prepared as described previously (Morino et al., 2005) were analyzed with an LC-PDA system (Shimadzu LC-10Avp). The Cadenza CD-C18 column (3 μm, 250 × 4.6 mm) was subjected to elution at 38°C and a flow rate of 0.85 mL/min with a mobile phase of acetonitrile/0.02% aqueous trifluoroacetic acid using a gradient of 0/100 (v/v) at 0 min, 1.8/98.2 at 10 min, 4.5/95.5 at 27.5 min, 30/70 at 60 min, and 95/5 at 75 min. The detection wavelength was set at UV190-400 nm. Data were using LCMS-Solution version 3.0 software (Shimadzu).
LC-MS/MS Analysis
Sample extracts (5 μL) prepared for nontargeted metabolic profiling analysis were analyzed with an HPLC system (Agilent 1100 series) coupled with a triple-stage mass spectrometer (API3000; Applied Biosystems/MDS Sciex). The analytical conditions were as follows: for HPLC, 2.0 × 75-mm column (Cadenza CD-C18; Imtact); solvent system, acetonitrile (0.1% formic acid)/water (0.1% formic acid); gradient program, 5/95 (v/v) at 0 min, 60/40 at 11 min, 98/2 at 12 min, 98/2 at 13 min, 5/95 at 13.1 min, and 5/95 at 20 min; flow rate, 0.25 mL/min; temperature, 35°C. The mass spectrometer was operated with turbo ion spray interface in the positive ion mode under the following conditions: nebulizer gas flow, 14 L/min; curtain gas flow, 10 L/min; collision gas flow, 6 L/min; ion spray voltage, 5500 V; and temperature, 500°C, declustering potential, 36 V; focusing potential, 300 V; entrance energy, 10 eV; collision energy, 15 or 40 eV; and collision cell exit potential, 12 V. The detection mode of positive product ion scan was used, in which the fragmentation of precursor ion was monitored. Data were processed with Analyst 1.3 software (Applied Biosystems).
Structure Determination for Metabolites That Accumulate in Mtr1 Callus Tissue
Mtr1 callus tissue (∼74 g fresh weight) was subjected to extraction with 1.0 liters of methanol:water (80:20, v/v) containing 0.2% acetic acid, and the resulting extract (3.1 g) was subjected to HPLC with an octadecyl silica gel column. Eight fractions were collected to give compounds 1 (<0.1 mg), 2 (5.1 mg), 3 (<0.1 mg), 4 (<0.1 mg), 5 (5.0 mg), 6 (<0.1 mg), 7 (0.2 mg), and 8 (2.3 mg). Structures of 1, 2, 3, 5, and 6 were deduced from comparison of MS/MS as well as one- and two-dimensional nuclear magnetic resonance spectra (1H-NMR, 13C-NMR, heteronuclear multiple bond correlation, and heteronuclear multiple quantum correlation) with those of authentic samples or published data (data not shown). Physicochemical data for three metabolites (4, 7, and 8) are as follows. For 2′-O-β-glucosylrosine, (cinnamyl 2-O-β-d-glucopyranosyl-β-d-glucopyranoside, 4), a colorless amorphous solid: UV λmaxH2O nm, 204, 252; [α]D30, 4.5 (water; c0.16); ESI-MS (positive ion mode), mass-to-charge ratio (m/z) 476 [M+NH4]+, 481 [M+Na]+. Fast atom bombardment--high-resolution mass spectrometry (FAB-HRMS): m/z 481.1693 [M+Na]+, calculated for C21H30O11Na, 481.1686. For 2′-O-β-glucosyl-6′-malonylrosine, (cinnamyl 2-O-β-d-glucopyranosyl-6-O-malonyl-β-d-glucopyranoside, 7), a colorless amorphous solid: UV λmaxH2O nm, 211, 254; [α]D30, 3.5 (water; c0.16); ESI-MS (positive ion mode), m/z 562 [M+NH4]+, 567 [M+Na]+. FAB-HRMS: m/z 545.1877 [M+H]+, calculated for C24H33O14, 545.1870. For 6′-O-malonylrosine (cinnamyl 6′-O-malonyl-β-d-glucopyranoside, 8), a colorless amorphous solid: UV λmaxMeOH nm, 206, 251; [α]D30, 16.3 (methanol; c0.25); ESI-MS (positive ion mode), m/z 400 [M+NH4]+, 405 [M+Na]+. FAB-HRMS: m/z 405.1172 [M+Na]+, calculated for C18H22O9 Na, 405.1162.
NMR data of these compounds are presented in Supplemental Table 3 online.
Genetic Analysis of Mtr1
For genetic mapping of mtr1-D, F2 and F3 populations obtained from Mtr1 and the indica variety Kasalath were tested for 5MT sensitivity. A candidate gene for mtr1-D was cloned from Mtr1 callus cDNA by PCR with the putative PDT-specific primers 5′-CGCTGCAGATGGTTTCCCCTTCGCTTCGG-3′ and 5′-CCGAGCTCTCATGCTTCACTGACATCGGTAGG-3′ (underlined sequences indicate the adapter region), which were designed on the basis of sequence data in the Rice Genome Automated Annotation System (http://ricegaas.dna.affrc.go.jp). The 1095-bp nucleotides of Mtr1 corresponding to the putative PDT gene (AK066428) of Nipponbare were compared with Norin 8 by sequence analysis.
Rice Transformation
The cDNA for the wild-type PDT gene (PDTWT) from Norin 8 or for the mutant gene (PDTS298I) from Mtr1 was joined to the maize ubiquitin gene promoter (Toki et al., 1992) for functional analysis in transformed callus lines (see Supplemental Figure 1A online). Transformation was performed as described previously (Urushibara et al., 2001).
Preparation of Recombinant Rice PDT Proteins
Expression plasmids were constructed for the synthesis of rice PDT proteins in a cell-free translation system (see Supplemental Methods online). Wild-type and mutant PDT proteins lacking the predicted chloroplast transit peptide were synthesized with COOH-terminal hexahistidine tags with the use of a wheat embryo cell-free system and the corresponding wild-type (pEUPDTΔN42) and mutant (pEUPDTS298IΔN42) plasmids. The proteins were then purified as described previously (Madin et al., 2000; Kanno et al., 2004).
Assay of ADT Activity
Arogenate was prepared by enzymatic synthesis and purified (see Supplemental Methods online). ADT activity was determined by monitoring the accumulation of Phe with the use of reversed-phase HPLC after derivatization with o-phthalaldehyde. Enzyme solutions were incubated with or without Phe for 10 min at 30°C before initiation of the reaction by addition of arogenate to the enzyme solutions. Enzyme and arogenate solutions contained 100 mM Hepes-KOH, pH 8.2, 5% glycerol, 0.1 mM EDTA, and 2 mM DTT. Reaction mixtures were incubated at 30°C, after which the reaction was stopped by transfer of the tubes to liquid nitrogen. The mixtures were stored at −80°C until immediately before analysis. Each sample (4 μL) was mixed with 1 μL of 0.5 mM leucine (internal control) and 25 μL of o-phthalaldehyde reagent (0.5% o-phthalaldehyde, 10% methanol, and 2% 2-mercaptoethanol in 0.4 M borate-KOH, pH 10.4). After derivatization for 5 min at 25°C, 20 μL of the mixture were injected into a Lichrosorb RP-18 column (GL Science), which was then subjected to elution at a flow rate of 0.9 mL/min with 20 mM potassium phosphate, pH 7.0:methanol (2:3, v/v). o-Phthalaldehyde derivatives were detected by measurement of fluorescence (excitation, 360 nm; emission, 455 nm).
Assay of PDT Activity
The reaction mixture (50 μL) contained 100 mM HEPES-KOH, pH 8.2, 5% glycerol, 0.1 mM EDTA, 2 mM DTT, 0.1 mM prephenate, and 20 nM enzyme. After incubation at 30°C, the reaction was stopped by the addition of 100 μL of 1.5 M NaOH, and phenylpyruvate synthesis was determined by measurement of absorbance at 320 nm.
Feeding with External Phe
Mtr1 or Norin 8 callus tissue was cultured for 2 weeks on 2N6 medium containing 0 or 300 μM Phe with or without 100 μM 5MT. Nipponbare callus tissue was cultured for 2 weeks on 2N6 medium containing 0, 300, or 600 μM Phe with or without 25 μM 5MT. Nipponbare tissue was then homogenized as described above for the quantification of free aromatic amino acids, and the concentration of 5MT in the extract was determined by HPLC coupled with ESI-MS (LCMS-2010) as described previously (Matsuda et al., 2005). Seeds of Nipponbare were germinated in test tubes containing MS medium supplemented with 0 or 300 μM Phe, Trp, or Tyr and with or without 300 μM 5MT; they were evaluated for shoot and root elongation after culture for 2 weeks. The quantification of free Trp in leaves and roots was performed as described above for callus tissue.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL/DDBJ data libraries under the following accession number: MTR1 (AB300404), Nipponbare PDT (AK066428), ADT2 in Arabidopsis (At3g07630), Anabaena (YP_321803), Bacillus (NP_846881), Erwinia (YP_051439), Escherichia (YP_541919), Prochlorococcus (YP_398092), Photorhabdus (NP_928576), Synechococcus (YP_476903), Xanthomonas (NP_636961), and Yersinia (NP_991795).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Sensitivity to 5MT in Seedlings of Norin 8 and Mtr1 Grown on MS Medium.
Supplemental Figure 2. Generation of Transformed Callus Lines Expressing Wild-Type (Ubipro:PDTWT) or Mutant (Ubipro:PDTS298I) Forms of Rice PDT.
Supplemental Figure 3. Substrate Specificity for Dehydratase Activity of Rice PDT.
Supplemental Figure 4. Effect of the S298I Mutation on Feedback Inhibition of the ADT Activity of Rice PDT by Phe.
Supplemental Figure 5. Phylogenetic Relations among Putative PDTs of Higher Plants as well as P-Proteins and PDTs of Bacteria or Fungi.
Supplemental Figure 6. Quantitative RT-PCR Analysis of Trp Synthetase α-Subunit (TSA) and β-Subunit (TSB) Genes in Calli of Norin 8 and Mtr1.
Supplemental Table 1. Primer and Linker Sequences for Construction of Expression Plasmids for the Synthesis of Rice PDT Proteins in a Cell-Free System.
Supplemental Table 2. Primer Sequences for Quantitative RT-PCR Analysis of TSA, TSB, and Tubulin Genes.
Supplemental Methods.
Supplemental References.
Supplemental Data Set 1. Data File Corresponding to Alignment in Supplemental Figure 5.
Supplementary Material
Acknowledgments
We thank Atsushi Ishihara and Derek B. Goto for helpful discussion as well as Minako Sakurai, Sayuri Ohta, and Tomoko Miyakawa for technical assistance. This work was supported by CREST of the Japan Science and Technology Agency.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Kyo Wakasa (k3wakasa@nodai.ac.jp).
Online version contains Web-only data.
References
- Achnine, L., Blancaflor, E.B., Rasmussen, S., and Dixon, R.A. (2004). Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16 3098–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, S., Weisburg, W.G., and Jensen, R.A. (1990). Evolution of aromatic amino acid biosynthesis and application to the fine-tuned phylogenetic positioning of enteric bacteria. J. Bacteriol. 172 1051–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate, N.J., Orr, J., Ni, W., Meromi, A., Nadler-Hassar, T., Doerner, P.W., Dixon, R.A., Lamb, C.J., and Elkind, Y. (1994). Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc. Natl. Acad. Sci. USA 91 7608–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongaerts, J., Kramer, M., Muller, U., Raeven, L., and Wubbolts, M. (2001). Metabolic engineering for microbial production of aromatic amino acids and derived compounds. Metab. Eng. 3 289–300. [DOI] [PubMed] [Google Scholar]
- Bonner, C., and Jensen, R. (1987). Prephenate aminotransferase. Methods Enzymol. 142 479–487. [DOI] [PubMed] [Google Scholar]
- Carlson, J.E., and Widholm, J.M. (1978). Separation of two forms of anthranilate synthetase from 5-methyltryptophan-susceptible and -resistant cultured Solarium tuberosum cells. Physiol. Plant. 44 251–255. [Google Scholar]
- Cho, M.H., et al. (2007). Phenylalanine biosynthesis in Arabidopsis thaliana. Identification and characterization of arogenate dehydratases. J. Biol. Chem. 282 30827–30835. [DOI] [PubMed] [Google Scholar]
- d'Amato, T.A., Ganson, R.J., Gaines, C.G., and Jensen, R.A. (1984). Subcellular localization of chorismate-mutase isoenzymes in protoplasts from mesophyll and suspension-cultured cells of Nicotiana silvestris. Planta 162 104–108. [DOI] [PubMed] [Google Scholar]
- Dixon, R.A., and Paiva, N.L. (1995). Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide, T.A., Crewther, P., and Davidson, B.E. (1972). Chorismate mutase-prephenate dehydratase from Escherichia coli K-12. II. Kinetic properties. J. Biol. Chem. 247 4447–4452. [PubMed] [Google Scholar]
- Eberhard, J., Ehrler, T.T., Epple, P., Felix, G., Raesecke, H.R., Amrhein, N., and Schmid, J. (1996). Cytosolic and plastidic chorismate mutase isozymes from Arabidopsis thaliana: Molecular characterization and enzymatic properties. Plant J. 10 815–821. [DOI] [PubMed] [Google Scholar]
- Frey, M., Chomet, P., Glawischnig, E., Stettne, C., Grun, S., Winklmair, A., Eisenreich, W., Bacher, A., Meeley, R.B., Briggs, S.P., Simcox, K., and Gierl, A. (1997). Analysis of a chemical plant defense mechanism in grasses. Science 277 696–699. [DOI] [PubMed] [Google Scholar]
- Gjetting, T., Petersen, M., Guldberg, P., and Güttler, F. (2001). Missense mutations in the N-terminal domain of human phenylalanine hydroxylase interfere with binding of regulatory phenylalanine. Am. J. Hum. Genet. 68 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, G.C., Flick, M.B., Gherna, R.L., and Jensen, R.A. (1982). Biochemical diversity for biosynthesis of aromatic amino acids among the cyanobacteria. J. Bacteriol. 149 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton, T.A., and Cornish, E.C. (1995). Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 7 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howles, P.A., Sewalt, V., Paiva, N.L., Elkind, Y., Bate, N.J., Lamb, C., and Dixon, R.A. (1996). Overexpression of L-phenylalanine ammonia-lyase in transgenic tobacco plants reveals control points for flux into phenylpropanoid biosynthesis. Plant Physiol. 112 1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao, P., Sanjaya, Su, R.C., Teixeira da Silva, J.A., and Chan, M.T. (2007). Plant native tryptophan synthase beta 1 gene is a non-antibiotic selection marker for plant transformation. Planta 225 897–906. [DOI] [PubMed] [Google Scholar]
- Im, S.W., and Pittard, J. (1971). Phenylalanine biosynthesis in Escherichia coli K-12: Mutants derepressed for chorismate mutase P-prephenate dehydratase. J. Bacteriol. 106 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara, A., Asada, Y., Takahashi, Y., Yabe, N., Komeda, Y., Nishioka, T., Miyagawa, H., and Wakasa, K. (2006). Metabolic changes in Arabidopsis thaliana expressing the feedback-resistant anthranilate synthase α subunit gene OASA1D. Phytochemistry 67 2349–2362. [DOI] [PubMed] [Google Scholar]
- Jimenez, N., Gonzalez-Candelas, F., and Silva, F.J. (2000). Prephenate dehydratase from the aphid endosymbiont (Buchnera) displays changes in the regulatory domain that suggest its desensitization to inhibition by phenylalanine. J. Bacteriol. 182 2967–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, E., Zamir, L.O., and Jensen, R.A. (1986). Chloroplasts of higher plants synthesize L-phenylalanine via L-arogenate. Proc. Natl. Acad. Sci. USA 83 7231–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno, T., Kasai, K., Ikejiri-Kanno, Y., Wakasa, K., and Tozawa, Y. (2004). In vitro reconstitution of rice anthranilate synthase: Distinct functional properties of the alpha subunits OASA1 and OASA2. Plant Mol. Biol. 54 11–22. [DOI] [PubMed] [Google Scholar]
- Kao, Y.Y., Harding, S.A., and Tsai, C.J. (2002). Differential expression of two distinct phenylalanine ammonia-lyase genes in condensed tannin-accumulating and lignifying cells of quaking aspen. Plant Physiol. 130 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai, K., Kanno, T., Akita, M., Ikejiri-Kanno, Y., Wakasa, K., and Tozawa, Y. (2005). Identification of three shikimate kinase genes in rice: Characterization of their differential expression during panicle development and of the enzymatic activities of the encoded proteins. Planta 222 438–447. [DOI] [PubMed] [Google Scholar]
- Kobe, B., Jennings, I.G., House, C.M., Michell, B.J., Goodwill, K.E., Santarsiero, B.D., Stevens, R.C., Cotton, R.G., and Kemp, B.E. (1999). Structural basis of autoregulation of phenylalanine hydroxylase. Nat. Struct. Biol. 6 442–448. [DOI] [PubMed] [Google Scholar]
- Kreps, J.A., Ponappa, T., Dong, W., and Town, C.D. (1996). Molecular basis of α-methyltryptophan resistance in amt-1, a mutant of Arabidopsis thaliana with altered tryptophan metabolism. Plant Physiol. 110 1159–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps, J.A., and Town, C.D. (1992). Isolation and characterization of a mutant of Arabidopsis thaliana resistant to alpha-methyltryptophan. Plant Physiol. 99 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan, T.M. (1995). Alkaloid biosynthesis—The basis for metabolic engineering of medicinal plants. Plant Cell 7 1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last, R.L., Bissinger, P.H., Mahoney, D.J., Radwanski, E.R., and Fink, G.R. (1991). Tryptophan mutants in Arabidopsis: The consequences of duplicated tryptophan synthase β genes. Plant Cell 3 345–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Last, R.L., and Fink, G.R. (1988). Tryptophan-requiring mutant of the plant Arabidopsis thaliana. Science 240 305–310. [DOI] [PubMed] [Google Scholar]
- Li, J., Chen, S., Zhu, L., and Last, R.L. (1995. a). Isolation of cDNAs encoding the tryptophan pathway enzyme indole-3-glycerol phosphate synthase from Arabidopsis thaliana. Plant Physiol. 108 877–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., and Last, R.L. (1996). The Arabidopsis thaliana trp5 mutant has a feedback-resistant anthranilate synthase and elevated soluble tryptophan. Plant Physiol. 110 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Zhao, J., Rose, A.B., Schmidt, R., and Last, R.L. (1995. b). Arabidopsis phosphoribosylanthranilate isomerase: Molecular genetic analysis of triplicate tryptophan pathway genes. Plant Cell 7 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberles, J.S., Thorolfsson, M., and Martinez, A. (2005). Allosteric mechanisms in ACT domain containing enzymes involved in amino acid metabolism. Amino Acids 28 1–12. [DOI] [PubMed] [Google Scholar]
- Madin, K., Sawasaki, T., Ogasawara, T., and Endo, Y. (2000). A highly efficient and robust cell-free protein synthesis system prepared from wheat embryos: Plants apparently contain a suicide system directed at ribosomes. Proc. Natl. Acad. Sci. USA 97 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda, F., Yamada, T., Miyazawa, H., Miyagawa, H., and Wakasa, K. (2005). Characterization of tryptophan-overproducing potato transgenic for a mutant rice anthranilate synthase α-subunit gene (OASA1D). Planta 222 535–545. [DOI] [PubMed] [Google Scholar]
- Miao, S., Duncan, D.R., and Widholm, J. (1988). Selection of regenerable maize callus cultures resistant to 5-methyl-DL-tryptophan, S-2-aminoethyl-L-cysteine and high levels of L-lysine plus L-threonine. Plant Cell Tissue Organ Cult. 14 3–14. [Google Scholar]
- Mobley, E.M., Kunkel, B.N., and Keith, B. (1999). Identification, characterization and comparative analysis of a novel chorismate mutase gene in Arabidopsis thaliana. Gene 240 115–123. [DOI] [PubMed] [Google Scholar]
- Morino, K., Matsuda, F., Miyazawa, H., Sukegawa, A., Miyagawa, H., and Wakasa, K. (2005). Metabolic profiling of tryptophan-overproducing rice calli that express a feedback-insensitive α subunit of anthranilate synthase. Plant Cell Physiol. 46 514–521. [DOI] [PubMed] [Google Scholar]
- Nester, E.W., and Jensen, R.A. (1966). Control of aromatic acid biosynthesis in Bacillus subtilis: Sequential feedback inhibition. J. Bacteriol. 91 1594–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi, K.K., and Fink, G.R. (1992). Two anthranilate synthase genes in Arabidopsis: Defense-related regulation of the tryptophan pathway. Plant Cell 4 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi, K.K., Last, R.L., Fink, G.R., and Keith, B. (1993). Suppressors of trp1 fluorescence identify a new Arabidopsis gene, TRP4, encoding the anthranilate synthase β subunit. Plant Cell 5 1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, N., Pierson, D.L., and Jensen, R.A. (1977). Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J. Biol. Chem. 252 5839–5846. [PubMed] [Google Scholar]
- Pohnert, G., Zhang, S., Husain, A., Wilson, D.B., and Ganem, B. (1999). Regulation of phenylalanine biosynthesis. Studies on the mechanism of phenylalanine binding and feedback inhibition in the Escherichia coli P-protein. Biochemistry 38 12212–12217. [DOI] [PubMed] [Google Scholar]
- Radwanski, E.R., Zhao, J., and Last, R.L. (1995). Arabidopsis thaliana tryptophan synthase α: Gene cloning, expression and subunit interaction. Mol. Gen. Genet. 248 657–667. [DOI] [PubMed] [Google Scholar]
- Ranch, J.P., Rick, S., Brotherton, J.E., and Widholm, J.M. (1983). Expression of 5-methyltryptophan resistance in plants regenerated from resistant cell lines of Datura innoxia. Plant Physiol. 71 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippert, P., and Matringe, M. (2002). Molecular and biochemical characterization of an Arabidopsis thaliana arogenate dehydrogenase with two highly similar and active protein domains. Plant Mol. Biol. 48 361–368. [DOI] [PubMed] [Google Scholar]
- Rose, A.B., Casselman, A.L., and Last, R.L. (1992). A phosphoribosylanthranilate transferase gene is defective in blue fluorescent Arabidopsis thaliana tryptophan mutants. Plant Physiol. 100 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siehl, D.L., and Conn, E.E. (1988). Kinetic and regulatory properties of arogenate dehydratase in seedlings of Sorghum bicolor (L.) Moench. Arch. Biochem. Biophys. 260 822–829. [DOI] [PubMed] [Google Scholar]
- Toki, S., Takamatsu, S., Nojiri, C., Ooba, S., Anzai, H., Iwata, M., Christensen, A.H., Quail, P.H., and Uchimiya, H. (1992). Expression of a maize ubiquitin gene promoter-bar chimeric gene in transgenic rice plants. Plant Physiol. 100 1503–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozawa, Y., Hasegawa, H., Terakawa, T., and Wakasa, K. (2001). Characterization of rice anthranilate synthase α-subunit genes OASA1 and OASA2. Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant of OASA1. Plant Physiol. 126 1493–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushibara, S., Tozawa, Y., Kawagishi-Kobayashi, M., and Wakasa, K. (2001). Efficient transformation of suspension-cultured rice cells mediated by Agrobacterium tumefaciens. Breed. Sci. 51 33–38. [Google Scholar]
- Wakasa, K., Hasegawa, H., Nemoto, H., Matsuda, F., Miyazawa, H., Tozawa, Y., Morino, K., Komatsu, A., Yamada, T., Terakawa, T., and Miyagawa, H. (2006). High-level tryptophan accumulation in seeds of transgenic rice and its limited effects on agronomic traits and seed metabolite profile. J. Exp. Bot. 57 3069–3078. [DOI] [PubMed] [Google Scholar]
- Wakasa, K., and Widholm, J.M. (1987). A 5-methyltryptophan resistant rice mutant, MTR1, selected in tissue culture. Theor. Appl. Genet. 74 49–54. [DOI] [PubMed] [Google Scholar]
- Wakasa, K., and Widholm, J.M. (1991). Rice mutants resistant to amino acids and amino acid analogs. In Biotechnology in Agriculture and Forestry 14 Rice. Y.P.S. Bajaj, ed (Berlin: Springer-Verlag), pp. 304–315.
- Warpeha, K.M., Lateef, S.S., Lapik, Y., Anderson, M., Lee, B.S., and Kaufman, L.S. (2006). G-protein-coupled receptor 1, G-protein Gα-subunit 1, and prephenate dehydratase 1 are required for blue light-induced production of phenylalanine in etiolated Arabidopsis. Plant Physiol. 140 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten, R., and Sederoff, R. (1995). Lignin biosynthesis. Plant Cell 7 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm, J.M. (1972). Cultured Nicotiana tabacum cells with an altered anthranilate synthetase which is less sensitive to feedback inhibition. Biochim. Biophys. Acta 261 52–58. [DOI] [PubMed] [Google Scholar]
- Zhao, G., Xia, T., Fischer, R.S., and Jensen, R.A. (1992). Cyclohexadienyl dehydratase from Pseudomonas aeruginosa. Molecular cloning of the gene and characterization of the gene product. J. Biol. Chem. 267 2487–2493. [PubMed] [Google Scholar]
- Zhao, Y., Christensen, S.K., Fankhauser, C., Cashman, J.R., Cohen, J.D., Weigel, D., and Chory, J. (2001). A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291 306–309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.