Abstract
Controlling blood glucose levels within acceptable limits is crucial to the long-term health of patients with type 2 diabetes, and patient involvement is a vital element in achieving this goal. The benefits of patient education and chronic disease management tools cannot be underestimated as many patients will require initiation of insulin therapy to achieve glycemic targets. The wide choice of insulin formulations and the ever-expanding range of delivery methods now available make insulin administration easier, less painful, more discreet, and more accurate than ever before, thus providing important tools to overcome barriers to insulin initiation and improve achievement of glycemic goals. In addition, exciting developments in technology for self-monitoring of blood glucose have increased the potential for optimal glycemic control. This review discusses how these approaches can help patients manage their diabetes.
Introduction
Insulin remains key to the management of hyperglycemia in patients with type 2 diabetes; the early addition of insulin to oral therapy in these patients is recognized as an effective option that can help improve glycemic control and contribute to more favorable outcomes.[1–3] A wide variety of insulin formulations are available (Table 1)[4,5]; these have been reviewed extensively elsewhere.[6]
Table 1.
| Insulin | Onset of action | Peak | Duration |
|---|---|---|---|
| Rapid acting (analog) | |||
| aspart | 10–20 minutes | 1–3 hours | 3–5 hours |
| lispro | 15–30 minutes | 0.5–2.5 hours | 3–6.5 hours |
| glulisine | 10–15 minutes | 1–1.5 hours | 3–5 hours |
| Short-acting (human) | |||
| Regular | 30–60 minutes | 1–5 hours | 6–10 hours |
| Intermediate (human) | |||
| NPH | 1–2 hours | 6–14 hours | 16–24+ hours |
| Long-acting (analog) | |||
| detemir | 0.8–2 hours | No significant peak | Up to 24 hours |
| glargine | 1.1 hours | No significant peak | Up to 24 hours |
| Premixed (analog) | |||
| 70% APS/30% aspart | 10–20 minutes | 1–4 (2.4) hours† | Up to 24 hours |
| 75% NPL/25% lispro | 10–30 minutes | 1–6.5 (2.6) hours† | Up to 24 hours |
| 50% NPL/50% lispro | 10–30 minutes | 0.8–4.8 (2.3) hours† | Up to 24 hours |
| Premixed (human) | |||
| 70% NPH/30% regular | 30–60 minutes | 1.5–16 (4.4) hours† | Up to 18–24 hours |
| 50% NPH/50% regular | 30–60 minutes | 2–5.5 (3.3) hours† | Up to 18–24 hours |
Range (mean)
NPH = neutral protamine Hagedorn; APS = aspart protamine suspension; NPL = neutral protamine lispro
Adapted with permission from Allen.[4]
This article considers the insulin delivery systems currently available and discusses how these and other measures, such as glucose monitoring and chronic disease management tools, can help address individual barriers to effective insulin therapy. By helping patients implement suitably informed therapeutic regimens, primary care physicians will best be able to help patients meet the challenges posed by diabetes.
Among those challenges is the trend towards unhealthy eating habits and low levels of exercise in the West that over the past 20 years or more has contributed to a substantial increase in obesity rates and, concurrently, the incidence of type 2 diabetes.[7–9] Add to this the ever-increasing numbers of elderly patients and the worrying number of young people with type 2 diabetes, and we are faced with a serious public health problem.[10,11]
In the US alone, approximately 21 million people – 7.0% of the population – have diabetes, with type 2 diabetes accounting for about 90–95% of all diagnosed cases.[12] The potential burden of long-term hyperglycemia and variability in plasma glucose levels can lead to severe complications. In experimental systems, glycemic variability and hyperglycemic conditions are associated with increased production of reactive oxygen species, which cause endothelial cell damage.[13] In addition, the work of Monnier and colleagues[14] demonstrated that glycemic variability is associated with higher expression levels of markers of cardiovascular damage. However, retrospective studies by Kilpatrick and colleagues[15] and Service and O'Brien[16] indicate that glycemic excursions may not be as important as long-term hyperglycemia. Taken together, these data still indicate that the consequences of poor glycemic control may be microvascular and macrovascular complications which, in turn, are associated with increased morbidity and mortality.[10,11]
Clearly, keeping blood glucose levels within acceptable limits is crucial to the long-term health of patients with type 2 diabetes. To this end, both the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) and American College of Endocrinology/American Association of Clinical Endocrinologists (ACE/AACE) have produced guidelines for achieving and maintaining glucose levels as close as possible to the normal range.[2,17] The ADA recommends that analog insulin therapy be initiated when patients' glycosylated hemoglobin (HbA1c) levels reach > 8.5%. Target HbA1c recommendations by the AACE and ADA are ≤ 6.5% and < 7%, respectively; however the ADA recommends levels < 6% in individuals not experiencing hypoglycemia.[17–20] Various key studies have confirmed that keeping glucose levels below recommended levels can substantially reduce the risk of complications.[2,3,17,19–22] The guidelines recommend that patients initially try to control their condition with lifestyle changes and oral hypoglycemic agents; however, the progressive nature of type 2 diabetes means that most patients will eventually require insulin therapy.[17,23]
The introduction of new insulin analogs signaled a major step towards the possibility of achieving optimal glycemic control. However, to fully meet the challenge of type 2 diabetes, not only do insulins have to match the action of endogenous insulin as closely as possible, but they must also be formulated and delivered in a way that will encourage patient adherence. This need is being met by an ever-expanding range of insulin delivery devices, which facilitate discreet and accurate administration of insulin doses and improve compliance. The way in which such developments can help primary care physicians achieve optimal glycemic control in type 2 diabetes is discussed here.
Patient Involvement Crucial to Achieving Glycemic Targets
With the growing number of people diagnosed with type 2 diabetes, it is more important than ever that patients become actively involved in their own treatment as there is unfortunately a low rate of aggressive treatment initiation and adjustment following abnormal blood glucose readings. As a consequence of this “clinical inertia,” many patients with type 2 diabetes are not meeting the recommended glycemic goals stated above.
The DAWN study clearly showed that patients achieved better diabetes control if they had a good relationship with their healthcare provider and felt more involved in the decision-making process.[24] To increase patient involvement, education programs need to be implemented, and tools need to be developed for chronic disease management. A multi-disciplinary approach to meeting the educational and psychosocial needs of diabetes patients is recommended in treatment guidelines and has been shown to significantly improve outcomes.[2,17,24,25]
The key aim of education is to help patients manage their diabetes and become “experts” on their own condition.[26] It is essential that patients are well informed and confident enough to self-adjust food intake, exercise, or insulin dose in response to glucose levels, and understand the consequences of not doing so.[26] A CDC report showed that daily self-monitored blood glucose (SMBG) measurements were performed by only 58% of patients as of 2003, having risen from 36% in 1994.[27] Saudek and colleagues[28] reviewed results from trials in which SMBG measurement was conducted, and they concluded that glucose monitoring exerted a positive outcome on glycemic control.
Log Books
An extremely effective way of improving self-management is to use log books (paper-based or electronic) in which patients chart their blood glucose levels (fasting plasma glucose [FPG] and postprandial levels), carbohydrate intake and level of activity throughout the day, making a note of any dose alterations. Log books currently available from healthcare teams and/or various Web sites all have limitations, and there is a need for a more targeted approach. Ideally, a log book would be specific to the type of insulin regimen being used (basal, basal-bolus, premixed or continuous subcutaneous infusion [CSII]), because different regimens require different testing patterns, and they would also have sections where the physicians could insert comments.
Patients send a summary of their readings by mail or email to their physician or other healthcare provider at the end of each month so that he or she can assess the level of control and adherence. Any patient with poor glycemic control can then be contacted and an appointment made to adjust treatment accordingly; for example, if a patient receiving only basal insulin has consistently high postprandial glucose levels, they can be considered for a premixed analog regimen (or a prandial insulin can be added). Conversely, if a patient on a premixed formulation needs a more flexible regimen because of unpredictable meal times, they could be switched to a basal-bolus analog regimen.
Patients should be able to actively engage with their physician or healthcare provider when deciding on treatment options. An effective log book system, combined with education, training, and feedback, can help overcome the human factors that contribute to suboptimal control of glucose levels. The system should be seen as an extension of treatment to increase patient involvement and not just as a means of collecting data.
Telemedicine
A major change that has been gathering momentum over the past 2 decades is the increase in the number of people with access to computers and the Internet who feel comfortable exchanging data by electronic means. This has facilitated the greater use of telemedical care in diabetes.[29] Telemedicine is rapidly maturing and the technology has proven to be sound, effective, cost-effective and practical.[29] A number of small studies have shown that electronic sharing of data between healthcare professionals and/or patients improves glycemic control and reduces costs.[30–33]
Further evidence for the usefulness of telemedicine comes from a randomized trial in older, ethnically diverse patients with type 2 diabetes.[34] In this trial, case management using standard care was compared with care via a home telemedicine unit (Web-enabled computer for videoconferencing, remote glucose monitoring, Web-based communication with nurse case managers, and Web site education). After 1 year, mean HbA1c levels were significantly lower in the telemedicine group.
Insulin Delivery
For many years, the vial and syringe were the standard means of delivering insulin; however, the fact that not all patients were comfortable with this method had serious implications for compliance and glycemic control. The situation has significantly improved with the development of alternative means of delivery (Table 2).[35–39] Although they may initially be more expensive than the vial and syringe combination, it is important to note that these delivery devices offer key advantages in terms of reduced complications and their ensuing costs, as a result of high patient satisfaction and adherence, and ease of dose adjustment.[35,40–42] Furthermore many, if not most, healthcare plans cover insulin pen devices with little additional cost to the patient. Approximately 80% to 90% of health plan formularies, including Medicare, cover prefilled insulin injection systems.[43–45]
Table 2.
| Device | Advantages | Disadvantages |
|---|---|---|
| Prefilled pen, eg, FlexPen (Novo Nordisk, Bagsvaerd, Denmark), Humalog Pen (Eli Lilly and Co, Indianapolis, Indiana), SoloSTAR (Aventis Pharma Holding GmbH, Frankfurt, Germany) | Less wastage of pen contents vs vial/syringe | Initially can be more expensive than vial/syringe |
| Discreet | Cannot mix insulins | |
| Appears less “medical” | Possibility of air bubbles | |
| Injection may be more comfortable than vial and syringe | ||
| Less time consuming | ||
| Refrigeration not required | ||
| Easy to use | ||
| Accurate dosing | ||
| Disposable | ||
| Reusable pen, eg, NovoPen (Novo Nordisk), HumaPen MEMOIR (Eli Lilly and Co), OptiClik (Aventis Pharma Holding GmbH) | Discreet | As above |
| Sturdier than prefilled pens | Need to change cartridges (time consuming and less convenient than prefilled pens) | |
| Injection may be more comfortable than vial and syringe | ||
| Accurate dosing | ||
| Dosers, eg, InnoLet (Novo Nordisk) | Easy to use | As above |
| Accurate dosing | Not currently available with insulin analogs | |
| Suitable for patients with visual/dexterity problems | ||
| Disposable | ||
| Insulin pump | Uses only rapid-acting insulin (most consistent profile) | Pump and supplies expensive |
| Most accurate dosing | Undetected interruptions in insulin delivery may occur, with possible increased risk of ketoacidosis | |
| Allows very flexible lifestyle | May cause discomfort as worn continuously | |
| Closest to replacing body's own insulin | Needs high patient motivation, involvement, and commitment to use | |
| Jet injectors | Needle-free | May cause bruising |
| Single component | Potential for decreased amount of absorbed insulin | |
| May benefit patients with severe insulin-induced lipoatrophy | Requires weekly cleaning | |
| Risk of infection | ||
| May be less comfortable than needle-based devices | ||
| Not suited to intermediate- or long-acting insulins |
Insulin Pens and Dosers
Since their introduction in 1985, insulin pens have become increasingly sophisticated. Pens are available in 2 different forms, with either replaceable cartridges or disposable prefilled cartridges. All types of insulin are available for use in these devices (Table 3).
Table 3.
Insulin Analog Formulations Available in Pen and Doser Devices
| Device | Insulin | Manufacturer |
|---|---|---|
| Disposable (prefilled) pens | ||
| FlexPen | Levemir, NovoLog, NovoLog Mix 70/30 | Novo Nordisk |
| Lilly Prefilled Pen | Humalog, Humalog Mix 70/30, Humalog Mix 75/25, Humalog Mix 50/50, Humulin N | Eli Lilly |
| SoloSTAR | Lantus, Apidra | Sanofi-Aventis |
| Durable (cartridge) pens | ||
| NovoPen 3 | Levemir, NovoLog, NovoLog Mix 70/30 | Novo Nordisk |
| NovoPen Junior | Levemir, NovoLog, NovoLog Mix 70/30 | Novo Nordisk |
| HumaPen LUXURA | Humalog, Humulin | Eli Lilly |
| HumaPen MEMOIR | Humalog | Eli Lilly |
| OptiClik | Lantus, Apidra | Sanofi-Aventis |
| Dosers | ||
| InnoLet | Novolin N, Novolin R, Novolin 70/30 | Novo Nordisk |
Modern devices are very easy to use; they have many features that facilitate accurate dosing, such as an audible click when the dose is dialed, single-unit dose increments, 2-way dose setting, clear dials showing the selected dose, and automatic zeroing after administration. Since they are discreet, portable and have extremely short needles, such devices help overcome many of the barriers that patients have to insulin therapy and are often preferred to vial and syringe delivery (Figure 1).[40] In a study by Graff and colleagues,[46] 90% of patients rated the NovoFine 30 needle as practically pain free.
Figure 1.
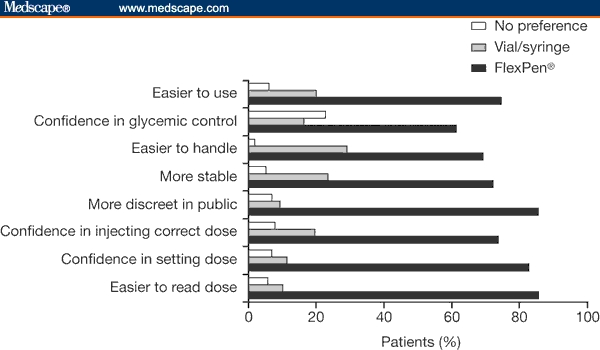
Patient preferences in insulin delivery options.[53] Republished from: Korytkowski M, Bell D, Jacobsen C, Suwannasari R; FlexPen Study Team. A multicenter, randomized, open-label, comparative, 2-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836-2848. Copyright 2003, with permission from Excerpta Medica, Inc.
Pens and dosers are particularly useful for people whose coordination or vision is impaired (although more dexterity is required for the cartridge option than for the prefilled pens), or for people who are away from home during the day, such as schoolchildren and those who work. The fact that this form of delivery is used by only 15% of patients in the US, compared with 80–90% in Europe, indicates that there is a strong need to educate both physicians and patients regarding the benefits of insulin delivery systems. As there are few reimbursement issues with most private health plans and coverage for most pens by Medicare,[43] this discrepancy may be due to inertia or lack of knowledge by both physicians and patients.
A questionnaire-based study showed that patients preferred FlexPen (Novo Nordisk; Bagsvaerd, Denmark) to all previously used treatments and delivery systems for convenience, flexibility, and quality of life; insulin-naive patients reported the greatest perceived improvements.[47] Similarly, a multinational evaluation of HumaPen (Eli Lilly and Co; Indianapolis, Indiana) reported that more than 90% of previous syringe and pen users preferred the pen device over their previous injection method, rating several specific features, including dose correction and readability, as “easy” or “very easy.”[48]
In a comparison of FlexPen, Humulin Pen (Eli Lilly and Co.) and OptiSet (Aventis Pharma; Frankfurt, Germany), both patients and healthcare professionals rated FlexPen as significantly better than the other 2 devices (P < .05) with respect to confidence in patients' ability to use the device (Figure 2); patients also felt that FlexPen would lead to greater patient adherence.[49] In another study, patient preference for FlexPen over Humalog Pen (Eli Lilly and Co.) was demonstrated for all 16 parameters investigated, including readability, injecting, and overall ease of use.[50]
Figure 2.
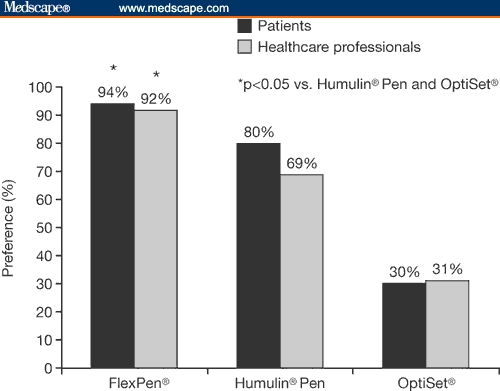
Comparison of confidence in patients' ability to use 3 insulin pen devices, as rated by patients and healthcare professionals.[49] This figure was generated from data published in: Lawton B, Berg B. Comparative evaluation of FlexPen, a new prefilled insulin delivery system, among patients and healthcare professionals. Diabetes. 2001;50:A440. (Publisher: American Diabetes Association, US)
Ease of dose selection is particularly important for elderly patients who may have impaired vision or reduced manual dexterity. A study comparing vial and syringe delivery with a prefilled pen in patients with diabetes aged over 60 years reported that 92% found preselection of dose easier with the pen after 2 weeks of use.[51]
An extensive data review spanning 20 years concluded that NovoPen (Novo Nordisk) provided more accurate dosing than conventional delivery, producing equivalent, and in some cases superior, glycemic control without increasing hypoglycemia.[52] In one crossover study comparing FlexPen with a conventional vial and syringe combination, while HbA1c reduction was equivalent between groups, a greater proportion of FlexPen patients reported confidence in their ability to achieve glycemic control (61% vs 16%; n = 121).[53]
InnoLet (Novo Nordisk) is a prefilled doser that may also be preferred to the standard vial and syringe.[41] It has many of the features of an insulin pen, but has the largest dosing dial available and a large grip. InnoLet is designed to rest securely against the skin, to help minimize tremor or movement at the needle tip, and to help prevent patients from injecting too deeply. Its ergonomic design and large numbers mean that the doser is particularly suited to patients with visual impairment and/or dexterity problems; however, only human insulin formulations are available for use with InnoLet.
Insulin Pumps
Since being launched more than 40 years ago, CSII pumps have been improved and have become an effective method for intensive glycemic management; they are recognized by the ADA as an acceptable therapeutic option.[39] While pumps are more commonly used in patients with type 1 diabetes, they are increasingly being considered for type 2 patients.
CSII pumps are now the size of a pager and can be worn discreetly. They typically deliver rapid-acting insulin through an indwelling subcutaneous catheter, which must be changed every 48–72 hours. Implanted pumps have also been developed to deliver insulin directly to the portal venous system via the peritoneal cavity, bypassing the liver in much the same way as physiological insulin. A disadvantage of implantable pumps is that they need to be changed surgically every year, or even more frequently.[54]
The CSII pump delivers continuous subcutaneous basal insulin over 24 hours, with the patient pushing a button to administer additional bolus doses before or after each meal. Doses can be adjusted according to the patient's needs. Choosing which patients will benefit from CSII is a major challenge to diabetes caregivers, as a great deal of training and commitment is required from patients and/or those who care for them.[55]
CSII has proven to be an effective option in the treatment of patients with type 1 and 2 diabetes. CSII has significantly reduced the incidence of serious hypoglycemia over 2 years (P = .005) and improved quality of life parameters in patients with type 1 diabetes switching from intensified basal-bolus insulin therapy.[56] Results in type 2 diabetes have also been encouraging. In 132 patients with type 2 diabetes, CSII with insulin aspart was equivalent to multiple daily injection (MDI) therapy with insulin aspart and neutral protamine Hagedorn (NPH) insulin for glycemic control after 42 weeks; however, the majority of patients preferred CSII and they had a significantly greater improvement in overall treatment satisfaction (P < .001).[57] The incidence of nocturnal hypoglycemia tended to be lower with CSII (16%) compared with MDI (22%), whereas the overall incidence of hypoglycemia was similar in both groups.[57] In another small study, CSII treatment with high-strength insulin proved effective in 9 patients who had uncontrolled type 2 diabetes and insulin resistance despite prior intensive insulin regimens; HbA1c was significantly decreased after 3 months (P = .026) and there were no clinically significant episodes of hypoglycemia.[58]
Data from various meta-analyses in patients with type 1 diabetes have shown that CSII improves overall diabetic control and reduces the risk of hypoglycemic episodes compared with MDI regimens; it seems to be particularly suited to patients with higher baseline HbA1c as well as those with poor or unpredictable glycemic control.[59–62]
The advances made in continuous blood glucose monitoring systems, discussed later in this article, have further enhanced the high level of glycemic control that can be achieved with insulin pump therapy.
Jet Injectors
Jet injectors deliver a high-pressure stream of insulin into the subcutaneous tissue. However, they are not widely used and an ADA statement recommends that they “should not be viewed as a routine option for use in patients with diabetes.”[63] Their advantages and disadvantages are listed in Table 2.
Inhaled Insulin
The first inhaled human insulin powder, Exubera (Pfizer Inc; New York, NY), was approved in 2006 for use in the US and Europe.[64] Exubera was a potential replacement for mealtime bolus injections of subcutaneous insulin, but it was not a replacement for injected long-acting insulin. Onset of action was faster than that of the short-acting regular insulin but similar to that of rapid-acting insulin analogs. The duration of action for Exubera was comparable to that of regular human insulin[65]; it also demonstrated efficacy equivalent to conventional human insulin regimens and was equally well tolerated. However, it was recently withdrawn from the US market due to low adoption rates and poor sales as a consequence of the doser being too large and cumbersome, a high incidence of cough, and a small, nonprogressive decline in forced expiratory volume.[66,67]
Development of a second inhaled insulin formulation, the AERx insulin Diabetes Management System (AERx iDMS; Aradigm Corporation; Hayward, California/Novo Nordisk), has also been discontinued. There were no safety issues involved in the decision; it was concluded that an inhaled, rapid-acting insulin was unlikely to offer significant clinical or convenience benefits over injections of modern insulin analogs with pen devices.[68]
Self-Monitoring of Blood Glucose: a Key Factor in Glycemic Control
Despite the welcome improvements in insulin pharmacokinetic profiles and delivery devices, if patients do not regularly check their blood glucose levels and, more importantly, adjust their therapy, optimum glycemic control can remain elusive. The importance of closely monitoring both fasting and postprandial glucose levels to maintain good glycemic control cannot be underestimated. Overall diabetes control is better reflected by postprandial glucose measurements than by fasting glycemia, although the importance of the latter increases as diabetes worsens.[69–71]
The ACE/AACE acknowledges that diabetes is primarily a self-managed disease and that SMBG is a critical factor in the management of diabetes, allowing patients and healthcare professionals to make informed decisions and provide ongoing feedback to patients about nutrition and physical activity.[2] The American Association of Diabetes Educators recommends that all healthcare providers encourage patients with diabetes to use SMBG, regardless of whether they are receiving insulin, oral agents, or both.[72] The ADA states that insulin-treated type 2 diabetes patients require more frequent SMBG testing than non-insulin users.[17]
Although practice varies among healthcare professionals, SMBG should ideally be recorded at least 4 times a day – before meals and before bed; patients can also benefit from obtaining additional postprandial readings.[73] As a minimum, patients with type 2 diabetes should perform SMBG measurements at least 4 times per week, 2 while fasting and 2 postprandially.[73]
The clinical value of regular SMBG monitoring has been demonstrated in 2 large studies. Karter and colleagues[74] reported that patients with pharmacologically treated type 2 diabetes who performed daily self-monitoring had HbA1c levels that were significantly lower than those who monitored less frequently (P < .0001) (Figure 3).
Figure 3.
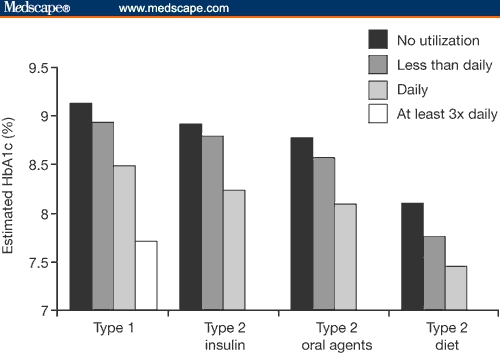
Adjusted HbA1c levels by frequency of self-monitoring in patients with type 1 or type 2 diabetes.[74] Karter AJ, Ackerson IM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes register. Am J Med. 2001;111:1–9. Copyright Elsevier 2001.
In an epidemiological cohort study involving 3268 patients with type 2 diabetes followed for a mean follow-up of 6.5 years, the total rate of fatal and nonfatal events was lower in SMBG patients than in non-SMBG patients: 2.7 vs 4.6% (P = .004) and 7.2 vs 10.4% (P = .002), respectively.[75] However, conflicting results from the Fremantle Diabetes Study suggest that further research is needed.[76]
Modern blood glucose monitoring meters have made it easier for patients to adhere to testing schedules, because they are simpler to use, more compact, less intrusive, and less painful than ever before. Some devices are noninvasive, while others allow blood sampling from sites other than the finger. There are more than 20 types of meters available, varying in size, weight, and memory. Most of the recent models have a data port, enabling electronic transfer of results to a computer, and involve only a 1- or 2-step process.[73, 77–80]
Continuous Blood Glucose Monitoring
Continuous blood glucose monitoring (CBGM) devices are also available (Table 4), and there have been exciting developments in this area in the past few years. CBGM meters provide maximal information about changes in blood glucose levels, allowing the patient to make informed decisions about their insulin dose adjustments.[81]
Table 4.
Examples of Continuous Blood Glucose Monitoring Devices Available[81]
| Device | Special features | Manufacturer |
|---|---|---|
| Continuous Glucose Monitoring System (CGMS) Gold | First product on the market, avoids arm implantation | Medtronic |
| GlucoWatch G2 Biographer | Needle-free, real-time readings, out-of-range alerts | Cygnus |
| Guardian Telemetered Glucose Monitoring System | Out-of-range alarm, avoids arm implantation | Medtronic |
| REAL-Time System | Real-time wireless sensor for use with an insulin pump | Medtronic |
| GlucoDay | Real-time readings, infrequent calibrations | Menarini Diagnostics Research |
| DexCom STS continuous glucose monitoring system | Real time sensor | Dexcom Inc |
| Pendra | Noninvasive, measures glucose in blood, not interstitial fluid | Pendragon Medical |
| FreeStyle Navigator Continuous Glucose Monitoring System | Out-of-range alarm, avoids abdominal wall implantation | Abbott |
Adapted with permission from Klonoff.[81]
The MiniMed Paradigm REAL-Time System (Medtronic MiniMed Inc; Northridge, California) is the first product to combine an insulin pump with a wireless continuous interstitial glucose monitor similar to that of the Guardian device (Medtronic MiniMed Inc.). When inserted under the skin, the device takes glucose readings from interstitial fluid every 5 minutes, helping patients take immediate corrective or preventive action to maintain healthy glucose levels.
Real-time CBGM has been associated with beneficial effects on glycemic control in clinical studies. In a 12-week observational study, significant reductions in HbA1c of 0.4% (P < .0001) were achieved in 140 outpatients using a real-time sensor device (STS System; DexCom Inc; San Diego, California) to supplement SMBG data; improvements occurred regardless of whether patients had type 1 or 2 diabetes or whether they were being treated by MDI or CSII.[82] Similarly successful results were obtained in 10 children (median age of 14.5 years) with type 1 diabetes who had used an insulin pump for more than a year and were studied over 4 weeks using a sensor-augmented pump system (MiniMed Paradigm REAL-Time System).[83] There was a mean decrease in HbA1c from 8.1% to 7.8%, a mean of 3.2 changes per patient in the treatment regimen, and a reduction in hypoglycemic episodes; none of the patients were overwhelmed or confused by the system.[83]
Future developments could include a closed-loop system in which integrated pump/monitor systems would automatically control insulin levels and act as an “artificial pancreas.”[32]
Barriers and Solutions to Effective Therapy
Of course, all the advances that have been made in the treatment of type 2 diabetes count for nothing if patients have a psychological resistance to initiating insulin therapy. Future efforts to improve the quality of diabetes control must therefore focus on overcoming barriers to both insulin treatment and effective dosing. The developments in insulin delivery discussed above can help achieve these goals and thus increase the potential for achieving HbA1c targets.
The main problems with initiation and maintenance of insulin therapy, along with solutions to these concerns, are outlined below.
Barrier: Belief That Insulin Causes Complications or Death
Solution: Endogenous insulin secretion is mimicked more closely by insulin analogs than by human insulin preparations. The convenient dose timings allowed by insulin analogs and by the use of delivery devices increase the likelihood of compliance and optimum glycemic control and so help reduce the potential for diabetes-related complications.[36,40,41,84]
Barrier: Fear of Hypoglycemia and Weight Gain
Solution: Both rapid and long-acting analogs are associated with lower rates of hypoglycemia compared with human insulins.[19,85–89] This may also help reduce the potential for weight gain as patients are less likely to snack if the fear of hypoglycemia is reduced.[88,90]
The basal analogs detemir and, to a lesser extent, glargine are associated with less weight gain than NPH insulin.[89,91]
Barrier: Perception of Insulin Treatment as Complicated and Difficult
Solution: Rapid-acting analogs can be injected within 15 minutes of mealtimes rather than at least 30 minutes before as with regular human insulin, while long-acting basal analogs need usually be given only once daily, at the same time each day.
Modern delivery devices with modern insulins make administration simpler, less painful, and more accurate, even for people with visual impairment and/or dexterity problems. Pens have the added advantage of being simpler to use and more discreet compared with a vial and syringe, and they have been proven to improve adherence.[40]
Dosage simplification has also been associated with improved compliance,[92] and this can be achieved in patients with regular eating patterns by the use of premixed insulin analog formulations.
Barrier: Human Factors
Solution: Patients are more likely to achieve optimal glycemic control if they have a good understanding of their condition and the importance of regular monitoring and feedback. Patient education, log books and telemedicine systems can help achieve this understanding.
Future Perspectives
The treatment of diabetes is continually evolving and a variety of new insulin delivery options are currently under investigation. An oral enteric insulin capsule formulation has produced promising results in phase 2 testing,[93] while an oral spray for buccal absorption has also shown potential as an add-on therapy for use with oral agents.[94]
Other delivery systems under development focus on intranasal, transdermal, ocular, and rectal routes, while innovative treatment solutions such as islet cell transplantation, gene therapy, and an antidiabetic vaccine are also being actively explored.[35,95,96]
Conclusions
The long-term benefits to patients with diabetes of maintaining optimal glycemic control within recommended limits has now been firmly established in key landmark studies.[2,3,17,19–22] Fortunately for patients, the development of the new insulin analogs, combined with innovative insulin devices and SMBG systems, makes this goal more achievable than ever before. The involvement and cooperation of patients is crucial to attaining these goals, and the benefits of patient education, together with the use of chronic disease management tools such as log books, cannot be underestimated.
The contribution of insulin analogs to glycemic control is further enhanced by the range of delivery devices in which they are available. The most widely used of these, prefilled pens and cartridge pens, help ensure compliance by allowing patients to administer their insulin discreetly, simply, and accurately. Exciting developments in SMBG technology, including an integrated pump/CBMG system, have also increased the potential for optimal glycemic control.
Insulin therapy has evolved, and by informing and reassuring patients about currently available treatment options, healthcare professionals can break down many of the barriers to achieving optimal glycemic control.
Acknowledgements
Jackie Mayne and Emma Campbell of Bioscript Stirling Ltd provided writing and editing support funded by Novo Nordisk.
Footnotes
Reader Comments on: Diabetes Care – Insulin Delivery in a Changing World See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at alan.marcus@medtronic.com or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
References
- 1.Riddle MC. Timely initiation of basal insulin. Am J Med. 2004;116:3S–9S. doi: 10.1016/j.amjmed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Lebovitz HE, Austin MM, Blonde L, et al. ACE/AACE consensus conference on the implementation of outpatient management of diabetes mellitus: consensus conference recommendations. Endocr Pract. 2006;12:6–12. doi: 10.4158/EP.12.S1.6. [DOI] [PubMed] [Google Scholar]
- 3.Stratton IM, Adler AI, Neil AW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen J. Insulins. Pharmacist's Letter/Prescriber's Letter. 2006;22:1–8. [Google Scholar]
- 5.Mooradian AD, Bernbaum M, Albert SG. Narrative review: a rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125–134. doi: 10.7326/0003-4819-145-2-200607180-00010. [DOI] [PubMed] [Google Scholar]
- 6.Levy P. Insulin analogs or premixed insulin analogs in combination with oral agents for treatment of type 2 diabetes. MedGenMed. 2007;9:12. [PMC free article] [PubMed] [Google Scholar]
- 7.Burke JP, Williams K, Gaskill SP, et al. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from Antonio Heart Study. Arch Intern Med. 1999;159:1450–1456. doi: 10.1001/archinte.159.13.1450. [DOI] [PubMed] [Google Scholar]
- 8.Hardy LR, Bell RA. An epidemiological perspective on type 2 diabetes among adult men. Diabetes Spectrum. 2004;17:208–214. [Google Scholar]
- 9.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Prevalence of diagnosed diabetes by age, United States, 1980–2005. 2007 Available at: http://www.cdc.gov/diabetes/statistics/prev/national/figbyage.htm. Accessed January 3, 2008.
- 11.Miller JL, Silverstein JH. The management of type 2 diabetes mellitus in children and adolescents. J Ped Endocrinol Metab. 2005;18:111–123. doi: 10.1515/JPEM.2005.18.2.111. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. National Diabetes Fact Sheet. United States: 2005. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. Accessed December 12, 2007. [Google Scholar]
- 13.Risso A, Mercuri F, Quagliaro L, et al. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab. 2001;281:E924–E930. doi: 10.1152/ajpendo.2001.281.5.E924. [DOI] [PubMed] [Google Scholar]
- 14.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 15.Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 16.Service FJ, O'Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia. 2001;44:1215–1220. doi: 10.1007/s001250100635. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association. Standards of medical care in diabetes – 2007. Diabetes Care. 2007;30:S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 18.Grant RW, Buse JB, Meigs JB, University HealthSystem Consortium (UHC) Diabetes Benchmarking Project Team Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care. 2005;28:337–442. doi: 10.2337/diacare.28.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 20.ACE/AACE Diabetes Road Map Task Force. Road map for the prevention and treatment of type 2 diabetes. doi: 10.4158/EP.13.3.260. September 2005. Available at: http://www.aace.com/meetings/consensus/odimplementation/roadmap.pdf. Accessed December 12, 2007. [DOI] [PubMed]
- 21.Feld S, Hellman R, Dickey RA, et al. The American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management: 2002 update. Endocr Pract. 2002;8:40–82. [Google Scholar]
- 22.Shichiri M, Kishikawa H, Ohkubo Y, et al. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23:B21–B29. [PubMed] [Google Scholar]
- 23.Palumbo P. The case for insulin treatment early in type 2 diabetes. Cleve Clin J Med. 2004;71:385–405. doi: 10.3949/ccjm.71.5.385. [DOI] [PubMed] [Google Scholar]
- 24.Rubin RR, Peyrot M, Siminerio LM, International DAWN Advisory Panel Health care and patient-reported outcomes: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Care. 2006;29:1249–1255. doi: 10.2337/dc05-2494. [DOI] [PubMed] [Google Scholar]
- 25.Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695–700. doi: 10.2337/diacare.24.4.695. [DOI] [PubMed] [Google Scholar]
- 26.Funnell MM. Overcoming obstacles: collaboration for change. Eur J Endocrinol. 2004;151:T19–T22. doi: 10.1530/eje.0.151t019. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Age-adjusted rates of annual dilated-eye exam, daily self-monitoring of blood glucose, foot examination in the last year, and doctor visit for diabetes in the last year per 100 adults with diabetes, United States, 1994–2004. Available at: http://www.cdc.gov/diabetes/statistics/preventive/tx.htm. Accessed December 12, 2007.
- 28.Saudek CD, Derr RL, Kalyani RR. Assessing glycemia in diabetes using self-monitoring blood glucose and hemoglobin A1c. JAMA. 2006;295:1688–1698. doi: 10.1001/jama.295.14.1688. [DOI] [PubMed] [Google Scholar]
- 29.Klonoff DC. Diabetes and telemedicine: s the technology sound, effective, cost-effective and practical? Diabetes Care. 2003;26:1626–1628. doi: 10.2337/diacare.26.5.1626. [DOI] [PubMed] [Google Scholar]
- 30.Wojcicki JM, Ladyzynski P, Krzymien J, et al. What we can really expect from telemedicine in intensive diabetes treatment: results from 3-year study on type 1 pregnant diabetic women. Diabetes Tech Ther. 2001;3:581–589. doi: 10.1089/15209150152811207. [DOI] [PubMed] [Google Scholar]
- 31.Rutten GEHM, Maaijen J, Valkenburg ACH, et al. The Utrecht Diabetes project: telemedicine support improves GP care in Type 2 diabetes. Diabet Med. 2001;18:459–463. doi: 10.1046/j.1464-5491.2001.00491.x. [DOI] [PubMed] [Google Scholar]
- 32.Hernando ME, García G, Gómez EJ, et al. Intelligent alarms integrated in a multi-agent architecture for diabetes management. Trans Instit Measurement Control. 2004;26:185–200. [Google Scholar]
- 33.Chase HP, Pearson JA, Wightman C, et al. Modem transmission of glucose values reduces the costs and need for clinic visits. Diabetes Care. 2003;26:1475–1479. doi: 10.2337/diacare.26.5.1475. [DOI] [PubMed] [Google Scholar]
- 34.Shea S, Weinstock RS, Starren J, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically undeserved patients with diabetes mellitus. J Am Med Inform Ass. 2006;13:40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flood T. Advances in insulin delivery systems and devices: beyond the vial and syringe. Insulin. 2006;1:99–108. [Google Scholar]
- 36.Flood TM. Appropriate use of insulin analogs in an increasingly complex type 2 diabetes mellitus (T2DM) landscape. J Fam Pract. 2007;56:S1–S12. [PubMed] [Google Scholar]
- 37.Grajower MM, Fraser CG, Holcombe JH, et al. How long should insulin be used once a vial is started? Diabetes Care. 2003;26:2665–2669. doi: 10.2337/diacare.26.9.2665. [DOI] [PubMed] [Google Scholar]
- 38.Robertson KE, Glazer NB, Campbell RK. The latest developments in insulin injection devices. Diabetes Educ. 2000;26:135–152. doi: 10.1177/014572170002600114. [DOI] [PubMed] [Google Scholar]
- 39.American Diabetes Association. Continuous subcutaneous insulin infusion. Diabetes Care. 2004;27:S110. doi: 10.2337/diacare.27.2007.s110. [DOI] [PubMed] [Google Scholar]
- 40.Korytkowski M, Niskanen L, Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27:S89–S100. doi: 10.1016/j.clinthera.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 41.Stockl K, Ory C, Vanderplas A, et al. An evaluation of patient preference for an alternative insulin delivery system compared to standard vial and syringe. Curr Med Res Opin. 2007;23:133–146. doi: 10.1185/030079906X159524. [DOI] [PubMed] [Google Scholar]
- 42.Lee WC, Balu S, Cobden D, et al. Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther. 2006;28:1712–1725. doi: 10.1016/j.clinthera.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Medicare. Formulary finder for prescription drug plans. Available at: http://formularyfinder.medicare.gov/formularyfinder. Accessed December 12, 2007.
- 44.Levemir formulary coverage. Available at: http://www.levemir-us.com/hcp/payingCoverage.asp. Accessed December 12, 2007.
- 45.Reimbursement information. Paying for NovoLog Mix 70/30. Available at: http://www.novologmix70-30.com/reimbursement.asp. Accessed December 12, 2007.
- 46.Graff MR, McClanahan MA. Assessment by patients with diabetes mellitus of two insulin pen delivery systems versus a vial and syringe. Clin Ther. 1998;20:486–496. doi: 10.1016/s0149-2918(98)80058-1. [DOI] [PubMed] [Google Scholar]
- 47.Rubin RR, Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27:2495–2497. doi: 10.2337/diacare.27.10.2495. [Erratum in: Diabetes Care. 2004;27:3032] [DOI] [PubMed] [Google Scholar]
- 48.Martin JM, Llewelyn JA, Ristic S, et al. Acceptability and safety of a new 3.0 ml re-usable insulin pen (HumaPen) in clinical use. Diabetes Nutr Metab. 1999;12:306–309. [PubMed] [Google Scholar]
- 49.Lawton B, Berg B. Comparative evaluation of FlexPen, a new prefilled insulin delivery system, among patients and healthcare professional. Diabetes. 2001;50:A440. [Google Scholar]
- 50.Niskanen L, Jensen LE, Rastam J, et al. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther. 2004;26:531–540. doi: 10.1016/s0149-2918(04)90055-0. [DOI] [PubMed] [Google Scholar]
- 51.Coscelli C, Lostia S, Lunetta M, et al. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 52.Rex J, Jensen KH, Lawton SA. A review of 20 years' experience with the Novopen family of insulin injection devices. Clin Drug Invest. 2006;26:367–401. doi: 10.2165/00044011-200626070-00001. [DOI] [PubMed] [Google Scholar]
- 53.Korytkowski M, Bell D, Jacobsen C, et al. FlexPen Study Team A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 54.Einhorn D. Advances in diabetes for the millennium: insulin treatment and glucose monitoring. MedGenMed. 2004;6:8. [PMC free article] [PubMed] [Google Scholar]
- 55.Schade DS, Valentine V. Are insulin pumps underutilized in type 1 diabetes? No. Diabetes Care. 2006;29:1453–1455. doi: 10.2337/dc06-0468. [DOI] [PubMed] [Google Scholar]
- 56.Linkeschova R, Raoul M, Bott U, et al. Less severe hypoglycaemia, better metabolic control, and improved quality of life in Type 1 diabetes mellitus with continuous subcutaneous insulin infusion (CSII) therapy: an observational study of 100 consecutive patients followed for a mean of 2 years. Diabet Med. 2002;19:746–751. doi: 10.1046/j.1464-5491.2002.00713.x. [DOI] [PubMed] [Google Scholar]
- 57.Raskin P, Bode BW, Marks JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003;26:2598–2603. doi: 10.2337/diacare.26.9.2598. [DOI] [PubMed] [Google Scholar]
- 58.Lane WS. Use of U-500 regular insulin by continuous subcutaneous insulin infusion in patients with type 2 diabetes and severe insulin resistance. Endocr Pract. 2006;12:251–256. doi: 10.4158/EP.12.3.251. [DOI] [PubMed] [Google Scholar]
- 59.Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. Br Med J. 2002;324:1–6. doi: 10.1136/bmj.324.7339.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care. 2003;26:1079–1087. doi: 10.2337/diacare.26.4.1079. [DOI] [PubMed] [Google Scholar]
- 61.Retnakaran R, Hochman J, Hans deVries J, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27:2590–2596. doi: 10.2337/diacare.27.11.2590. [DOI] [PubMed] [Google Scholar]
- 62.Nahata L. Insulin therapy in pediatric patients with type 1 diabetes: continuous subcutaneous infusion versus multiple daily injections. Clin Pediatr (Phila) 2006;45:503–508. doi: 10.1177/0009922806290565. [DOI] [PubMed] [Google Scholar]
- 63.American Diabetes Association. Insulin administration. Diabetes Care. 2004;27:S106–S109. doi: 10.2337/diacare.27.2007.s106. [DOI] [PubMed] [Google Scholar]
- 64.Hollander PA. Evolution of a pulmonary insulin delivery system (Exubera) for patients with diabetes. MedGenMed. 2007;9:45. [PMC free article] [PubMed] [Google Scholar]
- 65.Rave K, Bott S, Heinemann L, et al. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care. 2005;28:1077–1082. doi: 10.2337/diacare.28.5.1077. [DOI] [PubMed] [Google Scholar]
- 66.Johnson S. Exubera blown off by Pfizer. San Jose Mercury News. October 19, 2007.
- 67.Skyler JS, Jovanovic L, Klioze S, et al. Inhaled Human Insulin Type 1 Diabetes Study Group Two-year safety and efficacy of inhaled human insulin (Exubera) in adult patients with type 1 diabetes. Diabetes Care. 2007;30:579–585. doi: 10.2337/dc06-1863. [DOI] [PubMed] [Google Scholar]
- 68.Nealon A. Pharmaceutical Business Review. Available at: http://www.novonordisk.com/include/asp/exe_news_attachment.pdf?sAttachmentGUID=1531cc83-f75c-4368-995e-f0667112304f. Accessed January 18, 2008.
- 69.Monnier L, Lapinski H, Colette C. Contributions of fasting and post-prandial plasma glucose increments to the overall diurnal hyperglycaemia of type 2 diabetes: variations with increasing levels of HbA1c. Diabetes Care. 2003;269:881–885. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 70.Woerle HJ, Pimenta WP, Meyer C, et al. Diagnostic and therapeutic implications of relationships between fasting, 2-hour postchallenge plasma glucose and haemoglobin A1c values. Arch Intern Med. 2004;164:1627–1632. doi: 10.1001/archinte.164.15.1627. [DOI] [PubMed] [Google Scholar]
- 71.Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997;20:1822–1826. doi: 10.2337/diacare.20.12.1822. [DOI] [PubMed] [Google Scholar]
- 72.American Association of Diabetes Educators. AADE position statement. Self-monitoring of blood glucose: benefits and utilization. Diabetes Educ. 2006;32:835–847. doi: 10.1177/0145721706295873. [DOI] [PubMed] [Google Scholar]
- 73.Benjamin EM. Self-monitoring of blood glucose: the basics. Clin Diabetes. 2002;20:45–47. [Google Scholar]
- 74.Karter AJ, Ackerson IM, Darbinian JA, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes register. Am J Med. 2001;111:1–9. doi: 10.1016/s0002-9343(01)00742-2. [DOI] [PubMed] [Google Scholar]
- 75.Martin S, Schneider B, Heinemann L, et al. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia. 2006;49:271–278. doi: 10.1007/s00125-005-0083-5. [DOI] [PubMed] [Google Scholar]
- 76.Davis WA, Bruce DG, Davis ME. Does self-monitoring of blood glucose improve outcome in type 2 diabetes? The Fremantle Diabetes Study. Diabetologia. 2007;50:510–515. doi: 10.1007/s00125-006-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta M, Vincze G, Lopez DA. Emerging technologies in diabetes care. US Pharmacist. 2007;11:11. [Google Scholar]
- 78.U.S. Food and Drug Administration. Glucose meters & diabetes management. Available at: http://www.fda.gov/diabetes/glucose.html. Accessed December 12, 2007.
- 79.Mastrototaro JJ, Cooper KW, Soundararajan G, et al. Clinical experience with an integrated continuous glucose sensor/insulin pump platform: a feasibility study. Adv Ther. 2006;23:725–732. doi: 10.1007/BF02850312. [DOI] [PubMed] [Google Scholar]
- 80.Gabbay RA. New developments in home glucose monitoring: minimizing pain. Can J Diabetes. 2003;27:271–276. [Google Scholar]
- 81.Klonoff DC. Continuous glucose monitoring. Diabetes Care. 2005;28:1231–1239. doi: 10.2337/diacare.28.5.1231. [DOI] [PubMed] [Google Scholar]
- 82.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1c with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Tech Ther. 2007;9:203–210. doi: 10.1089/dia.2007.0205. [DOI] [PubMed] [Google Scholar]
- 83.Halvorson M, Carpenter S, Kaiserman K, et al. A pilot trial in pediatrics with the sensor-augmented pump: combining real-time continuous blood glucose monitoring with the insulin pump. J Pediatr. 2007;150:103–105. doi: 10.1016/j.jpeds.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 84.White JR, Davis SN, Cooppan R, et al. Clarifying the role of insulin in type 2 diabetes management. Clin Diabetes. 2003;21:14–21. [Google Scholar]
- 85.Bretzel RG, Arnolds S, Medding J, et al. A direct efficacy and safety comparison of insulin aspart, human soluble insulin, and human premix insulin (70/30) in patients with type 2 diabetes. Diabetes Care. 2004;27:1023–1027. doi: 10.2337/diacare.27.5.1023. [DOI] [PubMed] [Google Scholar]
- 86.Peterson GE. Intermediate and long-acting insulins: a review of NPH insulin, insulin glargine and insulin detemir. Curr Med Res Opin. 2006;22:2613–2619. doi: 10.1185/030079906X154178. [DOI] [PubMed] [Google Scholar]
- 87.Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–3086. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 88.Raslova K, Bogoev M, Razc I, et al. Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract. 2004;66:193–201. doi: 10.1016/j.diabres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 89.Hermansen K, Davies M, Derezinski T, et al. A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin naive people with type 2 diabetes. Diabetes Care. 2006;29:1269–1274. doi: 10.2337/dc05-1365. [DOI] [PubMed] [Google Scholar]
- 90.Rosenstock J, Schwartz SL, Clark CM, Jr, et al. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631–636. doi: 10.2337/diacare.24.4.631. [DOI] [PubMed] [Google Scholar]
- 91.Dornhorst A, Luddeke HJ, Sreenan S, et al. PREDICTIVE Study Group Safety and efficacy of insulin detemir in clinical practice: 14-week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVE European cohort. Int J Clin Pract. 2007;61:523–528. doi: 10.1111/j.1742-1241.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 92.Kripalani S, Yao X, Haynes B. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167:540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 93.Clement S, Dandona P, Still JG, et al. Oral modified insulin (HIM2) in patients with type 1 diabetes mellitus: results from a phase I/II clinical trial. Metabolism. 2004;53:54–58. doi: 10.1016/j.metabol.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 94.Guevara-Aguirre J, Guevara M, Saavedra J, et al. Beneficial effects of addition of oral spray insulin (Oralin) on insulin secretion and metabolic control in subjects with type 2 diabetes mellitus suboptimally controlled on oral hypoglycemic agents. Diabetes Technol Ther. 2004;6:1–8. doi: 10.1089/152091504322783341. [DOI] [PubMed] [Google Scholar]
- 95.Xuan B, McClellan DA, Moore R, et al. Alternative delivery of insulin via eye drops. Diabetes Technol Ther. 2005;7:695–698. doi: 10.1089/dia.2005.7.695. [DOI] [PubMed] [Google Scholar]
- 96.Shaikh IM, Jadhav KR, Ganga S, et al. Advanced approaches in insulin delivery. Curr Pharm Biotechnol. 2005;6:387–395. doi: 10.2174/138920105774370599. [DOI] [PubMed] [Google Scholar]


