Abstract
Candida albicans vertebral osteomyelitis is rare. Three cases are presented. Without antifungal treatment, they developed spinal collapse and neurological deterioration within 3–6 months from the onset of symptoms. There was a delay of 4.5 and 7.5 months between the onset of symptoms and surgery. All patients were managed with surgical debridement and reconstruction and 12-week fluconazole treatment. The neurological deficits resolved completely. The infection has not recurred clinically or radiologically at 5–6 years follow-up. Although rare, Candida should be suspected as a causative pathogen in cases of spinal osteomyelitis. Without treatment the disease is progressive. As soon as osteomyelitis is suspected, investigations with MRI and percutaneous biopsy should be performed followed by medical therapy. This may prevent the need for surgery. However, if vertebral collapse and spinal cord compression occurs, surgical debridement, fusion and stabilisation combined with antifungal medications can successfully eradicate the infection and resolve the neurological deficits.
Keywords: Spinal osteomyelitis, Vertebral osteomyelitis, Candida albicans, Fungal infection
Introduction
Fungal osteomyelitis is rare. Connor in 1928 first reported a patient with fungal Monilia psilosis osteomyelitis [4]. In 1932 Keating described four other patients with Monilia osteomyelitis involving long bone sites [14]. There was no report of Candida osteomyelitis in the literature till 1970 [22]. In a review of the literature, about 60% of Candida osteomyelitis occurred in the spine [11]. Early recognition of the disease may be delayed because of its unusual presentation. Once the vertebral destruction is evident on radiography the patient either has back pain with impending cord compression symptoms or develops various grades of neurological deficit. We report the radiological findings and progression of three cases of spinal candidiasis that were not treated for the fungal infection till late in the course of the disease. The outcome of combined surgical and medical treatment with 5–6 years follow-up is presented.
Materials and methods
Case 1
A 70-year-old female presented with neck, inter-scapular and right-sided radicular pain radiating to the fingers consistent with C6 dermatomal distribution. Neurological examination was normal. Radiographs revealed spondylo-arthropathy of the cervical spine at C5/6 and C6/7. She was treated with oral non-steroidal anti-inflammatory medications (NSAIDs) and cervical collar. Three months later she developed myelopathic symptoms with weakness of both lower limbs and an unsteady gait. On examination, there was generalised weakness of MRC grade 4 in all muscle groups and positive myelopathic signs in all extremities. The investigations’ results are summarised in Table 1.
Table 1.
Summary of investigations
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Haemoglobin measurement/s (g %) | 9 | 10/8.3 | 11/9.5 |
| Neutrophil count/s (109 per mm3) | 14 | 12.6/14.7 | 12.2/13.5 |
| ESR measurement/s (mm/h) | 40 | 120/125 | 27/32 |
| Blood cultures for bacteria including brucella and salmonella, PPD, PSA, CEA, CA19-9 | Negative | Negative | Negative |
| Biopsy | Intraoperative | Fluoroscopy-guided aspiration at 2 months and CT-guided biopsy at 5 months | Intraoperative |
| Biopsy cultures for Gram positive, Gram negative, anaerobes and mycobacterium. Ziehl–Nielsen acid-fast stain for tuberculosis | Negative | Negative | Negative |
| Candida cultures on Sabouraud dextrose agar (SDA) after 3 days at 30°C. Yeast isolates identified by the germ tube test in rabbit plasma and confirmed by assimilation of carbon sources utilising the API 20C (BioMerieux, Mercy l’Etoile, France) | Moderate growth of C. albicans | Aspiration culture: negativeCT-guided biopsy: yeast-like cells on gram stain and moderate growth of C. albicans | Moderate growth of C. albicans |
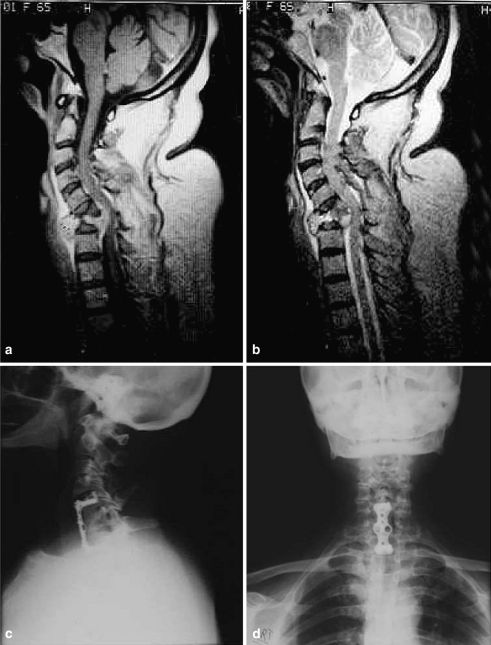
Plain radiographs and MRI confirmed C6 osteolysis and collapse with spinal cord compression (Fig. 1a). She refused surgery for 6 weeks during which she had deteriorated neurologically by one grade power (now MRC grade 3). Further MRI confirmed increased abscess and spinal cord compression (Fig. 1b). She underwent C6 corpectomy and debridement of cheesy material and infected granulation tissue. Anterior cervical fusion with iliac crest bone graft and locked plate stabilisation was performed. She was treated empirically with intravenous cefuroxime and gentamycin till Candidal infection was confirmed 3 days later.
Fig. 1.
Case 1. a Gadolinium-enhanced T1-weighted MRI image. There are diffuse soft tissue masses anterior and posterior to the vertebral body with significant compression of the spinal cord at C6. b T2-weighted MRI image performed 6 weeks after previous scan. There is no abnormal signal in the discs adjacent to the affected vertebral body. c, d Lateral and AP cervical radiograph 5 years after surgery
She was subsequently treated with intravenous fluconazole 400 mg/day for 2 weeks then orally for further 10 weeks. At 6 weeks she had recovered her neurological deficit with complete return of motor function in all the limbs and was walking normally. She had remained asymptomatic since with no recurrence of infection clinically or radiologically 5 years after surgery (Fig. 1c, d).
Case 2
A 65-year-old male farmer with insulin-dependent diabetes mellitus was admitted with malaise and back pain of gradual onset. There was no history of fever and night sweats. On clinical examination he was tender over his lower thoracic spine but there were no neurological abnormalities. The investigations’ results are summarised in Table 1. Radiographs of thoraco-lumbar spine did not reveal any lesion. The patient was discharged home with analgesia and iron.
Two months later he presented with persistent back pain and weight loss. Magnetic resonance imaging (MRI) revealed bone destruction of the adjacent margins of T11 and T12 and disorganised intervertebral disc. Needle aspiration was performed but failed to reveal any organism. The patient was treated empirically for possible bacterial osteomyelitis with imipenem and tetracycline but his back pain persisted.
Three months later, he presented with bilateral L2–L4 myotomal weakness of MRC grade 4 power. A follow-up MRI of the thoraco-lumbar spine revealed further bony destruction with anterior wedging of T11/12. There was evidence of paraspinal collection with epidural compression of the dural sac.
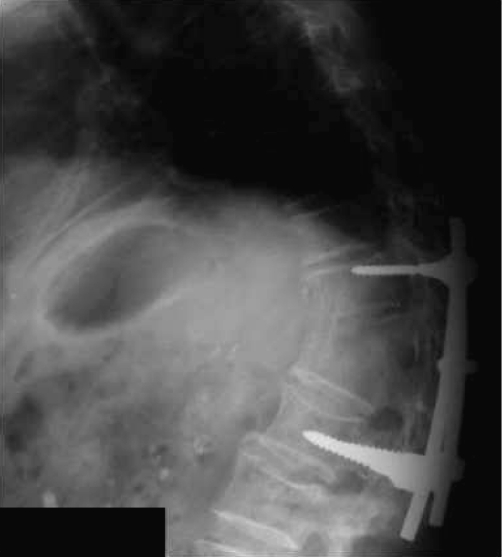
A computerised tomography (CT) guided needle aspiration revealed a thick, brownish yellow pus-like material and cultures confirmed Candidal infection. The patient was treated with intravenous fluconazole (400 mg/day) for 2 weeks prior to surgical debridement and posterior instrumented fusion from T10 to L1. The patient had recovered completely before discharge from hospital 2 weeks later. He subsequently continued oral fluconazole for 3 months. He had remained asymptomatic since with no recurrence of infection clinically or radiologically 6 years after surgery (Fig. 2).
Fig. 2.
Lateral thoraco-lumbar radiograph 6 years after surgery in case 2
Case 3
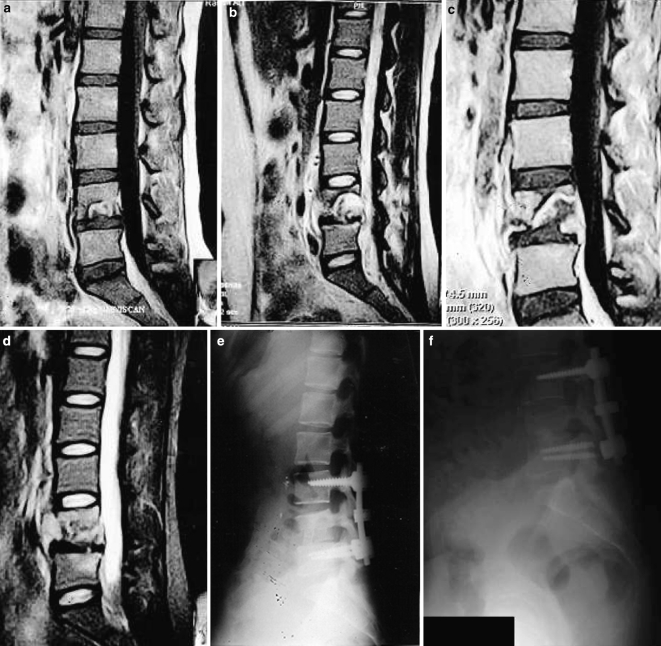
A 31-year-old female presented with a 3-month history of low back pain and right-sided sciatica radiating to the foot. There were no objective neurological deficits. The investigations’ results are summarised in Table 1. Radiographs of the lumbosacral spine were reported as normal. Subsequent MRI scan revealed a well-circumscribed lesion in the L4 vertebral body that was reported by a radiologist as a Schmorl’s node (Fig. 3a). She was treated with NSAIDs and physiotherapy. She continued to have persistent low back pain.
Fig. 3.
Case 3. a Gadolinium-enhanced T1-weighted MRI image showing increased signal in the inferior and posterior parts of the L4 vertebral body. b T2-weighted MRI image performed 3 months after previous scan. There is loss of anterior and posterior vertebral body heights with pathological fracture through the centre of the body. There is destruction of the L4 lower endplate. c, d Gadolinium-enhanced T1- and T2-weighted MRI just before surgery showing further collapse of the L4 vertebral body and spinal canal narrowing. There is increased signal in the affected vertebral body on both images. There is no significant involvement of the adjacent discs. There is anterior abscess formation. e Lateral lumbar radiograph immediately following surgery. f Lateral lumbar radiograph 5 years after surgery
Three months later she presented with severe pain radiating to both legs and inability to stand due to pain. On examination, straight leg raising was limited to 30° on the right and 45° on the left. There were no neurological deficits other than MRC grade 4 power in tibialis anterior muscle (L4). On repeat MRI, a pathologic lesion in L4 vertebral body was recognised (Fig. 3b).
She was offered surgical treatment but she refused that for 6 weeks. MRI scan was repeated before surgery (Fig. 3c, d). Through an anterior approach, she underwent L4/L5 debridement, decompression and iliac crest bone grafting at L4/L5. Pus in the psoas sheath was evacuated and irrigated. Through a posterior approach, she underwent pedicle screw instrumentation without fusion from L3 to L5 (Fig. 3e). The intra-operative cultures confirmed Candidal infection. She was treated with intravenous fluconazole 400 mg/day for 2 weeks followed by oral fluconazole for further period of 10 weeks. The legs motor weakness and pain resolved completely. On her last radiographs obtained 5 years after surgery (Fig. 3f), there is metalwork failure and she has mild low back pain but she continues to have no leg pain or neurologic deficits.
Discussion
Candida species are low virulence organisms that inhabit the skin and mucous membrane of humans. There is increasing incidence of disseminated and deep-seated Candida infections in immuno-compromised hosts [7, 23]. However, the fungal infection has been rarely suggested as cause of vertebral osteomyelitis [13]. In 1998, Anderamahr reported a case of lumbar spondylitis due to Candida and reviewed 31 adult cases with vertebral osteomyelitis from the literature [2]. Since then, further 14 cases with vertebral involvement were reported [9, 10, 18, 21, 23, 24], including three cases of Candida albicans vertebral osteomyelitis and discitis following laminectomy [18] which were attributed to wearing artificial nails by an operating room technician who was scrubbed for all these patients.
All our patients were not immuno-compromised although one was 70 year old and another was diabetic. There was no focus of Candidal infection apart from the spine in any of our patients. All had come from a suburban or rural community in Lebanon, eastern Mediterranean where tuberculosis and brucellosis are endemic, but all investigations for these and other bacterial infections were negative.
There are no non-invasive diagnostic tests or typical radiological findings in spinal candidiasis. Symptoms like low-grade fever, malaise and weight loss are non-specific. Similarly, anaemia, neutrophilia and raised ESR are also non-specific, representing a chronic inflammatory process although markedly elevated ESR is suspicious of infection. Before vertebral collapse occurred in our cases, the MRI features included absence of disc hyperintensity and preservation of the internuclear cleft on T2 weighted images [24] in addition to increased signal intensity on gadolinium-enhanced T1-weighted images.
The delay of anti-fungal medical or surgical treatment in our cases allowed us to evaluate the natural course of the disease. Candida affects the spinal segments probably through haematogenous dissemination [2, 7, 19] and once a focus develops in the vertebral body it continues an indolent course. In our cases, vertebral collapse and neurological deficits became apparent within 3–6 months from the onset of symptoms.
The delay in diagnosis between the onset of symptoms to the diagnosis of vertebral candidiasis has been reported as ranging from 1 month to several years [11, 22], with an average of 3.3 months. The reasons for delayed suspicion and diagnosis in our cases include: (a) Delay in presentation: case 3 presented 3 months after onset of symptoms. (b) Delay in investigations: An MRI was not obtained for 2–3 months after onset of symptoms in cases 1 and 2. A high index of suspicion and immediate MRI would have confirmed the pathologic lesions before vertebral collapse and neurological compromise developed. (c) Failure of percutaneous aspiration to confirm diagnosis: in case 2, the initial aspiration was negative for any organism and the patient was treated empirically with antibiotics for suspected bacterial infection till a further MRI and CT-guided biopsy confirmed Candidal infection 3 months later. Immediately repeating a large-calibre core percutaneous or open biopsy may have confirmed the diagnosis much earlier. (d) Failure of correct recognition of pathologic lesion on MRI: The first MRI in case 3 (Fig. 2a) was wrongly interpreted as “Schmorl’s” node in L4 causing further delay of three more months before the pathologic lesion was correctly diagnosed on a second MRI. If the initial MRI scan in case 3 was accurately interpreted as a pathologic lesion, the patient may have been successfully treated conservatively.
As soon as osteomyelitis is suspected, investigations with MRI and percutaneous biopsy should be performed followed by medical therapy. This may halt the progression of bony destruction and prevent the need for surgical treatment [1, 23]. Surgery is primarily indicated for failure of or relapse after conservative treatment, significant spinal collapse and neurological deficits [3].
Unfortunately, the infection was not suspected till vertebral collapse with spinal cord compression occurred in cases 1 and 2 and surgical treatment was then the best management to recover the neurological deficits [8]. In case 3, the L4 vertebra was severely affected with mild collapse and associated anterior and posterior abscesses and L4 weakness. The management options included conservative treatment with biopsy, medical therapy and bed rest; posterior surgical stabilisation and transpedicular biopsy with or without fusion; or anterior debridement of the abscesses, tissue diagnosis and fusion combined with posterior stabilisation. Combined anterior and posterior surgery was performed although there is controversy regarding the use of instrumentation in the presence of and for the treatment of spinal infection. However, there are multiple reports of the use of anterior debridement and bone grafting in combination with anterior or posterior instrumentation followed by intravenous antibiotics for fungal, pyogenic or tuberculous discitis and vertebral osteomyelitis [5, 12, 15, 17, 20]. Some authors have recommended primary reconstruction for all pyogenic infections of the spine to maximise eradication of the infection [6] and others have recommended surgery for aiding in diagnosis as well as decompressing and stabilising the spine at the same time [16]. At 5-year’s follow-up radiographs, the instrumentation has failed. This is likely due to the fact that instrumentation was performed at two levels but fusion was performed only at one level and the instrumentation was not removed before metalwork failure. Despite metalwork failure in that case, the use of instrumentation in combination with debridement and antifungal therapy was successful in eradicating the infection and resolving the neurological deficits in all cases.
Conclusions
Although rare, Candida should be suspected as a causative pathogen in cases of spinal osteomyelitis. It can occur without any predisposing risk factors. Without treatment the disease is progressive and leads to vertebral destruction and spinal cord and neural compression. As soon as osteomyelitis is suspected, investigations with MRI and percutaneous biopsy should be performed followed by medical therapy. This may halt the progression of bony destruction and prevent the need for surgery. However, if vertebral collapse and spinal cord compression occurs, surgical debridement, fusion and stabilisation combined with medical therapy can successfully eradicate the infection and resolve the neurological deficits.
References
- 1.Almekinders LC, Greene WB (1991) Vertebral Candida infections. A case report and review of the literature. Clin Orthop 267:174–178 [PubMed]
- 2.Andermahr J, Isenberg J, Prokop A, Rehm KE (1998) Candida spondylitis. Case report and review of literature [article in German]. Unfallchirurg 101:955–959 [DOI] [PubMed]
- 3.Burns J, Hemker T, Dahmen G (1986) Fungal spondylitis. Acta Orthop Scand 57:563–565 [DOI] [PubMed]
- 4.Conner CL (1928) Monilia from osteomyelitis. J Infect Dis 43:108–116
- 5.Curran MP, Lenke LG (1996) Torpulosis glabrata spinal osteomyelitis involving two contiguous vertebrae: a case report. Spine 21:866–870 [DOI] [PubMed]
- 6.Dietze DD Jr, Fressier RG, Jacob RP (1997) Primary reconstruction for spinal infections. J Neurosurg 86:981–989 [DOI] [PubMed]
- 7.Edwards JE, Turkel SB, Elder HA, Rand RW, Guze LB (1975) Haematogenous Candida osteomyelitis: report of three cases and review of literature. Am J Med 59:89–94 [DOI] [PubMed]
- 8.Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA (1983) Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg [Am] 65:19–29 [PubMed]
- 9.El-Zaatari MM, Hulten K, Fares Y et al (2002) Successful treatment of Candida albicans osteomyelitis of the spine with fluconazole and surgical debridement: case report. J Chemother 14:603–606 [DOI] [PubMed]
- 10.Garbino J, Schnyder I, Lew D et al (2003) An unusual cause of vertebral osteomyelitis: Candida species. Scand J Infect Dis 35:288–291 [DOI] [PubMed]
- 11.Gathe JC, Harris RL, Garland B, Bradshaw MW, Williams TW (1987) Candida osteomyelitis: report of five cases and review of literature. Am J Med 82:927–937 [DOI] [PubMed]
- 12.Hassan MG (2003) Anterior plating for lower cervical spine tuberculosis. Int Orthop 27:73–77 [DOI] [PubMed]
- 13.Hirschmann JV, Everett ED (1976) Candida vertebral osteomyelitis: case report and review of literature. J Bone Joint Surg [Am] 58:573–575 [PubMed]
- 14.Keating PM (1932) Fungus infection of bone and joint. South Med J 25:1072–1076
- 15.Korovessis P, Pestsinis G, Koureas G, Zacharatos S (2005) Posterior transcanal lumbar interbody fusion for septic vertebral fracture pseudarthrosis and sitting imbalance. Spine 30:E255–E258 [DOI] [PubMed]
- 16.Owen PG, Willis BK, Benzel EC (1992) Torulopsis glabrata vertebral osteomyelitis. J Spinal Disord 5:370–373 [DOI] [PubMed]
- 17.Ozgen S, Naderi S, Ozek MM, Pamir MN (2004) A retrospective review of cervical corpectomy: indications, complications and outcome. Acta Neurochir (Wien) 146:1099–1105 [DOI] [PubMed]
- 18.Parry MF, Grant B, Yukuna M et al (2001) Candida osteomyelitis and diskitis after spinal surgery: an outbreak that implicates artificial nail use. Clin Infect Dis 32:352–357 [DOI] [PubMed]
- 19.Pohjola-Sintonen S, Ruutu P, Tallroth K (1984) Hematogenous Candida spondylitis—a case report. Acta Med Scand 215:85–87 [PubMed]
- 20.Przybylski GJ, Sharan AD (2001) Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg 94(1 Suppl):1–7 [DOI] [PubMed]
- 21.Rodriguez D, Pigrau C, Almirante B et al (2003) Vertebral osteomyelitis due to Candida spp [article in Spanish]. Enferm Infecc Microbiol Clin 21:568–570 [DOI] [PubMed]
- 22.Waldvogel FA, Medoff G, Swartz MN (1970) Osteomyelitis: a review of clinical features, therapeutic consideration, and unusual aspects. N Engl J Med 282:198–206 [DOI] [PubMed]
- 23.Wang YC, Lee ST (2001) Candida vertebral osteomyelitis: a case report and review of the literature. Chang Gung Med J 24:810–815 [PubMed]
- 24.Williams RL, Fukui MB, Meltzer CC et al (1999) Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol 20:381–385 [PMC free article] [PubMed]