Abstract
Several choices are available for cervical interbody fusion after anterior cervical discectomy. A recent option is dense cancellous allograft (CS) which is characterized by an open-matrix structure that may promote vascularization and cellular penetration during early osseous integration. However, the biomechanical stability of CS should be comparable to that of the tricortical iliac autograft (AG) and fibular allograft (FA) to be an acceptable alternative to these materials. The purpose of this study was to compare the initial biomechanical stability of CS to that of AG and FA in a one-level anterior cervical discectomy and interbody fusion (ACDF) model. Twelve human cervical spines (C3–T1) were loaded in six modes of motion and evaluated under three conditions: (1) intact, (2) after ACDF using CS, AG, and FA in alternating sequences, and (3) after ACDF with anterior plating. Three reflective markers were placed on the adjacent vertebral bodies. Intervertebral motion was measured with a video-based motion-capture system (MacReflex, Qualisys, Sweden). Torques were applied to a maximum of 2.0 N m. The range-of-motion and neutral-zone values measured in each loading mode were compared. No graft material displayed significant differences in biomechanical stability in any of the tested loading modes, suggesting that the initial stability of CS is comparable to that of AG and FA. Anterior cervical plating significantly increased biomechanical stability in all modes.
Keywords: Biomechanical stability, Dense cancellous allograft, Tricortical iliac autograft, Fibular allograft, Anterior plating
Introduction
The Smith–Robinson technique for anterior cervical discectomy and fusion (ACDF) is widely accepted as an effective surgical treatment for patients with cervical degenerative disc disease or spondylosis. The addition of anterior cervical plate screw fixation to ACDF may provide the following advantages: (1) increased stability, (2) the prevention of graft-related complications, (3) an enhanced fusion rate, (4) the restoration of normal cervical lordosis, and (5) a decreased use of external rigid immobilization. Although there is debate regarding the use of anterior cervical plate fixation after one-level ACDF in the treatment of degenerative disease, recent retrospective studies illustrate the procedure’s safety and efficacy [16, 38, 40].
The best interbody fusions are characterized by biologic participation of the grafting materials. Because of its osteoinductive, osteoconductive, and osteogenic properties, autologous tricortical iliac crest (AG) graft has long been regarded as the gold standard for spinal fusion material [19]. However, AG requires a secondary surgical procedure for graft harvesting and is associated with not insignificant donor site morbidity [30, 32, 36]. Graft harvesting complications have been reported in up to 20% of patients undergoing ACDF with AG [22, 36].
The use of allograft or other graft substitutes can eliminate the second operation and its associated complications, and reduce operative time. Allograft is considered strongly osteoconductive but lacks osteoinductive and osteogenic properties. Concerns exist with respect to the source of allograft, the preservation and processing techniques utilized, and the risk of disease transmission [19, 43]. Although most clinical studies of cervical interbody grafts have reported that tricortical autografts result in a higher rate of radiological union and lower incidence of graft collapse than allograft, studies comparing autograft and allograft with anterior plating have not found significant differences between the two materials [1, 3, 7, 8, 19, 22, 28, 33, 34, 42–44]. Recent studies report the successful use of fibular allograft (FA) [3] in ACDF and instrumentation with acceptable rates of fusion and less postoperative pain [34, 43].
Considering the morbidity problems associated with autograft and the difficulty in achieving full cortical allograft incorporation, dense cancellous allograft may be a good alternative if it provides adequate stability. The Graftech™ dense cancellous cervical spacer (CS) is composed of allograft from load-bearing areas. Due to its trabecular bone structure, CS provides a larger surface area and open matrix for vascular and cellular penetration. When the trabecular spaces are filled with additional autograft or demineralized bone matrix, the spaces can enhance the osteoinductive signal. Although more than 25,000 dense cancellous allografts have been implanted over the past 2 years, no biomechanical stability studies of the material have been conducted. Balabhadra et al. [2] analyzed fusion success in 98 patients who underwent ACDF with CS and anterior plating. Successful fusion was observed in 96% of these patients at 12 months, and the average subsidence for a single-level ACDF was 2.0 mm.
The purpose of this study was to compare the initial biomechanical stability of CS in a one-level ACDF cadaver model to that of AG and FA, and to determine if anterior cervical plate screw fixation significantly alters this stability.
Materials and methods
Cadaveric specimen preparation and fixation
Twelve fresh human cadaveric cervical spines (C3–T1) were obtained from Science Care Anatomical (Phoenix, AZ, USA). The ages of the seven male and five female specimens were 63.1±6.1 years (range 52–76). Anteroposterior and lateral radiographs of the specimens were performed to exclude bony abnormalities, and bone mineral density measurements were obtained using a dual-energy X-ray absorptiometry scan (DEXA; Hologic QDR 4500A, Hologic Inc, Waltham, MA, USA). The bone mineral density of the C5–C6 level was 0.55±0.08 g/cm2 (range 0.44–0.69 g/cm2). The specimens for biomechanical testing were stored at −20°C until thawed to room temperature at the time of testing. They were kept moist during all procedures. The attached musculature was removed, with care taken to preserve the joint capsules, ligaments, discs, and bony structures. Specimen casting was accomplished by drilling and fixing several screws into the C3–C4 and C7–T1 end segments, which were primarily potted in polymethylmethacrylate (PMMA, COE tray plastic, GC America, Alsip, IL, USA) followed by secondary potting in polyester resin (Bondo, Atlanta, GA, USA). The potting fixtures for the C3–C4 and C7–T1 were attached to the upper and lower spine fixtures of the MTS loading frame (MTS 858 Minibionix, Eden Prairie, MN, USA), respectively. In this position, the correct orientation and motions between C4–C5, C5–C6, and C6–C7 were preserved.
Operative techniques
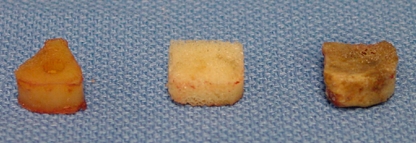
One-level anterior discectomy using the Smith–Robinson technique and interbody grafting, with and without anterior plate instrumentation, was performed at C5–C6. A rectangular window was cut in the anterior longitudinal ligament and anterior annulus. Pituitary forceps and curettes were used to perform a radical discectomy until the posterior longitudinal ligament was visualized. All possible compressive lesions, including the posterolateral portions of the hypertrophied uncinate processes and the posterior longitudinal ligament, were removed using a high-speed pneumatic drill and 1-mm Kerrison punches. Using a Caspar distractor system, a pre-shaped interbody graft was inserted with preservation of proper annular tension by disc height distraction of less than 2 mm [25]. Because both types of allograft were made by a freeze-drying process, the allografts were rehydrated prior to insertion. The grafts were placed just behind the anterior cortical margin of the adjacent vertebral bodies. The sizes of the inserted graft materials for the CS was 6–7 mm height (H) × 11 mm width (W) × 14 mm depth (D); AG, 6–7 mm (H) × 10 mm (W) × 13 mm (D); and FA, 6–7 mm (H) × 11 mm (W) × 11 mm (D) (Fig. 1). After testing the ACDF with an interbody graft alone, a rigid anterior plate (DOC plate system, DePuy Spine, Cleveland, OH, USA) was placed with 12- to 14-mm length screws.
Fig. 1.
Cervical interbody graft materials: freeze-dried fibular allograft (left), dense cancellous allograft (center), and tricortical iliac autograft (right)
Biomechanical testing
Stability was tested in six modes of motion: flexion, extension, right and left lateral bending, and right and left axial rotation. C4–C7 was loaded nondestructively using the MTS biomechanical testing machine (MTS 858 Minibionix, Eden Prairie, MN, USA). Nondestructive tests were performed under flexion (2.0 N m), extension (2.0 N m), lateral bending (2.0 N m), and axial rotation (2.0 N m) with an applied axial preload of 20 N. Angular displacements between C5 and C6 were captured using a video-based motion-capture system (MACReflex, Qualisys Inc, Sweden) by placing reflective markers on C5 and C6. In order to stabilize the viscoelastic effect, each mode of testing was performed three times with only the results of the third test being used. In each mode of loading, the range of motion (ROM) and neutral zone (NZ) were determined. The ROM was defined as the angular deformation in all directions at maximum load; the NZ was defined as the difference at zero load between the angular positions in all directions of the loading and unloading phases. ROM and NZ values for each specimen were determined under the following conditions: (1) intact; (2) after ACDF using CS, AG, and FA; and (3) after ACDF plus additional cervical fixation at C5–C6 with a rigid plate.
Statistical analysis
Because the number of specimens was limited and the data could not be assumed to be normally distributed, the average ROM and NZ values of each specimen group were determined. Nonparametric statistical methods were employed to ascertain statistically significant differences between the treatment groups. Paired comparisons between treatment groups were made by the use of the Wilcoxon pair tests. Statistical significance was set at P<0.05.
Results
For all specimens, the mean and standard deviation of ROM and NZ values are shown in Table 1. The ROM and NZ values for each individual specimen after treatment were normalized to those of their intact spine. Mean and standard deviation values of the ROM and NZ normalizations are shown in Figs. 2 and 3, respectively.
Table 1.
Biomechanical testing results of cervical interbody grafts with and without anterior plating
| Types of mode | Spinal specimen C5–3;-C6 motion | ||||||
|---|---|---|---|---|---|---|---|
| Intact | CS | CSP | AG | AGP | FA | FAP | |
| Flexion | |||||||
| ROM | 7.40±3.22 | 6.82±2.69 | 2.28±1.35*,** | 6.11±3.21 | 2.32±1.90*,** | 5.47±1.42 | 2.00±1.33*,** |
| NZ | 3.25±2.69 | 3.19±2.26 | 0.57±0.46*,** | 2.67±1.84 | 0.79±1.19*,** | 2.20±1.18 | 0.66±0.92*,** |
| Extension | |||||||
| ROM | 3.59±1.83 | 4.97±1.78* | 1.66±1.56*,** | 3.73±1.51 | 1.87±1.43*,** | 3.98±2.59 | 1.96±1.47*,** |
| NZ | 1.86±1.69 | 2.03±1.32 | 1.03±1.33*,** | 1.63±1.17 | 0.92±0.94*,** | 1.97±1.76 | 1.43±1.45* |
| Lateral bending | |||||||
| ROM | 4.70±1.31 | 5.25±3.31 | 3.53±2.96*,** | 4.35±2.06 | 3.54±2.15* | 4.48±3.21 | 3.62±2.94* |
| NZ | 1.58±0.97 | 2.04±1.60 | 1.30±1.41 | 1.70±1.15 | 1.31±1.02 | 1.74±1.21 | 1.32±1.45 |
| Axial rotation | |||||||
| ROM | 5.82±2.64 | 6.60±3.52 | 3.56±2.93*,** | 5.58±2.13 | 2.71±1.15*,** | 5.59±2.93 | 3.95±2.42* |
| NZ | 1.72±1.04 | 2.73±2.08 | 1.15±1.47*,** | 2.14±1.56 | 0.72±0.51*,** | 2.44±2.47 | 1.33±1.29** |
All data are presented as the mean ± standard deviation of the angle in degrees
*Significant difference between the intact spine and the corresponding treatment (P<0.05); **Significant difference between plating treatment and respective graft alone (CS vs. CSP, AG vs. AGP, FA vs. FAP) (P<0.05). No significant differences between plating treatments (CSP vs. AGP vs. FAP) (P<0.05) and between stand alone interbody grafts (CS vs. AG vs. FA) (P<0.05) were found
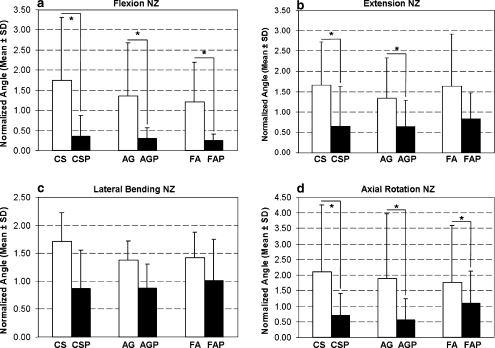
Fig. 2.
Normalized C5–C6 range of motion (ROM) for three different cervical interbody grafts without and with anterior plating in a flexion, b extension, c lateral bending, and d axial rotation. Significant differences (P<0.05) are shown with an asterisk
Fig. 3.
Normalized C5–C6 neutral zone (NZ) for three different cervical interbody grafts without and with anterior plating in a flexion, b extension, c lateral bending, and d axial rotation. Significant differences (P<0.05) are shown with an asterisk
ACDF with grafts alone (CS, AG, FA)
In the flexion mode, there were no significant differences in either ROM or NZ between the ACDF procedures with CS, FA, and AG and no significant differences in either ROM or NZ compared to the intact spine.
In the extension mode, there were no significant differences in either ROM or NZ between the ACDF procedures with CS, FA, and AG. With the exception of the ROM value for CS, which was significantly higher than that of the intact spine, no significant differences were found between any of the ROM and NZ values associated with the ACDF procedures without plating and those of the intact spine.
With respect to the ROM and NZ values measured in both the lateral bending and axial rotation modes, stability did not vary significantly between ACDF procedures or between these procedures and the intact spine.
ACDF with anterior plating (CSP, AGP, FAP)
In the flexion mode, all ACDF with plating regardless of graft imparted significantly more stability than was achieved by their respective unplated counterparts. All plating results in significantly more stability than that exhibited by the intact spine.
In the extension mode, both ROM and NZ values indicated that all ACDF procedures with anterior plating resulted in significantly more stability than that measured in the intact spine. In addition, ROM values indicated that plating (CSP, AGP, and FAP) imparted significantly more stability than did their respective counterparts without plating (CS, AG, and FA); NZ values suggested that CS and AG supplemented with plating are significantly more stable than CS and AG alone; however, FA with plating did not impart significantly more stability than FA alone.
In the lateral bending mode, when compared to the intact spine, ROM results indicate that all ACDF procedures with grafting and anterior plating resulted in significantly increased stability; however, NZ results did not indicate significantly more stability versus unplated and intact spines.
In the axial rotation mode, all ACDF with plating regardless of graft were more stable by NZ values. ROM values indicated that CS and AG supplemented with plating were significantly more stable than CS and AG alone; however, FA with plating did not impart significantly more stability than FA alone. All plating resulted in significantly more stability than was exhibited by the intact spine except for NZ of the FAP group.
Discussion
The Smith–Robinson technique for ACDF using autograft (AG) has long yielded favorable clinical outcomes [4, 5, 11, 12], but the harvest of AG can cause complications [11, 22, 30, 32, 36]. Allografts (FA, CS) do not cause harvest morbidity but only have osteoconductive properties [39]. Radiographic union can be delayed; graft collapse is more common [45]; and vascularization is decreased [21]. Allograft processing has also been reported to decrease graft bending and torsional strength [26]. Despite these concerns, excellent fusion rates using allografts has been reported [3, 7, 8, 19, 22, 28, 33, 34, 42–44].
The failure load of a motion segment indicates the required strength of a cervical interbody graft. The strength of a vertebra-disc–vertebra motion segment ranges from 1,500 to 1,800 N, and approximately 88% of this load, between 1,320 N and 1,584 N of axial compression, is transmitted through the disc space [14, 27, 41]. Smith et al. [37] reported that iliac crest autograft was able to bear an average axial load of 3,230 N (range 430 N–8,112 N). In the same study, fresh frozen FA had a mean load to failure of 12,617 N (range 6,163 N–17,749 N). GraftechTM dense cancellous allografts (CS) have an average failure strength of 1,786 N (range 956 N–2,614 N) (unpublished data, Osteotech, 2003). Though stronger, FA have little cancellous tissue, which might delay fusion, whereas CS are strong enough but have a trabeculated structure that may assist fusion.
Allografts are widely used for the ACDF procedure, but because of the risk of pseudoarthrosis, many surgeons routinely add instrumentation [1, 6, 13, 19, 22, 33, 34, 37, 44]. The anterior cervical plate can increase the immediate postoperative stability and thus enhance fusion [9, 15], decrease graft-related complications, and maintain sagittal balance [18, 38, 40]. The plate not only acts as a buttress, preventing graft extrusion, but it also decreases the extent of graft collapse and subsidence, preventing postoperative kyphosis formation [18, 22]. It can share the axial load at the fusion segment and eliminate the need for a rigid cervical orthosis [29].
Although a static plate yields a stiffer construct, some experts have expressed concerns that rigid constraints may stress-shield the graft, leading to osteopenia and delayed union or nonunion. Dynamic plating was developed to address this. Improved graft loading has been demonstrated even with subsidence; however, some biomechanical testing has indicated that without an intact posterior longitudinal ligament, dynamic plates may exhibit significant increases in ROM and potential failure [23].
In vitro biomechanical studies of the immediate interbody graft stability of ACDF have been reported [17, 20, 24, 31, 35]. Schulte et al. [31] examined the biomechanical stability of the human cadaveric cervical spine following C5–C6 ACDF using the Smith–Robinson approach, and found that after AG insertion, the ROM decreased by 17.5% in flexion, 45.9% in extension, 37.9% in lateral bending, and 39.4% in axial rotation. The addition of an anterior cervical plate resulted in 70% motion reduction in all load modalities. Maciejczak et al. [20] conducted a similar study of C5–C6 discectomy using the Cloward technique, and found that interbody fusion using AG only increased the immediate postoperative stiffness of an operated segment in the flexion and lateral bending modes. In the present study, the Smith–Robinson approach was performed at the C5–C6 level. None of the three graft materials alone significantly decreased motion, which may reflect the fact that the posterior longitudinal ligament and uncovertebral hypertrophied spurs were removed and the specimens had low bone mineral density.
Shimamoto et al. [35] performed cervical reconstruction with an interbody fusion cage, autograft, and an anterior locking plate. In the flexion/extension mode, the plate resulted in significantly lower ROM than the cage or the autograft, and ROM for the cage and the autograft were significantly higher than for the intact spine. Plating also lowered ROM in axial rotation, but had no significant effect in the lateral bending mode. Our results are consistent with these findings. Combining any of the three ACDF procedures with anterior plating significantly reduced ROM and NZ values relative to the intact spine, with the exception of the NZ values in the lateral bending mode and the FAP NZ value in the axial rotation mode.
As is the case in most biomechanical studies of the spine, the majority of the cadaveric specimens were older and had low bone mineral density. Because many patients who receive the ACDF procedure tend to be older, however, the findings may approximate the biomechanical effects on the typical patient population. Additionally, all musculature that would normally stabilize the spine in vivo had to be removed for testing. This study is limited in the few number of specimens for each type of graft though with more specimens, the variation may be decreased, but the results would likely be similar. We also did not test all the important biomechanical forces to which the graft would be exposed. For example, we only used 20N of arbitrary preload that may not replicate physiologic loading. Further, we did not explore fatigue loading testing, which is an important determinant of the long-term stability of grafts.
Conclusions
The present study demonstrated that without anterior cervical plating, the ACDF procedure did not provide increased stability compared to the intact spine, regardless of the graft material used. However, stability was generally increased in all but the lateral bending mode when any of the three ACDF procedures with grafting were accompanied by anterior plating. Overall, the stability of CS was comparable to that of AG and FA making it a suitable graft material for one-level ACDF with anterior plating while possibly having superior biological properties.
Abbreviations
- ACDF
Anterior cervical discectomy and interbody fusion
- AG
Tricortical iliac autograft
- AGP
ACDF with AG followed by application of an anterior cervical plate
- CS
Dense cancellous allograft cervical spacer
- CSP
ACDF with CS followed by application of an anterior cervical plate
- FA
Freeze-dried fibular allograft
- FAP
ACDF with FA followed by application of an anterior cervical plate
- NZ
Neutral zone
- ROM
Range of motion
- SD
Standard deviation
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s00586-006-0146-4
References
- 1.Alvarez JA, Hardy RW Jr (1999) Anterior cervical discectomy for one- and two-level cervical disc disease: the controversy surrounding the question of whether to fuse, plate, or both. Crit Rev Neurosurg 9:234–251 [DOI] [PubMed]
- 2.Balabhadra RSV, Kim DK, Zhang HY (2004) Anterior cervical fusion using dense cancellous allografts and dynamic plating. Neurosurgery 54:1405–1411 [DOI] [PubMed]
- 3.Bishop RC, Moore KA, Hadley MN (1996) Anterior cervical interbody fusion using autogeneic and allogeneic bone graft substrate: a prospective comparative analysis. J Neurosurg 85:206–210 [DOI] [PubMed]
- 4.Bohlman HH, Emery SE, Goodfellow DB, Jones PK (1993) Robinson anterior cervical discectomy and arthrodesis for cervical radiculopathy. Long-term follow-up of one hundred and twenty-two patients. J Bone Joint Surg Am 75:1298–1307 [DOI] [PubMed]
- 5.Brodke DS, Zdeblick TA (1992) Modified Smith–Robinson procedure for anterior cervical discectomy and fusion. Spine 17:S427–S430 [DOI] [PubMed]
- 6.Buttermann GR, Glazer PA, Bradford DS (1996) The use of bone allografts in the spine. Clin Orthop 324:75–85 [DOI] [PubMed]
- 7.Cauthen JC, Kinard RE, Vogler JB, Jackson DE, DePaz OB, Hunter OL, Wasserburger LB, Williams VM (1998) Outcome analysis of noninstrumented anterior cervical discectomy and interbody fusion in 348 patients. Spine 23:188–192 [DOI] [PubMed]
- 8.Cherry C (2002) Anterior cervical discectomy and fusion for cervical disc disease. Aorn J 76:998–1004, 1007, 1008; quiz 1009–1012 [DOI] [PubMed]
- 9.Connolly PJ, Esses SI, Kostuik JP (1996) Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord 9:202–206 [PubMed]
- 11.DePalma AF, Rothman RH, Lewinnek GE, Canale ST (1972) Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet 134:755–758 [PubMed]
- 12.Emery SE, Bohlman HH, Bolesta MJ, Jones PK (1998) Anterior cervical decompression and arthrodesis for the treatment of cervical spondylotic myelopathy. Two to seventeen-year follow-up. J Bone Joint Surg Am 80:941–951 [DOI] [PubMed]
- 13.Geer CP, Papadopoulos SM (1999) The argument for single-level anterior cervical discectomy and fusion with anterior plate fixation. Clin Neurosurg 45:25–29; discussion 21 [PubMed]
- 14.Goel VK, Clausen JD (1998) Prediction of load sharing among spinal components of a C5–C6 motion segment using the finite element approach. Spine 23:684–691 [DOI] [PubMed]
- 15.Heidecke V, Rainov NG, Burkert W (1998) Anterior cervical fusion with the Orion locking plate system. Spine 23:1796–1802; discussion 1803 [DOI] [PubMed]
- 16.Kaiser MG, Haid RW Jr, Subach BR, Barnes B, Rodts GE Jr (2002) Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery 50:229–236; discussion 236–238 [DOI] [PubMed]
- 17.Kandziora F, Pflugmacher R, Schafer J, Born C, Duda G, Haas NP, Mittlmeier T (2001) Biomechanical comparison of cervical spine interbody fusion cages. Spine 26:1850–1857 [DOI] [PubMed]
- 18.Katsuura A, Hukuda S, Imanaka T, Miyamoto K, Kanemoto M (1996) Anterior cervical plate used in degenerative disease can maintain cervical lordosis. J Spinal Disord 9:470–476 [DOI] [PubMed]
- 19.Khan S, Sama A, Sandhu HS (2001) Bone graft substitutes in spine surgery. Curr Opin Orthpaedics 12:216–222 [DOI]
- 20.Maciejczak A, Ciach M, Radek M Jr, Radek A, Awrejcewicz J (2001) Immediate stiffness of the C5–C6 segment after discectomy with the Cloward technique: an in vitro biomechanical study on a human cadaveric model. Neurosurgery 49:1399–1408 [DOI] [PubMed]
- 21.Malloy KM, Hilibrand AS (2002) Autograft versus allograft in degenerative cervical disease. Clin Orthop 394:27–38 [DOI] [PubMed]
- 22.Martin GJ Jr, Haid RW Jr, MacMillan M, Rodts GE Jr, Berkman R (1999) Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine 24:852–858; discussion 858–859 [DOI] [PubMed]
- 23.Mohr RA, Brodke DS (2005) Fixed versus dynamic cervical plates: how to choose the proper plate. Curr Opin Orthopaedics 16:194–199 [DOI]
- 24.Natarajan RN, Chen BH, An HS, Andersson GB (2000) Anterior cervical fusion: a finite element model study on motion segment stability including the effect of osteoporosis. Spine 25:955–961 [DOI] [PubMed]
- 25.Olsewski JM, Garvey TA, Schendel MJ (1994) Biomechanical analysis of facet and graft loading in a Smith–Robinson type cervical spine model. Spine 19:2540–2544 [DOI] [PubMed]
- 26.Pelker RR, Friedlaender GE, Markham TC (1983) Biomechanical properties of bone allografts. Clin Orthop 174:54–57 [PubMed]
- 27.Pintar FA, Yoganandan N, Voo L (1998) Effect of age and loading rate on human cervical spine injury threshold. Spine 23:1957–1962 [DOI] [PubMed]
- 28.Portnoy HD (2001) Anterior cervical discectomy and fusion. Surg Neurol 56:178–180 [DOI] [PubMed]
- 29.Rapoff AJ, O’Brien TJ, Ghanayem AJ, Heisey DM, Zdeblick TA (1999) Anterior cervical graft and plate load sharing. J Spinal Disord 12:45–49 [PubMed]
- 30.Schnee CL, Freese A, Weil RJ, Marcotte PJ (1997) Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine 22:2222–2227 [DOI] [PubMed]
- 31.Schulte K, Clark CR, Goel VK (1989) Kinematics of the cervical spine following discectomy and stabilization. Spine 14:1116–1121 [DOI] [PubMed]
- 32.Seiler JG III, Johnson J (2000) Iliac crest autogenous bone grafting:donor site complications. J South Orthop Assoc 9:91–97 [PubMed]
- 33.Shapiro S (1996) Banked fibula and the locking anterior cervical plate in anterior cervical fusions following cervical discectomy. J Neurosurg 84:161–165 [DOI] [PubMed]
- 34.Shapiro S, Connolly P, Donnaldson J, Abel T (2001) Cadaveric fibula, locking plate, and allogeneic bone matrix for anterior cervical fusions after cervical discectomy for radiculopathy or myelopathy. J Neurosurg 95:43–50 [DOI] [PubMed]
- 35.Shimamoto N, Cunningham BW, Dmitriev AE, Minami A, McAfee PC (2001) Biomechanical evaluation of stand-alone interbody fusion cages in the cervical spine. Spine 26:E432–E436 [DOI] [PubMed]
- 36.Silber JS, Anderson DG, Daffner SD, Brislin BT, Leland JM, Hilibrand AS, Vaccaro AR, Albert TJ (2003) Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 28:134–139 [DOI] [PubMed]
- 37.Smith MD, Cody DD (1993) Load-bearing capacity of corticocancellous bone grafts in the spine. J Bone Joint Surg Am 75:1206–1213 [DOI] [PubMed]
- 38.Troyanovich SJ, Stroink AR, Kattner KA, Dornan WA, Gubina I (2002) Does anterior plating maintain cervical lordosis versus conventional fusion techniques? A retrospective analysis of patients receiving single-level fusions. J Spinal Disord Tech 15:69–74 [DOI] [PubMed]
- 39.Vaccaro AR, Cirello J (2002) The use of allograft bone and cages in fractures of the cervical, thoracic, and lumbar spine. Clin Orthop 394:19–26 [DOI] [PubMed]
- 40.Wang JC, McDonough PW, Endow K, Kanim LE, Delamarter RB (1999) The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord 12:467–471 [DOI] [PubMed]
- 41.White A III, Panjabi MM (1990) Clinical biomechanics of the spine, 2nd edn. JB Lippincott-Raven, Philadelphia
- 42.Whitecloud TS III (1999) Modern alternatives and techniques for one-level discectomy and fusion. Clin Orthop 359:67–76 [DOI] [PubMed]
- 43.Wigfield CC, Nelson RJ (2001) Nonautologous interbody fusion materials in cervical spine surgery: how strong is the evidence to justify their use? Spine 26:687–694 [DOI] [PubMed]
- 44.Young WF, Rosenwasser RH (1993) An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine 18:1123–1124 [DOI] [PubMed]
- 45.Zdeblick TA, Ducker TB (1991) The use of freeze-dried allograft bone for anterior cervical fusions. Spine 16:726–729 [DOI] [PubMed]