Abstract
A randomized clinical trial compared two materials used to prevent epidural scarring after microdiscectomy. To determine whether ADCON®-L Gel (ALG) or Preclude Spinal Membrane® (PSM) was more effective in preventing scarring, reducing pain, and improving quality of life postoperatively. Postdiscectomy syndrome may result from epidural scarring. Various materials have been used in attempts to prevent this problem, but none have provided optimal results. Previous laboratory and clinical studies have found ALG and PSM to be effective, but none compared the two materials. Thirty-one patients undergoing primary microdiscectomy were randomly assigned to receive either ALG or PSM. Postoperatively, patients were evaluated by magnetic resonance imaging (MRI), with contrast, for volume and rostral–caudal extent of scar tissue and nerve root involvement. Back and leg pain and quality of life were assessed by neurologic examinations and standardized patient surveys. Findings at any reoperations were recorded. Results in the PSM (n = 18) and ALG (n = 13) groups were compared statistically. No operative or postoperative complications occurred. Two patients in each group required reoperation. MRI at 6 months showed no, mild or mild-moderate scarring in most patients, with no significant differences between the ALG and PSM groups in scar volume and extent or nerve root involvement. Neurologic examinations and patient surveys showed substantial reductions in pain over time in both groups but no significant differences between groups. PSM was easy to see and remove at reoperation. PSM and ALG are equally effective in preventing epidural scarring associated with postdiscectomy syndrome.
Keywords: Postdiscectomy syndrome, Microdiscectomy, Epidural scarring, ADCON®-L Gel, Preclude spinal membrane®
Introduction
Scarring at and around neurogenic structures that results in postdiscectomy syndrome is a common and difficult-to-manage complication of discectomy or other spinal decompressive procedure [1, 3, 11, 12, 16, 19, 21, 26, 29, 30, 33]. The pain associated with this syndrome can be severe enough to lead to an inability to work, hospitalization, and possibly additional operations that result in great patient discomfort and general socioeconomic losses [12, 23]. Thus, for several years, much emphasis has been laid on preventing epidural scar tissue formation.
Many materials have been used in attempts to prevent epidural scarring, chiefly by providing a barrier to invasion of the spinal canal by scar tissue. These include autologous fat, Silastic®, Gelfoam®, carbohydrate polymers, Dacron®, and methacrylate [6]. Autologous fat grafts have been the most widely used material for several decades. Recently, however, fat grafts have become less popular because of reports describing fibrous tissue penetration of the grafts and other complications resulting in cauda equina syndrome [6, 27]. Thus, the search for a safe and effective exogenous material has been given new momentum.
Previous experimental and clinical studies demonstrated the efficacy of two relatively new materials in preventing epidural scarring [2, 4–6, 9, 12, 16, 23–25, 28, 35]. One of these materials is a membrane consisting of a special form of expanded polytetrafluoroethylene (ePTFE) that has one virtually nonporous surface (1 μm microstructure) and one porous surface (22 μm microstructure) [Preclude Spinal Membrane® (PSM); W.L. Gore and Associates, Inc., Flagstaff, AZ, USA]. The other material is a resorbable (within 3–4 weeks) material composed of porcine gelatin and a polyglycan ester in phosphate-buffered saline [ADCON®-L Gel (ALG), Gliatech, Cleveland, OH, USA] Investigations comparing these two materials are lacking, so we conducted a randomized prospective study of results achieved with PSM and ALG in patients undergoing primary microdiscectomy. The study included both objective and subjective outcome measures.
Materials and methods
All patients consecutively admitted to our institutions for primary microdiscectomy between April 2000 and February 2001 who signed an informed consent form were considered for enrollment. Patients were included in the study if they were between 18 and 70 years of age and not pregnant, had not previously undergone surgery at the operation site or level, and had no infection, healing disorder, or contraindication to evaluation by magnetic resonance imaging (MRI) using contrast material.
Patients were randomly assigned to receive ALG or PSM at the time surgery was scheduled. For each eligible patient, a sealed envelope containing an assignment to one of the groups was opened and the surgical procedure then scheduled accordingly. At operation, a hemostatic agent was used if necessary but was removed before insertion of ALG or PSM. ALG was used according to the manufacturer’s instructions. The PSM was placed directly over the dura mater and nerve roots so the edges of the material were under the ligamentum flavum and lamina. This implantation technique differs slightly from the one recommended by the manufacturer, but we have found it to be safe and effective in a series of more than 600 cases.
Postoperatively, patients underwent neurologic examinations during follow-up visits at about 4–6 weeks and 3–6 months after surgery. At the 6-month visit, patients also underwent MRI, with contrast, of the operation site for evaluations of epidural scarring by a radiologist blinded to the type of material inserted at discectomy. Both the volume and the rostral–caudal extent of any epidural scarring were assessed. For the volume evaluation, scarring was classified as none (no scarring on the dural surface), mild (scarring on less than 25% of the surface), mild-moderate (scarring on 25–50% of the surface), moderate-extensive (scarring on 50–75% of the surface), or extensive (scarring on more than 75% of the surface). The rostral–caudal extent of scarring was determined by comparison to the length of the original defect. Thus, none was defined as no visible scarring, mild as scarring extending for less than 25% of the original defect length, mild-moderate as scarring extending for 25–50% of the defect length, moderate-extensive as scarring extending for 50–75% of the defect length, and extensive as scarring extending beyond 75% of the defect length. The MRI films were also examined to determine whether scarring involved the nerve root.
Patients’ subjective assessments of the outcome of their surgical procedure with respect to leg and back pain and quality of life were obtained by using semiquantitative surveys based on the Roland–Morris disability scoring system [30] and the SF-12 physical and mental health summary scales [34]. Back and leg pain were rated before surgery and at every follow-up visit. The SF-12 survey was administered preoperatively and 6 months after surgery. The scales for patients with at least one missing item on the SF-12 questionnaire were set to missing in accordance with the recommendations in the SF-12 handbook [34].
For each patient, demographic, operative, and postoperative data (MRI, neurologic examination, and survey results; complications; and reoperations) were recorded and compiled. Statistical analyses comparing variables in the ALG and PSM groups used the intention-to-treat principle and included χ2, t, Fisher exact, and Wilcoxon tests, as appropriate. A P value less than 0.05 was considered to represent a significant difference.
Results
A total number of 31 patients underwent primary microdiscectomy within the 10-month study period. Eighteen of these patients received a PSM and 13 were given ALG. Patient demographic characteristics and intraoperative and postoperative data in each group (spinal levels repaired, operating time, whether adhesions were present, whether a hemostatic agent was used, length of hospitalization, and number of reoperations) are shown in Table 1. There were no significant differences between the PSM and ALG groups in any variable recorded. No patient had a neural injury or other complication during surgery, and there were no cerebrospinal fluid (CSF) leaks, infections, or other complications postoperatively.
Table 1.
Demographic, operative, and postoperative data for patients in the preclude spinal membrane (PSM) and ADCON-L Gel (ALG) groups
| Variable | PSM group (n = 18) | ALG group (n = 13) |
|---|---|---|
| Mean age, year (range) | 41 (29–66) | 41 (25–70) |
| Sex: M/F | 12/6 | 11/2 |
| Mean weight, kg (range) | 81 (59–110) | 77 (61–95) |
| Mean height, cm (range) | 177 (162–194) | 176 (164–191) |
| Spinal procedure: single/multiple, n | 17/1 | 10/3 |
| Spinal level repaired, n | ||
| Lumbar-sacral | 8 | 6 |
| Lumbar-lumbar | 8 | 5 |
| Thoracic-thoracic | 1 | 0 |
| Not available | 1 | 2 |
| Mean operating timea, min (range) | 90 (40–130) | 89 (65–145) |
| Patients with adhesions (n) | 13 | 5 |
| Hemostatic agent used (n) | 16 | 8 |
| Mean hospital stay, days (range) | 8 (3–20) | 7 (3–12) |
| Complications (n) | 0 | 0 |
| Reoperations (n) | 2 | 2 |
None of the differences between the groups were significant on χ2, t, Wilcoxon, or Fisher exact tests
aThe OR times are the total time from the beginning to the end of anaesthesia
The mean ( ± SD) size of the PSM implants used was 3.3 ± 1.8 cm2. In no case were sutures or other devices used to attach a membrane; rather, each PSM was held in place by the hydrostatic force between it and the neurogenic structures. The mean amount of ALG used was 1.7 ± 0.7 ml.
The results of the MRI-based assessments of the volume and extent of epidural scar tissue in the PSM and ALG groups at 6 months after surgery are shown in Table 2. In the volume assessment, most patients in both groups were found to have mild scarring; no extensive scarring was observed in either group. The ALG group had higher percentages of patients with mild-moderate and moderate-extensive scarring, but none of the differences between the two groups were significant. In the evaluation of the rostral–caudal extent of scarring, some patients in the PSM group had no scarring and none had moderate-extensive scarring. No patient in either group had extensive scarring. Again, however, none of the differences between groups were significant. Nerve root involvement was observed in four patients in each group (P = 1.0 on Fisher exact test).
Table 2.
Results of MRI assessments of volume and rostral–Caudal extent of scarring in the PSM (n = 17) and ALG (n = 13) groups 6 months after microdiscectomy
| Assessment/group | Degree of scarring | |||
|---|---|---|---|---|
| None | Mild | Mild–moderate | Moderate–extensive | |
| Volume of scarring | ||||
| PSM group | 0 | 12 (71%) | 3 (18%) | 2 (12%) |
| ALG group | 0 | 7 (54%) | 3 (23%) | 3 (23%) |
| Extent of scarring | ||||
| PSM group | 1 (6%) | 10 (59%) | 6 (35%) | 0 |
| ALG group | 0 | 7 (54%) | 2 (15%) | 4 (31%) |
Values are numbers (%) of patients. Results were not available for one patient in the PSM group. None of the differences between the groups were significant (Wilcoxon two-sample test; P = 0.34 for volume and P = 0.21 for extent of scarring)
The postoperative clinical neurologic examinations found that back pain was absent at the first follow-up visit (4–6 weeks after surgery) in four patients in the PSM group and six in the ALG group. Six months postoperatively, six patients in the PSM group and five in the ALG group had no back pain. Leg pain was absent 4–6 weeks postoperatively in eight patients in the PSM group and seven in the ALG group. Six months after surgery, nine patients in the PSM group and four in the ALG group had no leg pain. None of the differences between groups were significant (Wilcoxon two-sample test).
The Roland–Morris disability scores for back and leg pain in the PSM and ALG groups are shown in Table 3. Both back and leg pain decreased substantially over time in the two groups. None of the differences between groups were significant (Wilcoxon two-sample test). Results of the SF-12 survey are shown in Table 4. There were no significant differences between groups. However, within each group, both physical and mental health variables had improved significantly by 6 months after surgery.
Table 3.
Roland–Morris disability scores for back and leg pain at different assessment times in the PSM and ALG groups
| Assessment time/group | Mean ± SD score | |
|---|---|---|
| Back pain | Leg pain | |
| Preoperatively | ||
| PSM group (n = 18) | 10.0 ± 8.7 | 13.5 ± 7.8 |
| ALG group (n = 13) | 15.0 ± 5.8 | 12.2 ± 7.6 |
| 4–6 weeks postoperatively | ||
| PSM group (n = 16) | 8.8 ± 7.0 | 2.9 ± 4.7 |
| ALG group (n = 13) | 6.1 ± 5.3 | 5.2 ± 6.5 |
| 3 months postoperatively | ||
| PSM group (n = 17) | 6.6 ± 7.0 | 4.3 ± 6.3 |
| ALG group (n = 13) | 5.8 ± 5.3 | 3.8 ± 4.4 |
| 6 months postoperatively | ||
| PSM group (n = 17) | 5.7 ± 6.2 | 4.0 ± 7.4 |
| ALG group (n = 13) | 3.3 ± 4.6 | 4.6 ± 6.9 |
None of the differences between the groups were significant
Table 4.
Mean ( ± SD) SF-12 physical and mental health scores in the PSM and ALG groups
| Assessment/assessment time | PSM group | ALG group |
|---|---|---|
| Physical health | ||
| Preoperatively | 31.7 ± 8.5 (n = 14) | 32.4 ± 7.8 (n = 11) |
| 6 months postoperatively | 42.6 ± 9.0 (n = 17) | 39.9 ± 10.3 (n = 13) |
| Mental health | ||
| Preoperatively | 34.9 ± 8.5 (n = 14) | 38.1 ± 11.4 (n = 11) |
| 6 months postoperatively | 51.1 ± 11.2 (n = 17) | 49.5 ± 13.4 (n = 13) |
None of the differences between the groups were significant, but within each group, there were significant improvements at 6 months in both physical health (P = 0.049 in the PSM group and P = 0.03 in the ALG group) and mental health (P = 0.002 in the PSM group and P = 0.02 in the ALG group; Wilcoxon test)
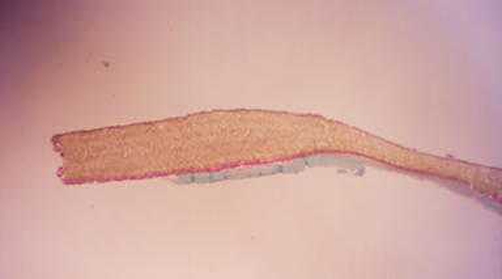
Four patients (two in each group) required reoperation because of relapse (three cases) or spinal instability (one case). At reoperation in the two patients in whom a PSM had been implanted, the membrane was easily seen lying over the neurogenic structures (Fig. 1). In both cases, the PSM was easy to remove. Histologic studies of the PSM explants showed no scar tissue on the nonporous surface (Fig. 1 left) that had been against the dura and fibroblasts within the material on the porous side (Fig. 1 right), indicating slight tissue in growth.
Fig. 1.
Histologic image showing the inner, nonporous surface (up) and the outer, porous surface (bottom) of a PSM that had been implanted for 6 months. While there is no scar tissue visible on the inner surface, abundant fibroblasts are present within the material on the outer surface (magnification 33× Milligan’s trichrome stain)
Discussion
In this first comparative study on the scar preventing materials PSM and ALG both were found to show comparable results in objective and subjective outcome measures generally used to demonstrate effectiveness in preventing postdiscectomy syndrome. Most of the studies of fibrosis-related problems after discectomy have focused on assessments of the degree of epidural scarring. This emphasis developed because of research [1, 3, 4, 19, 32] on an association between the degree of scarring and the amount of postoperative pain. In the current study, MRI using contrast was used to provide objective outcome variables. This assessment method was previously found to be very sensitive in evaluating postoperative scarring processes [31, 32]. In our study, MRI showed no excessive scarring in either the PSM or the ALG group and only a few cases of moderate-extended scarring in both the scar-volume and scar-extent analyses. Our investigation also included an evaluation of subjective results derived from standardized patient surveys. This assessment revealed that patients in the PSM and ALG groups had similar, substantial reductions in pain and improvements in quality of life after surgery.
Our study did not include a placebo group, i.e. a group of patients that received none of the scar preventing materials. Given the fact however that a large number of articles investigating the effectiveness of either PSM or ALG proved their superiority over placebo [2, 4–6, 9, 12, 16, 23–25, 28, 35] and because of the substantial disadvantage for patients when not treated with scar preventing materials we thought it to be unethical to introduce such a placebo design into our study. For estimating the effectiveness therefore we had to rely on older efficacy studies. DiFazio et al. [5], in a study in dogs, were among the first to demonstrate the efficacy of ePTFE membranes in minimizing epidural scar tissue. Subsequently, a prospective clinical study by Mohsenipour et al. [24] compared scarring after discectomy in patients in whom a PSM was used with that in patients not given a membrane. MRI investigations 3–6 months after surgery showed peridural or epidural scarring in all patients who did not receive a PSM but in only one patient who did. Carbohydrate polymers derived from animal tissue have also provided promising results. In particular, ALG has been found to be effective in preventing epidural scarring in a large number of studies [3, 4, 6, 19, 25]. In our study also no histological signs of scarring at reoperation in two patients in whom ALG was applied during their previous spinal procedure were found and only minimal scar-volume and scar-extent on MRI evaluation was detected in the remaining patients.
Very few head on comparative trials between various scar preventative measures exist and of these prospective randomized trials are even scarcer. Of the latter there are two experimental studies in dogs, one showing the benefits of radiation in combination with surgery compared to surgery alone [7] and one showing fat grafts to be superior to Gelfoam [26]. In human however two prospective randomized studies showed that Gelfoam was as effective as fat grafts in preventing scar formation [15, 18], but radiotherapy in combination with Gel was superior to radiotherapy alone [8]. Finally ALG with morphine compound was better in preventing postoperative pain than plain ALG [20]. Our study however is the first to compare the efficacy of the two newer products namely PSM and ALG suggesting that they are equally effective. Studies like that are imminently important to help the surgeons in choosing between two methods with proven efficacy on evidence based ground.
When faced with equal efficacy the making of decision may now rely on side effect profiles and costs. There were no complications in either group in our study. However, ALG, contrary to initial experimental data [28], has been associated in up to 18.5% of patients with CSF leaks [8] at dural lacerations not observable interoperatively [17] and with a 2% incidence of healing problems at the dura and suturing sites [8]. The possible consequences of the former is a chronic leakage of CSF leading to intracranial hypotension syndrome [14]. In addition adverse haemodynamic reactions including tachycardia and hypotension were found to occur in 3.3% of patients treated with ALG [13, 22]. This phenomenon might be explained by small but significant systemic absorption of ALG from the insertion side leading to myocardial depression and vasodilatation. Indeed, we have had some problems with ALG in patients not in this study and now use this material only with great caution. In major decompressive procedures with laminectomy and in reoperations, we apply only small amounts. Furthermore, all appliances that come in contact with ALG are immediately replaced. In extensive decompressions, ALG is often used in combination with PSM.
In contrast to ALG, implants made of ePTFE, including PSM, have been widely and safely used in numerous surgical applications for many years without major complications [24] and the results of our slightly modified implantation technique seem to be in keeping with these findings if not somewhat better. The manufacturer of PSM suggests inserting the membrane dorsally into the laminectomy or laminotomy wound. We feel, however, that this might allow a layer of blood to form between the neurologic structures and the PSM and attract pluripotent cells to form scar tissue around the membrane. Therefore, we place the PSM in direct contact with the dura and nerves, allowing hydrostatic adhesion to occur between the nonporous surface of the material and these structures and preventing pooling of blood between them. Histological findings of a lack of fibroblast ingrowth at the nonporous surface of the membrane at reoperation in our two patients (Fig. 1) seem to support this assumption. Macroscopically, in addition to an absence of scar tissue we have found the membrane to be fixed chiefly at the edge of the neurogenic structures. The PSM was also easy to see, facilitating the reoperation procedure. The minimization of scarring may prevent damage to the dura and nerves during any subsequent surgical intervention. However, despite a seemingly better adverse event profile of PSM, the overall experience in the application of PSM is not as long standing and not as widespread as is the case of ALG and there are also fewer studies on PSM than on ALG. In this way yet unknown rare but severe side effects might still be emerging with increased use of PSM.
PSM may have a financial advantage over ALG. In Europe, a 6 × 6 cm (36 cm2) patch of PSM currently costs about Euro 299. However, because the average size employed was 3.3 cm2, each patch could be divided up for use in different procedures. For example, it is possible to use one 6 × 6 cm patch for two unisegmental laminectomies. Moreover, because PSM can be re-sterilized up to three times, a patch can be divided for use in several separately performed procedures. Alternatively, smaller patches of PSM can be purchased; for instance, a 3 × 3 cm configuration is available for Euro 132. ALG comes in two sizes. The smaller, 1 g size is sufficient for a discectomy and costs about Euro 196 (the 3 g size is Euro 279), but it is single-use only. Recently, however, ALG has become unavailable. Of course, any material that decreases the risk of postdiscectomy syndrome must be considered to be cost effective because of the substantial expenses involved in treating the disorder [25]. In addition, minimization of epidural scarring associated with complications at reoperation will result in cost savings.
Conclusion
In summary, this randomized clinical trial, which is the first to compare results of PSM and ALG, two effective epidural scar preventing agents, shows equal outcome measures of the 2 materials at 6 months after primary discectomy in both MRI-based objective assessments and subjective patient surveys. In conjunction with already published data this suggests that the use of either of these materials may play a major role in preventing postdiscectomy syndrome. From the authors point of view the PSM may have a slight advantage over PSM with respect to complications and cost.
Acknowledgments
We would like to express our gratitude to W.L. Gore & Associates, Inc., Flagstaff, AZ, USA for their assistance in providing the materials needed for the investigations.
References
- 1.Abdou MS, Hardy RW Jr (1999) Epidural fibrosis and the failed back surgery syndrome: history and physical findings. Neurol Res 21(Suppl 1):S5–S8 [DOI] [PubMed]
- 2.Barbera J, Gonzalez J, Esquerdo J, Broseta J, Barcia-Salorio JL (1978) Prophylaxis of the laminectomy membrane—an experimental study in dogs. J Neurosurg 49:419–424 [DOI] [PubMed]
- 3.BenDebba M, Alphen van A, Long DM (1999) Association between peridural scar and activity-related pain after lumbar discectomy. Neurol Res 21(Suppl 1):S37–S42 [DOI] [PubMed]
- 4.Brotchi J, Pirotte B, De Witte O, Levivier M (1999) Prevention of epidural fibrosis in a prospective series of 100 primary lumbo-sacral discectomy patients: follow-up and assessment at re-operation. Neurol Res 21(Suppl 1):S47–S50 [DOI] [PubMed]
- 5.DiFazio FA, Nichols J, Pope MH, Frymorer JW (1995) The use of expanded polytetrafluoroethylene as an interpositional membrane after lumbar laminectomy. Spine 9:986–991 [DOI] [PubMed]
- 6.Geisler FH (1999) Prevention of peridural fibrosis: current methodologies. Neurol Res 21(Suppl 1):S9–S22 [DOI] [PubMed]
- 7.Gerszten PC, Moossy JJ, Flickinger JC, Gerszten K, Kalend A, Martinez AJ (2000) Inhibition of peridural fibrosis after laminectomy using low-dose external beam radiation in a dog model. Neurosurgery 46(6):1478–1485 [DOI] [PubMed]
- 8.Gerszten PC, Moossy JJ, Flickinger JC, Welch WC (2003) Low-dose radiotherapy for the inhibition of peridural fibrosis after reexploratory nerve root decompression for postlaminectomy syndrome. J Neurosurg 99(Suppl 3):271–277 [DOI] [PubMed]
- 9.Hadani M, Ram Z, Horowitz A, Shacked I (1993) Silicon prevents post laminectomy epidural root adhesions—an experimental study in rats. Acta Neurochir (Wien) 123:153–156 [DOI] [PubMed]
- 10.Hieb LD, Stevens DL (2001) Spontaneous postoperative cerebrospinal fluid leaks following application of anti-adhesion barrier gel. Spine 26:748–751 [DOI] [PubMed]
- 11.Ivanic GM, Pink TP, Homann NC, Scheitza W, Goyal S (2001) The post-discectomy syndrome—aetiology, diagnosis, treatment, prevention. Arch Orthop Trauma Surg 121:494–500 [DOI] [PubMed]
- 12.Ivanic GM, Wild A, Pink TP, Homann NC (2002) Prevention of epidural fibrosis with a nonabsorbable membrane. Unfallchirurg 105:483–485 [DOI] [PubMed]
- 13.Kalogrianitis S, Barrett P, Shackleford I (2001) ADCON-L and hypotension during lumbar microdiscectomy. Br J Anaesth 87:770–771 [DOI] [PubMed]
- 14.Kuhn J, Hofmann B, Knitelius HO, Coenen HH, Bewermeyer H (2005) Bilateral subdural haematomata and lumbar pseudomeningocele due to a chronic leakage of liquor cerebrospinalis after a lumbar discectomy with the application of ADCON-L gel. J Neurol Neurosurg Psychiatry 76:1030–1033 [DOI] [PMC free article] [PubMed]
- 15.Lapis I, Horvath G (2002) Effectiveness of autologous free fat graft and of Spongostan in preventing scar formation after microdiscectomy. Ideggyogy Sz 5:371–374 [PubMed]
- 16.LaRocca H, Macnab I (1974) The laminectomy membrane—studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br 56B:545–550 [PubMed]
- 17.Le AX, Rogers DE, Dawson EG, Kropf MA, De Grange DA, Delamarter RB (2001) Unrecognized durotomy after lumbar discectomy—a report of four cases associated. with the use of ADCON®-L. Spine 1:115–118 [DOI] [PubMed]
- 18.MacKay MA, Fischgrund JS, Herkowitz HN, Kurz LT, Hecht B, Schwartz M (1995) The effect of interposition membrane on the outcome of lumbar laminectomy and discectomy. Spine 20(16):1793–1796 [DOI] [PubMed]
- 19.Maroon JC, Abla A, Bost J (1999) Association between peridural scar and persistent low back pain after lumbar discectomy. Neurol Res 21(Suppl 1):S43–S36 [DOI] [PubMed]
- 20.Mastronardi L, Pappagallo M, Tatta C (2005) The Oxiplex/SP gel-morphine compound after lumbar microdiscectomy in the management of postoperative pain. Report of 20 cases. Surg Neurol 64(1):75–79 [DOI] [PubMed]
- 21.McAuley D, Russell C, Farling P (2004) Adcon-L gel and intraoperative hypotension during lumbar discectomy. Br J Neurosurg 18(2):180–182 [DOI] [PubMed]
- 22.McCulloch JA, Young PH (1998) Control of bleeding in microsurgery in essentials of spinal microsurgery. Lippincot–Raven, New York, pp 69–87
- 23.McKinley DS, Shaffer LM (1999) Cost effectiveness evaluation of ADCON®-L adhesion control gel in lumbar surgery. Neurol Res 21(Suppl 1):S67–S71 [DOI] [PubMed]
- 24.Mohsenipour I, Daniauz M, Aichner F, Twerdy K (1998) Prevention of local scar formation after operative discectomy for lumbar disc herniation. Acta Neurochir 140:9–13 [DOI] [PubMed]
- 25.Porchet F, Lombardi D, Preux de J, Pople IK (1999) Inhibition of epidural fibrosis with ADCON®-L: effect on clinical outcome one year following re-operation for recurrent lumbar radiculopathy. Neurol Res 21(Suppl 1):S51–S60 [DOI] [PubMed]
- 26.Pospiech J, Pajonk F, Stolke D (1995) Epidural scar tissue after Spinal surgery: an experimental study. Eur Spine J 4:213–219 [DOI] [PubMed]
- 27.Prusick VR, Lint DS, Bruder J (1988) Cauda equina syndrome as complication of free epidural fat-grafting. J Bone Joint Surg 70-A:1256–1258 [PubMed]
- 28.Robertson JT, Meric AL, Dohan FC, Schweitzer JB, Wujek JR, Ahmad S (1993) The reduction of postlaminectomy peridural fibrosis in rabbits by a carbohydrate polymer. J Neurosurg 79:89–95 [DOI] [PubMed]
- 29.Roland M, Morris R (1983) A study of the natural history of back pain; part I: development of a reliable and sensitive measure of disability in low-back pain. Spine 2:141–144 [DOI] [PubMed]
- 30.Roland M, Morris R (1983) A study of the natural history of back pain; part II: development of a guideline for trials of treatment in primary care. Spine 2:145–150 [DOI] [PubMed]
- 31.Ross JS, Obduchowski N, Modic TM (1999) MR evaluation of epidural fibrosis proposed grading system with intra- and inter-observer variability. Neurol Res 21(Suppl 1):S23–S26 [DOI] [PubMed]
- 32.Ross JS, Robertson JT, Frederickson RCA, Petrie JL, Obuchowski N, Modic MT, de Tribolet N (1996) Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. Neurosurgery 4:855–863 [DOI] [PubMed]
- 33.Spencer DL (1999) The anatomical basis of sciatica se condary to herniated lumbar disc: a review. Neurol Res 21(Suppl 1):S30–S36 [DOI] [PubMed]
- 34.Ware J Jr, Kosinski M, Keller SD (1996) A 12-item short-form health survey construction of scales and preliminary tests of reliability and validity. Med Care 34:220–233 [DOI] [PubMed]
- 35.Wujek JR, Ahmad S, Harel A, Maier KH, Roufa D, Silver J (1991) A carbohydrate polymer that effectively prevents epidural fibrosis at laminectomy sites in the rat. Exp Neurol 114:237–245 [DOI] [PubMed]