Abstract
It is believed that disc degeneration (DD) is, in general, only mildly associated with low back pain (LBP). MRI-identified Modic changes (MC), probably a late stage of DD, are relatively strongly associated with LBP but it is not known if people with MC also have a specific clinical profile. The purpose of this study was to investigate if the clinical findings differ in people with Modic changes (MC) as compared to those with only degenerative disc findings or none at all. In a population-based sample of 412 40-year-old Danes, information was collected independently with MRI, questionnaires and clinical examination. Three subgroups of people were created: those with both DD and MC, those with only DD, and those with neither DD nor MC. The clinical pattern was investigated for each subgroup in order to test the assumption that the clinical picture differs in the three groups. It was expected that people with both DD and MC would have a more pronounced clinical profile than those with only DD who, in turn, would differ from those with neither of these two MRI findings. Our findings were generally in concordance with our expectations. MC constitutes the crucial element in the degenerative process around the disc in relation to LBP, history, and clinical findings. People with DD and no MC only vaguely differ from those without. People with LBP and MC may deserve to be diagnosed as having specific LBP.
Keywords: Modic changes, Disc degeneration, Low back pain, Physical examination, Clinical history, Diagnosis, MRI
Background
A new imaging diagnosis appears to have been identified. In 1988, Modic et al. described and validated an MRI-detected vertebral anomaly that can be defined as signal changes in the vertebral bone extending from the vertebral endplate, the so-called Modic changes (MC). MC is strongly linked with disc degeneration (DD) [16]. According to Modic, the first stage of these changes (type 1) reflects hyper-vascularity of the vertebral body and endplates as a result of inflammation. Type 2 consists of fatty replacements of the red bone marrow, as documented by material harvested during lumbar surgery [23].
MC is associated with low back pain (LBP) in the general population [31] and observation from our out-patients’ clinic is that a large proportion of patients with persistent LBP have MC and in an in-house clinical study, patients with sciatica who were treated conservatively were three times as likely to report LBP if they had developed MC at 14 months follow-up as those who had not [1].
It therefore appears that MC is a clinical entity, possibly a late step in a degenerative process [16], which deserves further study. For example, it would be interesting to find out if MC results in a specific clinical picture which makes it easily recognizable or if there are circumstances in the clinical history that can point to its aetiology. No studies appear to have been published, in which relations between MC and the clinical picture were specifically investigated.
Such possible relations were studied using a unique database consisting of 412 40-year olds from the general Danish population, on whom there were extensive data on lumbar MRI findings, history, and clinical examination tests [14].
The overall aim of this study was to investigate if people with MC have a different clinical profile from those with DD only.
Methods
Methodological considerations
Our assumption was that MC, if it were a clinical entity on its own, would be discernable when it is contrasted against those without MC. However, if MC is a late event in a degenerative process, this picture might be diluted if other degenerative findings are present in the non-MC group, because these might have a similar clinical history and perhaps similar clinical findings. To study this issue we looked at the pattern of associations between the clinical picture and MRI findings of MC and/or DD in three subgroups, of descending “severity”, as described:
The first group consisted of all people with both DD and MC (group 1).
The second group consisted of all people with DD but no MC (group 2).
The third group consisted of all those with neither DD nor MC (group 3).
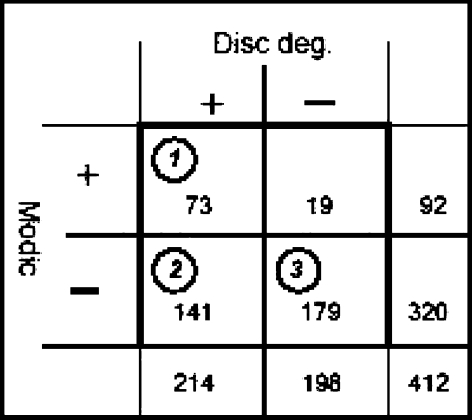
A fourth group of people with only MC was considered but as this group consisted of only 19 persons we did not include these (see Fig. 1 for prevalence in each group). We assumed that the prevalence of positive findings among the variables obtained from clinical history and clinical examination would differ in the three groups. We expected that it would be strongest in group 1 and become gradually weaker in groups 2 and 3. However, we did not have any precise expectations as to which type of variables would best fit this pattern.
Fig. 1.
Cross-tabulation of disc degeneration and Modic changes. The figures in circles denote the three groups used in the study
Variables of interest and the rationale for their choice
The following variables, related to clinical history and clinical examination, were investigated for the following reasons:
Clinical history
Total number of days with LBP in the past year. MC is likely to be a persistent condition and we would expect it to be linked with more persistent LBP.
The prevalence of LBP in the past week, month, and year. MC is expected to produce symptoms resulting in a relatively high prevalence of LBP but not necessarily in the week or month of the survey.
Health-care contacts because of LBP in the past year. MC is likely to be sufficiently painful and persistent to result in contacts with the health-care sector.
Non-trivial LBP. This variable was created to consist of people with LBP for altogether at least 30 days in the past year and evidence of some sort of consequence, because MC is likely to be relatively severe.
Frequency of previous periods of LBP problems. MC is probably the late stage of a slow degenerative process [16, 23]. Therefore more than one period of LBP is likely to have occurred.
Functioning as measured with SF-36. LBP caused by MC is likely to be severe enough to have functional repercussions.
A history of sick leave or reduced activities at work or during leisure time. LBP caused by MC is likely to be severe enough to have repercussions on various physical activities.
Type of work. Vertebral deterioration or delayed recovery may occur more easily in people with physically demanding jobs [20].
Vibrations at work. Vibrations may cause micro-trauma to the bony structure of the vertebral body and in this way facilitate the development of MC [8].
Physical activity in leisure time. See rationale for item 8.
Changes in level of physical activity. People with MC are likely to have reduced their level of physical activity because of LBP.
Smoking may reduce the strength of the vertebral bone, thus accelerating the development of MC or delaying the healing process [9, 12].
Body mass index. Excessive body weight may accelerate the development of MC or delay its recovery [19].
Clinical examination
Hypolordosis (“military spine”) may result in a suboptimal “suspension” effect [13] because excessive body weight is transmitted through the anterior part of the vertebral segment, which may precipitate disc and/or vertebral injuries or make the pain more obvious when the MC is already present.
Pain on lumbar movement. If MC consists of micro-fractures in the lumbar vertebrae with an ensuing inflammatory response, this would make lumbar movements painful, either directly in the lumbar vertebrae or indirectly from muscular defence reactions in the lumbar area [11].
Lumbar pain provocation test. MC is likely to be obviously painful when challenged directly and manually [21].
Lumbar pain tolerance. See rationale for item 16.
Neuromuscular control. Poor neuromuscular control may result in aberrant transitory movements [26], which may contribute to the development of MC, but LBP because of MC may also result in poor neuromuscular control.
Activation of lumbar multifidus muscles. These muscles are believed to play a major role in maintaining the muscular control of the lumbar vertebral segments [4, 24]. Pain is believed to alter muscular function resulting in lack of control [10].
An overview of all variables from history and clinical examination is shown in Tables 1 and 2.
Table 1.
Variables obtained from the questionnaire (clinical history)
| Variable name (coded) | Further description | Prevalence n (%) |
|---|---|---|
| Low back pain | ||
| Duration last year [17] | ||
| 1–7 days LPB | Totally 1–7 days of LBP | 91 (22) |
| 8–30 days LBP | Totally 8–30 days of LBP | 96 (23) |
| > 30 days LBP | Totally more than 30 days with LBP | 102 (25) |
| LBP week (y/n) | “Have you had trouble with the lowest part of your back... (picture provided) during the past 7 days?” [18] | 131 (32) |
| LBP month (y/n) | “Yes” to the above question or “... during the past month?” [18] | 175 (42) |
| LBP year (y/n) | “Yes” to any of the above questions or “Have you had trouble with the lowest part of your back... during the past 12 months?” [18] | 284 (69) |
| Seeking care (y/n) | “Yes” to any of the questions: “Have you sought care during the past year due to trouble with the lowest part of your back? (Please select the items that best applies to you): (a) general practitioner, (b) emergency service, (c) specialist, (d) out-patient clinic, (e) hospitalised, (f) chiropractor, (g) physical therapist, (h) other treatment” [17] | 114 (28) |
| More than one previous episode of LBP (y/n) | 271 (66) | |
| Non-trivial LBP (y/n) | LBP for more than 30 days during the last year with at least one consequence (seeking care or reduced time at work/leisure time), or previous episodes with a mean duration of more than 6 weeks or self-reported disc herniation [14] | 124 (30) |
| Self-reported disc herniation (y/n) | 20 (5) | |
| Work and life style | ||
| Heavy physical workload (y/n) | Heavy physical work, heavy lifting either now or previously for more than 10 years [17] | 105 (25) |
| Vibrations (y/n) | Exposed to vibrations for more than 2 h/week now or previously [17] | 66 (16) |
| Body mass index | Based on self-reported height and weight (kg weight/m height2) | Mean (SD): 25.1 (4.3) |
| High-level leisure time activity (y/n) | Active in sports/hard physical load in leisure time activity at least 3 h/week or participating in competitive sports [17] | 149 (36) |
| Heavy smokers (y/n) | More than 20 cigarettes a day [28] | 71 (17) |
| Functioning | ||
| Downtime work (y/n) | Reduced physical activity at work due to low back problems [17] | 64 (16) |
| Downtime leisure time (y/n) | Reduced physical activities in leisure time due to low back problems [17] | 71 (17) |
| Sick leave (y/n) | Any days off work within the past year due to low back problems [17] | 82 (20) |
| Reduced sports activity (y/n) | Have reduced sports activities | 54 (14) |
| Physical function (SF36) (0–100) | SF-36 questionnaire [2] | Mean [95% CI]: 90 [89; 92] |
Table 2.
Variables obtained from the clinical tests
| Variable name | Defined from | Prevalence n (%) |
|---|---|---|
| Flat back (y/n) | Inspection of lumbar curvature | 90 (22) |
| Pain on movement (y/n) | Person reporting pain on at least one lumbar movement of lateral flexion, flexion, and extension | 86 (21) |
| Lumbar pain provocation test (y/n) | Report of pain on one or more levels when pressure was applied over transverse processes of the lumbar vertebrae | 144 (35) |
| Lumbar pain provocation test (y/n) | Levels where pain was reported when pressure was applied over transverse processes of the lumbar vertebrae | 715 (29)a |
| Inability to control lumbar movement (y/n) | Patients were categorised as being able to control lumbar movement or not based on the therapist’s interpretation of the performance of six exercises [32] | 85 (21) |
| Activation of multifidus muscles (can/cannot) | Inability to voluntarily activate lumbar multifidus muscles (LMM) based on palpation of contraction after therapist’s instruction [29] | 210 (51) |
| Lumbar pain tolerance (0.0–16.0 kg/cm2) | The tolerated pressure in kg/cm2 applied to the spinous process of L4 [6] | Mean [95% CI]: 10.2 [9.8; 10; 6] |
aFor all lumbar levels
Study participants and procedures
In the study, 412 adults aged 40 years (199 men and 213 women) were included. On the day of study they filled in questionnaires, had an MRI of the lumbar spine, and were submitted to a physical examination. The study has been described in detail previously [14, 31] and its relevant aspects are described below. For a description of the strengths and weaknesses of the study refer to PhD thesis [14].
Data collection and research variables
MRI
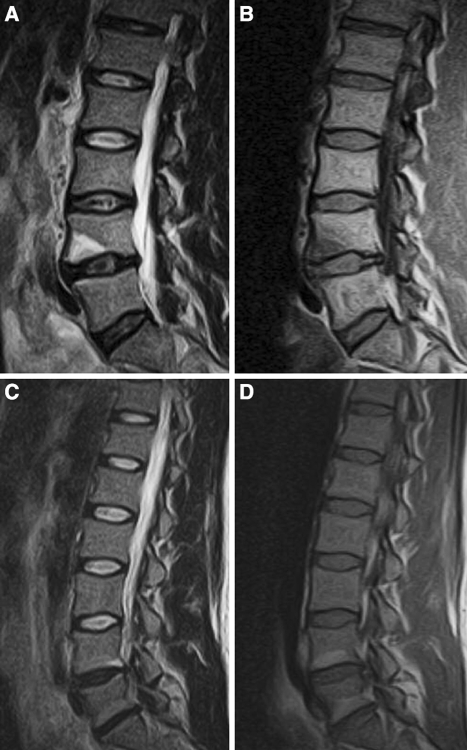
MRI was performed with an open low field 0.2 T MR unit (Magnetom Open Viva, Siemens AG, Erlangen, Germany). For this study, T1- and T2-weighted sagittal and axial T1-weighted sequences were used [31]. All images were described by an experienced radiologist according to a set protocol. DD was defined if there was reduced disc height [7, 33] (grade 2 or 3) or disc signal [27, 33] grade 3. MC was defined as either areas of high signal extending from the vertebral endplates on T2 and low signal on T1-weighted images (type 1), areas of high signal on both T1 and T2-weighted images (type 2), and areas of low signal on both T1 and T2-weighted images were classified as type 3 [23]. The quality of the images was excellent (examples given in Fig. 2). The reliability for this rating has been shown to be satisfactory in an intra- and inter-observer reproducibility study (for disc parameters Kw 0.56–0.87 [31], for MC percentage of agreement 98% and Kw 0.6) [14]. An overview of the MRI variables is shown in Table 3.
Fig. 2.
Examples of images from this study population. Modic type 1: high signal on T2-weighted images (a) and low signal on T1-weighted images (b). Modic type 2: high signal on T2 (c) and high signal on T1 (d)
Table 3.
Variables obtained for MRI and the definitions of “disc degeneration” and “Modic changes”
Clinical history
Questions used to obtain the clinical history were selected from previously used questionnaires in Denmark. For references, please see Table 1.
Physical examination
All participants underwent a thorough physical examination. For this report we used information on lumbar curvature, pain on active movement, a graded lumbar pain provocation test [21], lumbar pain threshold [5, 6], neuromuscular control [15, 32] as judged by the therapist, and a test of the ability to activate the lumbar multifidus muscles [29]. An overview of the variables is given in Table 2.
The majority (78%) of the physical examinations on the study subjects was performed by the principal author and the remaining by two physiotherapists (6 and 16%, respectively), who were trained in the procedures and closely supervised by the principal author.
Statistical methods
The prevalence rates for dichotomous variables and the mean values for continuous variables were listed in each of the three MRI groups. The hypothesis that the difference between groups 1 and 2 was larger than the difference between groups 2 and 3 was tested using a Walds test after a logistic regression.
Because our study was based on pre-existing data, our choice of variables was limited and opportunistic. It was therefore not considered suitable to bring this analysis any further, for example in order to identify best models or specify diagnostic values. Accordingly, we did not perform any additional multivariate analyses.
Stata 8 (Stata Corporation, 2003, Stata Statistical Software: Release 8.2, College Station, TX, USA) was used for the statistical analyses, which were performed by PK and LK.
Results
Descriptive data
The proportion of persons with the two MRI-defined diagnoses (MC yes/no and DD yes/no) is shown in Fig. 1 and Table 3 and the prevalence of the clinical findings is shown in Tables 1 and 2.
Group 1 (MC and DD) consisted of 73 persons, group 2 (DD and not MC) of 141 persons, and group 3 (neither DD and nor MC) was made up of 179 persons.
Different prevalence estimates in the three MRI groups
Our findings were in concordance with our assumption that there would be a difference between the three MRI groups (Table 4). Thus, out of the 23 variables tested, group 1, as compared to the other two groups, had the highest prevalence estimate for all but one of the variables. Furthermore, there was a pattern of declining frequency counts from group 1 through group 2 to group 3 for all but 5 of the 23 variables tested. This decline was statistically significant in 15 cases.
Table 4.
Overview of the prevalence of the investigated variables in the three different MRI groups
| Variable | MC and DD, group 1 (%) | DD only, group 2 (%) | Clean, group 3 (%) | MC + DD versus DD only > DD only versus clean, P value |
|---|---|---|---|---|
| Low back pain | ||||
| LBP week | 47.9 | 34.0 | 22.9 | 0.0013 |
| LBP month | 58.9 | 43.3 | 35.8 | 0.0029 |
| LBP year | 91.8 | 70.9 | 57.5 | 0.0000 |
| Seeking care | 42.5 | 28.4 | 21.8 | 0.0032 |
| Non-trivial LBP | 53.4 | 30.5 | 20.7 | 0.0000 |
| History | ||||
| Prev. episodes LBP | 86.3 | 70.9 | 54.2 | 0.0003 |
| Prev. disc hern. | 11.0 | 5.7 | 2.2 | 0.0142 |
| Work and life style | ||||
| Heavy phys. work | 47.9 | 29.8 | 25.1 | 0.0008 |
| Vibrations | 20.5 | 17.1 | 13.7 | 0.2819 |
| Body mass indexa | 25.14 | 25.26 | 24.83 | 0.8542 |
| High lv. leisure | 42.5 | 31.9 | 38.0 | 0.2273 |
| Heavy smokers | 26.0 | 15.6 | 15.1 | 0.0320 |
| Functioning | ||||
| Downtime work | 27.4 | 14.2 | 11.2 | 0.0021 |
| Downtime leisure | 27.4 | 16.3 | 13.4 | 0.0115 |
| Sick leave | 32.9 | 21.3 | 15.1 | 0.0054 |
| Red. sports act. | 14.1 | 12.4 | 14.3 | 0.8651 |
| Physical func.b | 12.26 | 9.26 | 8.41 | 0.1244 |
| Clinical tests | ||||
| Flat back | 23.6 | 20.6 | 22.3 | 0.6886 |
| Pain on movement | 39.7 | 19.9 | 14.5 | 0.0000 |
| Lumbar PPT | 45.2 | 37.6 | 29.2 | 0.0567 |
| Therapist rated | 24.3 | 19.7 | 21.8 | 0.5169 |
| Activation of LMM | 39.7 | 51.1 | 56.7 | 0.0303 |
| Lumbar pain tol.c | 11.34 | 10.27 | 9.80 | 0.0124 |
akg weight/m height2
bPhysical function (SF36) reduction in score
cMean pressure kg/cm2
Specific clinical profile for people with both DD and MC
The back pain reporting pattern was more marked for people in group 1 than in the other two groups. Regardless of whether the recall period was for the past week, month or year, group 1 would have the highest estimate and almost all of them reported having had LBP some time in the past year. They were also more likely to recall having had more than one episode in the past, to have sought care for LBP, to report to have been diagnosed with a discal hernia, and non-trivial LBP was by far most common in this group.
Group 1 was also characterized by having more people who had reduced their activities at both work and home. Heavy physical work was most commonly reported in this group but there was no association with vibrations at work or high-level physical activities at leisure time.
Heavy smokers were almost twice as common in group 1 as in both groups 2 and 3. The mean of BMI was almost equal in the three groups.
Out of the six clinical examination variables, three followed the expected pattern. Thus it was about twice as common for people in group 1 to report pain on lumbar movement as those in group 2. There was a small but significant difference in the amount of pain tolerated by people in group 1. It was also slightly more common for people in group 1 to be able to activate their multifidus muscle on command.
Discussion
Our study approach is not common and should not be confused with a typical predictor study. The aim of our study was not to specifically identify the clinical profile of people with MC but mainly to investigate if MC is a separate entity, one that deserves to be treated as a potential diagnostic subgroup with specific clinical consequences. In a population-based sample of 412 40-year-old Danes, we found that MC, indeed, has a specific clinical profile.
We performed a simple analysis to test the assumption that people with DD and MC have a different clinical picture from those with only DD and, in particular, from those who have neither DD nor MC. Our results confirmed this theory. In other words, DD in combination with MC has a specific clinical profile but DD on its own more resembles the group with neither DD nor MC.
The results of our study indicate that MC deserves further study.
In addition, we believe that our results may provide “the missing link” in the long-lasting debate on the clinical importance of DD. Is DD irrelevant because it is commonly present in asymptomatic people also [3] or is it clinically important because it is consistently more frequent in people [25] with LBP? Our contribution to this issue is the discovery that DD on its own, at least in 40-year-old Danes, was not characterized by a specific pain profile and was unremarkable in relation to the clinical picture. We therefore surmise that DD, per se, is a fairly quiet disorder but it constitutes a true clinical entity when MC is present. Thus in studies of LBP, in which comparisons are made of people with and without DD, the outcome would depend on the proportion of people with MC. If this proportion is high, there would be a stronger association with LBP than if it is low.
Unfortunately, in our study the number of people with MC on its own, without DD, was too small (n = 19) to make analyses on this group feasible. However, looking at the prevalence in this group (not shown), they clearly seemed to assemble groups 2 and 3 more than group one. Therefore, our conclusions are only pertinent to MC in combination with DD.
All studies have weak points, and ours is that we performed a large number of tests on a study sample that has already been explored from other angles. The potential consequence of this could be that a number of chance findings may appear. However, because we choose not to interpret our findings in detail but in relation to an overall pattern, the occasional chance finding is of limited consequence. Another potential point of critique could be the choice of variables included in this study. It is our belief that the pattern that we have found is likely to appear with a large number of other co-variates of LBP as well. The literature is full of items that have been found to be marginally associated with LBP. It is likely that some of these have the same properties as the ones we tested in the present study.
The important strengths of our study (apart from its size, the fact that our study sample is fairly representative of the general Danish population, and the validity of the MRI variables) are that we dealt with a population-generated study sample and that the study participants were all of the same age. In addition, the MRI was executed under highly controlled circumstances using a protocol exclusively designed for research purposes resulting in high-quality images.
In conclusion, the results of this explorative study indicate that MC in combination with DD is an entity on its own which is different from DD without MC, and that it deserves further study. In particular, it would be relevant to investigate the factors that initiate or accelerate the development of MC.
References
- 1.Albert HB, Manniche C (2005) Modic changes the prevalence and relationship to lumbar disc herniation. A possible new pathogenesis of low back pain (abstract, 7th annual meeting of the Spine Society of Europe, Barcelona, Spain). Eur Spine J 14(suppl. 1), ref. type: abstract
- 2.Bjørner JB, Damsgaard MT, Watt T, Bech P, Rasmussen NK, Kristensen TS, Modvig J, Thunedborg K (1997) Dansk manual til SF36. Et spørgeskema om helbredsstatus
- 3.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW (1990) Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 72(3):403–408 [PubMed]
- 4.Bogduk N (1997) Clinical anatomy of the lumbar spine and sacrum, 3rd edn. Churchill Livingstone, London
- 5.Buchanan HM, Midgley JA (1987) Evaluation of pain threshold using a simple pressure algometer. Clin Rheumatol 6(4):510–517 [DOI] [PubMed]
- 6.Chung S-C, Kim J-H, Kim H-S (1993) Reliability and validity of the pressure pain thresholds (PPT) in the TMJ capsules by electronic algometer. J Craniomandib Pract 11(3):171–176 [DOI] [PubMed]
- 7.Eyre D, Nemya P, Buckwalter J, Caterson N, Heinegard D, Oegema T, Pearce R, Pope M, Urban J (1989) Intervertebral disk: basic science perspectives. In: Frymoyer JW, Gordon SL (eds) New perspectives on low back pain. American Academy of Orthopaedic Surgeons, Park Ridge, pp 147–207
- 8.Hansson T, Holm S (1991) Clinical implications of vibration-induced changes in the lumbar spine. Orthop Clin North Am 22(2):247–253 [PubMed]
- 9.Heliovaara M (1989) Risk factors for low back pain and sciatica. Ann Med 21(4):257–264 [DOI] [PubMed]
- 10.Hodges PW, Moseley GL (2003) Pain and motor control of the lumbopelvic region: effect and possible mechanisms. J Electromyogr Kinesiol 13(4):361–370 [DOI] [PubMed]
- 11.Indahl A, Kaigle A, Reikeras O, Holm S (1995) Electromyographic response of the porcine multifidus musculature after nerve stimulation. Spine 20(24):2652–2658 [DOI] [PubMed]
- 12.Iwahashi M, Matsuzaki H, Tokuhashi Y, Wakabayashi K, Uematsu Y (2002) Mechanism of intervertebral disc degeneration caused by nicotine in rabbits to explicate intervertebral disc disorders caused by smoking. Spine 27(13):1396–1401 [DOI] [PubMed]
- 13.Kendall FP, McCreary EK (1993) Muscles: testing and function, 4th edn. Lippincott Williams & Wilkins, Baltimore
- 14.Kjaer P (2004) Low back pain in relation to lumbar spine abnormalities as identified by magnetic resonance imaging. Faculty of Health Sciences, University of Southern Denmark
- 15.Kjaer P, Jones R, Moeller H, Enoch F (2006) Inter-observer agreement in rating five tests designed to evaluate lumbo-pelvic control (submitted)
- 16.Kjaer P, Korsholm L, Bendix T, Sorensen JS, Leboeuf-Yde C (2006) Modic changes—their associations with other lumbar MRI findings and their attribution to low back pain (submitted) [DOI] [PMC free article] [PubMed]
- 17.Kuorinka I, Jonsson B, Kilbom A, Biering-Sorensen F, Andersson G, Jørgensen K (1987) Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl Ergon 18:233–237 [DOI] [PubMed]
- 18.Leboeuf-Yde C, Kyvik KO (1998) At what age does low back pain become a common problem? A study of 29,424 individuals aged 12–41 years. Spine 23(2):228–234 [DOI] [PubMed]
- 19.Liuke M, Solovieva S, Lamminen A, Luoma K, Leino-Arjas P, Luukkonen R, Riihimaki H (2005) Disc degeneration of the lumbar spine in relation to overweight. Int J Obes (Lond) 29(8):903–908 [DOI] [PubMed]
- 20.Luoma K, Riihimaki H, Raininko R, Luukkonen R, Lamminen A, Viikari-Juntura E (1998) Lumbar disc degeneration in relation to occupation. Scand J Work Environ Health 24(5):358–366 [DOI] [PubMed]
- 21.Maitland GD, Hengeveld E, Banks K, English K (2001) Maitland’s vertebral manipulation, 6th edn. Butterworth Heinemann, Oxford, pp 152–153
- 22.Modic MT, Masaryk TJ, Ross JS, Carter JR (1998) Imaging of degenerative disk disease. Radiology 168(1):177–186 [DOI] [PubMed]
- 23.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR (1988) Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology 166(1 Pt 1):193–199 [DOI] [PubMed]
- 24.Moseley GL, Hodges PW, Gandevia SC (2003) external perturbation of the trunk in standing humans differentially activates components of the medial back muscles. J Physiol 547(Pt 2):581–587 [DOI] [PMC free article] [PubMed]
- 25.Paajanen H, Erkintalo M, Parkkola R, Salminen J, Kormano M (1997) Age-dependent correlation of low-back pain and lumbar disc degeneration. Arch Orthop Trauma Surg 116(1–2):106–107 [DOI] [PubMed]
- 26.Panjabi MM (1992) The stabilizing system of the spine part II. Neutral zone and instability hypothesis. J Spinal Disord 5(4):390–396 [DOI] [PubMed]
- 27.Raininko R, Manninen H, Battie MC, Gibbons LE, Gill K, Fisher LD (1995) Observer variability in the assessment of disc degeneration on magnetic resonance images of the lumbar and thoracic spine. Spine 20(9):1029–1035 [DOI] [PubMed]
- 28.Rasmussen C (1998) [Lumbar disk prolapse. alcohol, tobacco and prognosis]. Ugeskr Laeger 160(36):5189–5192 [PubMed]
- 29.Richardson C, Jull G, Hodges P, Hides J (1999) Therapeutic exercise for spinal segmental stabilization in low back pain, 1st edn. Churchill Livingstone, London
- 30.Roberts N, Gratin C, Whitehouse GH (1997) MRI analysis of lumbar intervertebral disc height in young and older populations. J Magn Reson Imaging 7(5):880–886 [DOI] [PubMed]
- 31.Sorensen JS, Kjaer P, Jensen TS, Andersen P (2006) Low field magnetic resonance imaging of the lumbar spine: reliability of qualitative evaluation. Acta Radiol (in press) [DOI] [PubMed]
- 32.Van Dillen LR, Sahrmann SA, Norton BJ, Caldwell CA, Fleming DA, McDonnell MK, Woolsey NB (1998) Reliability of physical examination items used for classification of patients with low back pain. Phys Ther 78(9):979–88 [DOI] [PubMed]
- 33.Weishaupt D, Zanetti M, Hodler J, Boos N (1998) MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology 209(3):661–666 [DOI] [PubMed]