Abstract
Five-lamina (C3-7) procedure is the most popular cervical laminoplasty and there have been no studies on the most appropriate number of laminae to be opened. We prospectively reduced the range of laminoplasty from C3-7 to C3-6 in 2002 and compared the outcome of C3-6 laminoplasty (n=37) to that of C3-7 laminoplasty (n=28). In both groups, neurological gain was satisfactory, radiographic changes were minimal, and postoperative MRI indicated sufficient expansion of the dura and the spinal cord. Average operating period was significantly shorter, and length of the operative wound was significantly less in the C3-6 group than in the C3-7 group. Postoperative axial neck pain was significantly rarer after C3-6 laminoplasty than after C3-7 laminoplasty (5.4% vs. 29%, P=0.015). Due to its simplicity and various benefits, C3-6 laminoplasty is a promising alternative to conventional C3-7 laminoplasty for treatment of multisegmental compression myelopathy.
Keywords: Myelopathy, Axial neck pain, Laminoplasty, Complication, Seventh cervical vertebra
Introduction
Developmental narrow canal of the spine predisposes patients to cervical myelopathy, irrespective of pathology, which can include cervical spondylosis, ossification of posterior longitudinal ligament (OPLL) and herniated nucleus proposus. Laminoplasty has a long history as a reliable procedure for release of the spinal cord under such conditions [3, 6]. For cervical spines with a lordotic alignment, posterior shift and concomitant escape of the cord from anterior lesions have been presumed to be major elements of the decompression produced by this procedure [1, 12]. Although many variations of laminoplasty have been developed, the 5-lamina (C3-7) procedure has been the most popular procedure. [3, 6, 8] Due to disastrous complications of 1- or 2-lamina resection observed in the early days of laminectomy, many surgeons refrain from limiting the decompression range of laminoplasty. But is it always necessary to open 5 laminae (C3-7) to fully release the spinal cord? Is it ever inappropriate to consider limiting the range of decompression? The spinal cord is rarely affected at C6/7 in patients with degenerative spinal disease. [2] It is not known whether dorsal migration of the spinal cord is greater after C3-7 laminoplasty than after C3-6 procedure. We hypothesized that it would be beneficial to preserve the large spinous process of C7 as a fulcrum for neck muscles. In anatomical terms, Pal and Routal [9] demonstrated that the C7 lamina plays a critical role in maintaining the stability of the cervical spine. Furthermore, some of the problems inherent in laminoplasty, such as axial neck pain, may be prevented by preserving the C7 spinous process and its muscle and ligament attachments. Axial neck pain is one of the most notorious complications associated with cervical laminoplasty. The incidence of axial pain around neck and shoulder was reported as much as 60–80% of patients who had undergone laminoplasty [4, 7]. Since 2002, we have prospectively performed C3-6 laminoplasty for all patients with compression myelopathy, instead of C3-7 laminoplasty. In the present study, we compared these two procedures clinically and radiologically.
Materials and methods
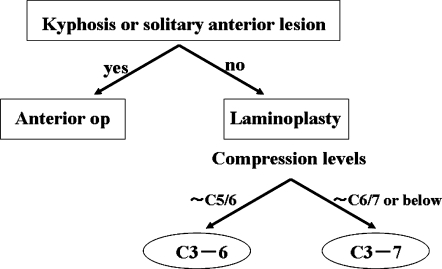
In our institute, the decision as to which surgical procedure should be performed to treat compression myelopathy is performed as follows. Patients with cervical kyphosis or a solitary anterior lesion without narrow spinal canal are operated on via an anterior approach, and all other patients are treated using laminoplasty. C3-6 laminoplasty was the standard procedure for all patients other than those with cord compression at C6/7 or lower levels, who were treated with 5-lamina (C3-7) or longer laminoplasty (Fig. 1). Based on these criteria, 74 patients have undergone cervical decompression surgery since 2002: 1 patient underwent anterior surgery; 5 patients underwent C3-7 laminoplasty; and 68 patients underwent C3-6 laminoplasty. Of the 68 patients who underwent C3-6 laminoplasty, 37 patients with cervical spondylotic myelopathy (CSM) who had a follow-up period longer than 1 year were enrolled in the present study as the C3-6 group. The C3-7 group consisted of 28 patients with CSM who underwent C3-7 laminoplasty before 2002. The C3-6 and C3-7 groups were comparable as to the age at surgery and gender ratio (Table 1).
Fig. 1.
Criteria for deciding between anterior surgery and laminoplasty for compression myelopathy. Patients with cervical kyphosis>15° or solitary anterior lesion are treated by anterior surgery, and all other patients are treated with laminoplasty. When cord compression is restricted to levels above C5/6, C3-6 laminoplasty is used. When cord compression includes C6/7 or lower levels, C3-7 or longer laminoplasty is performed
Table 1.
Demographics of the two groups
| C3-6 | C3-7 | ||
|---|---|---|---|
| Age (years) | 61.7 (31–87) | 61.2 (44–79) | NS |
| Sex (M:F) | 26:11 | 22:6 | NS |
| Diseases | CSM | CSM | |
| Total | 37 | 28 |
The C3-6 and C3-7 groups were comparable as to the age at surgery and gender ratio
CSM Cervical spondylotic myelopathy; NS Not significant
The laminoplasty we used was a modification of unilateral open-door procedure developed by Itoh [6]. The procedure preserves all of the bilateral muscles attached to the C2 spinous process and deep extensor muscles attached to subaxial spinous processes on the hinged side [5]. All patients wore a simple soft collar for 2 weeks postoperative. The only difference between the C3-7 procedure and C3-6 laminoplasty was that the C7 lamina was exposed and opened in the C3-7 procedure. The operating period, estimated intraoperative blood loss, length of the operative wound in the neutral position, and surgical morbidity were assessed for each patient. The clinical and radiological data were all collected prospectively. For the first 6 months after surgery, patients were asked to visit our clinic monthly. After that, they were asked to visit every 2 months; patients were examined on their neurological status and interviewed regarding the axial pain. Neurological status was assessed using the Japan Orthopaedic Association (JOA) score [14]. Patients were asked how severe their axial pain was and how they treated it. When a patient was attending local clinics asking for the pain control or buying any medicine at drugstores, the detail of the treatment or prescription was recorded. Those interviews allowed the precise grading of the patients’ axial pain as described subsequently. Axial neck pain was graded as severe (analgesics or injection of anesthetics to the painful muscles regularly needed), moderate (physical therapy including massage or thermotherapy for the painful muscles regularly needed) or mild (no treatment needed) [5]. Severe or moderate axial pain persisting for more than 1 week during the first 1 month after surgery was considered to constitute early axial pain. Severe or moderate axial pain persisting for more than 1 month after surgery was considered to constitute late axial pain. Although the C3-7 group had a longer average follow-up period than the C3-6 group, axial pain was exclusively examined for the first 1 year postoperative in each patient.
Flexion, extension and neutral lateral radiographs were taken on each visit. Vertebral slippage (≥3 mm) and cervical spine alignment (categorized as lordosis, straight or kyphosis) were assessed from preoperative and 12 months postoperative lateral radiographs. Antero-posterior diameters of the cord and dura were measured at each disc level on midsagittal T2-weighted magnetic resonance images (MRI) 2–4 weeks after surgery. Length of the operative wound was measured by tracing the metal staples for skin closure on the lateral radiograph in neutral position. The diameters or distances were all measured on the digital images of MRI or radiograph that were taken from the server and displayed on the monitor. Measurements were performed by a spine surgeon (SH) not directly involved with the surgery using the special software (NE-PACS Enterprise version 3.0, NEC, Tokyo, Japan). Statistical analysis was performed using Student’s t test and JMP statistical computer software version 4.0 (SAS Institute, Cary, NC, USA), with P values less than 0.05 considered to indicate statistical significance.
Results
Averaged JOA score improved from 11.3 to 14.6 after C3-6 laminoplasty, and improved from 10.8 to 14.3 after the C3-7 procedure. There was no difference in neurological gain after surgery between the groups. None of the patients in either group worsened postoperatively. Average operating period was significantly shorter in the C3-6 group than in the C3-7 group (113 min vs. 129 min, P=0.0082). There was no significant difference in estimated intraoperative blood loss between the C3-6 and C3-7 group (146 g vs. 158 g, P=0.65). Length of the operative wound was significantly less in the C3-6 group than in the C3-7 group (97 mm vs. 129 mm, P<0.0001). Deltoid muscle weakness, more than 1 grade decrease in manual muscle testing, also known as C5 palsy, developed postoperatively in three patients in the C3-6 group and one patient in the C3-7 group, with no significant difference in incidence between the groups (3/37 vs. 1/28, P=0.63). Deltoid muscle weakness recovered well, spontaneously in all patients other than one patient in the C3-7 group, who required anterior surgery to release the C5 nerve root. Additional surgery was performed in one patient in the C3-7 group: for nerve root impingement by dropped lamina. Both the patients who eventually underwent revision surgery showed satisfactory results.
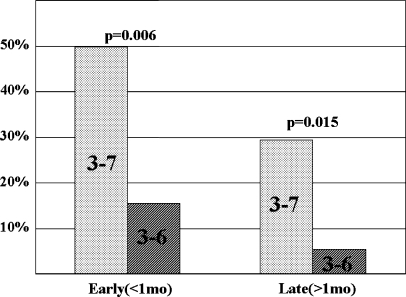
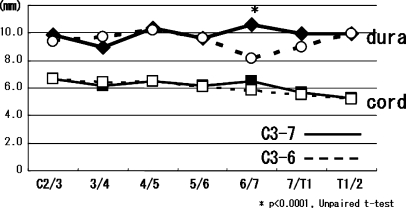
Early axial pain was significantly rarer in the C3-6 group (16%, 6/37) than in the C3-7 group (50%, 14/28) (Fisher’s exact probability test, P=0.006). Late axial pain developed in two patients in the C3-6 group (5.4%, 2/37) and eight patients in the C3-7 group (29%, 8/28); the difference was statistically significant (2/37 vs. 8/28, Fisher’s exact probability test, P=0.015; Fig. 2). Cervical alignment slightly changed in one patient in the C3-7 group (from straight to lordosis) and three patients in the C3-6 group; in two of the three patients in the C3-6 group, cervical alignment changed from straight to lordosis, while one patient showed alignment change from lordosis to straight. One patient in the C3-6 group (3 mm at C3/4) and two patients in the C3-7 group (3 mm at C3/4 and 3 mm at C4/5) developed vertebral slippage postoperatively, but this did not cause clinical symptoms. At all disc levels, there was no difference in antero-posterior diameter of the spinal cord between the two groups. The only significant difference in antero-posterior diameter of the dura occurred at disc level C6/7, where it was significantly smaller in the C3-6 group (8.2 mm vs. 10.6 mm, P<0.0001 ). However, there was sufficient subarachnoid space for the spinal cord at C6/7 (Fig. 3).
Fig. 2.
Incidence of early and late axial pain after laminoplasty. Axial neck pain was significantly rarer after C3-6 laminoplasty than after C3-7 procedure both in the early postoperative period (<1 month) and late postoperative period (>1 month)
Fig. 3.
Anteroposterior diameter of the dura and spinal cord at each disc level. There was no significant difference in antero-posterior diameter of the spinal cord between the two groups. The only significant difference in antero-posterior diameter of the dura occurred at the C6/7, where it was significantly smaller in the C3-6 group. However, there was sufficient subarachnoid space for the spinal cord at C6/7
Discussion
Ratliff et al. [11] have reviewed all English-language articles that deal with cervical laminoplasty. Among the 69 papers they reviewed, the operated range of laminoplasty was unclear in 36 papers. In the 33 papers in which the range of laminoplasty was precisely recorded, 5 or more laminae were opened in 911 patients (89.1%), and 111 patients (10.9%) underwent C3-6 or smaller laminoplasty. None of the papers reviewed described differential indications for C3-6 and C3-7 laminoplasty. Thus, the present paper appears to be the first report of prospective comparison between C3-6 and C3-7 laminoplasty for patients with compression myelopathy.
The operative period was significantly shorter for C3-6 laminoplasty. This may be due to the fact that much time is required to make gutters at C7, which has the largest lamina in the subaxial cervical spine. The lamina index (height × thickness) is 3 times larger at C7 than at C5 [9]. The operative wound was significantly smaller for C3-6 laminoplasty in the present study. Although a surgeon’s bias is a factor in the size of the skin incision, the larger operative wound of C3-7 laminoplasty may be due to the fact that the spinous process of C7 is longer than other cervical spinous processes, as reported by Panjabi et al. [10] Accordingly, the skin incision must be extended further than expected to reach the lamina of C7 (which is located relatively deep) in C3-7 laminoplasty. The shorter operative period and operative wound of C3-6 laminoplasty provide many benefits to patients.
Axial neck pain is often sufficiently severe to be a continuous chief complaint among patients who have undergone laminoplasty [4]. Although the exact pathology of axial neck pain is unclear, many surgeons suspect that neck muscles are directly involved in this complication. The severity of axial neck pain is increased in the sitting or upright position and decreased in the supine position. Downward displacement of the upper extremities may induce axial pain in the sitting position. The trapezius and rhomboideus minor muscles connecting the C7 spinous process and the scapula are stretched by adduction of the scapula with downward displacement of the upper extremity. We hypothesized that preservation of the C7 spinous process and the origin of the trapezius and rhomboideus minor muscles on the C7 spinous process could reduce the pain. This hypothesis proved to be true, as the incidence of axial pain was less in the C3-6 group than in the C3-7 group, both in the early and late postoperative periods. Certainly many tissues other than the trapezius or rhomboideus minor muscles are dissected in laminoplasty, and we do not know which tissues play the key role in producing axial neck pain following laminoplasty. However, the extremely low incidence of axial pain after C3-6 laminoplasty in the present study, compared to previous reports, may warrant a significant attention.
There are two main concerns about C3-6 laminoplasty: (1) Is the spinal cord fully released from the anterior lesions, allowing maximum neurological recovery? (2) Is the operative gain maintained throughout the remaining life of the patient? In the present study, the neurological results were satisfactory for C3-6 laminoplasty; there was no difference in the increase in the JOA score between the C3-6 and C3-7 groups. Furthermore, there was no difference in the increase in the diameter of the spinal cord between the two groups, although postoperative MRI indicated that the diameter of the dural sac at C6/7 was significantly smaller in the C3-6 group. Because the dural sac expands following opening of laminae from C3 to C6, it often appears to be relatively compressed by the intact C7 lamina (Fig. 4a, b). However, there is still enough subarachnoid space for the spinal cord, which has a small diameter at C6/7. Accordingly, we concluded that both the neurological and radiological results of laminoplasty from C3 to C6 are comparable to those of C3-7 laminoplasty.
Fig. 4.
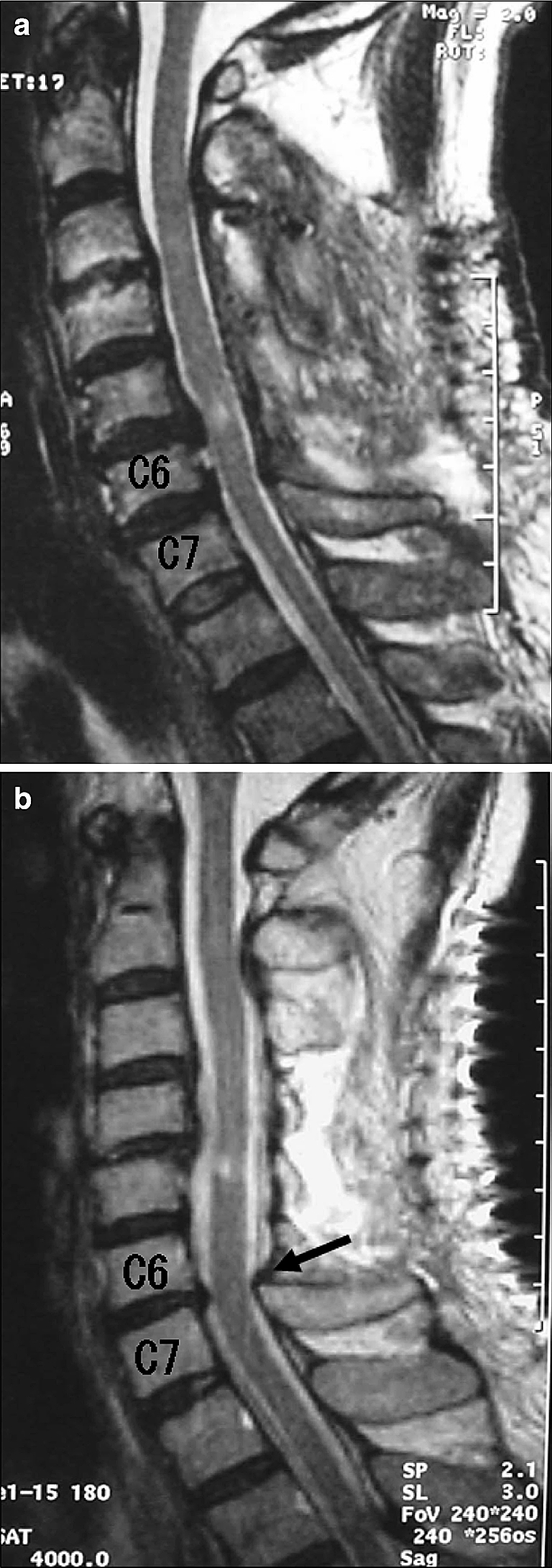
Two patterns of MRI after C3-6 laminoplasty. a Antero-posterior diameter of the dura is uniform throughout the entire cervical spine. b Extreme expansion of the dura over the decompression area causes relative impingement at C6/7 (arrow), although the spinal cord is fully released at the same level
There is a need to verify the reliability of C3-6 laminoplasty in long-term follow-up studies. However, range of motion at C6/7 is small [13] and antero-posterior canal stenosis is rare at C6/7 [2]. Serious problems are not likely to occur at C6/7, which is adjacent to the operative level in C3-6 laminoplasty. There has been no report in which adjacent stenosis required additional surgery after laminoplasty, despite the fact that thousands of patients undergo laminoplasty every year.
Conclusions
In order to preserve the C7 lamina and spinous process, C3-6 laminoplasty was prospectively performed for patients with compression myelopathy, as an alternative to the conventional C3-7 procedure. The operative time was significantly shorter, and the operative wound was smaller for C3-6 laminoplasty than for C3-7 laminoplasty. Axial neck pain was significantly rarer after C3-6 laminoplasty than after C3-7 laminoplasty. The results of the current study indicate that C3-6 laminoplasty should take over C3-7 laminoplasty with significantly lower incidence of axial neck pain and other various benefits.
References
- 1.Aita I, Hayashi K, Wadano Y, Yabuki T (1998) Posteiror movement and enlargement of the spinal cord after cervical laminoplasty. J Bone Joint Surg (Br) 80B:33–37 [DOI] [PubMed]
- 2.Hayashi H, Okada K, Hashimoto J, Tada K, Ueno R (1988) Cervical spondylotic myelopathy in the aged patient. A radiographic evaluation of the aging changes in the cervical spine and etiologic factors of myelopathy. Spine 13:618–625 [PubMed]
- 3.Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K (1981) Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine 6:354–364 [DOI] [PubMed]
- 4.Hosono N, Yonenobu K, Ono K (1996) Neck and shoulder pain after laminoplasty. A noticeable complication. Spine 21:1969–1973 [DOI] [PubMed]
- 5.Hosono N, Sakaura H, Mukai Y, Ishii T, Yoshikawa H (2005) En bloc laminoplasty without dissection of paraspinal muscles. J Neurosurg Spine 3:29–33 [DOI] [PubMed]
- 6.Itoh T, Tsuji H (1985) Technical improvements and results of laminoplasty for compressive myelopathy in the cervical spine. Spine 10:729–736 [DOI] [PubMed]
- 7.Kawaguchi Y, Kanamori M, Ishihara H, Gejo R, Yoshino O (1999) Axial symptoms after en-bloc cervical laminoplasty. J Spinal Disord Tech 12:392–395 [PubMed]
- 8.Kawai S, Sunago K, Doi K, Saika M, Taguchi T (1988) Cervical laminoplasty (Hattori’s method). Procedure and follow-up results. Spine 13:1245–1250 [DOI] [PubMed]
- 9.Pal GP, Routal RV (1996) The role of the vertebral laminae in the stability of the cervical spine. J Anat 188:485–489 [PMC free article] [PubMed]
- 10.Panjabi MM, Duranceau J, Goel V, Oxland T, Takata K (1991) Cervical human vertebrae—quantitative three-dimensional anatomy of the middle and lower regions. Spine 16:861–869 [DOI] [PubMed]
- 11.Ratliff JK, Cooper PR (2003) Cervical laminoplasty a critical review. J Neurosurg (Spine 3) 98:230–238 [DOI] [PubMed]
- 12.Sodeyama T, Goto S, Mochizuki M, Takahashi J, Moriya H (1999) Effect of decompression enlargement laminoplasty for posterior shifting of the spinal cord. Spine 24:1527–1532 [DOI] [PubMed]
- 13.White III AA, Panjabi MM (1990) Kinematics of the spine. In: White III AA, Panjabi MM (eds) Clinical biomechanics of the spine. 2nd edn, JB Lippincott Company, Philadelphia, pp 85–125
- 14.Yonenobu K, Hosono N, Iwasaki M, Asano M, Ono K (1992) Laminoplasty versus subtotal corpectomy—a comparative study of results in multisegmental cervical spondylotic myelopathy. Spine 17:1281–1284 [DOI] [PubMed]