Abstract
Electrical stimulation therapies have been used for more than 30 years to enhance spinal fusions. Although their positive effects on spinal fusions have been widely reported, the mechanisms of action of the technologies were only recently identified. Three types of technologies are available clinically: direct current, capacitive coupling, and inductive coupling. The latter is the basis of pulsed electromagnetic fields and combined magnetic fields. This review summarizes the current concepts on the mechanisms of action, animal and clinical studies, and cost justification for the use of electrical stimulation for spinal fusions. Scientific studies support the validity of electrical stimulation treatments. The mechanisms of action of each of the three electrical stimulation therapies are different. New data demonstrates that the upregulation of several growth factors may be responsible for the clinical success seen with the use of such technologies.
Keywords: Electrical stimulation, Electromagnetic field, Direct current, Capacitive coupling, Growth factors
Introduction
A common challenge to spinal fusion procedures is a postoperative failure to fuse, known as a nonunion or a pseudarthrosis. Despite the technologies that are available today, nonunion rates can still be as high as 40%. Certain risk factors are associated with a higher chance of a nonunion. Risk factors include smoking, a past medical history of a previously failed fusion, diabetes, obesity, multilevel arthrodesis, and the use of certain medications. Electrical stimulation is one of the therapies available to increase the success rates of spinal fusions.
Electrical stimulation has been used for over 30 years to enhance spinal fusions. Since the earliest reported clinical use in 1974 by Dwyer et al. [19], there has been a growing interest in clinical and scientific studies of these technologies. Three types of electrical stimulation technologies have been FDA approved for clinical use: direct current (DC), capacitive coupling (CC), and inductive coupling (IC) such as pulsed electromagnetic fields (PEMF) and combined magnetic fields (CMF).
Positive results have been widely reported on the use of these electrical stimulation therapies to promote bone healing. However, the mechanisms of action of these treatments have been elucidated only recently. New data show that the mechanisms of action involve the upregulation of several growth factors. There are similarities and differences between the electrical stimulation technologies, which are examined in this review.
This review summarizes the current concepts on the mechanisms of action and evaluates the effectiveness of electrical stimulation therapies as demonstrated in animal and clinical studies. Similarities and differences between the technologies and cost justification of their uses for spinal fusions are also examined. This information will help physicians choose which, if any, type of electrical stimulation therapy to use to better treat the patient.
Mechanisms of action
The potential use of electrical stimulation for bone healing first came from the observations of bone tissue electrical properties by Yasuda, Bassett, and Becker in the 1950s and 1960s [2, 3, 68]. When bone is mechanically strained, electrical potentials are generated; electronegative potentials are found in areas of compression and electropositive potentials in areas of tension. The significance of these observations is that the generation of these electric fields may form the basis by which bone remodels in response to mechanical stimuli (Wolff’s Law). Bone forms in the electronegative regions and resorbs in the electropositive regions. These strain-generated potentials arise from the piezoelectric properties of the collagen matrix and electro-kinetic effects referred to as streaming potentials. Electric fields are also generated at injury sites in soft tissue and bone (injury-induced potentials) and at areas of active bone formation such as at the growth plates of developing limbs (bio-potentials) [57].
Since these endogenous electric fields modulate bone cell activities, various electrical stimulation devices have been designed to deliver these fields to enhance bone formation. Three types of electrical stimulation devices have received FDA approval for treating spinal fusions. They are DC electrical stimulation, IC such as PEMFs and CMFs, and CC electrical stimulation. The DC technology requires surgical implantation of the device whereas IC and CC technologies are noninvasive methods of producing electric fields at the fusion site. All of these technologies can also be utilized as adjuncts to surgical procedures using bone grafts.
Studies using a wide variety of cultures and in vivo animal models have been carried out to elucidate the mechanisms of action behind these therapies (Table 1). This section of the article evaluates these studies first with DC, IC, and then CC stimulation.
Table 1.
Mechanisms of action of electrical stimulation technologies
| Study | Technique | Model | Results |
|---|---|---|---|
| Fredericks et al. [24] | DC | Rabbit posterolateral spinal fusion | Upregulates BMP-2, -6, and -7 mRNA |
| Bodamyali et al. [5] | DC | Calvarial organ culture | ↓ Oxygen concentration, ↑ pH and produces hydrogen peroxide.↓ oxygen concentration: ↑osteoblastic activity [12].↑ pH: ↑ osteoblastic activity and ↓ osteoclastic activity.Hydrogen peroxide: stimulates macrophages to produce VEGF [15] |
| Smith et al. [61] | PEMF | Rat cremaster muscle | Elicits arteriolar vasodilation |
| Ryaby et al. [58] | CMF | Rat fracture callus culture | ↑ IGF-II levels |
| Fitzsimmons et al. [20, 21] | CMF | Human osteosarcoma-derived osteoblast-like cell culture | ↑ IGF-II levels and receptors |
| Brighton et al. [11] | CMF | Mouse osteoblastic cell-line MC3T3-E1 | ↑ Cytosolic Ca2+ concentration from Ca2+ release from intracellular stores |
| Fredericks et al. [23] | CC | Rabbit posterolateral spinal fusion | Upregulates BMP-2, -4, -6, -7, TGF-β1, FGF-2, and VEGF mRNA (preliminary data) |
| Lorich et al. [45] | CC | Rat calvarial bone cell culture and mouse osteoblastic cell-line MC3T3-E1 | ↑ PGE2. ↑ cytosolic Ca2+ concentration from transmembrane Ca2+ translocation via voltage-gated calcium channels |
| Zhuang et al. [69] | CC | Mouse osteoblastic cell-line MC3T3-E1 | ↑ TGF-β1 mRNA |
DC direct current, PEMF pulsed electromagnetic field, CMF combined magnetic field, CC capacitive coupling,↑ increases, ↓ decreases, BMP bone morphogenetic protein, VEGF vascular endothelial growth factor, IGF insulin-like growth factor, TGF transforming growth factor, FGF fibroblast growth factor, PGE2 prostaglandin E2, Ca2+ calcium ion
Direct current electrical stimulation
The DC stimulation device consists of cathodes connected to a hermetically sealed power supply which is also the anode. It is an implantable device in which the titanium cathode is implanted at the site of fusion such as over decorticated transverse processes in posterolateral fusions, and the anode/power supply is positioned in the soft tissue at least 8 cm away from the cathodes. A constant localized current produced at the fusion site generates a biologically effective field of influence of approximately 5–8 mm from the cathode. Various configurations of the device, such as straight, wave and mesh, are available to maximize contact with the decorticated bone and graft material. Treatment usually lasts for a minimum of 6 months postimplantation, after which the power supply may be explanted at the discretion of the surgeon.
The mechanism of action behind DC stimulation involves upregulation of a number of osteoinductive factors, which are normal physiologic regulators of bone formation. Using a rabbit posterolateral intertransverse process spinal fusion model, Fredericks et al. [24] investigated the effect of DC stimulation on the expression of bone related factors. This rabbit model has been found to closely mimic the surgical procedure performed in humans (intertransverse process arthrodesis) with similar nonunion outcome rates [6]. An L4–L5 fusion with autograft was carried out bilaterally on rabbits treated either with or without DC. DC stimulation was observed to upregulate specific temporal and spatial gene expression of osteoinductive growth factors bone morphogenetic protein (BMP)-2, -6, and -7, relative to control expression and it did so by enhancing the normal physiologic expressions of these factors. These results support Morone’s findings on the importance of the upregulation of various growth factors at a specific time and location to attain successful fusion, and that each BMP has its own functions and is not interchangeable [50]. In addition, since the normal physiologic expression of the growth factors is enhanced, treatment with DC stimulation does not have the challenges observed with clinical application of single growth factors which requires implantation of high initial doses to retain the desired effective dose for the therapeutic time period. Short residence time of the applied growth factor, ectopic bone formation, bone resorption, and antibody formation against the single growth factor have been reported following intraoperative applications of single growth factors [16, 26, 39, 40, 47, 54, 66].
The electrochemical reactions that occur at the cathode also contribute to the mechanism of action of DC stimulation. Faradic reactions at the cathode lower oxygen concentration, increase pH, and produce hydrogen peroxide [5]. The primary faradic reaction is
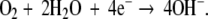 Decrease in oxygen concentration has been found to enhance osteoblastic activity while increase in pH increases osteoblastic activity and decreases osteoclastic activity [12]. In addition, hydrogen peroxide stimulates macrophages to release vascular endothelial growth factor (VEGF) which is an angiogenic factor crucial for bone healing [15].
Decrease in oxygen concentration has been found to enhance osteoblastic activity while increase in pH increases osteoblastic activity and decreases osteoclastic activity [12]. In addition, hydrogen peroxide stimulates macrophages to release vascular endothelial growth factor (VEGF) which is an angiogenic factor crucial for bone healing [15].
Inductive coupling
The PEMF device, a noninvasive technology, consists of external current-carrying coils driven by a signal generator. A magnetic field is produced which induces a secondary electric field at the fusion site. The coils are worn across the area of spinal fusion for about 3–8 h/day for 3–6 months. Patient compliance affects the efficacy of this therapy [49]. The CMF device differs from that of the PEMF device in that it is made up of a time-varying magnetic field superimposed on a static magnetic field.
Most investigations on the mechanism of action of IC technology have been performed with the PEMF signal which is FDA approved for fracture healing but not spinal fusion [1, 4, 8, 11, 29, 43, 44, 51, 64]. There is only one study using the PEMF signal that is clinically approved for spinal fusions [61] and a few studies with CMF [11, 20, 21, 58]. In assessing these studies, it must be noted that different PEMF signals have different effects and that the spine does not fuse in the same manner as a fracture heals; the latter may involve callus formation as in the case of endochondral ossification.
Thus, focusing on just the signals which have been FDA approved for spinal fusions, PEMF has been shown to elicit arteriolar vasodilation in a rat cremaster muscle model [61]. CMF stimulation, on the other hand, has been found to increase insulin-like growth factor II (IGF-II) in rat fracture callus [58], and the IGF-II levels and receptors in human osteosarcoma-derived osteoblast-like cells [20, 21]. These results suggest that IGF-II may help mediate the increase in bone cell proliferation, and CMF may regulate the ability of osteoblast-like cells to respond to the growth factor by modulating receptor availability.
The biochemical pathway mediating the effects of CMF on bone cell proliferation was defined using mouse osteoblast-like cell cultures [11]. The results showed that the pathway involves the release of calcium ions from intracellular stores, which increases cytosolic calcium concentration and activates calmodulin, leading to enhanced bone cell proliferation. A dose response was also observed with cells treated with CMF or inactive devices for 0, 30 min, 2, 6, and 24 h. Cell proliferation increased with increasing treatment time for the CMF groups. A dose–response effect was similarly observed for CMF in in vivo studies using rabbit osteotomy/ostectomy models [52, 67].
Capacitive coupling
Capacitive coupling electrical stimulation is another noninvasive therapy clinically used to enhance spinal fusions. The CC device consists of electrodes with conductive gel that are connected to an alternating current signal generator. The electrodes are placed paraspinally on the skin and produce an electric field at the fusion site.
Positive effects of CC on spinal fusions have been found to involve the upregulation of osteopromotive factors. Using the same rabbit posterolateral spinal fusion model as described by Boden et al. [6], Fredericks et al. [23] investigated the effects of CC on the expression of bone related factors on spinal fusions. Preliminary results showed that CC stimulation upregulates specific temporal and spatial gene expressions of growth factors BMP-2, BMP-4, BMP-6, BMP-7, transforming growth factor (TGF)-β1, fibroblast growth factor (FGF)-2 and VEGF, and it does so by enhancing the normal physiologic expressions of the growth factors.
Lorich et al. [45] investigated the biochemical pathway mediating the response of bone cells to CC stimulation and found it to involve the calcium signal transduction pathway. Rat calvarial bone cells and mouse bone cell-lines were cultured and treated with CC device or inactive device. With the use of signal transduction inhibitors, the effects on cell proliferation and prostaglandin E2 (PGE2) production were analyzed. The biochemical pathway mediating the positive effects of CC stimulation on bone healing involves transmembrane calcium translocation via voltage-gated calcium channels, causing an increase in cytosolic calcium concentration which activates calmodulin, leading to enhanced bone cell proliferation. Phospholipase A2 is also activated resulting in subsequent increase in PGE2. Zhuang et al. [69] further showed that CC stimulation promotes synthesis of TGF-β1 via the calcium–calmodulin pathway.
Capacitive coupling stimulation of osteoblast-like cells similarly demonstrated a dose–response effect with increased cell proliferation with increasing treatment time [11]. When compared to the dose response of IC technologies, CC stimulation resulted in greater enhancement of cell proliferation. This greater response with CC may be due to the unlimited external source of calcium ions available to the cells compared to the limited intracellular source of calcium ions involved in IC stimulation [11].
Animal studies
Direct current electrical stimulation
In 1986, Nerubay et al. [53] carried out an experimental double-blind swine study that showed enhanced rates of posterior fusion success with DC. Using a canine posterior facet fusion model, Kahanovitz and Arnoczky [32] similarly reported the effectiveness of DC in enhancing fusion success. All DC-treated animals achieved solid fusions compared to none in the control group. Fredericks et al. investigated the upregulation of growth factors by DC using a rabbit posterolateral spinal fusion model, and demonstrated solid fusions in all sites treated with DC. Bilateral fusions were observed in all animals (100% fusion success) in the DC-stimulated group at 21 and 28 days postoperative, compared to 0 and 33% in the autograft alone control group [24].
Several studies have also demonstrated that DC stimulation enhances fusion success in a dose-dependent manner. Using a rabbit posterolateral spinal fusion model with autograft, France et al. [22] observed increased healing rates and fusion mass strength with 60 μA current DC stimulation compared to 20 μA stimulation and to control unstimulated groups. In the 60 μA group, faster fusion formation was obtained, as confirmed by histology, radiography, and biomechanical tests. When used in combination with coralline hydroxyapatite bone substitute, Bozic et al. [9] showed that 100 μA DC stimulation was significantly better than autograft alone. These results indicate the possibility of eliminating the morbidity associated with bone harvests from the iliac crest by combining bone substitutes with DC stimulation as a treatment in place of autograft. Using a sheep interbody fusion model with an electrified titanium cage, Toth et al. [65] showed 100% fusion rate with 100 μA DC stimulation compared to 27% in the unstimulated group. The increase in speed of fusion with increasing current density of DC stimulation was also demonstrated by Dejardin et al. [17] using a canine facet fusion model. All these studies demonstrate that DC stimulation enhances fusion success in a dose-dependent manner.
Inductive coupling
The results on the use of PEMF for spinal fusions are not as conclusive as those for DC. A number of spinal fusion studies showed no significant differences between the PEMF-treated groups and the control groups. The first controlled experimental spinal fusion study with PEMF, carried out using a canine posterior spinal fusion model, demonstrated no statistically significant difference in fusion rates between PEMF and unstimulated control groups, despite observations of possible early accelerated healing response [34]. No enhancement of fusion success rates with PEMF was again observed in another canine posterior spinal fusion study, this time, using a different PEMF signal [35]. In a rabbit posterolateral fusion model, increases in stiffness, area under the load–displacement curve and load to failure of the fusion mass in the stimulated group versus control group were observed [27]. However, there was no difference in the fusion rates between the groups. A histological posterior fusion study with rats indicated enhanced bone callus formation with PEMF initially but the observed histological pattern became similar to that of controls after 8 weeks [30]. Furthermore, an investigation on the effects of PEMF on instrumentation-assisted posterolateral fusion using beagles showed a 17% increase in bone mineral density of the vertebral bodies of animals fused with instrumentation but again no statistical improvement in bone mineral density with PEMF [31]. With regards to CMF, there are no published scientific in vivo animal studies documenting the use of CMF for spinal fusions.
Capacitive coupling
Positive effects of CC stimulation on fracture healing have led to studies on the use of CC stimulation as an adjunct to spinal fusions. Using a castration-induced osteoporotic rat model, various CC fields and treatment durations were tested with the electrodes placed paraspinally at T11 and L4 levels [10]. The results showed that the lowest μA signal tested in this study (60 kHz, 100 μA signal) worked best. Castration-induced osteoporosis was significantly reversed and bone mass/unit volume was restored. It is interesting to note that since this is the lowest signal tested in the study, it is probable that a lower CC signal than that tested would also be effective.
Fredericks et al. [23] investigated the effects of CC on spinal fusion and the expression of noted bone related factors using a rabbit posterolateral intertransverse spinal fusion model. An L4–L5 fusion with autograft was carried out bilaterally on rabbits treated either with or without CC stimulation. It was found that at 21 days, two out of three rabbits had unilateral fusions in the CC-treated group compared to one out of three rabbits in the control group. At 28 days, one rabbit had bilateral fusion and two rabbits had unilateral fusion in the CC group, in contrast to two out of three rabbits in the control group with unilateral fusion. None of the rabbits in the control group had bilateral fusion.
Electric field distributions using CC stimulation were also determined in the spine and soft tissues for the rat [13] and the human [14]. Using a three-dimensional, anatomically based, finite element model of the human trunk from vertebral levels T5 to L5, current density distributions in the human spine during CC stimulation were evaluated by Carter et al. [14]. The model included fat, ribs, lung, intervertebral discs, spinal cord, muscle, cortical and trabecular bone of the spine, and other tissues as would be found at the respective levels. Variations in the distribution of induced current density depending on tissue resistivity and placements and configurations of electrodes were observed. The presence of fat contributed to higher current densities in the spine area. The generated electric field increased with increasing tissue resistivity.
The number and placement of electrodes and the output signal required to generate current densities and electric fields in the human spine of the levels shown to be effective in restoring bone mass were determined from these animal studies. Consequently, the CC device in clinical use consists of a pair of electrodes with a signal output of the same frequency but higher current than that modeled by Carter et al. [14].
Clinical studies
Electrical stimulation therapies have been used clinically to treat spinal fusions (Table 2). The primary methods for determination of fusion success in a clinical setting are based on radiographic grading and clinical function. Most studies evaluate fusion success with radiographic evidence alone. Fusion success rates defined by clinical function or both radiographic and clinical evidence are otherwise stated. Original criteria for fusion success rates in the clinical studies presented here have not been altered.
Table 2.
Clinical studiesa
| Study | Technique | Model | Number of patients | Results |
|---|---|---|---|---|
| Kane [36] | DC | Multicenter posterior spinal fusions | 82 | 91.5% fusion success in DC group versus 80.5% in control group |
| Kane [36] | DC | Randomized, prospective, posterior spinal fusions, high-risk patients | 31 | 81% fusion success versus 54% control |
| Kane [36] | DC | Prospective, open, posterior spinal fusions, high-risk patients | 116 | 93% fusion success |
| Meril [48] | DC | Prospective, controlled anterior and posterior lumbar interbody fusions with allograft | 122 | 93% fusion success versus 75% control. Subset of patients who were smokers: 92% success rate versus 71% control. No internal fixation: 91% versus 65% control |
| Rogozinski and Rogozinski [56] | DC | Instrumentation-assisted lumbosacral fusions | 94 | 96% fusion success versus 85% control |
| Kucharzyk [38] | DC | Prospective, instrumentation-assisted posterior high-risk lumbar spinal fusions | 130 | 95.6% fusion success versus 87% control. Smokers: 83% success rate versus 66% control |
| Tejano et al. [63] | DC | Prospective, open, multilevel posterior lumbar spinal fusions without instrumentation, long-term follow-up | 118 | 91–93% fusion success in a median 5-year follow-up period |
| Simmons [59] | PEMF | Posterior lumbar interbody fusions | 13 | 77% success rate |
| Mooney [49] | PEMF | Prospective, randomized, double-blind interbody lumbar spinal fusions | 195 | 92% success rate versus 65% control. At 4-year follow-up: 67% success rate versus 49% control |
| Di Silvestre and Savini [18] | PEMF | Posterolateral lumbosacral spinal fusion | 31 | 96% fusion success |
| Bose [7] | PEMF | Posterolateral lumbar high-risk fusions with instrumentation | 48 | 97.9% fusion success |
| Marks [46] | PEMF | Lumbar spinal fusions | 61 | Mean 15.6-month follow-up period: 97.6% fusion success versus 52.6% control |
| Linovitz et al. [42] | CMF | Prospective, multicenter, randomized, double-blind, posterolateral lumbar spinal fusions without instrumentation | 201 | 64% success rate versus 43% control. Appeared effective only in women. Fusion rates were not enhanced in men |
| Goodwin et al. [28] | CC | Multicenter, randomized, double-blind, prospective, lumbar spinal fusions | 179 | 85% success rate versus 65% control. Both radiographic and clinical criteria |
DC direct current, PEMF pulsed electromagnetic field, CMF combined magnetic field, CC capacitive coupling
aPeer-reviewed publications
A large multicenter clinical study using DC to enhance spinal fusions was first reported in 1988 [36]. The results of three independent studies were published in this article. In the first study, 82 patients underwent posterior spinal fusion with DC stimulation. The results were compared to that of a historical control group of 150 patients with fusion alone without DC. It was found that the DC group had a statistically significant higher success rate of 91.5% compared to 80.5% in the control group. The second study was a randomized, prospective, controlled clinical study on the use of DC stimulation on high-risk patients undergoing posterior spinal fusions. The patient population consisted of those with previous failed fusions, patients with grade II or worse spondylolisthesis, patients requiring multiple level fusions, and patients with other risk factors such as obesity, smoking, and diabetes. The DC-stimulated group had an 81% fusion success rate compared to 54% in the control group. The third study evaluated 116 patients from the same “difficult to fuse” population in an uncontrolled clinical study with DC for posterior spinal fusion. A 93% fusion rate was reported.
In 1994, Meril [48] reported the results of patients undergoing anterior and posterior lumbar interbody fusions with allograft. The fusion success rate of the DC-stimulated group was found to be 93%, compared to 75% in the control group. In a subset of patients who were smokers, the stimulated group had a 92% success rate versus 71% in the nonstimulated group. Cases without internal fixation had a 91% success rate in the stimulated group compared to 65% in the control group.
Other clinical studies focused on the use of DC for posterolateral fusions. Rogozinski and Rogozinski [56] carried out a study on patients undergoing posterior spinal fusion with pedicle screw instrumentation with and without DC. Patients in the DC group were found to have a higher success rate of 96% from clinical and radiographic assessments compared to 85% in the control group. A similar study evaluating the use of DC in patients with pedicle-screw-assisted posterior fusions showed a 95.6% success rate in the experimental group as evaluated clinically and radiographically versus 87% in the control unstimulated group [38]. Smokers were also found to heal better with a success rate of 83% in the DC group compared to 66% in the control group. DC-assisted spinal fusions resulted in statistically increased clinical successes with higher fusion grades, thus supporting the concomitant use of DC and instrumentation. The beneficial effects of DC have also been demonstrated in patients undergoing spinal fusions without instrumentation. A prospective clinical study of 118 patients with multilevel posterior spinal arthrodesis without pedicle instrumentation and treated with DC showed success rates that varied between 91 and 93% in a median 5-year follow-up period [63].
Clinical efficacy of PEMF was first reported in 1985. The study, which consisted of 13 patients with established pseudarthrosis who had undergone posterior lumbar interbody fusion, showed 77% of patients with healed interbody pseudarthrosis [59]. Lee, on the other hand, reported a 67% success rate of patients treated for posterior pseudarthrosis with PEMF [41]. The fusion rates were found to be dependent on patient compliance in wearing the PEMF unit. Noncompliant patients had fusion rates that were similar to unstimulated controls. Simmons et al. [60] also evaluated the use of PEMF for primary posterolateral spinal fusions and showed a fusion rate of 71% which is significantly lower than that demonstrated with DC in patients undergoing primary posterolateral fusions.
The first multicenter clinical study with PEMF was carried out in 1990 with 195 patients who underwent primary posterior or anterior lumbar interbody fusions [49]. Posterolateral fusions were not evaluated. In this study, the radiographic criteria for fusion required only 50% graft incorporation, which results in an overall success rate of 92% making it similar to that of Kane’s overall success rate with DC. The control group had a 65% success rate. Subsequent 4-year follow-up of the patients revealed a decrease by approximately 25% in the longer term success rates, for a fusion rate of 67% compared to 49% in the control group.
With regards to posterolateral spinal fusion, Di Silvestre and Savini [18] carried out an uncontrolled study of 31 patients demonstrating encouraging preliminary results at 4-month time period. In an uncontrolled study of 48 high-risk fusion patients with PEMF and instrumentation, Bose [7] showed a 97.9% radiographic fusion rate and an overall clinical assessment of good to excellent of 83.4%.
Marks examined 61 patients with discogenic low-back pain who underwent lumbar fusion with or without PEMF [46]. Of the 42 patients treated with PEMF with a mean follow-up period of 15.6 months, the radiographic fusion rate was 97.6% compared to 52.6% in the unstimulated control group.
There is only one published clinical study on the use of CMF for spinal fusions [42]. The study was carried out with 201 patients undergoing noninstrumented posterolateral spinal fusions and showed an overall success rate of 64% in the CMF-treated group compared to 43% in the control group. CMF appeared to be effective only in women; fusion rates were not enhanced in men.
The largest and most comprehensive study evaluating the use of CC as an adjunct to spinal fusion is a multicenter randomized double-blind study carried out by Goodwin et al. [28]. In this study, the fusion is considered a success only if it meets the success criteria of both radiographic and clinical assessments, thus making the criteria more stringent. The overall fusion success rates were found to be statistically higher in the CC-stimulated group (85%) compared to in the control group (65%).
Cost analysis
The cost effectiveness of using adjunctive electrical stimulation devices was evaluated in 1996 [33]. Kahanovitz et al. examined a large database of patients undergoing posterolateral spinal fusions with and without DC and pedicle screw instrumentation, as well as the costs incurred in caring for the patients after discharge. The study used a carefully constructed framework consisting of well-defined clinical, functional, and economic patient outcomes as supported by evidence from randomized and prospective multicenter clinical trials, data obtained through epidemiological surveillance of lumbar fusion patients and peer-reviewed studies. Published reports using DC showed 90% or more radiographic fusion success in difficult-to-fuse patients and clinical assessments with improved patient outcomes. Lower mean inpatient day counts at 24 months after discharge were observed with DC patients with and without instrumentation (1.79 days for patients with DC and 0.73 days with DC and instrumentation) compared to controls (3.02 days for patients with no adjuncts and 3.08 days with instrumentation alone). The mean inpatient costs were similarly lower in the DC group with and without instrumentation (US $3,637 for DC stimulation alone and US $796 for DC with instrumentation) versus controls (US $6,110 for patients with no adjuncts and US $6,735 with instrumentation alone). The results showed that patients treated with DC with and without instrumentation had significant cost savings over those fused without DC.
An evaluation by the National Blue Cross Blue Shield Association’s Medical Advisory Panel of DC treatment for spinal fusions in 1992 similarly concluded that DC as an adjunct to spinal surgery improves the outcomes of patients at high risk for pseudarthrosis [33]. DC was found by the panel to meet all five distinct criteria necessary to receive coverage for a medical device: (1) DC has received final approval from the appropriate government regulatory bodies, (2) the scientific evidence permits conclusions regarding the effect of DC on health outcomes, (3) DC improves the net health outcome, (4) DC is as beneficial as any established alternatives, and (5) the improvement is obtainable outside the investigational settings.
When compared to biologic treatments such as BMP-7 and BMP-2 (out of indication use) for posterolateral fusions, DC was also found to be most cost effective. Since only one DC device is used, the cost of DC treatment of US $5,075 remains the same for one to three or more level fusions. However, the cost of biologics doubles or triples from one to two to three level fusions, as the amount of material needed increases with multilevel fusions (currently the cost for BMP-7 is US $14,500 for a one level fusion, US $29,000 for two level, and US $43,500 for a three level fusion; BMP-2, based on an out of indication use at the efficacious dose of 40 mg per level, would increase from US $20,000 to US $60,000 for one to three level posterolateral fusions). This cost saving is also relevant for CC and IC treatments since only one CC or IC device is used for one or more level fusions.
Biological and biochemical effects of electrical stimulation on fusions
Recently, the mechanisms of action of electrical stimulation therapies have been elucidated. These studies support the validity of these treatment modalities to enhance success rates of spinal fusions. Electrical stimulation technologies—DC, IC, and CC—share a few similarities. These technologies upregulate a number of growth factors that are involved in normal bone healing. It is important to note that the upregulation of growth factors by DC and CC was determined mainly using spinal fusion models, whereas the IC mechanism of action was observed from fracture healing studies. Different PEMF signals have different effects. Since this review covers therapies for spinal fusions, only studies using the PEMF signal which is FDA approved for spinal fusions are reviewed and discussed. The healing of bone in spinal fusion also differs significantly from that of fracture healing; the latter may involve callus formation as in the case of endochondral ossification. DC stimulation upregulates mRNA for osteoinductive growth factors such as BMP-2, -6, and -7 which are known to stimulate bone and cartilage cell proliferation, differentiation, and extracellular matrix synthesis [24, 25]. IC CMF stimulation upregulates mRNA for IGF-II [20, 21, 58]. CC stimulation, on the other hand, upregulates the expression of factors TGF-β1 [69], and PGE2 [45], with preliminary results showing also the upregulation of BMP-2, -4, -6, -7, FGF-2, and VEGF [23, 25].
In addition to the upregulation of growth factors, the mechanism of action of DC stimulation is also related to the electrochemical reactions that occur at the cathode which lower local oxygen concentration, increase pH, and produce hydrogen peroxide [5]. The decrease in oxygen concentration enhances osteoblastic activity while an increase in pH increases osteoblastic activity and decreases osteoclastic activity [12]. The net result is thus bone formation. In addition, hydrogen peroxide, another faradic product that is produced, has been shown to stimulate macrophages to release VEGF, an angiogenic factor that is crucial for bone healing [15]. It is possible that the faradic products from the electrochemical reactions at the cathode result in the expression of osteopromotive factors such as BMP-2, -6, and -7 that are upregulated by DC. Parathyroid hormone, which stimulates bone resorption, has been observed to be associated with cytoplasmic acidification of osteoblast-like cells [55, 62]. On the other hand, prostaglandins, which are known to stimulate bone formation, have been found to involve increases in pH in the cytoplasm of mouse osteoblast-like cells [37].
In IC and CC stimulation, the biochemical pathway mediating the response of bone cells to both technologies involves an increase in intracellular calcium ion (Ca2+) concentration and subsequent activation of calmodulin, leading to enhanced cell proliferation. However, the source of Ca2+ for IC is different from that of CC. The CC signal acts at or within the bone-cell membrane, opening voltage-gated calcium channels, allowing extracellular Ca2+ to enter the cell. In contrast, IC brings about the release of Ca2+ from intracellular stores. Since the supply of Ca2+ in extracellular fluid is infinite compared to the limited intracellular store of Ca2+, it has been suggested that this factor may contribute to the enhanced cell proliferation observed by Brighton et al. [11] at 24 h with CC compared to IC CMF. These forms of electrical stimulation technologies demonstrate dose-dependent effects. IC PEMF stimulation has also been shown to elicit arteriolar vasodilation [61].
A number of studies show the effectiveness of growth factors on bone healing. However, recent studies have raised concerns about the application of single growth factors. Although carriers have improved delivery and maintenance of the growth factors locally, high initial doses are still needed to achieve the desired mean dose over the therapeutic time period. Growth factors remain locally at the site of application for a short time period, and ectopic bone formation, bone resorption, and antibody formation against the growth factors have been reported [16, 26, 39, 40, 47, 54, 66]. In addition, since this therapeutic approach delivers only a single growth factor, it is still dependent on the recruitment of cells and other growth factors, which have synergistic effects. Morone et al. [50] have shown that successful fusion requires a specific spatial and temporal expression of various growth factors, and that each BMP has its own functions and thus is not interchangeable. In contrast, both DC and CC electrical stimulation technologies do not have these challenges as they upregulate a number of growth factors throughout the treatment time, by enhancing the normal physiologic expression of most of the growth factors.
In assessing the efficacy of these electrical stimulation therapies, it is also important to note that there are differences in the manner spinal fusions heal physiologically and biomechanically. Anterior interbody fusions are revascularized through the decorticated vertebral bodies, and the graft materials placed in the interspace are under compressive loads. In contrast, revascularization in posterolateral fusion comes mostly from surrounding tissues with little compressive forces on the graft material used.
There are numerous studies showing the effectiveness of electrical stimulation therapies as adjuncts to spinal fusions. Studies with DC consistently showed positive results with DC treatment for both posterolateral and interbody fusions whereas the results with IC, mainly PEMF, have been inconsistent and CMF has no published animal studies documenting its use for spinal fusions. In addition, there are more clinical studies supporting the use of DC for spinal fusions compared to IC. DC therapy has also been shown to be particularly successful in treating difficult-to-fuse cases. There is only one CMF clinical study and that is to enhance noninstrumented posterolateral spinal fusions. The concept of CC therapy is relatively new. Animal studies with CC have shown positive effects for spinal fusions, with other studies focusing on optimizing the signal used for CC treatment. In addition to animal studies, a large multicenter randomized double-blind study with CC has demonstrated the efficacy of CC treatment for spinal fusions. DC is an implantable device, and patient compliance is not an issue, unlike noninvasive IC devices. CC, which is also a noninvasive device, is the smallest and lightest of the noninvasive technologies available, perhaps improving its patient compliance.
Conclusions
The clinical benefits of electrical stimulation as adjuncts to spinal fusions have become increasingly recognized over the last 30 years. Scientific studies have better defined the mechanisms of action, thus supporting the validity of these treatments. However, these adjunctive electrical stimulation therapies are not equally effective in enhancing fusion success. Basic science and clinical studies shows DC stimulation to be superior to IC particularly when used to treat posterior spinal fusions. Data on CC therapy also indicate advantages over IC particularly for posterolateral fusions. However, it is not as statistically beneficial as DC for posterior spinal fusions. The demonstration that these technologies create an upregulation of several synergistic growth factors may prove to be more cost efficacious than the application of a single growth factor for spinal fusions. More studies on the mechanisms of action of electrical stimulation would help in even further defining the specific biological and biochemical pathways of its effects on spinal fusions and also possibly indicating new applications for these technologies. New applications for electrical stimulation include soft tissue healing such as wound healing, diabetic ulcers, and neuropathy. As research is conducted into other aspects and applications of electrical stimulation therapies, electrical stimulation should prove to be even more useful in the future.
Footnotes
This review complies with current USA laws inclusive of ethics approval.
References
- 1.Aaron RK, Wang S, Ciombor DM (2002) Upregulation of basal TGFβ1 levels by EMF coincident with chondrogenesis—implications for skeletal repair and tissue engineering. J Orthop Res 20:233–240 [DOI] [PubMed]
- 2.Bassett CA, Becker RO (1962) Generation of electric potentials by bone in response to mechanical stress. Science 137:1063–1064 [DOI] [PubMed]
- 3.Bassett CA, Pawluk RJ, Becker RO (1964) Effects of electric currents on bone formation in vivo. Nature 204:652–654 [DOI] [PubMed]
- 4.Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, Abbott J, Blake DR, Stevens CR (1998) Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem Biophys Res Commun 250:458–461 [DOI] [PubMed]
- 5.Bodamyali T, Kanczler JM, Simon B, Blake DR, Stevens CR (1999) Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem Biophys Res Commun 264:657–661 [DOI] [PubMed]
- 6.Boden SD, Schimandle JH, Hutton WC (1995) An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine 20:412–420 [DOI] [PubMed]
- 7.Bose B (2001) Outcomes after posterolateral lumbar fusion with instrumentation in patients treated with adjunctive pulsed electromagnetic field stimulation. Adv Ther 18:12–20 [DOI] [PubMed]
- 8.Boyan BD, Simon BJ, Gan JC, MacDougall MJ, Lohmann CH, Schwartz Z (2005) EMF regulates growth factor synthesis by osteoblasts. In: Aaron RK, Bolander ME (eds) Physical regulation of skeletal repair. American Academy of Orthopaedic Surgeons, Rosemont, pp 201–207
- 9.Bozic KJ, Glazer PA, Zurakowski D, Simon BJ, Lipson SJ, Hayes WC (1999) In vivo evaluation of coralline hydroxyapatite and direct current electrical stimulation in lumbar spinal fusion. Spine 20:2127–2133 [DOI] [PubMed]
- 10.Brighton CT, Luessenhop CP, Pollack SR, Steinberg DR, Petrik ME, Kaplan FS (1989) Treatment of castration-induced osteoporosis by a capacitive coupled electrical signal in the rat vertebrae. J Bone Joint Surg Am 71:228–233 [PubMed]
- 11.Brighton CT, Wang W, Seldes R, Zhang G, Pollack SR (2001) Signal transduction in electrically stimulated bone cells. J Bone Joint Surg Am 83:1514–1523 [DOI] [PubMed]
- 12.Bushinsky DA (1996) Metabolic alkalosis decreases bone calcium efflux by suppressing osteoclasts and stimulating osteoblasts. Am J Physiol 271:F216–F222 [DOI] [PubMed]
- 13.Carter EL, Vresilovic EJ, Pollack SR, Brighton CT (1989) Field distributions in vertebral bodies of the rat during electrical stimulation: a parametric study. IEEE Trans Biomed Eng 36:333–345 [DOI] [PubMed]
- 14.Carter EL, Pollack SR, Brighton CT (1990) Theoretical determination of the current density distributions in human vertebral bodies during electrical stimulation. IEEE Trans Biomed Eng 37:606–614 [DOI] [PubMed]
- 15.Cho M, Hunt TK, Hussain MZ (2001) Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am J Physiol Heart Circ Physiol 280:H2357–H2363 [DOI] [PubMed]
- 16.Dawson EG (2003) Bone morphogenetic proteins BMPs. Letter. Spine J 3:87–88 [DOI] [PubMed]
- 17.Dejardin LM, Kahanovitz N, Arnoczky SP, Simon BJ (2001) The effect of varied electrical current densities on lumbar spinal fusion in dogs. Spine J 1:341–347 [DOI] [PubMed]
- 18.Di Silvestre M, Savini R (1992) Pulsing electromagnetic fields (PEMFs) in spinal fusion: preliminary clinical results. Chir Organi Mov 77:289–294 [PubMed]
- 19.Dwyer AF, Yau AC, Jefcoat KW (1974) Use of direct current in spine fusion. J Bone Joint Surg Am 56:442
- 20.Fitzsimmons RJ, Ryaby JT, Magee FP, Baylink DJ (1995) IGF-II receptor number is increased in TE-85 osteosarcoma cells by combined magnetic fields. J Bone Miner Res 10:812–819 [DOI] [PubMed]
- 21.Fitzsimmons RJ, Ryaby JT, Mohan S, Magee FP, Baylink DJ (1995) Combined magnetic fields increase insulin-like growth factor-II in TE-85 human osteosarcoma bone cell cultures. Endocrinology 136:3100–3106 [DOI] [PubMed]
- 22.France JC, Norman TL, Santrock RD, McGrath B, Simon BJ (2001) The efficacy of direct current stimulation for lumbar intertransverse process fusions in an animal model. Spine 26:1002–1008 [DOI] [PubMed]
- 23.Fredericks D, Petersen E, Bobst J, Gan J, Simon B, Nepola J (2004) Effects of capacitive coupling electrical stimulation on expression of growth factors in a rabbit posterolateral spine fusion model. North American Spine Society, Chicago
- 24.Fredericks DC, Petersen EB, Bobst JA, Gan JC, Simon BJ, Glazer P, Nepola JV (2006) Effects of direct current electrical stimulation on gene expression of osteopromotive factors in a posterolateral spinal fusion model. Spine (in press) [DOI] [PubMed]
- 25.Gan JC, Fredericks DC, Glazer PA (2004) Direct current and capacitive coupling electrical stimulation upregulates osteopromotive factors for spinal fusions. Orthop J Harvard Med School 6:57–59
- 26.Geesink RG, Hoefnagels NH, Bulstra SK (1999) Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br 81:710–718 [DOI] [PubMed]
- 27.Glazer PA, Heilmann MR, Lotz JC, Bradford DS (1997) Use of electromagnetic fields in spinal fusion. A rabbit model. Spine 22:2351–2356 [DOI] [PubMed]
- 28.Goodwin CB, Brighton CT, Guyer RD, Johnson JR, Light KI, Yuan HA (1999) A double-blind study of capacitively coupled electrical stimulation as an adjunct to lumbar spinal fusions. Spine 24:1349–1357 [DOI] [PubMed]
- 29.Guerkov HH, Lohmann CH, Liu Y, Dean DD, Simon BJ, Heckman JD, Schwartz Z, Boyan BD (2001) Pulsed electromagnetic fields increase growth factor release by nonunion cells. Clin Orthop 384:265–279 [DOI] [PubMed]
- 30.Guizzardi S, Di Silvestre M, Govoni P, Scandroglio R (1994) Pulsed electromagnetic field stimulation on posterior spinal fusions: a histological study in rats. J Spinal Disord 7:36–40 [DOI] [PubMed]
- 31.Ito M, Fay LA, Ito Y, Yuan MR, Edwards WT, Yuan HA (1997) The effect of pulsed electromagnetic fields on instrumented posterolateral spinal fusion and clinical related stress shielding. Spine 20:382–388 [DOI] [PubMed]
- 32.Kahanovitz N, Arnoczky SP (1990) The efficacy of direct current electrical stimulation to enhance canine spinal fusions. Clin Orthop 251:295–299 [PubMed]
- 33.Kahanovitz N, Pashos C (1996) The role of implantable direct current electrical stimulation in the critical pathway for lumbar spinal fusion. J Care Manage 6:2–8
- 34.Kahanovitz N, Arnoczky SP, Hulse D, Shires PK (1984) The effect of post-operative electromagnetic pulsing on canine posterior spinal fusions. Spine 9:273–279 [DOI] [PubMed]
- 35.Kahanovitz N, Arnoczky SP, Nemzek J, Shores A (1994) The effect of electromagnetic pulsing on posterior lumbar spinal fusions in dogs. Spine 19:705–709 [DOI] [PubMed]
- 36.Kane WJ (1988) Direct current electrical bone growth stimulation for spinal fusion. Spine 24:363–365 [DOI] [PubMed]
- 37.Kawase T, Orikasa M, Suzuki A (1991) Effects of prostaglandin E2 and F2α on cytoplasmic pH in a clonal osteoblast-like cell line, MOB 3–4. J Cell Physiol 146:141–147 [DOI] [PubMed]
- 38.Kucharzyk D (1999) A controlled prospective outcome study of implantable electrical stimulation with spinal instrumentation in a high-risk spinal fusion population. Spine 5:465–469 [DOI] [PubMed]
- 39.Lane JM (2001) BMPs: why are they not in everyday use? J Bone Joint Surg Am 83(Suppl 1 Pt 2):S161–S163 [PubMed]
- 40.Laursen M, Hoy K, Hansen ES, Gelineck J, Christensen FB, Bunger CE (1999) Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J 8:485–490 [DOI] [PMC free article] [PubMed]
- 41.Lee K (1989) Clinical investigation of the spinal stem system, open trail phase: pseudarthrosis stratum. American Academy of Orthopaedic Surgeons, Las Vegas
- 42.Linovitz RJ, Pathria M, Bernhardt M, Green D, Law MD, McGuire RA, Montesana PX, Rechtine G, Salib RM, Ryaby JT, Faden JS, Ponder R, Muenz LR, Magee FP, Garfin SA (2002) Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study (discussion 1389). Spine 27:1383–1389 [DOI] [PubMed]
- 43.Lohmann CH, Schwartz Z, Liu Y, Guerkov H, Dean DD, Simon B, Boyan BD (2000) Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Orthop Res 18:637–646 [DOI] [PubMed]
- 44.Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ, Boyan BD (2003) Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res 21:326–334 [DOI] [PubMed]
- 45.Lorich DG, Brighton CT, Gupta R, Corsetti JR, Levine SE, Gelb ID, Seldes R, Pollack SR (1998) Biochemical pathway mediating the response of bone cells to capacitive coupling. Clin Orthop 350:246–256 [PubMed]
- 46.Marks RA (2000) Spine fusion for discogenic low back pain: outcomes in patients treated with or without pulsed electromagnetic field stimulation. Adv Ther 17:57–67 [DOI] [PubMed]
- 47.McKay B, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine 27(16 Suppl 1):S66–S85 [DOI] [PubMed]
- 48.Meril AJ (1994) Direct current stimulation of allograft in anterior and posterior lumbar interbody fusions. Spine 19:2393–2397 [DOI] [PubMed]
- 49.Mooney V (1990) A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine 15:708–712 [DOI] [PubMed]
- 50.Morone MA, Boden SD, Hair G, Martin GJ, Racine M, Hutton WC (1998) Gene expression during allograft lumbar spine fusion and the effect of bone morphogenetic protein 2. Clin Orthop 351:252–265 [PubMed]
- 51.Nagai M, Ota M (1994) Pulsating electromagnetic field stimulates mRNA expression of bone morphogenetic protein-2 and -4. J Dent Res 73:1601–1605 [DOI] [PubMed]
- 52.Nepola JV, Fredericks D, Simon B, Abbott J (1996) Effect of exposure time on stimulation of healing in the rabbit tibial osteotomy model by a time varying pulsed electromagnetic field and by combined magnetic fields. Canadian Orthopaedic Research Society, Quebec City
- 53.Nerubay J, Margarit B, Bubis JJ, Tadmor A, Katznelson A (1986) Stimulation of bone formation by electrical current on spinal fusion. Spine 11:167–169 [DOI] [PubMed]
- 54.Poynton AR, Lane JM (2002) Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 27(16 Suppl 1):S40–S48 [DOI] [PubMed]
- 55.Reid IR, Civitelli R, Avioli LV, Hruska KA (1988) Parathyroid hormone depresses cytosolic pH and DNA synthesis in osteoblast-like cells. Am J Physiol Endocrinol Metab 255:E9–E15 [DOI] [PubMed]
- 56.Rogozinski A, Rogozinski C (1996) Efficacy of implanted bone growth stimulation in instrumented lumbosacral spinal fusion. Spine 21:2479–2483 [DOI] [PubMed]
- 57.Rubinacci A, De Ponti A, Shipley A, Samaja M, Karplus E, Jaffe LF (1996) Bicarbonate dependence of ion current in damaged bone. Calcif Tissue Int 58:423–428 [DOI] [PubMed]
- 58.Ryaby J, Fitzsimmons RJ, Khin NA, Culley PL, Magee FP, Weinstein AM, Baylink DJ (1994) The role of insulin-like growth factor II in magnetic field regulation of bone formation. Bioelectrochem Bioenerg 35:87–91 [DOI]
- 59.Simmons JW (1985) Treatment of failed posterior lumbar interbody fusion (PLIF) of the spine with pulsing electromagnetic fields. Clin Orthop 183:127–132 [PubMed]
- 60.Simmons JW, Hayes MA, Christensen DK, Dwyer AP, Koullsis CS, Kimmich SJ (1989) The effect of postoperative pulsing electromagnetic fields on lumbar fusion: an open trial phase study. North American Spine Society, Quebec
- 61.Smith TL, Wong-Gibbons D, Maultsby J (2004) Microcirculatory effects of pulsed electromagnetic fields. J Orthop Res 22:80–84 [DOI] [PubMed]
- 62.Sugimoto T, Kano J, Fukase M, Fujita T (1992) Second messenger signaling in the regulation of cytosolic pH and DNA synthesis by parathyroid hormone (PTH) and PTH-related peptide in osteoblastic osteosarcoma cells: role of Na+/H+ exchange. J Cell Physiol 152:28–34 [DOI] [PubMed]
- 63.Tejano NA, Puno R, Ignacio JMF (1996) The use of implantable direct current stimulation in multilevel spinal fusion without instrumentation. Spine 16:1904–1908 [DOI] [PubMed]
- 64.Tepper OM, Callaghan MJ, Chang EI, Galiano RD, Bhatt KA, Baharestani S, Gan J, Simon B, Hopper RA, Levine JP, Gurtner GC (2004) Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2 (Epub Jun 18). FASEB J 18:1231–1233 [DOI] [PubMed]
- 65.Toth JM, Seim HB, Schwardt JD, Humphrey WB, Wallskog JA, Turner AS (2000) Direct current electrical stimulation increases the fusion rate of spinal fusion cages. Spine 25:2580–2587 [DOI] [PubMed]
- 66.Uludag H, D’Augusta D, Palmer R, Timony G, Wozney J (1999) Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res 46:193–202 [DOI] [PubMed]
- 67.Weinstein AM, McLeod BR, Smith SD, Liboff AR (1990) Ion resonance tuned electromagnetic fields increase healing rate in ostectomized rabbits. Orthopaedic Research Society, New Orleans
- 68.Yasuda I (1953) Fundamental problems in the treatment of fracture. J Kyoto Med Soc 4:395–406
- 69.Zhuang H, Wang W, Seldes RM, Tahernia AD, Fan H, Brighton CT (1997) Electrical stimulation induces the level of TGF-β1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway. Biochem Biophys Res Commun 237:225–229 [DOI] [PubMed]


