Abstract
Choline is an essential nutrient for humans and its availability during pregnancy is important for optimal fetal development. The Food and Nutrition Board of the Institute of Medicine in the United States of America has set the adequate choline intake during pregnancy at 450 mg/day. There is limited data available on normal plasma choline concentrations in pregnancy. Moreover, there are neither documented studies of choline intake among pregnant women in the Jamaican population nor of free plasma choline concentrations during pregnancy. Sixteen women presenting to the antenatal clinic of the University Hospital of the West Indies (UHWI) at 10−15 weeks of gestation were selected for this pilot study. A food frequency questionnaire was administered to estimate frequency of consumption of foods rich in choline. Fasting blood samples were collected by venepuncture and plasma assayed for choline using liquid chromatography electrospray ionization isotopic dilution mass spectrometry. Most of the women reported consumption of diets that delivered less than the recommended choline intake (mean ± SEM, 278.5 ± 28.9 mg). Mean plasma choline concentration was 8.4 ± 0.4 μmol/L. This falls below the normal concentration (10 μmol/L) reported for individuals that are not pregnant and pregnant (14.5 μmol/L). The results of this study may be an indication that the choline included in the diet of pregnant women in Jamaica may not be adequate to meet both the needs of the mother and fetus and that further studies are warranted to determine clinical implications.
INTRODUCTION
Choline is a nutrient that is a precursor of the phosphatidylcholine and sphingomyelin, both major components of cell membranes and important in the proper triglyceride turnover from the liver and blood. In addition, it is an important methyl-group donor needed for homocysteine metabolism and is used to make acetylcholine, a neurotransmitter (1).
This nutrient is especially important during pregnancy when mother actively transports it to the fetus (2). In rodent models, optimal development of the brain is directly influenced by the availability of choline during gestation, and can influence lifelong memory function (3–7). Choline is synthesized in the liver through the action of phosphatidylethanolamine methyltransferase (PEMT) (8).
Endogenous biosynthesis is not adequate to meet the demands for choline in humans and diets deficient in choline cause fatty liver and liver cell death (9, 10). In the United States of America (USA), the Food and Nutrition Board of the Institute of Medicine of the National Academy of Sciences has set an adequate intake level for choline at 550 mg/day for males, 425 mg/day for non-pregnant females and 450 mg/day for pregnant women (9).
Dietary choline is supplied in the form of free choline, phosphatidylcholine (lecithin), phosphocholine, sphingomyelin and glycerolphosphocholine (11, 12). These choline derivatives can all be hydrolysed to produce choline. Some of the richest sources of choline include eggs, liver, chicken, fish, legumes, cruciferous vegetables, milk and soya products (11, 12). Also, betaine in the diet can provide methyl groups (like choline), but cannot be converted to choline to form phospholipids or acetylcholine (11) (Fig. 1). Many plant-based foods contain betaine (11, 12).
Fig. 1.
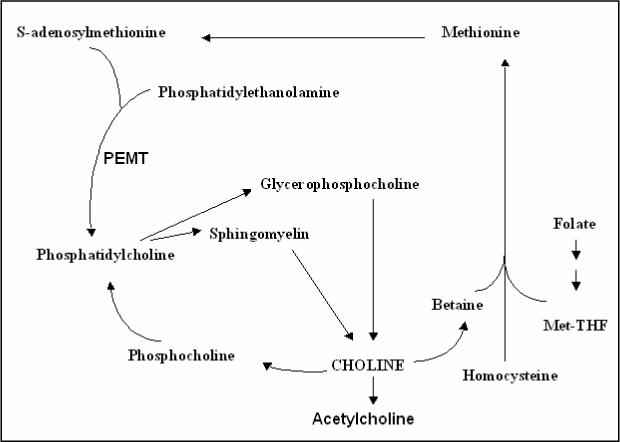
Diagram showing the functions of choline in the blood and liver.
Pregnancy is a time when the dietary choline supply may not match the increased demand for choline (2) and reserves of choline are depleted. One study reported that choline concentrations in serum are higher in pregnant women than in non-pregnant women (14.5 μmol/L vs 10.7 μmol/L, non-fasted) (13). The reason for this is unknown, but it could be related to increased dietary intake or increased endogenous synthesis.
Considering the importance of choline to overall fetal development and the lack of information available on maternal choline levels, the aim in this pilot study was to examine the plasma choline concentrations in a group of pregnant women in Jamaica and to assess their dietary choline intake.
SUBJECTS AND METHOD
Recruitment of subjects
Sixteen women at 10−15 weeks of gestation who attended the antenatal clinic of the University Hospital of the West Indies (UHWI), Jamaica, were recruited in this pilot study. It was decided to look only at women with normal pregnancies and therefore persons with a history of hypertension, diabetes or spontaneous abortions were excluded. The women were asked to identify their ethnicity. All participants reported compliance with standard iron and folic acid supplementation. This study was approved by the University Hospital of the West Indies/University of The West Indies Faculty of Medical Sciences Ethical Committee and the Institutional Review Board of the University of North Carolina at Chapel Hill, USA. All participants signed an informed consent form.
Assessment of choline intake
Choline content of foods was calculated using published data (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html). A food frequency questionnaire for consumption of the foods listed in Table 1 was filled out by each subject. The foods in this questionnaire were selected because they are common in Jamaica and represent high choline sources.
Table 1.
Selected foods from the Jamaican diet known to be rich sources of choline. Total choline consisted of glycerolphosphocholine (GPCho), phosphocholine (Pcho) phosphatidylcholine (PtdCho) and sphingomyelin (SM). Betaine is a metabolite produced from the demethylation of choline
| USDA code | Food | Free Choline | GPCho | PCho | PtdCho | SM LPC | Total Choline mg/100g | Betaine mg/100g |
|---|---|---|---|---|---|---|---|---|
| 01129 | Egg | 0.7 | 0.5 | 0.5 | 209.9 | 13.6 | 225.2 | 0.6 |
| 13327 | Liver | 56.7 | 77.9 | 11.8 | 247.8 | 24.1 | 418.3 | 5.6 |
| 16087 | Peanut | 17.6 | 1.3 | 1.8 | 31.8 | 0 | 52.5 | 0.6 |
| 16098 | Peanut butter | 25.8 | 1.1 | 0.7 | 38 | 0 | 65.6 | 0.4 |
| 01077 | Milk | 3.7 | 7.5 | 1.8 | 0.6 | 0.6 | 141.2 | 0.6 |
| 43261 | Yogurt | 3.3 | 7.8 | 2.0 | 1.9 | 1.4 | 16.4 | 0.7 |
| 01009 | Cheddar Cheese | 1.6 | 2.3 | 0.6 | 7.4 | 4.6 | 16.5 | 0.7 |
| 13497 | Beef | 1.9 | 1.6 | 0.2 | 64.5 | 9.1 | 77.3 | 12.8 |
| 10042 | Pork | 2.2 | 22.5 | 1.2 | 70.5 | 6.4 | 102.8 | 1.6 |
| 05006 | Chicken | 6 | 1 | 3.6 | 40.6 | 8.5 | 59.7 | 7.8 |
| 98016 | Fish | 21.4 | 1.2 | 2.5 | 53.7 | 4.1 | 82.9 | 25.3 |
| 16028 | Peas/Kidney Beans | 14.2 | 2.2 | 0.6 | 13.5 | 0 | 30.5 | 0.1 |
| 16120 | Soy Milk | 13.1 | 1.3 | 3.4 | 5.7 | 0 | 23.5 | 0.8 |
| 11091 | Broccoli | 8.5 | 1.3 | 9.3 | 21 | 0 | 40.1 | 0.1 |
| 11252 | Lettuce | 4.8 | 0 | 1.5 | 0.4 | 0 | 6.7 | 0.1 |
| 18064 | Wheat Bread | 11.5 | 3.7 | 0.3 | 3.1 | 0 | 18.6 | 85.2 |
Plasma collection
Subjects were required to be fasted overnight. Blood samples were collected by venepuncture at 10−15 weeks gestation. By this gestational period none of the subjects reported vomiting. The blood samples were centrifuged immediately for the collection of plasma and stored at −80°C until time for analysis, which was carried out at the University of North Carolina at Chapel Hill, USA.
Analysis of plasma
Choline and choline metabolites were extracted and analyzed using standardized methods described by Koc et al (14). Briefly, lipids were extracted from plasma (200 μl) with methanol:chloroform (2:1, by vol), vortexed vigorously and left at −20°C overnight. Samples were then centrifuged and the supernatant collected. The residue was re-extracted with methanol: chloroform: water (2:1:0.8, by vol). The supernatant from both extractions were combined. Chloroform (200 μl) and water (200 μl) were added to the supernatant to separate an aqueous and an organic phase. Quantification was done using liquid chromatography electrospray ionization isotopic dilution mass spectrometry. All samples were done in duplicates and final values reflect the average of these two readings.
RESULTS
Subject profile
All the women in this study were Afro-Caribbean (based on self-assessment) in ethnicity and had parity in the range of one to four (Table 2). Five subjects reported a history of chronic illness (asthma or manic depression) with and without pharmacological management.
Table 2.
Profile of subjects (n = 16)
| Subject Code | Age Yr | weight/ 12 wks gestation Kg | height cm | parity | BP(12−16 wks) mmHg | Hb g/dL | Comments |
|---|---|---|---|---|---|---|---|
| PL001 | 25 | 66.2 | 173.5 | 2 | 100/60 | 11.4 | bi-polar disorder, medication included lithium |
| PL002 | 24 | 97.6 | 164 | 100/80 | 11.3 | ||
| PL003 | 29 | 66.5 | 159.5 | 2 | 90/60 | 11.2 | asthmatic/ventolin |
| PL004 | 28 | 60.7 | 175.3 | 100/70 | 11.4 | ||
| PL005 | 29 | 72.4 | 159 | 2 | 120/80 | 11.9 | Asthmatic |
| PL006 | 28 | 72.8 | 170 | 2 | 120/70 | 12.2 | |
| PL007 | 18 | 55.6 | 166.5 | 1 | 100/60 | 12.3 | Asthmatic |
| PL008 | 20 | 48.3 | 162 | 1 | 100/60 | 18.4 | Asthmatic/spina bifida |
| PL009 | 34 | 67.6 | 160.5 | 2 | 100/70 | 11.4 | |
| PL010 | 38 | 91.7 | 173 | 4 | 120/70 | 13.1 | |
| PL011 | 29 | 92.1 | 178 | 1 | 100/60 | 11.8 | |
| PL012 | 20 | 62.2 | 164.7 | 1 | 100/90 | 12.2 | childhood chickenpox |
| PL013 | 21 | 97.6 | 154 | 2 | 120/70 | 10.6 | shellfish allergy |
| PL014 | 32 | 66.9 | 171 | 1 | 114/70 | 9.8 | |
| PL016 | 24 | 76.34 | 155.2 | 120/80 | 11.8 | ||
| PL017 | 18 | 70.9 | 165.2 | 1 | 120/70 | 12 |
Dietary choline consumption and plasma choline concentration
Most of the participants reported low consumption of the choline-rich foods selected from the Jamaican diet (Fig. 2). Two of the subjects showed adequate or close to adequate intake of choline (over 400 mg) from the consumption of known choline-rich foods. The mean (± SEM) dietary choline consumed was 278.5 mg ± 28.9 mg.
Fig. 2.
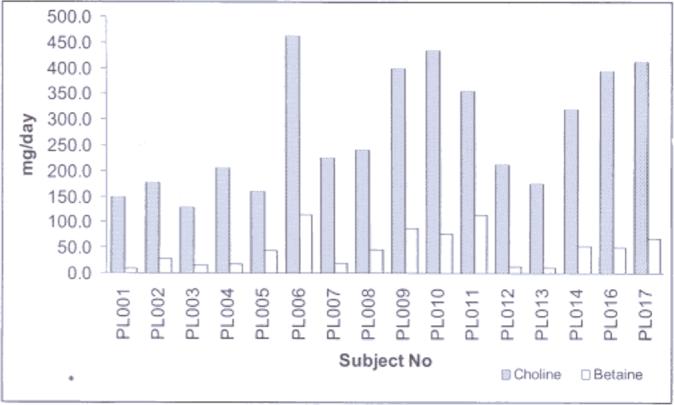
Graph showing daily consumption of choline and betaine for each subject. Choline bar represents free choline, phosphatidylcholine, phosphocholine, sphingomyelin and glycerolphosphocholine content. Most of the women reported a diet that provided less than 450 mg of choline (adequate allowance of choline in pregnancy).
The fasting plasma choline ranged between 6.8 to 11.2 μmol/L (Fig. 3) with a mean (± SEM) plasma choline concentration of 8.4 ± 0.4 μmol/L. In further analysis of the plasma choline concentrations, it was observed that subjects with a history of chronic illness, with or without being on medication showed significantly (student's t test, p < 0.01) higher plasma choline concentrations (9.7 ± 1.7 μmol/L, n = 5) compared to those not having chronic illnesses (7.7 ± 0.9 μmol/, n = 11).
Fig. 3.
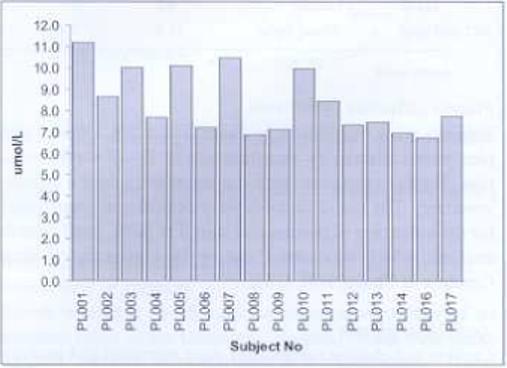
Graph showing the plasma choline concentration measured at 10−15 weeks of gestation for each participant. Plasma choline concentrations tended towards low to normal values (normal fasted plasma choline range from 7−20 μmol/L (9,15) with most subjects having low concentrations.
DISCUSSION
During pregnancy, large amounts of choline are delivered across the placenta for fetal development and maternal stores are depleted in rodent models (2). This suggests that dietary intake of choline is likely to be very important during pregnancy to ensure that normal plasma choline concentrations are maintained. In this study, an assessment of dietary choline intake was made using a food frequency questionnaire consisting of foods that are rich sources of choline. Although food frequency questionnaires are of limited accuracy, they have been validated as adequate for assessment of single micronutrient intake (15). In this study, there were however further limitations associated with an incomplete database for the choline content of many foods, as well as whether the values of choline in foods listed on the United States Department of Agriculture database are accurate for Jamaican foods.
The pilot study suggests that pregnant women in Jamaica are eating foods that may not be delivering adequate amounts of choline. Although the implications of inadequate choline in the diet have not been fully examined in humans, several animal studies show that in terms of memory development, the role of dietary choline during pregnancy is significant (3–7).
Normal plasma choline concentrations in fasted non-pregnant humans vary from 7−20 μmol/L (with most individuais having 10 μmol/L) (9, 16) and are controlled not only by the supply (endogenous and dietary), but also by the ability of all tissues to accumulate choline (17). The uptake and storage of choline by the liver is integral in this control. Choline is also significantly taken across the blood-brain barrier through a carrier mediate mechanism, the rate of which is positively dependent on plasma choline concentrations (17). Additionally, hormonal influences, namely cortisol, insulin and prolactin participate in maintaining plasma choline concentrations (18). Previous reports suggest that pregnant women have increased plasma choline concentration (14.5 μmol/L) when compared with non-pregnant women (13). Reasons for this elevation have not been fully determined, but occur, possibly to meet the increasing demands of the fetus including the increased accumulation in breast milk (19).
The authors found that pregnant women in Jamaica have low to normal plasma choline concentrations during the first trimester. The trend of more normal plasma choline concentrations in subjects with chronic illness was an interesting finding and although the observation was limited by numbers (and type of illness), the power obtained (0.78 at p = 0.05) from this pilot study suggest that future work should examine this association.
In conclusion, this is the first documented study evaluating plasma choline concentrations in the Jamaican population and suggests a need to assess further whether diets of this population ensure adequate plasma choline concentration during pregnancy. An extension of this study should also examine the implications of low plasma choline concentration including the significance it may have in ensuring healthy fetal brain development in humans.
ACKNOWLEDGEMENTS
This work was funded by grants from the National Institutes of Health (Fogarty Fellowship grant; DK 55865). Support for this work was also provided by grants from the NIH to UNC Clinical Nutrition research Unit (DK56350), Fulbright visiting researcher grant and funds provided by Caribbean Health Research Council.
REFERENCES
- 1.Zeisel SH. Choline: an essential nutrient for humans. Nutrition. 2000;16:669–71. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 2.Zeisel SH, Mar MH, Zhou ZW, Da Costa KA. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–54. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 3.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999;115:123–9. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 4.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 5.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–21. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 6.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–53. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 7.Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18:545–7. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- 8.Vance DE, Walkey CJ, Cui Z. Phosphatidylethanolamine N-methyltransferase from liver. Biochim Biophys Acta. 1997;1348:142–50. doi: 10.1016/s0005-2760(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 9.Institute of Medicine, and National Academy of Sciences USA . Dietary reference intakes for folate, thiamin, riboflavin, niacin, vitamin B12, panthothenic acid, biotin, and choline. National Academy Press; Washington DC: 1998. pp. 402–4. [PubMed] [Google Scholar]
- 10.Zeisel SH, Da Costa KA, Franklin PD, Alexander EA, Lamont JT, Sheard NF, et al. Choline, an essential nutrient for humans. FASEB J. 1991;5:2093–8. [PubMed] [Google Scholar]
- 11.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:1302–7. doi: 10.1093/jn/133.5.1302. [DOI] [PubMed] [Google Scholar]
- 12.Zeisel SH, Mar MH, Howe JC, Holden JM. Concentrations of choline-containing compounds and betaine in common foods. J Nutr. 2003;133:2918. doi: 10.1093/jn/133.5.1302. Erratum in. [DOI] [PubMed] [Google Scholar]
- 13.Ozarda Ilcol Y, Uncu G, Ulus IH. Free choline and phospholipid-bound choline cincentrations in serum and during pregnancy, after delivery and in newborns. Arch Physiol Biochem. 2002;110:393–9. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 14.Koc H, Mar MH, Ranasinghe A, Swenberg JA, Zeisel SH. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal Chem. 2002;74:4734–40. doi: 10.1021/ac025624x. [DOI] [PubMed] [Google Scholar]
- 15.Mason JB. Biomarkers of nutrient exposure and status in one carbon (methyl) metabolism. J Nutr. 2003;133:941–7. doi: 10.1093/jn/133.3.941S. [DOI] [PubMed] [Google Scholar]
- 16.Savendahl L, Mar MH, Underwood LE, Zeisel S. Prolonged fasting in humans results in diminished plasma choline concentrations but does not cause liver dysfunction. Am J Clin Nutr. 1997;66:622–5. doi: 10.1093/ajcn/66.3.622. [DOI] [PubMed] [Google Scholar]
- 17.Lockman PR, Allen DD. The transport of choline. Drug Dev Ind Pharm. 2002;28:749–71. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- 18.Ozarda Ilcol Y, Ozyurt G, Kilicturgay S, Uncu G, Ulus IH. The decline in serum choline concentration in humans during and after surgery is associated with the elevation of cortisol, adrenocorticotropic hormone, prolactin and beta-endorphin concentrations. Neurosci Lett. 2002;324:41–4. doi: 10.1016/s0304-3940(02)00171-4. [DOI] [PubMed] [Google Scholar]
- 19.Rillema JA. Hormone regulation of choline uptake and incorporation in mouse mammary gland explants. Exp Biol Med. 2004;229:323–6. doi: 10.1177/153537020422900406. [DOI] [PubMed] [Google Scholar]


