Abstract
Choline is derived not only from the diet, but also from de novo synthesis. It is important for methyl-group metabolism, the formation of membranes, kidney function, and neurotransmission. When deprived of dietary choline, most adult men and postmenopausal women develop signs of organ dysfunction (fatty liver or muscle damage) and have a decreased capacity to convert homocysteine to methionine. Choline is critical during fetal development, when it influences stem cell proliferation and apoptosis, thereby altering brain structure and function (memory is permanently enhanced in rodents exposed to choline during the latter part of gestation).
Keywords: brain development, choline, folate, hippocampus, memory
CHOLINE IS AN ESSENTIAL NUTRIENT
Choline consists of three methyl groups covalently attached to the nitrogen atom of ethanolamine, and is essential for normal functioning of all cells.1 A major use for choline is as a precursor for the synthesis of membranes. Phosphatidylcholine (sometimes called lecithin) is the predominant phospholipid (>50%) in most mammalian membranes; sphingomyelin is another important choline phospholipid. Only a small fraction of dietary choline is acetylated, catalyzed by the activity of choline acetyltransferase.2 The product is acetylcholine, an important neurotransmitter. The methyl groups of choline can be made available from one-carbon metabolism upon conversion to betaine,3 which cannot be reduced back to choline. The interrelationship of this pathway with folate and methionine metabolism is discussed below.
When deprived of dietary choline, most adult men and postmenopausal women develop signs of organ dysfunction (fatty liver or muscle damage)4,5; premenopausal women are relatively resistant to choline deficiency (unpublished data). The 1998 Institute of Medicine recommendations on dietary reference intakes included estimates of the Adequate Intake (AI) of choline required by humans (approximately half a gram a day).6 The USDA recently developed a database of choline content in foods (http://www.nal.usda.gov/fnic/foodcomp/Data/Choline/Choline.html), which shows that excellent sources of dietary choline include liver, eggs, and wheat germ. In foods, choline is found in free and in esterified forms (as phosphocholine, glycerophosphocholine, phosphatidylcholine, and sphingomyelin). There is some evidence that these forms of choline may have different bioavailability,7 because the lipid-soluble forms bypass the liver when absorbed from the diet, while the water-soluble forms enter the portal circulation and are mostly absorbed by the liver. Human milk is rich in choline compounds,8 and soy-derived infant formulas have lower total choline concentrations than do either human milk or bovine milk-derived formulas.8 Choline intake by humans on ad libitum diets for males and females averages between 8.4 and. 6.7 mg/kg of choline per day, respectively.9 However, Shaw et al.10 studied pregnant women in California and observed intakes that were less than half this amount in 25% of the women studied.
Choline is derived not only from the diet, but also from the de novo synthesis of phosphatidylcholine catalyzed by phosphatidylethanolamine N-methyltransferase (PEMT).11 This enzyme, which is the most active in the liver, uses S-adenosylmethionine as a methyl donor and forms a new choline moiety.12 When fed a diet deficient in choline, Pemt−/− mice developed fatty liver, severe liver damage, and died; a choline-supplemented diet prevented this13 and reversed hepatic damage if begun early enough.14 Pemt−/− mice have lower choline pools in liver despite being fed sufficient or supplemental amounts of dietary choline,15 suggesting that choline production by PEMT is a significant source of choline relative to dietary intake.
CHOLINE, FOLATE, AND METHIONINE METABOLISM ARE INTERRELATED
Choline, methionine and folate metabolism are metabolically interrelated at the point that homocysteine is methylated to form methionine. Thus, any requirement for dietary choline must be considered in relation to these other nutrients. Homocysteine can be methylated to form methionine16 by two parallel pathways, both of which lower homocysteine concentrations17 (Figure 1). In the first, vitamins B12 and folic acid are involved in a reaction catalyzed by methionine synthase.18 Deficiency of these nutrients19,20 or single nucleotide polymorphisms in the genes for the enzymes involved in this pathway18,20,21 can result in elevated plasma homocysteine concentrations. In addition, tetrahydrofolate is needed to scavenge one-carbon groups when betaine is metabolized.22 The alternative pathway for the methylation of homocysteine to form methionine is catalyzed by betaine homocysteine methyltransferase.23 Betaine, derived from dietary choline, is the methyl group donor in this reaction, and supplemental oral betaine can lower plasma homocysteine concentrations.24,25 We recently reported that humans who are depleted of choline have diminished capacity to methylate homocysteine and develop elevated homocysteine concentrations in plasma after a methionine loading test.4
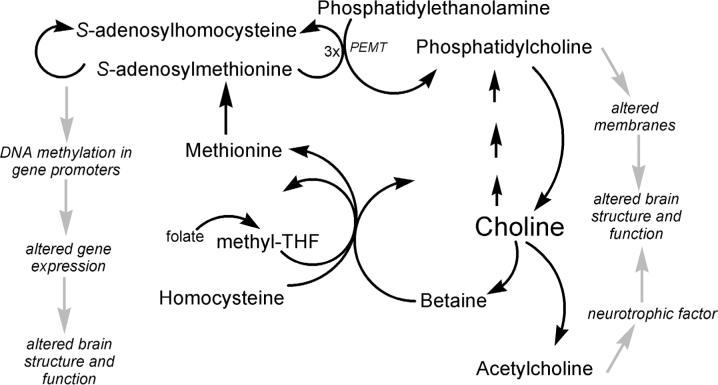
Figure 1.
Metabolism of choline and possible mechanisms for effects on brain structure and function. Choline is acetylated to form acetylcholine, which is a trophic factor for brain. Choline is phosphorylated and then used to form membranes that are required for brain function. Finally, choline is a methyl-group donor that can influence DNA methylation and gene expression, which can, in turn, alter brain structure and function. Methyl-THF = methyltetrahydrofolate.
CHOLINE IN PREGNANCY AND LACTATION
Pregnancy and lactation are times when the demand for choline is especially high; transport of choline from mother to fetus26,27 depletes maternal plasma choline in humans.28 Thus, the demand for this nutrient is so high that stores are depleted. Pregnant rats have diminished total liver choline compounds compared with non-mated controls and become as sensitive to choline-deficient diets as male rats29 Because milk contains a great deal of choline, lactation further increases maternal demand for choline, resulting in further depletion of tissue stores.8,29 Choline nutriture during pregnancy is especially important because it influences brain development in the fetus (see below), and because it is important for maintaining normal plasma homocysteine concentrations during pregnancy.30 High maternal homocysteine concentrations are associated with increased incidence of birth defects.31 Molloy et al.32 reported that, at the time of delivery, plasma choline and betaine concentrations increase in maternal plasma, and that this is highly correlated with an increase in plasma homocysteine concentrations. Molloy speculates that there are two mechanisms that could explain this correlation: 1) pregnancy depletes choline and betaine in the liver (perhaps in order to maintain plasma choline concentrations for delivery to the fetus), resulting in decreased methylation of homocysteine; or 2) pregnancy induces endogenous synthesis of choline via a pathway that also produces homocysteine. Whatever the mechanism, the supply of choline is critical during pregnancy. Mice that cannot form their own choline (Pemt−/−) abort pregnancies around 9 to 10 days after gestation unless fed supplemental choline (personal observation).11
THE FETUS LIVES IN A HIGH-CHOLINE ENVIRONMENT
Large amounts of choline are delivered to the fetus across the placenta, where choline transport systems pump it against a concentration gradient.27 Choline concentration in amniotic fluid is 10-fold greater than that present in maternal blood (unpublished observations). Plasma or serum choline concentrations are 6- to 7-fold higher in the fetus and newborn than they are in the adult.33,34 High levels of choline circulating in the neonate presumably ensure enhanced availability of choline to tissues. Neonatal rat brain efficiently extracts choline from blood,35 and increased serum choline in the neonatal rat is associated with 2-fold higher choline concentration in neonatal brain than is present later in life. Supplementing choline during the perinatal period further increases choline metabolite concentrations in blood and brain.36 There is a novel form of phosphatidylethanolamine-N-methyltransferase in neonatal rat brain that is extremely active12; this isoform is not present in adult brain. Furthermore, in the brains of newborn rats, S-adenosylmethionine concentrations are 40 to 50 nmol/g of tissue37—levels probably sufficient to enable the neonatal form of phosphatidylethanolamine-N-methyltransferase to maintain high rates of activity. As previously mentioned, human and rat milk provide large amounts of choline to the neonate. The existence of these multiple mechanisms, which ensure the availability of choline to the fetus and neonate, suggest that evolutionary pressures favored exposure to high concentrations of choline in utero.
CHOLINE AND BRAIN DEVELOPMENT IN UTERO
Choline is needed for normal neural tube closure in early pregnancy,38,39 and women in the lowest quartile for dietary choline intake had four times the risk (compared with women in the highest quartile) of having a baby with a neural tube defect.10 It is widely accepted that folate affects embryogenesis of the brain, and it is recommended that all women be supplemented with folate during the periconceptional period because this reduces the risk for these serious defects in brain development.6,40 Folic acid administered to women who had previously had a child with a neural tube defect lowered risk of recurrence by 72%.41 Choline and folate metabolism intersect at pathways for methyl-group donation (see earlier discussion), and it is reasonable to hypothesize that methylation reactions are the mechanism they share in common that influence neural tube closure. As discussed below, folate deficiency and choline deficiency have similar effects on stem cell proliferation and apoptosis in the brain.42,43
Choline and folate are also important in later periods of pregnancy, when the memory center of brain (the hippocampus) is developing. Maternal dietary choline supplementation or choline deficiency during late pregnancy are associated with significant and irreversible changes in hippocampal function in the adult rodent, including altered long-term potentiation (LTP)44-46 and altered memory.47-52 More choline (about four times dietary levels) during days 11 to 17 of gestation in the rodent increases hippocampal progenitor cell proliferation,53,54 decreases apoptosis in these cells,53,54 enhances LTP in the offspring when they become adult animals,44-46 and enhances visuo-spatial and auditory memory by as much as 30% in the adult animals throughout their lifetimes.47-49,51,52,55,56 Indeed, adult rodents have a decrement in memory as they age, and offspring exposed to extra choline in utero do not show this “senility.”49,55 Mothers fed choline-deficient diets during late pregnancy have offspring with diminished progenitor cell proliferation and migration and increased apoptosis in fetal hippocampus,53,54 insensitivity to LTP when they become adult animals,46 and decremented visuo-spatial and audtory memory.52
The effects of perinatal choline supplementation on memory were initially found using radial-arm maze tasks and in the Sprague-Dawley rat strain, but other laboratories have found similar results using other spatial memory tasks, such as the Morris water maze,57,58 passive avoidance paradigms,59 and measures of attention60 in other strains of rats such as Long-Evans61-63 and in mice.59 The effects of choline supplementation in utero were also detected in studies on effects of fetal alcohol exposure, where supplementation with choline attenuated behavioral alterations but not motor abnormalities.64,65 Thus, choline supplementation during a critical period in pregnancy causes lifelong changes in brain structure and function.
The mechanism whereby a choline supplement supplied to the dam results in a permanent change in memory of their offspring has not been fully understood. Though the initial hypothesis was that the effect of neonatal choline supplementation on memory is mediated by increased brain choline, with subsequent increased acetylcholine (a neurotransmitter and neurotrophic factor) release, the amounts of choline that accumulate in fetal brain after treatment of the pregnant dam are not likely of sufficient magnitude to enhance acetylcholine release.36 Rather, supplementing choline to dams results in significantly greater accumulation of phosphocholine and betaine in fetal brain than in fetuses of controls.36 Alternatively, the effects of choline on neuronal precursor cell proliferation, differentiation, and apoptosis likely underly the effects on memory. Using mouse neural precursors in vitro, we recently reported that choline deficiency induces significant changes in the expression of genes involved in cell-cycle progression and neuronal differentiation, with the net effect being reduced proliferation and increased neuronal and glial differentiation.66 The hypothesis of accelerated neuronal differentiation is supported in choline-deficient mouse fetal brain by in vivo studies showing increased expression of several early markers of neuronal and glial differentiation, including calretinin (a calcium-binding protein), MAP-1 (microtubule-associated protein), and vimentin (intermediate filament protein) within the hippocampus area.67
EPIGENETIC MECHANISMS FOR CHOLINE EFFECTS ON BRAIN DEVELOPMENT
The effects of choline on neural tube closure and on brain development could be mediated by changes in the expression of genes. Dietary choline deficiency not only depletes choline and choline metabolites in rats, but also decreases S-adenosylmethionine concentrations,68,69 with resulting hypomethylation of DNA.70,71 DNA methylation occurs at cytosine bases that are followed by a guanosine (CpG sites)72 and influences many cellular events, including gene transcription, genomic imprinting, and genomic stability.73-75 In mammals, about 60% to 90% of 5′CpG-3′ sites are methylated.76 Many coding and non-coding DNA regions have a higher incidence of CpG repeats than expected (CpG islands), and these islands are the main targets for methylation.75 Changes in dietary availability of methyl-groups (folate, methionine, and choline intakes) can induce stable changes in gene expression and resulting phenotype.77,78 When this modification occurs in promoter regions, gene expression is altered79; increased methylation is associated with gene silencing or reduced gene expression.76 In choline-deficient human neuroblastoma cells in culture, methylation of the CDKN3 gene promoter is decreased, resulting in the overexpression of this gene, which inhibits cell proliferation.80 In choline-deficient liver, there is hypomethylation of specific CCGG sites within several genes for which mRNA levels were increased, including c-myc, c-fos, and c-Ha-ras.81 Hypomethylation of CpG sites and c-myc gene overexpression occurs in hepatocellular carcinomas induced by a choline-deficient diet in rats.71 It is also reasonable that maternal diet during pregnancy could alter the methylation status of fetal DNA. For example, feeding pregnant Pseudoagouti Avy/a mouse dams a choline methyl-supplemented diet altered epigenetic regulation of agouti expression in their offspring, as indicated by increased agouti/mottling of their coats.77,82 It is clear that the dietary manipulation of methyl donors (either deficiency or supplementation) can have a profound impact upon gene expression and, by consequence, upon the homeostatic mechanisms that ensure the normal function of physiological processes.
Whether these findings in rodents apply to humans is not known. Of course, human and rat brains mature at different rates, with rat brain comparatively more mature at birth than the human brain. In humans, the architecture of the hippocampus continues to develop after birth, and by 4 years of age it closely resembles adult structure.83 This area of brain is one of the few areas in which nerve cells continue to multiply slowly throughout life.84,85 Are we varying the availability of choline when we feed infant formulas instead of milk? Does the form and amount of choline ingested contribute to variations in memory observed between humans? All of these are good questions worthy of additional research. The observation by Shaw et al.10 that women eating low-choline diets have a greatly increased risk for having a baby with a neural tube defect supports the suggestion that the basic research in rodents will be applicable to humans as well.
ACKNOWLEDGMENTS
This work was funded by grants from the National Institutes of Health (AG09525, DK55865, ES012997) and the US Department of Agriculture (2005-3520015247). Support for this work was also provided by grants from the NIH to the University of North Carolina Clinical Nutrition Research Unit (DK56350).
REFERENCES
- 1.Zeisel SH, Blusztajn JK. Choline and human nutrition. Ann Rev. Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 2.Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983;221:614–620. doi: 10.1126/science.6867732. [DOI] [PubMed] [Google Scholar]
- 3.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 4.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81:440–444. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.da Costa KA, Badea M, Fischer LM, Zeisel SH. Elevated serum creatine phosphokinase in choline-deficient humans: mechanistic studies in C2C12 mouse myoblasts. Am J Clin Nutr. 2004;80:163–170. doi: 10.1093/ajcn/80.1.163. [DOI] [PubMed] [Google Scholar]
- 6.Food and Nutrition Board . Institute of Medicine, Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. National Academies Press; Washington, DC: 1998. [March 27, 2006]. Available online at: http://www.nap.edu/openbook/0309065542/html/. [PubMed] [Google Scholar]
- 7.Cheng W-L, Holmes-McNary MQ, Mar M-H, Lien EL, Zeisel SH. Bioavailability of choline and choline esters from milk in rat pups. J Nutr Biochem. 1996;7:457–464. [Google Scholar]
- 8.Holmes-McNary M, Cheng WL, Mar MH, Fussell S, Zeisel SH. Choline and choline esters in human and rat milk and infant formulas. Am J Clin Nutr. 1996;64:572–576. doi: 10.1093/ajcn/64.4.572. [DOI] [PubMed] [Google Scholar]
- 9.Fischer LM, Scearce JA, Mar MH, et al. Ad libitum choline intake in healthy individuals meets or exceeds the proposed adequate intake level. J Nutr. 2005;135:826–829. doi: 10.1093/jn/135.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am J Epidemiol. 2004;160:102–109. doi: 10.1093/aje/kwh187. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Mar MH, Song J, Zeisel SH. Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res Dev Brain Res. 2004;149:121–129. doi: 10.1016/j.devbrainres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Blusztajn JK, Zeisel SH, Wurtman RJ. Developmental changes in the activity of phosphatidylethanolamine N-methyltransferases in rat brain. Biochem J. 1985;232:505–511. doi: 10.1042/bj2320505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walkey CJ, Yu L, Agellon LB, Vance DE. Biochemical and evolutionary significance of phospholipid methylation. J Biol Chem. 1998;273:27043–27046. doi: 10.1074/jbc.273.42.27043. [DOI] [PubMed] [Google Scholar]
- 14.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370:987–993. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkelstein JD. Pathways and regulation of homocysteine metabolism in mammals. Semin Thromb Hemost. 2000;26:219–225. doi: 10.1055/s-2000-8466. [DOI] [PubMed] [Google Scholar]
- 17.Olthof MR, van Vliet T, Verhoef P, Zock PL, Katan MB. Effect of homocysteine-lowering nutrients on blood lipids: results from four randomised, placebo-controlled studies in healthy humans. PLoS Med. 2005;2:e135. doi: 10.1371/journal.pmed.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisberg IS, Jacques PF, Selhub J, et al. The 1298A—>C polymorphism in methylenetetrahydrofolate reductase (MTHFR): in vitro expression and association with homocysteine. Atherosclerosis. 2001;156:409–415. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 19.Shelnutt KP, Kauwell GP, Chapman CM, et al. Folate status response to controlled folate intake is affected by the methylenetetrahydrofolate reductase 677C—>T polymorphism in young women. J Nutr. 2003;133:4107–1411. doi: 10.1093/jn/133.12.4107. [DOI] [PubMed] [Google Scholar]
- 20.Jacques PF, Bostom AG, Wilson PW, Rich S, Rosenberg IH, Selhub J. Determinants of plasma total homocysteine concentration in the Framing-ham Offspring cohort. Am J Clin Nutr. 2001;73:613–621. doi: 10.1093/ajcn/73.3.613. [DOI] [PubMed] [Google Scholar]
- 21.Watkins D, Ru M, Hwang HY, et al. Hyperhomocysteinemia due to methionine synthase deficiency, cblG: structure of the MTR gene, genotype diversity, and recognition of a common mutation, P1173L. Am J Hum Genet. 2002;71:143–153. doi: 10.1086/341354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mudd SH, Ebert MH, Scriver CR. Labile methyl group balances in the human: the role of sarcosine. Metabolism. 1980;29:707–720. doi: 10.1016/0026-0495(80)90192-4. [DOI] [PubMed] [Google Scholar]
- 23.Sunden S, Renduchintala M, Park E, Miklasz S, Garrow T. Betaine-Homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- 24.Steenge GR, Verhoef P, Katan MB. Betaine supplementation lowers plasma homocysteine in healthy men and women. J Nutr. 2003;133:1291–1295. doi: 10.1093/jn/133.5.1291. [DOI] [PubMed] [Google Scholar]
- 25.Wendel U, Bremer H. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 26.Sweiry JH, Yudilevich DL. Characterization of choline transport at maternal and fetal interfaces of the perfused guinea-pig placenta. J Physiol. 1985;366:251–266. doi: 10.1113/jphysiol.1985.sp015795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweiry JH, Page KR, Dacke CG, Abramovich DR, Yudilevich DL. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: studies with calcium and choline. J Devel Physiol. 1986;8:435–445. [PubMed] [Google Scholar]
- 28.McMahon KE, Farrell PM. Measurement of free choline concentrations in maternal and neonatal blood by micropyrolysis gas chromatography. Clin Chim Acta. 1985;149:1–12. doi: 10.1016/0009-8981(85)90267-0. [DOI] [PubMed] [Google Scholar]
- 29.Zeisel SH, Mar M-H, Zhou Z-W, da Costa K-A. Pregnancy and lactation are associated with diminished concentrations of choline and its metabolites in rat liver. J Nutr. 1995;125:3049–3054. doi: 10.1093/jn/125.12.3049. [DOI] [PubMed] [Google Scholar]
- 30.Velzing-Aarts FV, Holm PI, Fokkema MR, van der Dijs FP, Ueland PM, Muskiet FA. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am J Clin Nutr. 2005;81:1383–1389. doi: 10.1093/ajcn/81.6.1383. [DOI] [PubMed] [Google Scholar]
- 31.Hobbs CA, Cleves MA, Melnyk S, Zhao W, James SJ. Congenital heart defects and abnormal maternal biomarkers of methionine and homocysteine metabolism. Am J Clin Nutr. 2005;81:147–153. doi: 10.1093/ajcn/81.1.147. [DOI] [PubMed] [Google Scholar]
- 32.Molloy A, Mills J, Cox C, et al. Choline and homocysteine interrelations in umbilical cord and maternal plasma at delivery. Am J Clin Nutr. 2005;82:836–842. doi: 10.1093/ajcn/82.4.836. [DOI] [PubMed] [Google Scholar]
- 33.Zeisel SH, Wurtman RJ. Developmental changes in rat blood choline concentration. Biochem J. 1981;198:565–570. doi: 10.1042/bj1980565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozarda IY, Uncu G, Ulus IH. Free and phospholipid-bound choline concentrations in serum during pregnancy, after delivery and in newborns. Arch Physiol Biochem. 2002;110:393–399. doi: 10.1076/apab.110.5.393.11832. [DOI] [PubMed] [Google Scholar]
- 35.Cornford EM, Cornford ME. Nutrient transport and the blood-brain barrier in developing animals. Fed Proc. 1986;45:2065–2072. [PubMed] [Google Scholar]
- 36.Garner SC, Mar M-H, Zeisel SH. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J Nutr. 1995;125:2851–2858. doi: 10.1093/jn/125.11.2851. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman DR, Cornatzer WE, Duerre JA. Relationship between tissue levels of S-adenosylmethionine, S-adenosylhomocysteine, and transmethylation reactions. Can J Biochem. 1979;57:56–65. doi: 10.1139/o79-007. [DOI] [PubMed] [Google Scholar]
- 38.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology. 2001;64:114–122. doi: 10.1002/tera.1053. [DOI] [PubMed] [Google Scholar]
- 39.Fisher MC, Zeisel SH, Mar MH, Sadler TW. Perturbations in choline metabolism cause neural tube defects in mouse embryos in vitro. FASEB J. 2002;16:619–621. doi: 10.1096/fj.01-0564fje. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control Recommendations for the use of folic acid to reduce the number of cases of spina bifida and othe neural tube defects. Morbid Mortal Wkly Rep. 1992;41:1–7. [PubMed] [Google Scholar]
- 41.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the medical Research Council Vitamin Study. Lancet. 1991;338:131–137. [PubMed] [Google Scholar]
- 42.Craciunescu CN, Albright CD, Mar MH, Song J, Zeisel SH. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J Nutr. 2003;133:3614–3618. doi: 10.1093/jn/133.11.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craciunescu CN, Brown EC, Mar MH, Albright CD, Nadeau MR, Zeisel SH. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J Nutr. 2004;134:162–166. doi: 10.1093/jn/134.1.162. [DOI] [PubMed] [Google Scholar]
- 44.Pyapali G, Turner D, Williams C, Meck W, Swartzwelder HS. Prenatal choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J Neurophysiol. 1998;79:1790–1796. doi: 10.1152/jn.1998.79.4.1790. [DOI] [PubMed] [Google Scholar]
- 45.Montoya DA, White AM, Williams CL, Blusztajn JK, Meck WH, Swartzwelder HS. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res Dev Brain Res. 2000;123:25–32. doi: 10.1016/s0165-3806(00)00075-4. [DOI] [PubMed] [Google Scholar]
- 46.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 47.Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 48.Meck W, Williams C. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 49.Meck W, Williams C. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 50.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 51.Meck WH, Smith RA, Williams CL. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev Psychobiol. 1988;21:339–353. doi: 10.1002/dev.420210405. [DOI] [PubMed] [Google Scholar]
- 52.Meck WH, Williams CL. Choline supplementation during prenatal development reduces proactive interference in spatial memory. Brain Res Dev Brain Res. 1999;118:51–59. doi: 10.1016/s0165-3806(99)00105-4. [DOI] [PubMed] [Google Scholar]
- 53.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 54.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 55.Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- 56.Williams CL, Meck WH, Heyer DD, Loy R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998;794:225–238. doi: 10.1016/s0006-8993(98)00229-7. [DOI] [PubMed] [Google Scholar]
- 57.Schenk F, Brandner C. Indirect effects of peri- and postnatal choline treatment on place-learning abilities in rat. Psychobiology. 1995;23:302–313. [Google Scholar]
- 58.Brandner C. Perinatal choline treatment modifies the effects of a visuo-spatial attractive cue upon spatial memory in naive adult rats. Brain Res. 2002;928:85–95. doi: 10.1016/s0006-8993(01)03363-7. [DOI] [PubMed] [Google Scholar]
- 59.Ricceri L, Berger-Sweeney J. Postnatal choline supplementation in preweanling mice: sexually dimorphic behavioral and neurochemical effects. Behav Neurosci. 1998;112:1387–1392. [PubMed] [Google Scholar]
- 60.Mohler E, Meck W, Williams C. Sustained attention in adult mice is modulated by prenatal choline availability. Int J Comp Psychol. 2001;14:136–150. [Google Scholar]
- 61.Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 62.Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev Psychobiol. 1999;35:328–342. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 63.Tees RC, Mohammadi E, Adam TJ. Altering the impact of early rearing on the rat's spatial memory with pre- and postnatal choline supplementation. Soc Neurosci Abstr. 1999;17:1401. [Google Scholar]
- 64.Thomas JD, Garrison M, O'Neill TM. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Thomas JD, O'Neill TM, Dominguez HD. Perinatal choline supplementation does not mitigate motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2004;26:223–229. doi: 10.1016/j.ntt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 66.Niculescu MD, Craciunescu CN, Zeisel SH. Gene expression profiling of choline-deprived neural precursor cells isolated from mouse brain. Brain Res Mol Brain Res. 2005;134:309–322. doi: 10.1016/j.molbrainres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci. 2001;23:100–106. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- 68.Shivapurkar N, Poirier LA. Tissue levels of S-adenosylmethionine and S-adenosylhomocysteine in rats fed methyl-deficient, amino acid-defined diets for one to five weeks. Carcinogenesis. 1983;4:1051–1057. doi: 10.1093/carcin/4.8.1051. [DOI] [PubMed] [Google Scholar]
- 69.Zeisel SH, Zola T, daCosta K, Pomfret EA. Effect of choline deficiency on S-adenosylmethionine and methionine concentrations in rat liver. Biochem J. 1989;259:725–729. doi: 10.1042/bj2590725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Locker J, Reddy TV, Lombardi B. DNA methylation and hepatocarcinogenesis in rats fed a choline devoid diet. Carcinogenesis. 1986;7:1309–1312. doi: 10.1093/carcin/7.8.1309. [DOI] [PubMed] [Google Scholar]
- 71.Tsujiuchi T, Tsutsumi M, Sasaki Y, Takahama M, Konishi Y. Hypomethylation of CpG sites and c-myc gene overexpression in hepatocellular carcinomas, but not hyperplastic nodules, induced by a choline-deficient L-amino acid-defined diet in rats. Jpn J Cancer Res. 1999;90:909–913. doi: 10.1111/j.1349-7006.1999.tb00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holliday R, Grigg GW. DNA methylation and mutation. Mutat Res. 1993;285:61–67. doi: 10.1016/0027-5107(93)90052-h. [DOI] [PubMed] [Google Scholar]
- 73.Jaenisch R. DNA methylation and imprinting: why bother? Trends Genet. 1997;13:323–329. doi: 10.1016/s0168-9525(97)01180-3. [DOI] [PubMed] [Google Scholar]
- 74.Jones PA, Gonzalgo ML. Altered DNA methylation and genome instability: a new pathway to cancer? Proc Natl Acad Sci U S A. 1997;94:2103–2105. doi: 10.1073/pnas.94.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson KD, Wolffe AP. DNA methylation in health and disease. Nat Rev Genet. 2000;1:11–19. doi: 10.1038/35049533. [DOI] [PubMed] [Google Scholar]
- 76.Jeltsch A. Beyond Watson and Crick: DNA methylation and molecular enzymology of DNA methyltransferases. Chembiochem. 2002;3:382. doi: 10.1002/1439-7633(20020402)3:4<274::AID-CBIC274>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 77.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J Nutr. 2002;132:2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 78.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 80.Niculescu MD, Yamamuro Y, Zeisel SH. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J Neurochem. 2004;89:1252–1259. doi: 10.1111/j.1471-4159.2004.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christman JK, Sheikhnejad G, Dizik M, Abileah S, Wainfan E. Reversibility of changes in nucleic acid methylation and gene expression induced in rat liver by severe dietary methyl deficiency. Carcinogenesis. 1993;14:551–557. doi: 10.1093/carcin/14.4.551. [DOI] [PubMed] [Google Scholar]
- 82.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12:949–957. [PubMed] [Google Scholar]
- 83.Dani S, Hori A, Walter G, editors. Principals of Neural Aging. Elsevier; Amsterdam: 1997. [Google Scholar]
- 84.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 85.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]